Abstract
Spontaneous retroperitoneal parasitic leiomyomas are extremely rare and are mostly located in the pelvic cavity. Herein, we present a case of a small primary parasitic leiomyoma arising in the retroperitoneum. A 46-year-old Japanese woman presented with iron deficiency anemia. The patient had not undergone any abdominal surgeries. Transvaginal ultrasonography and MRI revealed multiple uterine myomas and an 18-mm, low-intensity mass adjacent to the uterus. The patient underwent a total laparoscopic hysterectomy. A solitary retroperitoneal tumor in the pouch of Douglas was resected. Histologic examination of the resected retroperitoneal tumor revealed a well-circumscribed benign leiomyoma. In this case, we suggest that retroperitoneal primary parasitic leiomyoma may have arisen independently of uterine leiomyoma. Further investigations of similar cases may fully elucidate the pathogenesis of primary retroperitoneal parasitic leiomyomas.
Keywords: laparoscopy, parasitic leiomyoma, peritoneal metaplasia, retroperitoneum, spontaneous development
Introduction
Uterine leiomyoma is the most common benign gynecological tumor, affecting 20-50% of women of reproductive age and is the leading cause of hysterectomy [1, 2]. These benign neoplasms comprise smooth muscle cells and surrounding fibrous connective tissues. In addition to traditional patterns of leiomyomatous growth in the uterus, some unusual extrauterine growth presentations have been reported in the literature [3].
Parasitic leiomyoma is defined as the ectopic implantation of a uterine leiomyoma that loses its connection with the uterus. It usually arises in the pelvic or intra-abdominal organs, including the pelvic peritoneum, abdominal wall, small intestine, colon, and omentum, receives an alternative blood supply from another source, and grows afterward [3-7]. Parasitic leiomyomas can be divided into primary, spontaneous, secondary, and iatrogenic types. Primary parasitic leiomyomas are conventionally seen as a rare subtype of pedunculated subserosal fibroids that develop into large stalks. They adhere to surrounding structures, such as the broad ligament or omentum, and develop an auxiliary blood supply. Thus, leiomyomas can grow after detaching from the uterus [3-5]. Alternatively, primary parasitic leiomyomas are known to develop de novo with metaplasia of the peritoneum [3].
Here, we report a case of a small primary parasitic leiomyoma arising in the retroperitoneum and discuss the pathogenesis of the spontaneous development of this rare tumor.
Case presentation
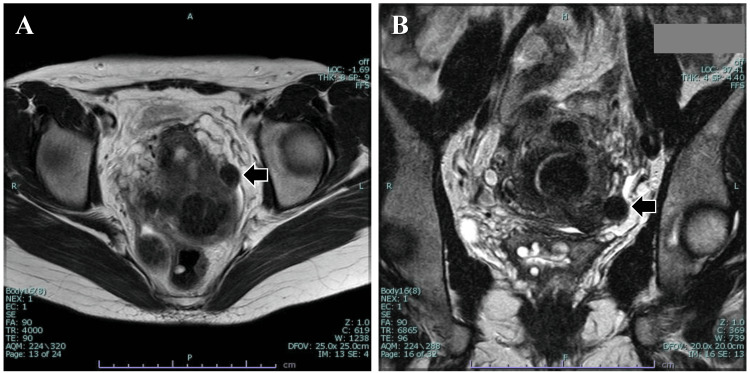
A 46-year-old Japanese woman (gravida 2, para 2) was referred to our hospital for a thorough examination of iron deficiency anemia. The patient had not undergone any abdominal surgeries. Transvaginal ultrasonography and MRI revealed multiple uterine myomas in subserous, intramural, and submucosal locations. Additionally, an 18-mm low-intensity mass adjacent to the uterus was detected (Figure 1). However, the continuity with the uterus remained unclear.
Figure 1. T2-weighted MRI images.
(A) Horizontal view. (B) Coronal view. The MRI illustrates an 18-mm low-intensity mass adjacent to the uterus (arrow). However, the continuity of the uterus is unclear.
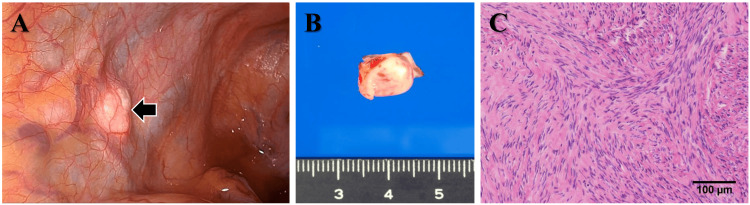
After three months of treatment with a gonadotropin-releasing hormone antagonist, the patient underwent a laparoscopic total hysterectomy. During surgery, a solitary retroperitoneal tumor was found on the left side of the pouch of Douglas (Figure 2A). There was no continuity between the retroperitoneal tumor and the uterus. A few subserous and intramural leiomyomas were observed in the uterine corpus. Both ovaries and fallopian tubes were normal. Histologic examination of the resected retroperitoneal tumor revealed a well-circumscribed benign leiomyoma (Figure 2B-2C).
Figure 2. Laparoscopic, macroscopic, and histologic findings of the retroperitoneal tumor.
(A) Laparoscopy reveals a retroperitoneal solid tumor on the left side of the pouch of Douglas (arrow). (B) The resected tumor is a white, well-circumscribed solid mass. (C) The resected tumor consists of whorled fascicles of benign smooth muscle cells separated by dense fibrovascular stroma.
The patient’s postsurgical course was unremarkable, and she showed no evidence of recurrence nine months after the laparoscopic surgery. Written informed consent was obtained from the patient.
Discussion
Lete I et al. [7] reviewed 274 patients with parasitic leiomyomas and found that 56% had no history of uterine surgery, whereas 44% had a history of hysterectomy or myomectomy. They estimated the prevalence of spontaneous and iatrogenic parasitic myomas to be 0.21% and 0.07%, respectively. The incidence of retroperitoneal parasitic leiomyoma is low. A PubMed search up to September 2011 revealed only 50 cases of retroperitoneal parasitic leiomyomas reported up to October 2008 [8-12]. Reportedly, 73% of retroperitoneal parasitic leiomyomas are located in the pelvic cavity [13].
These retroperitoneal parasitic leiomyomas may grow asymptomatically and can be diagnosed incidentally [14]. The most common symptom of a retroperitoneal parasitic leiomyoma is the palpation of a pelvic mass, which is present in almost 90% of patients [13]. Most published cases were large in size and surgically resected, with a high suspicion of malignancy or a misdiagnosis of subserous leiomyoma [15-18]. Most cases of small parasitic leiomyomas are found incidentally during other investigations or surgical procedures [19].
The pathogenesis of primary retroperitoneal parasitic leiomyoma remains controversial. The most accepted hypothesis involves the detachment of a pedunculated subserosal leiomyoma from the uterus. Alternatively, metaplasia of peritoneal mesothelial cells, retroperitoneal mesenchymal stem cells from embryonal remnants, and immature myocytes from the local vessel musculature are also considered potential sources for the development of de novo primary retroperitoneal parasitic leiomyomas [3, 17, 20]. These concepts appear to be suitable explanations for the present case because the leiomyoma was too small to have detached from the uterus.
Retroperitoneal parasitic leiomyomas are estrogen-dependent, slow-growing tumors with a low recurrence rate and are commonly reported in premenopausal women. We recommend that patients be followed up at least until menopause.
Conclusions
We report a case of a small primary parasitic leiomyoma arising in the retroperitoneum. It has been suggested that some retroperitoneal primary parasitic leiomyomas may arise independently of uterine leiomyomas. Further investigations of similar cases may fully elucidate the pathogenesis of primary retroperitoneal parasitic leiomyomas.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Kaei Nasu, Shunsuke Yasumi, Harunobu Matsumoto, Miho Sato, Eri Obata
Drafting of the manuscript: Kaei Nasu, Shunsuke Yasumi, Harunobu Matsumoto, Miho Sato, Eri Obata
Critical review of the manuscript for important intellectual content: Kaei Nasu, Harunobu Matsumoto, Miho Sato, Eri Obata
Supervision: Kaei Nasu, Harunobu Matsumoto
Acquisition, analysis, or interpretation of data: Harunobu Matsumoto, Miho Sato, Eri Obata
References
- 1.Epidemiology of myomas. Payson M, Leppert P, Segars J. Obstet Gynecol Clin North Am. 2006;33:1–11. doi: 10.1016/j.ogc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uterine fibroids. Bulun SE. N Engl J Med. 2013;369:1344–1355. doi: 10.1056/NEJMra1209993. [DOI] [PubMed] [Google Scholar]
- 3.Leiomyomas beyond the uterus: unusual locations, rare manifestations. Fasih N, Prasad Shanbhogue AK, Macdonald DB, et al. Radiographics. 2008;28:1931–1948. doi: 10.1148/rg.287085095. [DOI] [PubMed] [Google Scholar]
- 4.Parasitic myomas. Kho KA, Nezhat C. Obstet Gynecol. 2009;114:611–615. doi: 10.1097/AOG.0b013e3181b2b09a. [DOI] [PubMed] [Google Scholar]
- 5.Parasitic leiomyomas after laparoscopic surgery with morcellation. Leren V, Langebrekke A, Qvigstad E. Acta Obstet Gynecol Scand. 2012;91:1233–1236. doi: 10.1111/j.1600-0412.2012.01453.x. [DOI] [PubMed] [Google Scholar]
- 6.Uterine sarcomas and parasitic myomas after laparoscopic hysterectomy with power morcellation. Tan-Kim J, Hartzell KA, Reinsch CS, O'Day CH, Kennedy JS, Menefee SA, Harrison TA. Am J Obstet Gynecol. 2015;212:594. doi: 10.1016/j.ajog.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Parasitic leiomyomas: a systematic review. Lete I, González J, Ugarte L, Barbadillo N, Lapuente O, Álvarez-Sala J. Eur J Obstet Gynecol Reprod Biol. 2016;203:250–259. doi: 10.1016/j.ejogrb.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Three isolated, large retroperitoneal leiomyomas. Kang WD, Kim SM, Kim YH, Choi HS. Arch Gynecol Obstet. 2009;280:499–501. doi: 10.1007/s00404-009-0945-z. [DOI] [PubMed] [Google Scholar]
- 9.A rare cause of difficulty with sexual intercourse: large retroperitoneal leiomyoma. Yildiz F, Camuzcuoglu H, Toy H, Terzi A, Guldur ME. J Sex Med. 2009;6:3221–3223. doi: 10.1111/j.1743-6109.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- 10.Vaginal vault leiomyoma: 25 years after total abdominal hysterectomy. Yarci A, Bayramov V, Sükür YE, Yüce T, Berker B. J Minim Invasive Gynecol. 2010;17:116–117. doi: 10.1016/j.jmig.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Pelvic retroperitoneal leiomyoma. Lin HW, Su WC, Tsai MS, Cheong ML. Am J Surg. 2010;199:0. doi: 10.1016/j.amjsurg.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Benign metastasizing leiomyoma of external iliac vessels: an unusual case report. Scutiero G, Nappi L, Spada A, Bufo P, Greco P. Eur J Obstet Gynecol Reprod Biol. 2011;154:114–115. doi: 10.1016/j.ejogrb.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 13.Epidemiology, presentation, and management of retroperitoneal leiomyomata: systematic literature review and case report. Poliquin V, Victory R, Vilos GA. J Minim Invasive Gynecol. 2008;15:152–160. doi: 10.1016/j.jmig.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Retroperitoneal leiomyomatosis: a case report. Dursun P, Salman MC, Taskiran C, Yüce K, Ayhan A. Int J Gynecol Cancer. 2005;15:1222–1225. doi: 10.1111/j.1525-1438.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 15.Retroperitoneal parasitic leiomyoma causing unilateral ureteral obstruction. Zaitoon MM. J Urol. 1986;135:130–131. doi: 10.1016/s0022-5347(17)45543-1. [DOI] [PubMed] [Google Scholar]
- 16.Ultrasonographic and magnetic resonance imaging findings of a large asymptomatic retroperitoneal pelvic leiomyoma. Abulafia O, Sherer DM. Am J Obstet Gynecol. 1995;173:228–230. doi: 10.1016/0002-9378(95)90198-1. [DOI] [PubMed] [Google Scholar]
- 17.Leiomyoma in the space of Retzius: a report of 2 cases. Stutterecker D, Umek W, Tunn R, Sulzbacher I, Kainz C. Am J Obstet Gynecol. 2001;185:248–249. doi: 10.1067/mob.2001.114503. [DOI] [PubMed] [Google Scholar]
- 18.Giant pelvic retroperitoneal leiomyoma arising from the rectal wall. Sayer RA, Amundsen CL. Obstet Gynecol. 2003;101:1132–1134. doi: 10.1016/s0029-7844(03)00066-8. [DOI] [PubMed] [Google Scholar]
- 19.Parasitic myomas after laparoscopic surgery: an emerging complication in the use of morcellator? Description of four cases. Cucinella G, Granese R, Calagna G, Somigliana E, Perino A. Fertil Steril. 2011;96:0. doi: 10.1016/j.fertnstert.2011.05.095. [DOI] [PubMed] [Google Scholar]
- 20.Uterine smooth-muscle tumors with unusual growth patterns. Vaquero ME, Magrina JF, Leslie KO. J Minim Invasive Gynecol. 2009;16:263–268. doi: 10.1016/j.jmig.2009.01.013. [DOI] [PubMed] [Google Scholar]