Abstract
INTRODUCTION
Blood‐based biomarkers offer a promising approach for the detection of neuropathologies from repetitive head impacts (RHI). We evaluated plasma biomarkers of amyloid, tau, neurodegeneration, and inflammation in former football players.
METHODS
The sample included 180 former football players and 60 asymptomatic, unexposed male participants (aged 45–74). Plasma assays were conducted for beta‐amyloid (Aβ) 40, Aβ42, hyper‐phosphorylated tau (p‐tau) 181+231, total tau (t‐tau), neurofilament light (NfL), glial fibrillary acidic protein (GFAP), interleukin‐6 (IL‐6), Aβ42/p‐tau181 and Aβ42/Aβ40 ratios. We evaluated their ability to differentiate the groups and associations with RHI proxies and traumatic encephalopathy syndrome (TES).
RESULTS
P‐tau181 and p‐tau231(padj = 0.016) were higher and Aβ42/p‐tau181 was lower(padj = 0.004) in football players compared to controls. Discrimination accuracy for p‐tau was modest (area under the curve [AUC] = 0.742). Effects were not attributable to AD‐related pathology. Younger age of first exposure (AFE) correlated with higher NfL (padj = 0.03) and GFAP (padj = 0.033). Plasma GFAP was higher in TES‐chronic traumatic encephalopathy (TES‐CTE) Possible/Probable (padj = 0.008).
DISCUSSION
Plasma p‐tau181 and p‐tau231, GFAP, and NfL may offer some usefulness for the characterization of RHI‐related neuropathologies.
Highlights
Former football players had higher plasma p‐tau181 and p‐tau231 and lower Aβ42/ptau‐181 compared to asymptomatic, unexposed men.
Younger age of first exposure was associated with increased plasma NfL and GFAP in older but not younger participants.
Plasma GFAP was higher in participants with TES‐CTE possible/probable compared to TES‐CTE no/suggestive.
Keywords: chronic traumatic encephalopathy, college football, concussion, football, head trauma, National Football League, neurodegenerative disease, plasma biomarkers, repetitive head impacts, subconcussion, traumatic brain injury, traumatic encephalopathy syndrome
1. INTRODUCTION
Exposure to repetitive head impacts (RHI) is associated with mixed neuropathologies, including axonal and microvascular injury, neuroinflammation, white matter degeneration, and an increased burden of hyper‐phosphorylated tau (p‐tau) proteins. 1 , 2 , 3 , 4 , 5 , 6 RHI exposure is an environmental trigger for the development of the neurodegenerative tauopathy chronic traumatic encephalopathy (CTE), a link that has been studied mostly in former American football players. 5 , 6 There is indeed a dose–response relationship between exposure to RHI and CTE risk. 3 , 7 CTE is characterized by an accumulation of p‐tau proteins in neurons at the depths of the cortical sulci, clustering around small blood vessels. 5 , 8 CTE can be definitively diagnosed only through a post mortem neuropathological examination. 8 In vivo biomarkers are essential for early and accurate detection and diagnosis of neurodegenerative diseases. 9 , 10 Research diagnostic criteria have been proposed for the clinical presentation of CTE (i.e., traumatic encephalopathy syndrome, TES). 11 Validated in vivo biomarkers to detect CTE and other RHI‐related neuropathologies during life do not yet exist. 8
Neuroimaging and cerebrospinal fluid (CSF) assay analyses serve as the most investigated forms of biomarkers for CTE and RHI‐related neuropathologies. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Advancements in blood immunoassay techniques have allowed for the identification of blood‐based biomarkers that provide insight into neuropathological processes. 20 , 21 The feasibility, cost‐effectiveness, and scalability of blood‐based biomarkers make them ideal candidates for in vivo detection and monitoring of CTE and RHI‐related neuropathologies. Studies have examined acute changes in plasma biomarkers after traumatic brain injury (TBI) 22 or RHI, 23 but studies on the usefulness of plasma biomarkers to detect long‐term neuropathologies of exposure to RHI in large aging cohorts are scarce. This will be the largest study to examine a panel of plasma biomarkers in a cohort at risk for the development of CTE.
In CTE, plasma biomarkers of p‐tau may be ideal candidates for disease detection and differentiation, as assays for different epitopes now exist. Plasma p‐tau217, p‐tau181 and, to a lesser extent, p‐tau231 have been shown to have associations with Alzheimer's disease (AD) pathology. 24 , 25 , 26 There have been initial studies on plasma p‐tau epitopes in the setting of CTE. Participants with consensus‐diagnosed TES showed significantly higher levels of plasma p‐tau181 and p‐tau217 when compared to healthy controls. 27 However, the sample size was small and the effect was driven by participants who had comorbid beta‐amyloid (Aβ) pathology. 27
In addition to p‐tau, previous work found that participants exposed to RHI (n = 33) had higher levels of plasma neurofilament light (NfL, marker of axonal degeneration) and interleukin‐6 (IL‐6, marker of inflammation) compared with 59 healthy controls and 62 AD participants, respectively. 28 A case series of nine RHI‐exposed patients found higher plasma glial fibrillary acidic protein (GFAP), NfL and total tau (t‐tau) levels when compared to controls. 29 Greater exposure to RHI has been associated with increased levels of plasma t‐tau in both acute and longitudinal settings. 30 , 31 The preliminary associations between RHI and plasma NfL, IL‐6, and GFAP are concordant with the neuropathological literature that shows neuroinflammation and axonal injury in brain donors with RHI. 1 , 32 Markers of Aβ deposition, including Aβ40 and Aβ42, have been studied in AD and have become an object of interest in CTE research. 33 Despite the growing interest in plasma biomarkers, previous studies have not had sufficiently large or well‐characterized samples enriched for suspected CTE and they lacked the assessment of multiple plasma biomarkers, including different p‐tau epitopes.
The objective of this study was to evaluate the utility of plasma biomarkers of Aβ, p‐tau (181, 231), neurodegeneration (t‐tau), axonal injury (NfL), and inflammation (GFAP, IL‐6) among former American football players to examine long‐term RHI‐related neuropathological changes and the potential for detection of CTE. Our sample included participants from the Diagnostics, Imaging, and Genetics Network for the Objective Study and Evaluation of Chronic Traumatic Encephalopathy (DIAGNOSE CTE) Research Project. 34 The DIAGNOSE CTE Research Project is comprised of former college and professional American football players and similar‐aged asymptomatic men without exposure to RHI or TBI. We aimed to identify whether levels of plasma biomarkers differed between these two groups, as well as investigate whether plasma biomarkers correlate with proxies of RHI exposure, such as total years of play, position played, and age of first exposure (AFE) to football. We analyzed the association of these biomarkers to TES diagnoses.
2. METHODS
2.1. Sample
The sample included 240 males between 45 and 74 years of age who are part of the DIAGNOSE CTE Research Project. 34 The sample was comprised of 120 former professional football players, 60 former college football players, and 60 participants with no TBI or RHI exposure (i.e., asymptomatic unexposed [UE] participants). A full description of the inclusion and exclusion criteria is provided in a methodological paper 34 and the Supplementary Materials (Table S1). Former college football players must have played ≥ 6 years in organized football with ≥3 years at the college level. Former professional football players must have played ≥12 years of organized football with ≥3 seasons in college and ≥4 seasons professionally. For the former college and professional players, there were no enrollment criteria based on the presence or severity of cognitive or neuropsychiatric symptoms. A substantial number of former players reported subjective or objective cognitive symptoms, as described elsewhere. 35
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources (e.g., PubMed) as well as references of research articles. Repetitive head impacts (RHI) are the main risk factor for the later development of chronic traumatic encephalopathy (CTE), a neurodegenerative tauopathy that has been mainly studied and identified in American football players. RHI has commonly been studied in the literature using neuroimaging, such as magnetic resonance imaging (MRI) or positron emission tomography (PET). However, these methods are invasive and expensive. Blood‐based biomarkers offer a clinically promising, cost‐effective way to evaluate underlying neuropathologies of RHI, but as of yet, there have been no studies using a panel of multiple biomarkers in a large RHI‐exposed cohort. All relevant studies are appropriately cited.
Interpretation: Our findings led to an integrated hypothesis evaluating the usefulness of blood‐based biomarkers as a method to investigate RHI exposure‐related pathologies.
Future directions: Future studies are needed to expand on our knowledge of which blood‐based biomarkers most accurately reflect RHI exposure, especially as participants age and have neurological comorbidities, such as Alzheimer's disease.
Asymptomatic UE participants had no self‐reported history of exposure to RHI including participation in organized contact/collision sports, combat military service, physical violence, or other sources. In addition, the UE participants had no known TBIs, including any mild (including concussion), moderate or severe TBIs. They were required to have no history of psychiatric illness or cognitive impairment, a body mass index (BMI) greater than 24, and must have denied cognitive or neuropsychiatric symptoms at screening.
Participants were evaluated at one of four sites: Boston University Chobanian and Avedisian School of Medicine (Chobanian & Avedisian SOM) with MRI scans conducted at Brigham and Women's Hospital (BWH); Las Vegas Cleveland Clinic Lou Ruvo Center for Brain Health; Mayo Clinic Arizona with positron emission tomography (PET) scans conducted at Banner Alzheimer's Institute (BAI) in Phoenix; and New York University Langone Medical Center. Participants completed a 2‐day baseline evaluation that included neurocognitive testing, assessment of functional status, neuropsychiatric questionnaires, neurological assessments, and biomarker data collection in the form of an MRI, two types of PET scans (florbetapir amyloid PET, flortaucipir tau PET), a lumbar puncture and phlebotomy for a blood sample. Semi‐structured interviews and online questionnaires assessed demographics, as well as medical, psychiatric, and athletic history. Data on substance use and sleep were collected. Baseline data were collected between September 2016 and February 2020. All sites received approval from their Institutional Review Boards. All participants provided written informed consent and study procedures were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Diversity, equity, and inclusion (DEI) were addressed in this study through its enrollment of a diverse sample and an ongoing collaboration between the Retention Coordinator and the DEI team; areas of improvement regarding DEI are outlined in the discussion.
2.2. Plasma assays
A full description of blood processing protocols for the DIAGNOSE CTE Research Project is reported elsewhere. 34 Participants were instructed to fast for at least 12 h before blood sample collection. Study coordinators confirmed that participants had fasted before the venipuncture. A portion of whole blood was kept at room temperature and shipped to BU Chobanian & Avedisian SOM on the day of collection for DNA extraction for genetic and genomic analyses, including apolipoprotein (APOE) ε4 genotyping. All other blood was processed, aliquoted, and stored at ‐80 °C at the four participant evaluation sites within 90–120 min of collection, then batch shipped on dry ice overnight to VA Puget Sound to be stored in ‐70 °C freezers.
Plasma biomarkers were a priori selected for this study based on their application across neurodegenerative diseases: Aβ40, Aβ42, p‐tau181, p‐tau231, t‐tau, NfL, GFAP, and IL‐6. We also calculated the ratio of Aβ42/p‐tau181 and Aβ42/Aβ40. 36 These proteins were also selected based on their association with RHI‐related neuropathologies. 1 , 27 , 28 , 29 , 30 , 31 , 32 , 33 These are also inclusive of accepted biomarkers used for the detection and monitoring of AD and AD‐related diseases. 10 , 21 , 24 , 25 , 26 , 28 , 33 , 36 However, we acknowledge that this is not an exhaustive list of all plasma biomarkers.
Plasma assays for Aβ, NfL, and GFAP included the Simoa 4‐plex assay from Quanterix. Plasma assays for p‐tau proteins were measured by in‐house Simoa assays from the University of Gothenburg. 25 Plasma IL‐6 was analyzed using the V‐PLEX Proinflammatory Panel 1 from Meso Scale Diagnostics. All assays were analyzed in batches at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital in Mölndal, Sweden (University of Gothenburg).
2.3. Traumatic Encephalopathy Syndrome (TES)
TES is the research diagnostic criteria for the clinical syndrome of CTE. 11 A TES diagnosis requires the presence of substantial exposure to RHI and cognitive impairment and/or neurobehavioral dysregulation that are progressive and not otherwise accounted for by another condition. 11 Supportive features and functional status are used to determine provisional levels of certainty for CTE pathology in addition to RHI severity and the presence of core features. TES diagnoses and levels of certainty for CTE were made following a presentation of clinical, medical, and family history, as well as psychiatric and neuropsychological test data at multidisciplinary diagnostic consensus conferences. For this study, each participant was assigned a TES diagnosis (Yes/No) and level of certainty for CTE pathology, including TES‐CTE suggestive, TES‐CTE possible, and TES‐CTE probable. TES diagnoses were made without the use of biomarkers and consensus conferences integrated all other clinical, medical, and lifestyle data to guide clinical judgment of diagnoses.
2.4. Demographics, psychosocial, clinical, and athletic data
Participants completed comprehensive semi‐structured interviews providing data on demographics (e.g., age, education, racial identity); psychosocial and lifestyle history (e.g., educational attainment); medical and psychiatric history (e.g., substance use, diabetes, hypertension, sleep disorders); and athletic history (e.g., total years of football play, position played, AFE). Blood pressure measurements were obtained as was the participant's height and weight for calculation of BMI. Participants completed the World Health Organization's 10‐item alcohol use disorders identification test (AUDIT) to determine problematic alcohol use behaviors. 37 The revised Framingham Stroke Risk Profile (rFSRP) was calculated to reflect vascular and stroke risk factors in all participants. 38
2.5. Statistical analysis
All plasma biomarkers, excluding Aβ40 and Aβ42, were log‐transformed (natural log) due to non‐normal distribution of residuals. All analyses were adjusted for multiple comparisons using the Benjamini–Hochberg false discovery rate (FDR) method with significance set at an adjusted p‐value of 0.05. p‐values were corrected by the number of items per biomarker domain. All analyses controlled for the following covariates: age, race, BMI, rFSRP, and total AUDIT score. Analyses examining AFE also controlled for total years of play. Race was included as a covariate due to the association of Black racial identity with plasma biomarkers of amyloid 39 and CSF markers of tau. 13 While the reasons for the association between Black racial identity and these plasma biomarkers are unclear, it is best attributable to unmeasured social factors that we wanted to capture in the present study using race as a proxy. 35 , 40 , 41 BMI, rFSRP, and total AUDIT score were included as covariates due to their association with disease outcomes. 42 , 43 , 44 Analyses were completed using R Statistical Software (version 4.2.2).
There were minimal differences in levels of biomarkers between professional versus college level of football exposure (i.e., only t‐tau had a statistically significant but small effect). Due to the small effect sizes reported both here and in the literature, the two exposure levels were grouped for the statistical analysis. 15
The association of covariates with plasma biomarkers was analyzed using t‐tests (APOE4 genotype, race [self‐identified]) and Pearson correlation coefficient analyses for continuous variables (age, BMI, rFSRP, and total AUDIT score). The association of plasma biomarker concentrations and demographic variables was investigated in both the asymptomatic UE participants and former football player cohorts individually.
Analysis of covariance (ANCOVA) compared the concentration of each plasma biomarker between football players and asymptomatic UE participants. ANCOVA also compared the concentration of plasma biomarkers between levels of TES‐CTE certainty. To increase sample size and power, TES groups were condensed into TES‐No/TES‐CTE suggestive and TES‐CTE possible/TES‐CTE probable. There were also no significant differences in plasma biomarker concentrations between the TES‐No and TES‐CTE suggestive groups. Some significant group differences in concentrations of plasma GFAP across TES CTE levels of certainty were found, which is outlined in Table S2.
To further evaluate the diagnostic usefulness of the plasma biomarkers, we conducted binary logistic regressions and receiver operating characteristic (ROC) area under the curve (AUC) statistics to evaluate the accuracy of each biomarker in discriminating the football players from the asymptomatic UE participants. ROC and AUC analyses were also used to determine the accuracy of each biomarker in determining TES level of certainty. The AUC statistic was calculated with and without covariates included in models. For models with covariates, predicted probabilities from the binary logistic regressions were used for the AUC. The AUC, 95% confidence interval (CI), and associated p‐values are reported.
Among the former football players, nested multivariable regression models examined the associations between total years of football play and AFE with each plasma biomarker. Nested regression models included three levels: (1) demographic variables in model 1 (age, race), (2) medical variables in model 2 (BMI, rFSRP, AUDIT), and (3) RHI exposure variables in model 3 (total years of play, AFE). Models were performed consecutively and the change in R2 was examined. ANCOVA compared levels of plasma biomarkers between linemen versus non‐linemen position groups in former players. Linemen positions included offensive and defensive linemen. Non‐linemen positions included offensive backs and receivers, linebackers, defensive backs, and special teams. Participants were grouped this way because offensive and defensive linemen have been found to sustain significantly higher numbers of head impacts during football participation than non‐linemen positions. 3 , 45
As a post‐hoc analysis, models were repeated after excluding study participants who had a positive amyloid PET scan, defined by an average cortical standardized uptake value ratio (SUVR) of 1.10 or greater (centiloid values > 24.3). 46 This was done to rule out potential confounding from underlying AD.
3. RESULTS
3.1. Sample characteristics
A total of 166 football players and 51 asymptomatic UE participants were included in the analysis. Fourteen football players and five asymptomatic UE participants were excluded from the analysis due to missing plasma biomarkers and rFSRP data. Four asymptomatic UE participants included in the original baseline sample were excluded from the current analysis due to history information provided at the 4‐year remote follow‐up evaluation that contradicted their initial telephone screening and would have made them ineligible for inclusion. 34 One participant did not have a TES diagnosis and was excluded from TES analyses.
Participant characteristics by exposure group are summarized in Table 1. There were no significant differences between the exposure groups in age, education level, self‐reported race, APOE ε4 genotype, or incidence of diabetes and hypertension. The football players had a higher mean BMI and total AUDIT score than asymptomatic UE participants. Asymptomatic UE participants had a higher rFSRP mean.
TABLE 1.
Participant characteristics
| Parameter |
Football players n = 166 |
Asymptomatic UE participants n = 51 |
p‐value |
|---|---|---|---|
| Demographics | |||
| Age in years, mean (σ) | 57.5 (8.1) | 59.5 (8.5) | 0.14 |
| Formal education in years, mean (σ) | 16.8 (1.5) | 17.7 (3.5) | 0.33 |
| Education level (terminal degree n [%]) | <0.001 | ||
| High school/GED | 19 (11) | 5 (9.8) | |
| Bachelor's/Associates | 110 (66.2) | 24 (46.8) | |
| Master's or Doctorate | 37 (22.2) | 22 (43.6) | |
|
Self‐reported race Black n (%) |
56 (34) | 18 (35) | 0.84 |
| Medical history | |||
| BMI, mean (σ) | 32.4 (4.6) | 30.8 (4.8) | 0.02 |
| APOE ε4 n (%) | 50 (31) | 10 (20) | 0.16 |
| Hypertension n(%) | 70 (42) | 22 (43) | 0.90 |
| Diabetes n (%) | 12 (7.2) | 4 (7.8) | >0.99 |
| rFRSP mean (σ) | 0.03 (0.03) | 0.05 (0.04) | 0.002 |
| AUDIT mean (σ) | 5.0 (5.6) | 3.0 (3.2) | 0.008 |
| Exposure | |||
| Age at first exposure (years), mean (σ) | 11.0 (2.9) | * | * |
| Total years of play | 15.8 (4.3) | * | * |
| Position at the highest level played n (%) | |||
| Offensive lineman | 38 (23) | * | * |
| Offensive backs/receivers | 47 (28) | * | * |
| Defensive linemen | 19 (11) | * | * |
| Linebackers | 26 (16) | * | * |
| Defensive backs | 32 (19) | * | * |
| Special teams | 4 (2.4) | * | * |
| TES | |||
| TES yes n (%) | 106 (49) | * | * |
| TES level of certainty n (%) | |||
| TES no | 110 (51) | ||
| TES‐CTE suggestive | 32 (15) | ||
| TES‐CTE possible | 19 (8.8) | ||
| TES‐CTE probable | 55 (25) |
Abbreviations: %, percent; APOE, apolipoprotein E; AUDIT, alcohol use disorders identification test; BMI, body mass index; CTE, chronic traumatic encephalopathy; rFSRP, revised Framingham Stroke Risk Profile; TES, traumatic encephalopathy syndrome; UE, unexposed; σ , standard deviation.
*No data collected, no p‐values calculated.
In the full sample, 27.6% (n = 60) of participants were APOE ε4 carriers; ε4 carrier status was not significantly associated with any of the plasma biomarkers. Older age was significantly associated with higher Aβ40 (r = 0.296, padj ≤ 0.001), higher NfL (r = 0.426, padj ≤ 0.001), higher GFAP (r = 0.608, padj ≤ 0.001) higher IL‐6 (r = 0.231, padj = 0.001) and lower Aβ42/ Aβ40 ratio (r = −0.312, padj ≤ 0.001). Participants who identified as Black had significantly lower concentrations of Aβ40 (padj = 0.002) and Aβ42 (padj = 0.002), but higher Aβ42/p‐tau181 ratio (padj = 0.005) when compared to participants who did not identify as Black. Higher BMI was significantly associated with lower NfL (r = −0.308, padj ≤ 0.001) and GFAP (r = −0.305, padj ≤ 0.001). Higher rFSRP was significantly associated with higher Aβ40 (r = 0.247, padj ≤ 0.001), higher NfL (r = 0.243, padj ≤ 0.001), higher GFAP (r = 0.354, padj ≤ 0.001), higher IL‐6 (r = 0.213, padj = 0.002) and lower Aβ42/ Aβ40 ratio (r = −0.246, padj ≤ 0.001). Higher total AUDIT scores were significantly associated with lower p‐tau181 (r = −0.243, padj ≤ 0.001), lower p‐tau231 (r = −0.209, padj = 0.003), lower GFAP (r = −0.166, padj = 0.032) and higher Aβ42/p‐tau181 ratio (r = 0.174, padj = 0.022).
Concentrations of plasma biomarkers by exposure group are summarized in Table S3. Pearson correlation coefficient analysis showed that older age was associated with higher concentrations of Aβ40 (p‐value = 0.001), t‐tau (p‐value = 0.003), NfL (p‐value = 0.002), and GFAP (p‐value ≤ 0.001) in asymptomatic UE participants (Table S4a). Similarly, older age in former football players was associated with higher concentrations of Aβ40 (p‐value = 0.003), NfL (p‐value ≤ 0.001), GFAP (p‐value ≤ 0.001), and IL‐6 (p‐value = 0.001) (Table S4b). Other demographic variables also had associations with plasma biomarker levels in asymptomatic UE participants and former football players. A full summary of the results can be found in Table S4a/b.
3.2. Plasma biomarker levels in football players and asymptomatic UE participants
ANCOVA tests demonstrated that football players had significantly higher concentrations of p‐tau181 (est. marginal mean difference = −0.221, padj = 0.016), p‐tau231 (est. marginal mean difference = −0.212, padj = 0.016), and significantly lower Aβ42/p‐tau181 ratio (est. marginal mean difference = 0.545, padj = 0.004) compared to asymptomatic UE participants (Table 2 and Figure 1). There were no group differences for Aβ40 (est. marginal mean difference = 4.91, padj = 0.278), Aβ42 (est. marginal mean difference = 0.298, padj = 0.278), t‐tau (est. marginal mean difference = 0.069, padj = 0.647), NfL (est. marginal mean difference = −0.067, padj = 0.362), GFAP (est. marginal mean difference = 0.026, padj = 0.208), IL‐6 (est. marginal mean difference = 0.223, padj = 0.650), or the Aβ42/Aβ40 ratio (est. marginal mean difference = 0.001, padj = 0.582) (Figure 2).
TABLE 2.
Plasma biomarkers and exposure to RHI (football players vs. asymptomatic UE participants).
| Biomarker | Est. marginal mean diff. | padj [95% CI] |
|---|---|---|
| Amyloid | ||
| Aβ40 | 4.91 | 0.278 [−13.810, 3.985] |
| Aβ42 | 0.298 | 0.278 [−0.801, 0.205] |
| Tau | ||
| p‐tau181 | −0.221 | 0.016 [0.047, 0.395] |
| p‐tau231 | −0.212 | 0.016 [0.040, 0.383] |
| Neurodegeneration | ||
| t‐Tau | 0.069 | 0.647 [−0.364, 0.226] |
| Axonal injury | ||
| NfL | −0.067 | 0.362 [−0.067, −0.077] |
| Neuroinflammation | ||
| GFAP | 0.026 | 0.208 [0.139, 0.087] |
| IL‐6 | 0.223 | 0.650 [−0.491, 0.046] |
| Ratios | ||
| Aβ42/p‐tau181 | 0.545 | 0.004 [−0.890, −0.201] |
| Aβ42/Aβ40 | 0.001 | 0.582 [−0.006, 0.003] |
Note: A negative value indicates that the mean value of UE participants is lower than the mean value of football players.
Abbreviations: Aβ, beta‐amyloid; CI, confidence interval; Est. Marginal Mean Diff., estimated marginal mean difference, where a positive value indicates that the mean value of UE participants is higher than the mean value of football players; GFAP, glial fibrillary acidic protein; IL‐6, interleukin‐6; NfL, neurofilament light; p‐tau, hyper‐phosphorylated tau; RHI, repetitive head impacts; t‐tau, total tau; UE, unexposed.
Bolded results indicate significance.
FIGURE 1.
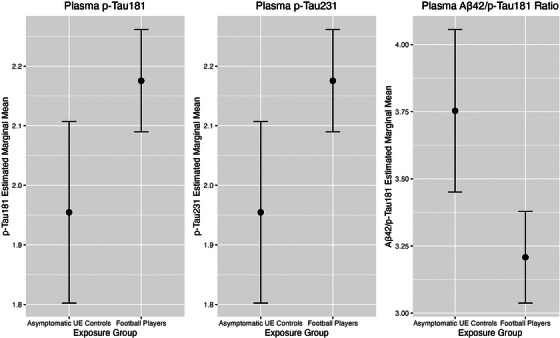
Estimated marginal means plot—pT181, pT231. ANCOVA compared concentrations of plasma biomarkers in football players and asymptomatic UE participants. Above figure shows the differences in estimated marginal means between football players and asymptomatic UE exposure groups for plasma p‐Tau181, p‐Tau231, and the Aβ42/p‐Tau181 ratio. All models adjusted for age, race, BMI, rFSRP, and total AUDIT score. Error bars at 95% CI. (a) p‐Tau181. (b) p‐Tau231. (c). Aβ42/p‐Tau181. ANCOVA, analysis of covariance; AUDIT, alcohol use disorders identification test; BMI, body mass index; CI, confidence interval; p‐tau, hyper‐phosphorylated tau; rFSRP, revised Framingham Stroke Risk Profile; UE, unexposed
FIGURE 2.
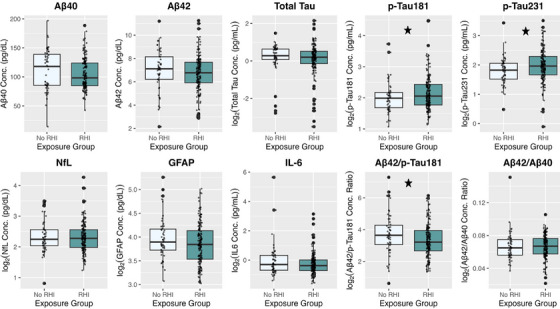
Plasma biomarker concentrations. Concentrations of plasma biomarkers after log‐transformation by ln() (if applicable) between football players (RHI) and asymptomatic UE participants (No RHI) exposure groups shown as boxplots. Individual data points are shown. Stars denote statistically significant results. Plasma biomarker concentrations are shown on the y‐axis. pg/dL, picograms per deciliter; pg/mL, picograms per milliliter, RHI, repetitive head impacts; UE, unexposed.
As shown in Figure 1, effect sizes for p‐tau181, p‐tau231, and Aβ42/p‐tau181 were small and there was observable overlap in concentration levels at the individual level between former football players and the asymptomatic UE men.
Unadjusted and adjusted binary logistic regression and ROC curve analysis evaluated the diagnostic usefulness of these plasma biomarkers (Table S5a/b and Figure 3). The unadjusted models' AUC values ranged from 0.48 to 0.61, representing poor discrimination. The adjusted model AUC ranged from 0.70 to 0.75, representing acceptable discrimination. The best performing and statistically significant biomarkers in the adjusted analysis were the Aβ42/p‐tau181 ratio (AUC = 0.75, padj = 0.008), p‐tau181 (AUC = 0.74, padj = 0.018), and p‐tau231 (AUC = 0.74, padj = 0.018). All adjusted models were significantly better than their unadjusted counterparts.
FIGURE 3.
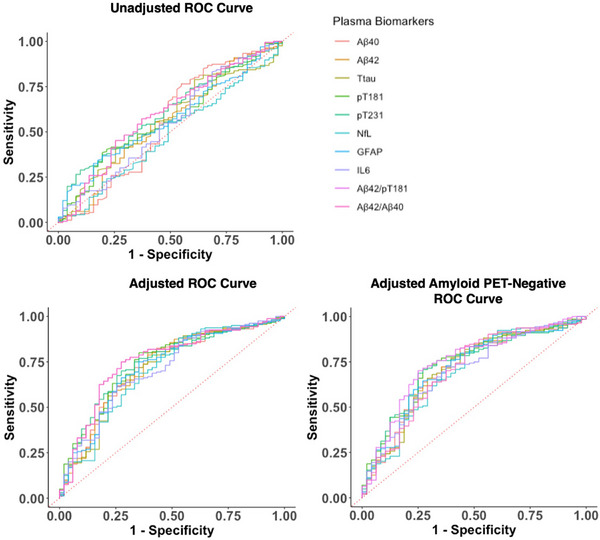
Plasma biomarker ROC curve analysis. ROC curve analysis of plasma biomarkers differentiating capability between football exposure groups (former players vs. asymptomatic UE participants). Binomial regression models were performed. Adjusted analysis included age, race, BMI, rFSRP and total AUDIT score. AUDIT, alcohol use disorders identification test; BMI, body mass index; rFSRP, revised Framingham Stroke Risk Profile; ROC, receiver operating characteristic; UE, unexposed.
A total of 10 former professional players, 7 former college players, and 3 asymptomatic UE participants had positive amyloid PET scans. Findings remained the same for both ANCOVA and ROC analyses of the association of plasma biomarkers with RHI exposure when the 20 participants with a positive amyloid PET were excluded. AUC values ranged from 0.69 to 0.74, with the best‐performing biomarkers still the Aβ42/p‐tau181 ratio (AUC = 0.74, padj = 0.008), p‐tau181 (AUC = 0.74, padj = 0.020), and p‐tau231 (AUC = 0.73, padj = 0.040). (Table S5c). AUC and ROC analyses of the association of plasma biomarkers and condensed TES levels also remained similar in the amyloid‐negative sample. AUC values ranged from 0.62 to 0.69 with the best‐performing biomarker still being GFAP (AUC = 0.69, padj = 0.040) (Table S5d).
3.3. Plasma biomarkers and TES‐CTE certainty
ANCOVA models compared TES‐No/TES‐CTE suggestive and TES‐CTE possible/probable groups on each plasma biomarker while controlling for covariates. Pairwise comparisons of estimated marginal means found significantly elevated concentrations of plasma GFAP (est. marginal mean difference = −0.157, padj = 0.008) in TES‐CTE possible/probable compared to TES‐No/TES‐CTE suggestive (Table 3 and Figure 4). Neither p‐tau181 (est. marginal mean difference = 0.081, padj = 0.553) nor p‐tau231 (est. marginal mean difference = 0.052, padj = 0.553) was found to be significantly different between TES‐CTE groups.
TABLE 3.
Pairwise comparisons of estimated marginal means for TES‐CTE certainty groups in football players
| Biomarker | Estimated marginal mean difference | 95% CI—lower | 95% CI—higher | padj |
|---|---|---|---|---|
| Amyloid | ||||
| Aβ40 | −4.75 | −3.405 | 12.905 | 0.350 |
| Aβ42 | −0.227 | −0.251 | 0.705 | 0.350 |
| Tau | ||||
| p‐tau181 | 0.081 | −0.255 | 0.093 | 0.553 |
| p‐tau231 | 0.052 | −0.226 | 0.121 | 0.553 |
| Neurodegeneration | ||||
| t‐Tau | 0.291 | −0.583 | 0.000 | 0.050 |
| Axonal injury | ||||
| NfL | −0.085 | −0.051 | 0.220 | 0.221 |
| Neuroinflammation | ||||
| GFAP | −0.157 | 0.052 | 0.261 | 0.008 |
| IL‐6 | −0.151 | −0.068 | 0.369 | 0.175 |
| Ratios | ||||
| Aβ42/p‐tau181 | −0.247 | −0.069 | 0.563 | 0.250 |
| Aβ42/Aβ40 | −0.000 | −0.004 | 0.004 | 0.870 |
Note: TES‐CTE certainty groups include TES‐No/TES‐CTE suggestive and TES‐CTE possible/TES‐CTE probable. Estimated marginal mean difference, a positive value indicates that the mean value of TES‐No/TES‐CTE suggestive is higher than the mean value of TES‐CTE possible/TES‐CTE probable players. A negative value indicates that the mean value of TES‐No/TES‐CTE suggestive is lower than the mean value of TES‐CTE possible/TES‐CTE probable players.
Abbreviations: Aβ, beta‐amyloid; CI, confidence interval; GFAP, glial fibrillary acidic protein; IL‐6, interleukin‐6; NfL, neurofilament light; p‐tau, hyper‐phosphorylated tau; t‐tau, total tau; TES‐CTE, traumatic encephalopathy syndrome‐chronic traumatic encephalopathy.
Bolded results indicate significance.
FIGURE 4.
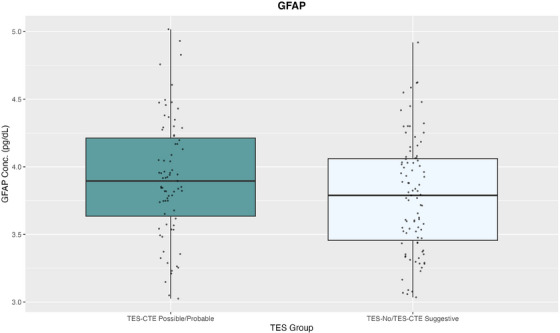
GFAP concentrations and TES‐CTE certainty. Concentrations of plasma GFAP after log‐transformation by ln() between TES‐CTE possible/probable (left) and TES‐No/TES‐CTE Suggestive (right). Individual data points are shown. Plasma GFAP concentrations are shown on the y‐axis. GFAP, glial fibrillary acidic protein; pg/dL, picograms per deciliter; TES‐CTE, traumatic encephalopathy syndrome‐chronic traumatic encephalopathy.
Unadjusted and adjusted binary logistic regression and ROC curve analysis also investigated the usefulness of plasma biomarkers in discriminating across TES level of certainty groups (Table S5e/f). The unadjusted models' AUC values ranged from 0.49 to 0.59, demonstrating poor discrimination. The adjusted model AUC ranged from 0.61 to 0.70, representing acceptable discrimination. T‐tau was significant in the unadjusted model (AUC = 0.59, padj = 0.032), but was not significant after adjustment for covariates. The only statistically significant biomarker in the adjusted analysis was GFAP (AUC = 0.70, padj = 0.008).
3.4. Associations between proxies of RHI exposure and plasma biomarkers
Nested regressions demonstrated significant associations between younger AFE and higher NfL (NfL Δ R2 = 0.033, B = −0.011, padj = 0.03) and GFAP (GFAP Δ R2 = 0.022, B = −0.008, padj = 0.033) (Figure 5). Findings remained the same when the 17 former players with a positive amyloid PET were excluded.
FIGURE 5.
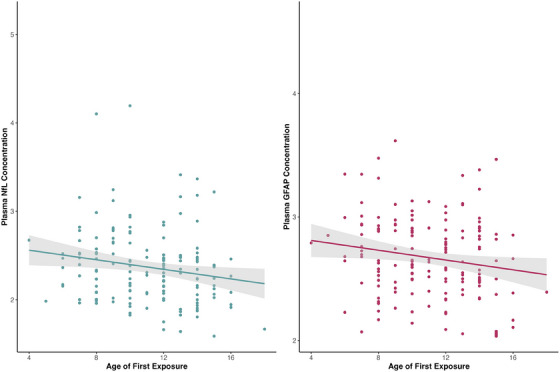
Association between plasma NfL, GFAP, and AFE. Results of regression models for starting age of football on both plasma NfL (left, blue; NfL Δ R2 = 0.033, B = −0.011, padj = 0.03) and GFAP (right, maroon; GFAP Δ R2 = 0.022, B = −0.008, padj = 0.033). These partial regression plots show the results with covariates taken into account and include a regression line with 95% CI. Covariates include age, race, BMI, rFSRP, total AUDIT score and total years of football play. AFE, age of first exposure; AUDIT, alcohol use disorders identification test; BMI, body mass index; CI, confidence interval; GFAP, glial fibrillary acidic protein; NfL, neurofilament light; rFSRP, revised Framingham Stroke Risk Profile.
Multivariable regression analysis found no significant associations between total years of play and plasma biomarkers in former football players. ANCOVA analyses found no significant differences in plasma biomarker levels between linemen versus non‐linemen position groups at the highest level of football played.
4. DISCUSSION
This study examined the usefulness of a panel of plasma biomarkers to detect RHI‐related neuropathologies in former American college and professional football players. Compared with asymptomatic UE men, the former American football players had higher levels of plasma p‐tau181 and p‐tau231 as well as a lower Aβ42/p‐tau181 ratio. The plasma p‐tau biomarkers had modest accuracy for discriminating the former football players from the asymptomatic UE men. There were no group effects for plasma Aβ40, Aβ42, t‐tau, NfL, GFAP, IL‐6, or Aβ42/Aβ40 ratio. Among the former football players, younger AFE to football was associated with higher GFAP, a measure of neuroinflammation, and NfL, a marker of axonal degeneration. Plasma GFAP was found to be increased in the TES‐CTE possible/probable group compared to the TES‐no/TES‐CTE suggestive. There were no other associations with TES‐CTE certainty diagnoses. All analyses controlled for age, race, BMI, rFSRP, and total AUDIT score. , , ,
4.1. Group differences in plasma biomarkers
Plasma biomarkers of p‐tau and the Aβ42/p‐tau181 ratio were higher and lower, respectively, in former football players compared with asymptomatic UE participants and demonstrated some discrimination accuracy when modeled with demographic, medical and genetic variables. There were no other biomarkers that demonstrated a significant difference between the two groups. Plasma p‐tau181 has been identified as a prognostic and discriminatory biomarker in AD. 47 , 48 P‐tau231 is also strongly associated with AD pathology, and has more robust associations with amyloid and tau PET biomarkers than p‐tau181 in the early stages of the disease. 25 , 49 Initial research of these p‐tau biomarkers in autopsy‐confirmed CTE found that p‐tau181 was significantly elevated in TES participants compared to healthy controls, but these findings were driven by the presence of Aβ pathology. 29 Taken together, these p‐tau biomarkers are optimal for the detection of AD neuropathological changes. Yet, we observed higher p‐tau181 and p‐tau231 biomarkers in participants who were predominantly amyloid PET‐negative, suggesting that their utility is not restricted to patients with AD. 27 Notably, the effect sizes were small with limited separation between the former football players and controls at the individual level. These results are consistent with those found in Canadian football players, where plasma p‐tau181 concentrations were higher in RHI‐exposed players than in healthy controls. 50 It is important to note that participants in this Canadian football players study, participants had an average age of 52. Tau is a phosphorylated protein that has 85 potential phosphorylation types, with 45 sites identified. 51 It is possible that other epitopes not examined here might have better specificity for the conformational changes of tau in RHI and CTE. 52 For example, post mortem neuropathological examinations of the dorsolateral frontal cortex of CTE‐ and AD‐confirmed cases found that p‐tau202 was significantly upregulated in high‐stage CTE whereas p‐tau396 was increased in AD. 53 Recent mass spectrometric methods have begun to detect novel p‐tau epitopes in plasma. 54 While p‐tau181 and p‐tau231 may be good biomarkers to help rule out or rule in the presence of other neurodegenerative diseases, like AD, investigation of alternative p‐tau epitopes will be necessary to definitively measure RHI or CTE‐specific plasma p‐tau.
There were no group differences in plasma markers of Aβ (Aβ40, 42), neurodegeneration, axonal injury, or inflammation. Previous studies have found neuroinflammation and neurodegeneration to be long‐term consequences of exposure to RHI. 1 , 18 , 55 , 56 , 57 Our null findings could be a result of the assays used and/or the sample and methods (e.g., recruitment design). The sample is relatively young and the presence and extent of the underlying disease are unknown and, if present, likely to be mild. These markers might not become elevated until later in the disease course. Consistent with the neuropathological descriptions of CTE, we would not necessarily expect changes in Aβ40 and Aβ42 as it is not a diagnostic feature of CTE. Most of the present sample is also amyloid PET‐negative. 46 However, we observed lower concentrations of Aβ40 and Aβ42 in the older former football players. A previous report of post mortem CSF found lower CSF Aβ42, but not Aβ40, in autopsy‐confirmed CTE including relative to cases with comorbid AD. 57 The authors hypothesized that changes in Aβ40 and Aβ42 in CTE might be related to impaired clearance mechanisms. This remains an area that needs further investigation.
4.2. Plasma biomarkers and TES
Plasma GFAP was higher in the TES‐CTE possible/probable group compared to TES‐No/TES‐CTE suggestive. The effect size was small. Only plasma GFAP was predictive of TES‐CTE possible/probable in an adjusted ROC/AUC analysis. The elevated plasma GFAP in the TES‐CTE possible/probable participants highlights the potential role of neuroinflammation and astrogliosis in CTE. 1 , 58 GFAP and similar markers might only be elevated in those at greatest risk for having underlying CTE pathology, potentially explaining why we did not observe an effect at the group level. Additionally, levels of serum GFAP have been found to be normal at 8‐months post TBI, but rose significantly after 5 years of follow‐up. 59 There are active biological processes related to brain injury still happening after several years; our cohort may be participating in research at the optimal time‐point to demonstrate elevated GFAP levels. Neither p‐tau181 nor p‐tau231 were associated with TES level of certainty. Interpretation of this finding is challenging given p‐tau181 and 231 might not be optimal for detection of CTE and the validity of 2021 TES research diagnostic criteria are unknown as they have yet to be compared against neuropathology. With these caveats, RHI likely leads to p‐tau and non‐p‐tau pathologies that lead to clinical symptoms. Additionally, participants in our cohort are relatively young; only 32 former football players are over the age of 65, and only 12 are over the age of 70. There may not be sufficient pathological p‐tau burden to manifest symptoms. Finally, the TES criteria were developed without biomarkers, and continued efforts to develop biomarkers will be important to improve the specificity of the criteria.
4.3. Metrics of exposure to RHI and plasma biomarkers
There were no significant associations between the plasma p‐tau biomarkers and years of American football play. This contradicts the neuropathological and tau PET literature that has found associations between years of play and markers of p‐tau. 16 The lack of associations present could be the result of p‐tau181 and p‐tau231 being better reflections of amyloid pathology than tau pathology. 60 , 61 It may also be due to the restricted range of exposure, as all participants are former elite highly exposed players. The present study did find that younger AFE was significantly associated with higher plasma NfL and GFAP. There were no significant associations between other plasma biomarkers and AFE. The literature surrounding younger AFE and later‐life neurological function is mixed. Younger AFE has not been associated with neurological outcomes in younger participants but has been in older symptomatic cohorts. 62 , 63 , 64 In older symptomatic participants, younger AFE is associated with white matter hyperintensities, smaller thalamic volumes, and white matter changes in the anterior corpus callosum on neuroimaging. 12 , 65 , 66 A working hypothesis is that AFE does not confer risk for p‐tau, but could lead to other types of pathologies (e.g., white matter injury, neuroinflammation) that decrease resiliency to neurodegenerative changes in older age.
4.4. Context of use of biomarkers in individuals with RHI
The United States Food and Drug Administration (FDA) and the National Institutes of Health (NIH) Biomarkers Working Group recognize seven contexts of use of biomarkers including risk/vulnerability, diagnosis, prognosis, pharmacodynamic, predictive, monitoring, and safety. 67 The observations from this study suggest that the p‐tau abnormalities observed may have limited diagnostic capability; however, the p‐tau and GFAP changes noted may have a role in risk assessment, prognosis, monitoring, or establishing a pharmacodynamic response to an intervention used in individuals with RHI. Discovery research is needed to establish diagnostic biomarkers for RHI or CTE within populations of persons with RHI.
4.5. Limitations
The present study is not without limitations. This was a cross‐sectional study, and longitudinal investigations on how plasma biomarker concentrations change over time would provide key insights into their diagnostic accuracy and utility. The recruitment design of this study is a limitation. Unlike the former football players, the UE participants were forced to be asymptomatic with no exposure to RHI. It is challenging to infer that any group differences observed are related to RHI exposure or other factors. In addition, the sample was made up of entirely male former American football players who were often symptomatic. This homogenous group of participants limits the generalizability of the results including to other contact and collision sport (CCS) athletes and former football players who played at lower levels. We also examined several indirect proxies of exposure to RHI including position group at the highest level of play. We did not examine positions played throughout the participant's career and the proxies examined do not fully account for the cumulative exposure to RHI of the participant, including other lifetime events, such as participation in other CCS.
This study also did not include clinicopathological correlation as there were no autopsies conducted to validate the presence of CTE or other neuropathologies. The p‐tau epitopes used demonstrated modest effects. Further evaluation of p‐tau epitopes beyond p‐tau181 and p‐tau231 is necessary to more accurately capture RHI‐related pathology, such as p‐tau217. 68 The plasma biomarkers chosen in this study were not fully inclusive of markers of other neurodegenerative diseases, such as alpha‐synuclein. 69 Future studies should incorporate a wider range of markers of neurodegenerative diseases to fully understand the incidence of disease comorbidity. Finally, plasma assays have evolved since the time of this study and thus newer generation p‐tau assays could demonstrate more promising results.
5. CONCLUSION
Plasma biomarkers of p‐tau181 and 231 epitopes, GFAP, and NfL may have usefulness for the detection and study of RHI‐related neuropathologies, but their diagnostic value remains unclear at this time. Biomarkers developed and established for AD are proving to have restricted utility in CTE and innovative fluid biomarker discovery for CTE and other RHI‐related neuropathologies, including proteomic analyses and analyses of different plasma p‐tau epitopes, are needed to improve diagnostic capabilities. Overall, blood‐based biomarkers are a promising, cost‐effective solution for in vivo monitoring of neuropathologic changes in disease.
CONFLICT OF INTEREST STATEMENT
L.J.B. is Editor‐in‐chief of the Journal of Neuro‐Ophthalmology. C.B. receives research support from the Ultimate Fighting Championship, Top Rank promotions, and Haymon Boxing. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant and on advisory boards for AC Immune, Acumen, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, and Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served on data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. E.P. has served as consultant, on scientific advisory boards, or on data monitoring committees for Eli Lilly, Avanir, Acadia, Roche, Regeneron, and ALPHA‐Cognition. EMR is a compensated scientific advisor for Alkahest, Alzheon, Aural Analytics, Denali, Green Valley, Retromer Therapeutics, and Vaxxinity, and a co‐founder of ALZPath. W.B.B. provides expert witness testimony in legal cases involving concussion and CTE. R.C.C. is a Senior Advisor to the NFL Head Neck & Spine Committee; Vice President, National Operating Committee on Standards for Athletic Equipment; and Chair, Scientific Advisory Committee, Co‐Founder, and Medical Director, Concussion Legacy Foundation. He is a member of the Medical Science Committee for the National Collegiate Athletic Association Student‐Athlete Concussion Injury Litigation, and he receives royalties for published books from Houghton Mifflin Harcourt. D.W.D. reports the following competing interests: Consulting: AEON, Amgen, Clexio, Cerecin, Cooltech, Ctrl M, Allergan, Alder, Biohaven, GSK, Linpharma, Lundbeck, Promius, Eli Lilly, eNeura, Novartis, Impel, Satsuma, Theranica, WL Gore, Nocira, XoC, Zosano, Upjohn (Division of Pfizer), Pieris, Praxis, Revance, Equinox. Honoraria: Clinical Care Solutions, CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Academy for Continued Healthcare Learning, Majallin LLC, Medlogix Communications, MJH Lifesciences, Miller Medical Communications, Southern Headache Society (MAHEC), WebMD Health/Medscape, Wolters Kluwer, Oxford University Press, Cambridge University Press. Research Support: Department of Defense, National Institutes of Health, Henry Jackson Foundation, Sperling Foundation, American Migraine Foundation, Patient Centered Outcomes Research Institute (PCORI). Stock Options/Shareholder/Patents/Board of Directors: Ctrl M (options), Aural analytics (options), ExSano (options), Palion (options), Healint (Options), Theranica (Options), Second Opinion/Mobile Health (Options), Epien (Options/Board), Nocira (options), Matterhorn (Shares/Board), Ontologics (Shares/Board), King‐Devick Technologies (Options/Board), Precon Health (Options/Board). Patent 17189376.1‐1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis. D.I.K. received royalties from Springer/Demos Publishing for a textbook on brain injury; serves as an expert witness in legal cases involving brain injury and concussion; receives a stipend from Encompass Health as program medical director for brain injury and chair of the annual Neurorehabilitation conference; and has received honoraria for a keynote address for the HealthSouth Annual Medical Directors Meeting. J.L.C. has provided consultation to Acadia, Actinogen, Acumen, AlphaCognition, Aprinoia, AriBio, Artery, Biogen, BioVie, Bristol‐Myers Squib, Cassava, Cerecin, Diadem, EIP Pharma, Eisai, GemVax, Genentech, GAP Innovations, Janssen, Jocasta, Karuna, Lighthouse, Lilly, Lundbeck, LSP/EQT, Merck, NervGen, Novo Nordisk, Oligomerix, Optoceutics, Ono, Otsuka, PRODEO, Prothena, ReMYND, Roche, Sage Therapeutics, Signant Health, Simcere, Suven, SynapseBio, TrueBinding, Vaxxinity, and Wren pharmaceutical, assessment, and investment companies. He owns the copyright of the Neuropsychiatric Inventory. E.M.R. is a compensated scientific advisor for Alkahest, Alzheon, Aural Analytics, Denali, Green Valley, Retromer Therapeutics, and Vaxxinity, and a co‐founder of ALZPath. R.A.S. is a member of the Board of Directors of King‐Devick Technologies, Inc. (Chicago, IL, USA), and he receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. (Lutz, FL, USA), and consulting fees from Eisai. M.L.A. receives royalties from Oxford University Press Inc and has received honorarium from the Michael J Fox Foundation for services unrelated to this study. He also reports research support from Life Molecular Imaging Inc and Rainwater Charitable Foundation Inc. A.E.M., J.R.G., Y.T., C.H.A., N.J.A., C.E.G., B.M., J.N.P., S.J.B., J.W., J.M., S.A., and M.E.S. have nothing to report. Author disclosures are available in the Supporting Information.
CONSENT STATEMENT
All human subjects provided the necessary informed consent to participate in this research study.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
Funding for this study came from the National Institute of Neurological Disorders and Stroke (NINDS) through a U01 Research Project Cooperative Agreement (U01NS093334). H.Z. is a Wallenberg Scholar and a Distinguished Professor at the Swedish Research Council supported by grants from the Swedish Research Council (#2023‐00356; #2022‐01018 and #2019‐02397), the European Union's Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG‐71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809‐2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF‐21‐831376‐C, #ADSF‐21‐831381‐C, #ADSF‐21‐831377‐C, and #ADSF‐24‐1284328‐C), the Bluefield Project, Cure Alzheimer's Fund, the Olav Thon Foundation, the Erling‐Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022‐0270), the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme–Neurodegenerative Disease Research (JPND2021‐00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI‐1003). K.B.is supported by the Swedish Research Council (#2017‐00915 and #2022‐00732), the Swedish Alzheimer Foundation (#AF‐930351, #AF‐939721, #AF‐968270, and #AF‐994551), Hjärnfonden, Sweden (#FO2017‐0243 and #ALZ2022‐0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF‐agreement (#ALFGBG‐715986 and #ALFGBG‐965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019‐466‐236), the Alzheimer's Association 2021 Zenith Award (ZEN‐21‐848495), the Alzheimer's Association 2022‐2025 Grant (SG‐23‐1038904 QC), La Fondation Recherche Alzheimer (FRA), Paris, France, and the Kirsten and Freddy Johansen Foundation, Copenhagen, Denmark. M.L.A. is supported by NINDS/NIA (RF1NS132290).
COLLABORATORS
1.
The DIAGNOSE CTE Research Project Current and Former Investigators and Key Personnel
Banner Alzheimer's Institute
Investigators
Kewei Chen, Ph.D.
Hillary Protas, Ph.D.
Eric Reiman, M.D. (mPI)
Yi Su, Ph.D.
Other Key Personnel
Connie Boker, M.B.A. (Director, Imaging Center Operations)
Boston University Chobanian & Avedisian School of Medicine
Investigators
Michael L. Alosco, Ph.D. Rhoda Au, Ph.D.
Robert C. Cantu, Ph.D.
Lindsay Farrer, Ph.D.
Robert Helm, M.D.*
Douglas I. Katz, M.D.
Neil Kowall, M.D.*
Ann McKee, M.D.
Jesse Mez, M.D.
Gustavo Mercier, M.D., Ph.D.*
James Otis, M.D. (now at UMass Chan Medical School‐Baystate)*
Robert A. Stern, Ph.D. (mPI)
Jason Weller, M.D.
Trainees
Tahlia Bragg, Ph.D. (Postdoctoral Fellow)
Jenna Groh (PhD Student)
Annalise Miner (PhD Student)
Diana Trujillo‐Rodriguez, B.Sc., M.Sc. (Ph.D. Student)
Suzan van Amerongen, M.D. (Research Fellow)
Boston University Project Coordinating Center Staff
Alondra Andino, B.A. (Project Administrative Manager)*
Kaleb Batten, B.A. (Administrative Coordinator)*
Shannon Conneely, B.A. (Site Coordinator)*
Courtney Diamond, M.B.A. (Project Manager)*
Tessa Fagle, B.A. (Research Assistant, Senior Follow‐Up Visit Coordinator)
Olivia Haller, B.A. (Recruitment Coordinator)*
Tennyson Hunt, M.B.A. (Project Administrative Manager)*
Nicole Gullotti, M.B.A. (Research Administrator)*
Minna Holleck, B.S. (Research Assistant)
Bailey Kossow, B.S. (Research Assistant, Follow‐Up Visit Coordinator)
Carrie Kugelmass, B.A. (Research Assistant, Follow‐Up Visit Coordinator)
Megan Mariani, B.S., B.A. (Project Manager)*
Brian Mayville, B.S. (Site Coordinator)*
Kathleen McLaughlin, B.A. (Research Assistant)*
Mary Nanna, B.A. (Retention Coordinator)*
Marty DiPopolo, B.S. (Retention Coordinator)*
Taylor Platt, M.P.H. (Recruitment Coordinator)*
Surya Pulukuri, B.A. (Research Assistant)*
Fiona Rice, M.P.H. (Project Manager)*
Madison Sestak, B.S. (Assistant Recruitment Coordinator)*
Irene Simkin, M.S. (Lab Manager, Molecular Genetics Core Facility)
Boston University School of Public Health
Investigators
Michael McClean, Sc.D.
Yorghos Tripodis, Ph.D.
Data Team Staff
Douglas Annis, M.S. (Systems Analyst)*
Christine Chaisson, M.P.H. (Leader of Data Management Sub‐team)*
Diane B. Dixon (Project Manager)
Carolyn Finney, B.A. (Data Manager)
Kerrin Gallagher, M.P.H. (Statistical Analyst)*
Kaitlin Hartlage, M.P.H. (Statistical Analyst)
Jun Lu, M.S. (Data Security and Technology Analyst)
Brett Martin, M.S. (Statistical Manager) Emmanuel Ojo, M.P.H. (Statistical Analyst)*
Joseph N. Palmisano, M.A., M.P.H. (Leader of Data Management Sub‐team)
Brittany Pine, B.A., B.S. (Statistical Analyst)
Janani Ramachandran, M.S. (Data Manager)*
Greta Schneider (Project Manager)
Trainees
Zachary Baucom, Ph.D. (now at BENlabs)
Fatima Tuz‐Zahra, M.S.
Eukyung Yhang, B.A.
Brigham and Women's Hospital
Investigators
Sylvain Bouix, Ph.D.
Jennifer Fitzsimmons, M.D.*
Alexander P. Lin, Ph.D.
Inga K. Koerte, M.D., Ph.D.
Ofer Pasternak, Ph.D.
Martha E. Shenton, Ph.D. (mPI)
Other Key Personnel
Tashrif Billah, M.S. (Software Engineer)
Holly Carrington, B.A. (Research Assistant)
Michael J. Coleman, M.A. (Senior Scientist)
Omar John, B.S. (Research Assistant)
Huijun Liao, B.S. (Study Coordinator)*
Maria Loy, M.B.A., M.P.H. (Senior Program Coordinator)
Elizabeth Rizzoni, B.A. (Research Assistant)*
Annelise Silva, B.S. (Research Assistant)*
Brynn Vessey, B.S. (Research Assistant)*
Trainees
Elena Bonke, M.S. (Ph.D. Student)
Katherine Breedlove, Ph.D. (Postdoctoral Research Fellow)
Eduardo Coello, Ph.D. (Postdoctoral Research Fellow)
Leonard Jung, (Ph.D. Student)
Vivian Schultz, M.D. (Postdoctoral Research Fellow)*
Tim L.T. Wiegand, (Ph.D. Student)
Cleveland Clinic Lou Ruvo Center for Brain Health
Investigators
Sarah Banks, Ph.D. (Now at University of California, San Diego)
Charles Bernick, M.D.
Jason Miller, Ph.D.
Aaron Ritter, M.D.* (Now at Hoag's Pickup Family Neurosciences Institute)
Marwan Sabbagh, M.D.* (Now at Barrow Institute)
Other Key Personnel
Raelynn de la Cruz, (Psychometrician)*
Jan Durant, (Psychometrician)*
Morgan Golceker (Site Coordinator)*
Nicolette Harmon, (Site Coordinator)*
Jaeson Kaylegian, (Psychometrician)*
Rachelle Long, (Site Coordinator)*
Christin Nance, (Psychometrician)*
Priscilla Sandoval (Site Coordinator)*
Miranda Staples, Ph.D. (Program Manager)*
George Washington University School of Medicine and Health Sciences
Investigator
Robert W. Turner, Ph.D. (DEI Team Leader)
Other Key Personnel
Emma F. Clark, B.A. (Research Assistant)*
Invicro (formerly Molecular NeuroImaging)
Investigator
Kenneth L. Marek, M.D.
Other Key Personnel
Andrew Serrano, M.B.A.
Mayo Clinic Arizona
Investigators
Charles H. Adler, M.D., Ph.D.,
David W. Dodick, M.D. (Now at Atria Academy of Science and Medicine) Yonas Geda, M.D., M.Sc. (Now at Barrow Neurological Institute)
Jennifer V. Wethe, Ph.D.
Other Key Personnel
Amy Duffy, (Site Coordinator)*
Bryce Falk, R.N.*
Marci Howard, (Psychometrician)*
Michelle Montague, (Psychometrician)*
Thomas Osgood, (Site Coordinator)*
National Institute of Neurological Disorders and Stroke (NINDS)
Debra Babcock, M.D., Ph.D. (Scientific Program Official)
Nsini Umoh, Ph.D. (TBI Program Director)
Patrick Bellgowan, Ph.D. (Administrative Program Official)*
New York University
Investigators
Hector Arciniega, Ph.D.
Laura Balcer, M.D., M.S.C.E.
William Barr, Ph.D.
Judith Goldberg, Sc.D.
Binu Joseph, M.B.B.S.
Ivan Kirov, Ph.D.
Yvonne Lui, M.D.
Charles Marmar, M.D.*
Thomas Wisniewski, M.D.*
Other Key Personnel
Alhassan Al‐Kharafi (Psychometrician)*
Allan George (Psychometrician)*
Lisena Hasanaj (Site Coordinator)*
Sammie Martin (Psychometrician)*
Edward Riley (Psychometrician)*
William Runge (Psychometrician)*
Liliana Serrano*
University of Gothenburg, Sweden
Nicholas Ashton, Ph.D.
Henrik Zetterberg, M.D., Ph.D.
Kaj Blennow, M.D., Ph.D.
University of Nevada, Las Vegas
Jeffrey L. Cummings, M.D., ScD (mPI)
Trainee
Grace Goodwin (PhD Student)
University of Washington and VA Puget Sound
Investigator
Jeffrey Iliff, Ph.D.
Gail Li, M.D., Ph.D.
Deidre Janssen, Ph.D.
James Meabon, Ph.D.
Elaine R. Peskind, M.D.
Juan Piantino, M.D. (at Oregon Health and Science University)
Abigail Schindler, Ph.D.
Ronald Thomas, Ph.D
Other Key Personnel
Elizabeth Colasurdo (Lab Manager)
Jane Shofer, M.S.
Washington University (CNDA)
Investigators
Daniel S. Marcus, Ph.D.
Other Key Personnel
Jenny Gurney, M.S.
Consultants
Richard Greenwald, Ph.D. (Simbex)*
Keith A. Johnson, M.D. (Massachusetts General Hospital)
*No longer involved in the project.
Miner AE, Groh JR, Tripodis Y, et al. Examination of plasma biomarkers of amyloid, tau, neurodegeneration, and neuroinflammation in former elite American football players. Alzheimer's Dement. 2024;20:7529–7546. 10.1002/alz.14231
REFERENCES
- 1. Cherry JD, Tripodis Y, Alvarez VE, et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun. 2016;4(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saltiel N, Tripodis Y, Menzin T, et al. Relative contributions of mixed pathologies to cognitive and functional symptoms in brain donors exposed to repetitive head impacts. Ann Neurol. 2024;95(2):314‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daneshvar DH, Nair ES, Baucom ZH, et al. Leveraging football accelerometer data to quantify associations between repetitive head impacts and chronic traumatic encephalopathy in males. Nat Commun. 2023;14(1):3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alosco ML, Stein TD, Tripodis Y, et al. Association of white matter rarefaction, arteriolosclerosis, and tau with dementia in chronic traumatic encephalopathy. JAMA Neurol. 2019;76(11):1298‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKee AC, Stein TD, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McKee AC, Stein TD, Huber BR, et al. Chronic traumatic encephalopathy (CTE): criteria for neuropathological diagnosis and relationship to repetitive head impacts. Acta Neuropathol. 2023;145(4):371‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mez J, Daneshvar DH, Abdolmohammadi B, et al. Duration of American football play and chronic traumatic encephalopathy. Ann Neurol. 2020;87(1):116‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Awasthi S, Spellman DS, Hatcher NG. Proteomic discovery and validation of novel fluid biomarkers for improved patient selection and prediction of clinical outcomes in Alzheimer's disease patient cohorts. Proteomes. 2022;10(3):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katz DI, Bernick C, Dodick DW, et al. National Institute of Neurological Disorders and stroke consensus diagnostic criteria for traumatic encephalopathy syndrome. Neurology. 2021;96(18):848‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alosco ML, Tripodis Y, Baucom ZH, et al. White matter hyperintensities in former American football players. Alzheimers Dement. 2023;19(4):1260‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alosco ML, Tripodis Y, Fritts NG, et al. Cerebrospinal fluid tau, Aβ, and sTREM2 in former National Football League Players: modeling the relationship between repetitive head impacts, microglial activation, and neurodegeneration. Alzheimers Dement. 2018;14(9):1159‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alosco ML, Tripodis Y, Koerte IK, et al. Interactive effects of racial identity and repetitive head impacts on cognitive function, structural MRI‐derived volumetric measures, and cerebrospinal fluid tau and Aβ. Front Hum Neurosci. 2019;13:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ly MT, Tuz‐Zahra F, Tripodis Y, et al. Association of vascular risk factors and CSF and imaging biomarkers with white matter hyperintensities in former American football players. Neurology. 2024;102(2):e208030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alosco ML, Su Y, Stein TD, et al. Associations between near end‐of‐life flortaucipir PET and postmortem CTE‐related tau neuropathology in six former American football players. Eur J Nucl Med Mol Imaging. 2023;50(2):435‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mantyh WG, Spina S, Lee A, et al. Tau positron emission tomographic findings in a former US football player with pathologically confirmed chronic traumatic encephalopathy. JAMA Neurol. 2020;77(4):517‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alosco ML, Mian AZ, Buch K, et al. Structural MRI profiles and tau correlates of atrophy in autopsy‐confirmed CTE. Alzheimers Res Ther. 2021;13(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stern RA, Adler CH, Chen K, et al. Tau positron‐emission tomography in former National Football League players. N Engl J Med. 2019;380(18):1716‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simrén J, Elmgren A, Blennow K, Zetterberg H. Fluid biomarkers in Alzheimer's disease. Adv Clin Chem. 2023;112:249‐281. [DOI] [PubMed] [Google Scholar]
- 21. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood‐based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66‐77. [DOI] [PubMed] [Google Scholar]
- 22. Shahim P, Politis A, van der Merwe A, et al. Time course and diagnostic utility of NfL, tau, GFAP, and UCH‐L1 in subacute and chronic TBI. Neurology. 2020;95(6):e623‐e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shahim P, Zetterberg H, Simrén J, et al. Association of plasma biomarker levels with their CSF concentration and the number and severity of concussions in professional athletes. Neurology. 2022;99(4):e347‐e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coomans EM, Verberk IMW, Ossenkoppele R, et al. A head‐to‐head comparison between plasma pTau181 and tau PET along the Alzheimer's disease continuum. J Nucl Med. 2023;64(3):437‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ashton NJ, Pascoal TA, Karikari TK, et al. Plasma p‐tau231: a new biomarker for incipient Alzheimer's disease pathology. Acta Neuropathol. 2021;141(5):709‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho‐tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asken BM, Tanner JA, VandeVrede L, et al. Plasma P‐tau181 and P‐tau217 in patients with traumatic encephalopathy syndrome with and without evidence of Alzheimer disease pathology. Neurology. 2022;99(6):e594‐e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asken BM, Tanner JA, Gaynor LS, et al. Alzheimer's pathology is associated with altered cognition, brain volume, and plasma biomarker patterns in traumatic encephalopathy syndrome. Alzheimers Res Ther. 2023;15(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asken BM, Tanner JA, VandeVrede L, et al. Multi‐modal biomarkers of repetitive head impacts and traumatic encephalopathy syndrome: a clinicopathological case series. J Neurotrauma. 2022;39(17‐18):1195‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neselius S, Zetterberg H, Blennow K, et al. Olympic boxing is associated with elevated levels of the neuronal protein tau in plasma. Brain Inj. 2013;27(4):425‐433. [DOI] [PubMed] [Google Scholar]
- 31. Alosco ML, Tripodis Y, Jarnagin J, et al. Repetitive head impact exposure and later‐life plasma total tau in former National Football League players. Alzheimers Dement. 2016;7:33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holleran L, Kim JH, Gangolli M, et al. Axonal disruption in white matter underlying cortical sulcus tau pathology in chronic traumatic encephalopathy. Acta Neuropathol. 2017;133(3):367‐380. [DOI] [PubMed] [Google Scholar]
- 33. Stein TD, Montenigro PH, Alvarez VE, et al. Beta‐amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015;130(1):21‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alosco ML, Mariani ML, Adler CH, et al. Developing methods to detect and diagnose chronic traumatic encephalopathy during life: rationale, design, and methodology for the DIAGNOSE CTE research project. Alzheimers Res Ther. 2021;13(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alosco ML, Barr WB, Banks SJ, et al. Neuropsychological test performance of former American football players. Alzheimers Res Ther. 2023;15(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martínez‐Dubarbie F, Guerra‐Ruiz A, López‐García S, et al. Accuracy of plasma Aβ40, Aβ42, and p‐tau181 to detect CSF Alzheimer's pathological changes in cognitively unimpaired subjects using the Lumipulse automated platform. Alzheimers Res Ther. 2023;15:163. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10544460/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Babor TF, Robaina K. The alcohol use disorders identification test (AUDIT): a review of graded severity algorithms and national adaptations. Int J Alcohol Drug Res. 2016;5(2):17‐24. [Google Scholar]
- 38. Dufouil C, Beiser A, McLure LA, et al. Revised Framingham stroke risk profile to reflect temporal trends. Circulation. 2017;135(12):1145‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schindler SE, Karikari TK, Ashton NJ, et al. Effect of race on prediction of brain amyloidosis by plasma Aβ42/Aβ40, phosphorylated tau, and neurofilament light. Neurology. 2022;99(3):e245‐e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393‐e423. [DOI] [PubMed] [Google Scholar]
- 41. Zahodne LB, Manly JJ, Smith J, Seeman T, Lachman ME. Socioeconomic, health, and psychosocial mediators of racial disparities in cognition in early, middle, and late adulthood. Psychol Aging. 2017;32(2):118‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pelcher I, Puzo C, Tripodis Y, et al. Revised Framingham stroke risk profile: association with cognitive status and MRI‐derived volumetric measures. J Alzheimers Dis. 2020;78(4):1393‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qu Y, Hu HY, Ou YN, et al. Association of body mass index with risk of cognitive impairment and dementia: a systematic review and meta‐analysis of prospective studies. Neurosci Biobehav Rev. 2020;115:189‐198. [DOI] [PubMed] [Google Scholar]
- 44. Heymann D, Stern Y, Cosentino S, Tatarina‐Nulman O, Dorrejo JN, Gu Y. The association between alcohol use and the progression of Alzheimer's disease. Curr Alzheimer Res. 2016;13(12):1356‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baugh CM, Kiernan PT, Kroshus E, et al. Frequency of head‐impact—related outcomes by position in NCAA division I collegiate football players. J Neurotrauma. 2015;32(5):314‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stern RA, Trujillo‐Rodriguez D, Tripodis Y, et al. Amyloid PET across the cognitive spectrum in former professional and college American football players: findings from the DIAGNOSE CTE research project. Alzheimers Res Ther. 2023;15(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baiardi S, Quadalti C, Mammana A, et al. Diagnostic value of plasma p‐tau181, NfL, and GFAP in a clinical setting cohort of prevalent neurodegenerative dementias. Alzheimers Res Ther. 2022;14(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moscoso A, Grothe MJ, Ashton NJ, et al. Longitudinal associations of blood phosphorylated tau181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurol. 2021;78(4):396‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meyer PF, Ashton NJ, Karikari TK, et al. Plasma p‐tau231, p‐tau181, PET biomarkers, and cognitive change in older adults. Ann Neurol. 2022;91(4):548‐560. [DOI] [PubMed] [Google Scholar]
- 50. Vasilevskaya A, Taghdiri F, Multani N, et al. Investigating the use of plasma pTau181 in retired contact sports athletes. J Neurol. 2022;269(10):5582‐5595. [DOI] [PubMed] [Google Scholar]
- 51. Noble W, Hanger DP, Miller CCJ, Lovestone S. The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol. 2013;4:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Falcon B, Zivanov J, Zhang W, et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019;568(7752):420‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stathas S, Alvarez VE, Xia W, et al. Tau phosphorylation sites serine202 and serine396 are differently altered in chronic traumatic encephalopathy and Alzheimer's disease. Alzheimers Dement. 2022;18(8):1511‐1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Montoliu‐Gaya L, Benedet AL, Tissot C, et al. Mass spectrometric simultaneous quantification of tau species in plasma shows differential associations with amyloid and tau pathologies. Nat Aging. 2023;3(6):661‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bernick C, Shan G, Ritter A, et al. Blood biomarkers and neurodegeneration in individuals exposed to repetitive head impacts. Alzheimers Res Ther. 2023;15(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Amerongen S, Pulukuri SV, Tuz‐Zahra F, et al. Inflammatory biomarkers for neurobehavioral dysregulation in former American football players: findings from the DIAGNOSE CTE research project. J Neuroinflammation. 2024;21:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Turk KW, Geada A, Alvarez VE, et al. A comparison between tau and amyloid‐β cerebrospinal fluid biomarkers in chronic traumatic encephalopathy and Alzheimer disease. Alzheimers Res Ther. 2022;14(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Babcock KJ, Abdolmohammadi B, Kiernan PT, et al. Interface astrogliosis in contact sport head impacts and military blast exposure. Acta Neuropathol Commun. 2022;10(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Newcombe VFJ, Ashton NJ, Posti JP, et al. Post‐acute blood biomarkers and disease progression in traumatic brain injury. Brain. 2022;145(6):2064‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Salvadó G, Ossenkoppele R, Ashton NJ, et al. Specific associations between plasma biomarkers and postmortem amyloid plaque and tau tangle loads. EMBO Mol Med. 2023;15(5):e17123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Therriault J, Vermeiren M, Servaes S, et al. Association of phosphorylated tau biomarkers with amyloid positron emission tomography vs tau positron emission tomography. JAMA Neurol. 2023;80(2):188‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Iverson GL, Terry DP, Caccese JB, Büttner F, Merz ZC. Age of first exposure to football is not associated with midlife brain health problems. J Neurotrauma. 2021;38(5):538‐545. [DOI] [PubMed] [Google Scholar]
- 63. Lempke LB, Walton SR, Brett BL, et al. Relating American football age of first exposure to patient‐reported outcomes and medical diagnoses among former National Football League Players: an NFL‐LONG study. Sports Med. 2023;53(5):1073‐1084. [DOI] [PubMed] [Google Scholar]
- 64. Alosco ML, Mez J, Tripodis Y, et al. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol. 2018;83(5):886‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schultz V, Stern RA, Tripodis Y, et al. Age at first exposure to repetitive head impacts is associated with smaller thalamic volumes in former professional American football players. J Neurotrauma. 2018;35(2):278‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stamm JM, Koerte IK, Muehlmann M, et al. Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J Neurotrauma. 2015;32(22):1768‐1776. doi: 10.1089/neu.2014.3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Califf RM. Biomarker definitions and their applications. Exp Biol Med. 2018;243(3):213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ashton NJ, Brum WS, Di Molfetta G, et al. Diagnostic accuracy of a plasma phosphorylated tau 217 immunoassay for Alzheimer disease pathology. JAMA Neurol. 2024;81:e235319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bruce HJ, Tripodis Y, McClean M, et al. American football play and Parkinson disease among men. JAMA Netw Open. 2023;6(8):e2328644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


