Abstract
The Alzheimer's Disease Neuroimaging Initiative (ADNI) has fostered collaboration among researchers around the world, catalyzing innovation and accelerating progress in the field. In Latin America, this initiative advanced the validation and development of Alzheimer's disease biomarkers for the first time in our region. In 2011, as part of the international ADNI, Argentina‐ADNI (Arg‐ADNI) was founded. The following years were characterized by strong support from entities such as the Alzheimer's Association, transforming into the emergence of several multinational studies focusing on prevention and diagnosis, and treatment of dementias. These studies are heirs to the tradition of Arg‐ADNI: the Dominantly Inherited Alzheimer Network, Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline, Longitudinal Early‐Onset Alzheimer's Disease Study, and Initiative for the Study of Down Syndrome and Alzheimer's Disease. These initiatives have contributed significantly to the development of regional research and serve as essential tools for health policy planning in Latin America.
Highlights
In Latin America, the Alzheimer's Disease Neuroimaging Initiative (ADNI) advanced the validation and development of Alzheimer's disease biomarkers for the first time in our region.
ADNI has been the basis that boosted several multinational studies focusing on both prevention and diagnosis, and treatment of dementias (Dominantly Inherited Alzheimer Network, LatAm‐FINGER, LEADS).
These initiatives with the support from the Alzheimer's Association have contributed significantly to the development of regional research
This is an example of collaboration between high‐income countries with low‐ and middle‐income countries aimed at global scientific development and inclusion.
Keywords: Alzheimer's disease, biomarkers, dementia
1. INTRODUCTION
Alzheimer's disease (AD) is the leading cause of dementia worldwide. It results in disability in the functional lives of patients and caregiver/family exhaustion. According to the World Health Organization, there are currently over 55 million dementia patients worldwide, with an estimated 78 million by the year 2030 and 139 million by 2050. 1 This will lead to a socioeconomic impact on global health systems. Consequently, in recent decades, there has been significant international interest from various organizations in implementing different policies to address this scenario. Among these initiatives, we can highlight the valuable contribution made by the Alzheimer's Disease Neuroimaging Initiative (ADNI) in the study of patients with AD.
ADNI is a pivotal longitudinal multicenter study aimed at advancing the understanding, detection, and tracking of AD. Launched over two decades ago, this pioneering public‐private partnership has significantly influenced AD research by facilitating the development and dissemination of clinical, imaging, genetic, and biochemical biomarkers for early detection and monitoring of AD progression. Through robust data‐sharing mechanisms, ADNI has fostered collaboration among researchers worldwide, catalyzing innovation and accelerating progress in the field. In Latin America, this initiative advanced the validation and development of AD biomarkers for the first time in the region, while establishing the groundwork for conducting other studies with similar structures (such as the Dominantly Inherited Alzheimer Network [DIAN], Longitudinal Early‐Onset Alzheimer's Disease Study [LEADS], Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability [FINGERS], etc.).
2. ARGENTINA‐ALZHEIMER'S DISEASE NEUROIMAGING INITIATIVE
Fleni (www.fleni.org.ar), one of the biggest neurological institutes in South America, was founded in 1959 in Buenos Aires, Argentina. From its origins, the Department of Cognitive Neurology, Neuropsychology, and Neuropsychiatry has developed as one of the leaders in dementia in Argentina. Based on resources available (positron emission tomography [PET] scan with cyclotron, brain magnetic resonance imaging [MRI]‐3T, molecular biology laboratory), in 2011 Fleni was included as the first ADNI site in Latin America (Argentina‐ADNI [Arg‐ADNI)) with the financial support of local funding granted by the Fleni Foundation. Arg‐ADNI is overseen by a management committee. The structural organization of Arg‐ADNI is comprised of five streams of research (Figure 1), each of which is directed by a stream leadership group. 2
FIGURE 1.
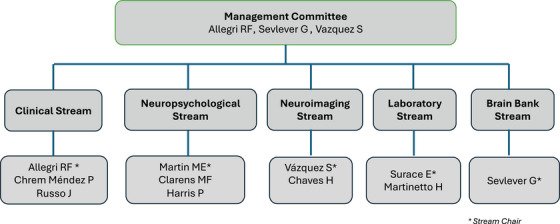
Structural organization of Arg‐ADNI. Arg‐ADNI, Argentina–Alzheimer's Disease Neuroimaging Initiative.
2.1. Arg‐ADNI's contributions to AD research
Summarizing Arg‐ADNI's contribution to the study of AD in the region proves challenging. Nonetheless, below we outline several significant aspects that have had and continue to have an impact on patient research.
Arg‐ADNI involved voluntary participation from community members who were attracted through various forms of advertising, including scientific forums, video interviews, and media press releases, among others. Patient assessment took place from 2012 to 2019. Each patient underwent medical monitoring for a duration of 60 months, involving a total of 4 medical visits. At baseline, a total of 56 participants were included. All underwent comprehensive clinical neurological and neuropsychological assessments, neuroimaging studies (MRI and PET scan), laboratory assessments, and cerebrospinal fluid (CSF) analysis including genetic evaluation.
RESEARCH IN CONTEXT
Systematic review: we describe the regional development of the Alzheimer's Disease Neuroimaging Initiative and other multinational network in dementia research.
Interpretation: These initiatives with the support from the Alzheimer's Association have contributed significantly to the development of regional research and serve as essential tools for health policy planning in Latin America.
Future directions: This model of collaboration between high‐income countries with low‐ and middle‐income countries must be replicate in other region of the world.
Based on the aforementioned criteria, the sample was divided into four groups: (1) normal controls (NC), (2) early mild cognitive impairment (MCI), (3) late MCI, and (4) AD dementia. 2 Table 1 summarizes the main demographic characteristics of the included patients.
TABLE 1.
Demographics and clinical characteristics of the participants.
| Feature |
HC n = 15 |
MCI n = 28 |
AD n = 13 |
F/χ2 | p‐value | p < 0.05* |
|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 64.9 ± 8.1 | 70.1 ± 6.8 | 73.1 ± 5.5 | 5.1 | 0.01 | b |
| Gender, men/women | 5/10 | 13/15 | 6/7 | 0.76 | 0.68 | |
| Education, years (mean ± SD) | 14.5 ± 3.4 | 13.1 ± 4.4 | 12.2 ± 3.9 | 1.26 | 0.29 | |
| Symptoms onset, years (mean ± SD) | NA | 2.9 ± 2.7 | 3.2 ± 3.7 | 0.08 | 0.78 | |
| MMSE (mean ± SD) | 29.2 ± 1.5 | 28.6 ± 1.3 | 22.0 ± 3.2 | 60.16 | < 0.001 | b, c |
| GDS‐15 (mean ± SD) | 1.7 ± 1.5 | 2.4 ± 2.3 | 1.5 ± 1.1 | 1.35 | 0.27 | |
| CDR (mean ± SD) | 0.0 ± 0.1 | 0.5 ± 0.0 | 0.7 ± 0.3 | 89.72 | <0.001 | a, b, c |
| Hachinski (mean ± SD) | 0.3 ± 0.8 | 0.2 ± 0.5 | 0.3 ± 0.5 | 0.37 | 0.69 | |
| Pharmacological Treatment | ||||||
| Total number, (mean ± SD) | 2.3 ± 2 | 4 ± 1.6 | 4.1 ± 1.6 | 5.85 | 0.01 | a, b |
| Acetylcholinesterase inhibitor, n | 0 | 13 | 10 | 17.69 | <0.001 | a, b |
| Memantine, n | 0 | 1 | 2 | 3.6 | 0.17 | |
| Antidepressants, n | 1 | 10 | 4 | 3.6 | 0.17 | |
| Antipsychotics, n | 0 | 2 | 5 | 2.98 | 0.23 | |
| APOE ε4 Carriers, % | 38.5 | 36.8 | 44.4 | 0.15 | 0.93 |
Abbreviations: AD, Alzheimer´s dementia; APOE ε4, apolipoprotein E gene ε4 allele; CDR, Clinical Dementia Rating; GDS, Geriatric Depression Scale; HC, healthy controls; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; SD, standard deviation.
Multiple comparisons: a = HC are different from AD; b = MCI are different from AD; c = HC are different from MCI.
Neurocognitive assessment is a fundamental tool in evaluating patients with cognitive impairment. There are countless cognitive batteries available for this purpose, varying in sensitivity, specificity, and language. Therefore, it was necessary to validate internationally used neurocognitive assessment batteries in our region to evaluate Arg‐ADNI patients. The Everyday Cognition (ECog) scale is utilized to assess cognitive functions and functionality in patients. Russo et al. validated this scale for Arg‐ADNI to differentiate between NC, MCI, and mild dementia due to AD. 3 ECog proved to be an effective tool for this purpose, even demonstrating superiority in evaluating functional changes in MCI patients compared to the Functional Assessment Questionnaire (FAQ) scale. Clarens et al. further illustrate the utility of neuropsychological tests in assessing patients with cognitive impairment, particularly in distinguishing patients with AD. 4 Cognitive assessments of patients with amyloid‐positive MCI (presence of amyloid in PET‐11C Pittsburgh Compound B [PiB] scan or low levels of amyloid beta [Aβ]42 in CSF) and amyloid‐negative MCI from the Arg‐ADNI cohort were compared. The delayed recall of the Rey Auditory Verbal Learning Test (RAVLT) emerged as the strongest predictor of amyloid presence, followed by total learning RAVLT and recognition RAVLT. These findings provide valuable insights for clinical decision‐making.
Not only was the regional validation of these cognitive tests important, but it also allowed us to subsequently observe the longitudinal progression of our cohort over time. Chrem‐Mendez et al. published the results of changes in the neuropsychological assessment of the participants at the 1‐year follow‐up. 5 The study included 50 patients (six patients from the baseline were withdrawn from the study). The three groups had the following number of participants: 15 NC, 24 subjects with MCI, and 12 with AD dementia. In the AD dementia group, 92.3% had positive biomarkers for AD, as did 20% in the NC group. Nearly half of the MCI participants had positive biomarkers for AD. After 1 year of follow‐up, the significant differences found at baseline in cognitive tests were similar, although individuals with AD dementia experienced functional worsening measured by the FAQ and CDR. The exception was semantic fluency, which showed greater decline between AD dementia and the other groups. The incorporation of biomarkers as a variable did not significantly alter the group findings. The annual conversion rate to dementia was 20%. Russo et al. investigated in the cohort the predictive capacity of memory recognition tests combined with delayed recall of episodic memory and CSF biomarkers for the conversion from MCI to AD over a 24‐month period. 6 , 7 , 8 CSF Aβ1‐42 levels, RAVLT delayed recall, and the combination of RAVLT delayed recall and d‐prime were predictive of conversion to AD. Figure 2 illustrates the proportion of each patient group during the follow‐up period.
FIGURE 2.
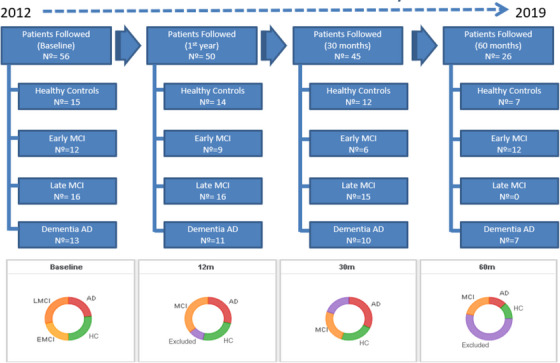
Follow‐up of Arg‐ADNI, comprising four visits over 5 years (60 months). AD, Alzheimer's disease; Arg‐ADNI, Argentina–Alzheimer's Disease Neuroimaging Initiative; EMCI, early MCI; HC, healthy controls; LMCI, late MCI; MCI, mild cognitive impairment.
On the other hand, the most significant novelty of this initiative was the assessment of these patients with AD biomarkers. Several insights were gained regarding this topic, and below, we will mention some of the results. The research by Surace et al. on CSF biomarkers of AD was one of the first of its kind in Latin America. 9 The study investigated amyloid protein, tau, and phosphorylated tau (p‐tau), demonstrating their significance in discriminating AD from frontotemporal dementia and their utility in predicting the conversion from MCI to AD. Chrem‐Mendez et al. published their findings regarding the presence of PET‐PiB across different AD phenotypes and healthy controls. 10 Only NC and patients with AD‐type dementia exhibited the most consistent results. Patients with the greatest discrepancies were those diagnosed with non‐amnestic MCI, primary progressive aphasia, and frontotemporal dementia. Allegri et al. published in 2019 and 2020 the results on the characteristics and biomarker evolution of the participants during the 60‐month follow‐up. 11 , 12 , 13 The baseline included the following numbers of patients: MCI = 23, AD dementia = 12, and NC = 12. All participants underwent studies for Aβ protein (in CSF or PET‐PiB), p‐tau (in CSF), and neurodegeneration (total tau [t‐tau] in CSF, fluorodeoxyglucose [FDG]‐PET, and structural MRI). They were grouped according to the amyloid/tau/neurodegeneration (ATN) biomarker scale. Initially, 91% of MCI patients were classified as A+T+N+, 20% of early MCI, 46% of late MCI, and 14% of controls. The percentages of patients with suspected non‐AD pathology (A−T−N+) were 8% for MCI, 20% for early MCI, 15% for late MCI, and 7% for controls. Finally, during the 5‐year follow‐up, the conversion rate to dementia from baseline MCI was 85% for A+T+N+ and 50% for A−T−N+. Harris et al. in 2015 published a work about the cognitive reserve and AB42 in patients with mild cognitive impairment from Arg‐ADNI. 14
TABLE 2.
Objectives and results of the main publications from Arg‐ADNI.
| Study | Author (year) | Objective | Results |
|---|---|---|---|
| Latin American Experience with Alzheimer's disease. Cerebrospinal Fluid Biomarkers | Surace et al. (2013) | The aim of the present study was to evaluate CSF AD biomarkers in their capacity to discriminate AD from frontotemporal dementia (FTD) and to predict progression from MCI to AD | The mean value of biomarkers and the ratios were not significantly different in the three main groups (AD, MCI, and FTD) because of the high dispersion observed in the MCI group. There were significant differences between the groups with AD and FTD in the biomarkers and ratios (Aβ42, p = 0.01; t‐tau, p = 0.04; p‐tau, p = 0.03). When the group with MCI was analyzed and divided according to clinical progression to AD over time, the mean value of Aβ42 was 355 pg/mL in those with progression, versus 800 pg/mL in those without AD progression, which was significantly different. |
| Concordance Between 11C‐PIB‐PET and Clinical Diagnosis in a Memory Clinic | Chrem Mendez et al. (2015) | The aim of this study is to determine the frequency of PiB amyloid findings in different diagnostic syndromes grouped into high and low probability pretest categories, taking into account pretest clinical assumption of the presence of AD‐related pathology | Only normal controls and AD dementia patients were the most consistent across clinical and molecular diagnostics. MCI, non‐logopenic PPA and FTD were the syndromic diagnoses that most discrepancies were found |
| Predicting episodic memory performance using different biomarkers: results from Argentina–Alzheimer's Disease Neuroimaging Initiative | Russo et al. (2016) | The objective was to describe baseline characteristics and to examine whether biomarkers related to AD physiopathology were associated with worse memory performance. | A total of 56 participants were included and underwent baseline evaluation. The three groups (CN, MCI, AD) were similar with respect to years of education and sex, and they differed in age (F = 5.10, p = 0.01). Mean scores for the baseline measurements of the neuropsychological evaluation differed significantly among the three groups at p < 0.001, showing a continuum in their neuropsychological performance. No significant correlations were found between the principal measures (long‐delay recall, PiB scan, left hippocampal volume, and APOEε4) and either age, sex, or education (p > 0.1). Baseline amyloid deposition and left hippocampal volume separated the three diagnostic groups and correlated with memory performance (p < 0.001). |
| Usefulness of Discriminability and Response Bias Indices for the Evaluation of Recognition Memory in Mild Cognitive Impairment and Alzheimer Disease | Russo et al. (2017) | To evaluate the recognition memory performance by using a yes/no procedure to examine the effect of discriminability and response bias measures in amnestic mild cognitive impairment (a‐MCI), AD dementia, and normal‐aging subjects. | Based on the proportions of correct responses (hits) and false alarms from the Rey Auditory Verbal Learning Test (RAVLT), discriminability (d') and response bias (C) indices from signal detection theory (SDT) were calculated. Results showed significant group differences for d' (F(2) = 83.26, p < 0.001), and C (F(2) = 6.05, p = 0.00). The best predictors of group membership were delayed recall and d' scores. The d' measure correctly classified subjects with 82.98% sensitivity and 91.11% specificity. |
| Adding Recognition Discriminability Index to the Delayed Recall Is Useful to Predict Conversion from Mild Cognitive Impairment to Alzheimer's Disease in the Alzheimer's Disease Neuroimaging Initiative | Russo et al. (2017) | The aim was to investigate whether recognition memory tasks in combination with delayed recall measure of episodic memory and CSF biomarkers can predict conversion from MCI to AD at 24‐month follow‐up. | Of the all included variables, CSF Aβ1‐42 levels, RAVLT Delayed Recall, and the combination of RAVLT Delayed Recall and d‐prime were predictive of progression to AD (χ2 = 38.23, df = 14, p < 0.001) |
| Utility of the Spanish version of the Everyday Cognition scale in the diagnosis of mild cognitive impairment and mild dementia in an older cohort from the Argentina‐ADNI | Russo et al. (2018) | To assess the Everyday Cognition (ECog) scale in cognitively intact controls (CN) and in patients with MCI and mild AD from the Argentina‐ADNI cohort to establish diagnostic accuracy. In addition, the sensitivity and specificity of the ECog was compared with the Functional Assessment Questionnaire (FAQ) scale to discriminate between the three groups. | The average total score on the ECog was significantly different across the three diagnostic syndromes (p < 0.05). The ECog was more sensitive than FAQ in discriminating between CN and MCI patients and between MCI and AD subjects. The ECog showed a strong correlation with FAQ, and moderate correlations with neuropsychological tests. Cronbach's alpha was 0.98. |
| Prognostic value of ATN Alzheimer biomarkers: 60‐month follow‐up results from the Argentine Alzheimer's Disease Neuroimaging Initiative | ` | To describe results of the amyloid, tau, neurodegeneration (ATN) research framework classification in the Argentine‐Alzheimer's Disease Neuroimaging Initiative (arg‐ADNI) cohort | A+T+N+ biomarker profile was identified at baseline in 91% of mild dementia patients, 20% of early MCI patients, 46% of late MCI patients, and 14% of control subjects. Suspected non‐AD pathophysiology (SNAP, A‐T‐N+) was found in 8% of mild dementia, 20% of early MCI, 15% of late MCI, and 7% of control subjects. Conversion rates to dementia after 5‐year follow‐up were 85% in A+T+N+ MCI patients and 50% in A‐T‐N+ patients |
| Neuropsychological profile of Alzheimer's disease based on amyloid biomarker findings results from a South American cohort | Clarens et al. (2020) | This study aimed to identify which neuropsychological tests best indicated underlying AD pathophysiology | Delayed recall score at 30 minutes on the RAVLT was the best predictor of amyloid pathology presence (AUC 0.68), followed by RAVLT total learning (AUC 0.66) and RAVLT Recognition (AUC 0.59) scores, providing useful cut off values in the clinical setting. |
| Biomarkers of Alzheimer's disease in mild cognitive impairment: Experience in a memory clinic from Latin America | Allegri et al. (2021) | This study aimed to investigate the role and prognosis of Alzheimer disease biomarkers in patients with MCI at a memory clinic in Latin America. | Amyloid pathology was observed in cerebrospinal fluid results in 18% of controls, 64% of patients with MCI, and 92% of patients with Alzheimer‐type dementia. Suspected non‐Alzheimer disease pathophysiology was found in 11% of controls, 6% of patients with MCI, and 8% of patients with Alzheimer‐type dementia. At 30 months of follow‐up, 45% of amyloid‐positive patients with MCI and 20% of amyloid‐negative patients with MCI showed progression to dementia. |
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; Arg‐ADNI, Argentina–Alzheimer's Disease Neuroimaging Initiative; APOE ε4, apolipoprotein E gene ε4 allele; AUC, area under the curve; CSF, cerebrospinal fluid; CN, cognitively normal; MCI, mild cognitive impairment; PET, positron emission tomography; PiB, 11C Pittsburgh Compound B; PPA, primary progressive aphasia; p‐tau, phosphorylated tau; RAVLT, Rey Auditory Verbal Learning Test; t‐tau, total tau.
In summary, the findings of Arg‐ADNI represented a significant advancement in regional AD research. It facilitated the validation of imaging and cerebrospinal fluid biomarkers, enabling their quantitative analysis. Additionally, the validation of neuropsychological assessments in Spanish was developed, providing a crucial tool not only in research but also in clinical practice. Table 2 summarizes the objectives and results of the main publications from Arg‐ADNI.
2.2. Challenges for developing ADNI in low‐and middle‐income countries
Some challenges encountered in the development of the Arg‐ADNI project in a low‐ and middle‐income country will be highlighted. First, the project did not receive any international or national funding. All expenses were covered by our center's benefactors' society, a philanthropic group. Second, due to the unprecedented nature of a project of this scale in our region, it was necessary to develop the human and technological resources required to execute this task. This included validating functional scales and neuropsychological tests, among others, as well as optimizing technological infrastructure for biomarker studies (PiB amyloid PET, tau‐PET, and FDG‐PET scans). Finally, methodological challenges such as long‐term patient follow‐up had to be managed. This necessitated constant motivation at each medical visit, encouraging patients to continue participating in an observational project that offered no financial or therapeutic benefits.
It is important to note that these difficulties are not unique to our center, and that was the reason that Fleni was the only ADNI center. Many institutions across Latin America face similar economic and technological disadvantages, making it very challenging for other centers to participate. Therefore, similar projects in other parts of the world, such as the United States or Japan, had greater participation compared to ours.
2.3. Arg‐ADNI as a catalyzer of scientific capability
In the years leading up to the ADNI project, the field of dementias in Argentina was in an intriguing potential state. While many of our scientists were training abroad in the newly developed diagnostic techniques, interest in developing our own expertise and contributing to the study of the disease in our region was growing. The proposal of ADNI allowed for the repatriation of scientists and the establishment of an organized program upon which a systematic approach to the study of AD and related disorders for both research and clinical practice could be built. Thus, in 2012, the Center for Memory and Aging at Fleni was born. This was a multidisciplinary center that combined four working groups: the Department of Cognitive Neurology, the Department of Neuroimaging, the Laboratory of Neurodegenerative Diseases, and the Center for Molecular Imaging. Each of these areas was responsible for developing new technologies that would be indispensable for the ADNI project but would also become the cornerstone of our clinical practice in the coming years.
The Molecular Imaging Center implemented a PET‐CT scanner and the installation of a cyclotron on‐site to produce PiB. At the time of this installation, it was the first PET scanner in Latin America for the application of molecular biomarkers. Over time, this center would become a producer of biomarkers for other PET centers in the region and the reference center for clinical studies, gradually adding the production of other radiopharmaceuticals such as florbetaben and florbetapir. In 2018, this center became the first to conduct tau‐PET imaging (using AV1451 tracer) in the region, an experience developed as an extension of the Arg‐ADNI protocol. This kind of development not only marked a milestone in the region but also served as a starting point to assess the potential for clinical applicability in the area.
In parallel, the neurodegenerative diseases laboratory developed a platform for measuring biomarkers in cerebrospinal fluid (Aβ, t‐tau, and p‐tau) in these subjects. This initiative received support and methodological validation from the Zetterberg Laboratory at the University of Gothenburg, Sweden, thus achieving international quality standards. This development quickly transitioned into a clinical application and became the primary molecular diagnostic source in Argentina and the surrounding regions for subjects with cognitive impairment.
One of the collateral benefits resulting from the development of Arg‐ADNI was the advancement in AD biomarkers. The experience gained in the utilization of amyloid and tau markers in CSF and PET imaging was a direct result of the work carried out by Arg‐ADNI. Additionally, the utilization of neuropsychological assessment batteries was refined, enhancing their use in daily medical care practice. This effort facilitated the development of novel diagnostic algorithms based on the learning derived from the study's outcomes.
2.4. Arg‐ADNI and its legacy in Latin America
A notable contribution of the Arg‐ADNI initiative lies in its synchronized and systematic integration of both imaging and molecular biomarkers within the framework of neuropsychological analysis and clinical diagnosis of specified populations. Such integration marked a groundbreaking development in the annals of cognitive neurology in Argentina, signifying a substantial leap in the quality of patient study methodologies. Moreover, this initiative leveraged its organizational foundations to establish institutional local biobanks, including repositories for brain specimens, CSF, and DNA, sourced from meticulously characterized populations.
We should emphasize the importance of Arg‐ADNI in AD research, as it spurred the development of further research projects at the regional level. The clinical experience from this study put our country on the map of clinical dementia research. Subsequent years were characterized by strong support from entities such as the Alzheimer's Association, which transformed into the emergence of several multinational studies focusing on both prevention and diagnosis, as well as treatment of dementias. These studies are heirs to the tradition of Arg‐ADNI: the DIAN, Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline (LatAm‐FINGERS), LEADS, Initiative for the Study of Down Syndrome and Alzheimer's Disease (IASDA), to name a few. Next, we will delve into what these initiatives entail.
3. DIAN
DIAN is an initiative of the Washington University School of Medicine in St. Louis, USA. It focuses on studying families with mutations in the deterministic genes of AD (PSEN1N, PSEN2, and APP), causing autosomal dominant Alzheimer's disease. These genes are termed deterministic because they guarantee that individuals who inherit the mutations will develop the disease at an early age, typically around 45 years old, with a range of onset between 30 and 60 years old, and with a near 100% chance of developing the disease (complete penetrance).
Currently, DIAN is divided into three main lines of research: DIAN‐Obs (observational) is dedicated to investigating, in the most comprehensive way possible, mutation carriers, comparing them with non‐carriers. DIAN‐TU (trial unit), started in 2012, aims to test new potential treatments. It has a dynamic design to test the cohort with the best possible treatment. Generally, it proposes to test more than one drug simultaneously in different parallel branches, and its arms are projected until 2028. Finally, there is a third line called DIAN‐EXR, whose main function is to disseminate information and invite new families to participate through a digital registry that allows direct communication between family members and researchers.
These initiatives were developed at Fleni, marking a regional scientific advancement. Currently, DIAN‐TU continues to explore novel drugs recently used in AD, such as monoclonal antibodies against cerebral Aβ. At the time of publication of this review, 15 patients in this cohort were enrolled in local research study protocols involving novel medications such as Lecanemab and E2814.
4. LatAm‐FINGERS
FINGERS was the first large long‐term randomized controlled trial to demonstrate that a multidomain lifestyle‐based intervention improving vascular risk factors and lifestyle‐related factors can preserve cognitive functioning and reduce the risk of cognitive decline among older adults at higher risk of dementia.
The positive results of the FINGERS trial on cognition have inspired other global initiatives to confirm its findings using similar approaches. In Latin America, the LatAm‐FINGERS is the first attempt to bring together Latin American countries in an intervention to reduce cognitive decline. Based on the Argentinean development in Arg‐ADNI and DIAN, Fleni in 2019 proposed to the Alzheimer Association to support a multinational Latin American platform for the prevention of dementia. LatAm‐FINGERS offers two ideal conditions for testing a multidomain intervention to prevent cognitive decline. First, the region has a higher prevalence of modifiable risk factors than Finland, and our hypothesis is that participants from Latin America will derive a more significant benefit from this intervention. Second, the regional population has unique sociocultural and ethnic characteristics that differ greatly from those of Finland.
For the first time, twelve Latin American countries are part of this collaborative study, comprising Argentina, Bolivia, Chile, Colombia, Costa Rica, Ecuador, Mexico, Peru, the Dominican Republic, Uruguay, and Brazil. This initiative is currently ongoing at the time of this publication.
Thanks to a grant from the Alzheimer's Association, the regional implementation of the LatAm‐FINGERS project brought forth a significant benefit: the establishment of a network comprising regional neurological institutions. This network commenced operating in unison, following a unified protocol, thus facilitating the exchange of data and nurturing an unparalleled spirit of collaboration in dementia prevention. This collaboration presents promising opportunities for further expansion through networking.
5. LEADS
LEADS, funded by the National Institute on Aging, aims to address several important gaps in AD and related dementia research. LEADS will explore the development of early‐onset AD and how it compares to the more common late‐onset Alzheimer's variant. LEADS is an observational study enrolling and following 500 participants with cognitive impairment and 100 cognitively normal participants aged 40 to 64 years at approximately 15 centers in the United States. Clinical, cognitive, imaging, biomarker, and genetic characteristics will be assessed. The primary goal of LEADS is to develop sensitive clinical measures and biomarkers for future clinical and research use. At the time of this publication, supported by the Alzheimer's Association, the local initiative of the project is being designed to be implemented in Fleni in 2024.
6. IASDA
IASDA is an initiative spearheaded at Fleni for studying AD in patients with Down syndrome (DS) supported by CONICET (Argentine National Agency for Research). AD not only occurs more frequently and at an earlier age in DS individuals than in the general population but also presents with an atypical clinical phenotype and an uncertain genetic risk factor and biomarker profile. The goal of this initiative is to investigate the clinical, genetic, and biomarker aspects of AD in DS. Currently, this research study is ongoing, actively recruiting patients, and inviting the community to participate.
7. CONCLUSION
Arg‐ADNI was an initiative that brought about a paradigm shift in AD research in Latin America. Given the region's socioeconomic, educational, genetic, and other characteristics, it is crucial to have an accurate description of the population's context. The diversity of these features makes the region unique, and drawing parallels between the results of other regions and the regional idiosyncrasies would be inaccurate. Its contribution to the international study of AD was pivotal, not only due to its own results but also as a driving force for other regional research projects such as DIAN, LatAm‐FINGERS, LEADS, and IASDA to name a few. These initiatives with the support from the Alzheimer's Association have contributed significantly to the development of regional research and serve as essential tools for health policy planning in Latin America. This is an example of collaboration between high‐income countries with low‐ and middle‐income countries aimed at global scientific development, inclusion, and the democratization of science.
CONFLICT OF INTEREST STATEMENT
P.C.M. reports grants paid to his institution from the Alzheimer's Association and Washington University in St. Louis. I.C. reports grants paid to his institution from the Alzheimer's Association. G.S. reports grants paid to his institution from the Alzheimer's Association. R.F.A. reports honoraria as a speaker from Tecnofarma, Biogen, Bago, Roche, and Novo Nordisk, and grants paid to his institution from the Alzheimer's Association and Washington University in St. Louis. The other authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All human subjects provided informed consent.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank the Alzheimer's Association and Fleni for the possibility of developing Arg‐ADNI.
Cubas Guillen JF, Chrem Méndez P, Surace E, et al. Argentina–Alzheimer's Disease Neuroimaging Initiative: pioneering Alzheimer's Research in Latin America and its Implications for Regional Advancement. Alzheimer's Dement. 2024;20:8153–8161. 10.1002/alz.14285
Jonathan Fernando Cubas Guillen and Patricio Chrem Méndez shared first authorship.
REFERENCES
- 1. World Health Organization (WHO) . Global Status Report on the Public Health Response to Dementia. World Health Organization (WHO); September 2021. https://www.who.int/publications/i/item/9789240033245 [Google Scholar]
- 2. Russo MJ, Gustafson D, Vázquez S, et al. Creation of the Argentina‐Alzheimer's disease neuroimaging initiative. Alzheimer's Dement. 2014;10:84‐87. doi: 10.1016/j.jalz.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 3. Russo MJ, Cohen G, Chrem Mendez P, et al. Utility of the Spanish version of the everyday cognition scale in the diagnosis of mild cognitive impairment and mild dementia in an older cohort from the Argentina‐ADNI. Aging Clin Exp Res. 2018;30:1167‐1176. doi: 10.1007/s40520-018-0899-8 [DOI] [PubMed] [Google Scholar]
- 4. Clarens MF, Crivelli L, Calandri I, et al. Neuropsychological profile of Alzheimer's disease based on amyloid biomarker findings results from a South American cohort. Appl Neuropsychol. 2020;29:345‐350. doi: 10.1080/23279095.2020.1756816 [DOI] [PubMed] [Google Scholar]
- 5. Méndez PC, Calandri I, Nahas F, et al. Argentina‐Alzheimer's disease neuroimaging initiative (Arg‐ADNI): neuropsychological evolution profile after one‐year follow up. Arq Neuropsiquiatr. 2018;76:231‐240. doi: 10.1590/0004-282x20180025 [DOI] [PubMed] [Google Scholar]
- 6. Russo MJ, Campos J, Vázquez S, Sevlever G, Allegri RF. Adding recognition discriminability index to the delayed recall is useful to predict conversion from mild cognitive impairment to Alzheimer's disease in the Alzheimer's disease neuroimaging initiative. Front Aging Neurosci. 2017;9:1‐7. doi: 10.3389/fnagi.2017.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russo MJ, Cohen G, Campos J, et al. Usefulness of discriminability and response bias indices for the evaluation of recognition memory in mild cognitive impairment and Alzheimer disease. Dement Geriatr Cogn Disord. 2017;43(1‐2):1‐14. doi: 10.1159/000452255 [DOI] [PubMed] [Google Scholar]
- 8. Russo MJ, Cohen G, Chrem Mendez P, et al. Predicting episodic memory performance using different biomarkers: results from Argentina‐Alzheimer's Disease Neuroimaging Initiative. Neuropsychiatr Dis Treat. 2016;12:2199‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Surace E, Cohen G, Chrem Méndez P, et al. Latin American experience with Alzheimer's disease cerebrospinal fluid biomarkers. J Am Geriatr Soc. 2013;61:1229‐1231. [DOI] [PubMed] [Google Scholar]
- 10. Patricio CM, Gabriela C, Julieta RM, et al. Concordance between 11C‐PIB‐PET and clinical diagnosis in a memory clinic. Am J Alzheimers Dis Other Demen. 2015;30:599‐606. doi: 10.1177/1533317515576387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allegri RF, Pertierra L, Cohen G, et al. A biological classification for Alzheimer's disease—amyloid, tau and neurodegeneration (A/T/N): results from the Argentine‐Alzheimer's Disease Neuroimaging Initiative. Int Psychogeriatr. 2019;31(12):1837‐1838. doi: 10.1017/S1041610219000085 [DOI] [PubMed] [Google Scholar]
- 12. Allegri RF, Chrem Méndez P, Calandri I, et al. Prognostic value of ATN Alzheimer biomarkers: 60‐month follow‐up results from the Argentine Alzheimer's Disease Neuroimaging Initiative. Alzheimer's Dement Diagnosis. Alzheimers Dement. 2020;12:e12026. doi: 10.1002/dad2.12026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allegri RF, Mendez Chrem P, Russo MJ, et al. Biomarkers of Alzheimer's disease in mild cognitive impairment: experience in a memory clinic from Latin America. Neurologia. 2021;36(3):201‐208. doi: 10.1016/j.nrl.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 14. Harris P, Fernandez Suarez M, Surace EI, et al. Cognitive reserve and aβ1‐42 in mild cognitive impairment (Argentina‐Alzheimer's Disease Neuroimaging Initiative). Neuropsychiatr dis Treat. 2015;11:2599‐2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


