Abstract
Blood‐based biomarkers (BBM) for Alzheimer's disease (AD) are being increasingly used in clinical practice to support an AD diagnosis. In contrast to traditional diagnostic modalities, such as amyloid positron emission tomography and cerebrospinal fluid biomarkers, BBMs offer a more accessible and lower cost alternative for AD biomarker testing. Their unique scalability addresses the anticipated surge in demand for biomarker testing with the emergence of disease‐modifying treatments (DMTs) that require confirmation of amyloid pathology. To facilitate the uptake of BBMs in clinical practice, The Global CEO Initiative on Alzheimer's Disease convened a BBM Workgroup to provide recommendations for two clinical implementational pathways for BBMs: one for current use for triaging and another for future use to confirm amyloid pathology. These pathways provide a standardized diagnostic approach with guidance on interpreting BBM test results. Integrating BBMs into clinical practice will simplify the diagnostic process and facilitate timely access to DMTs for eligible patients.
Keywords: Alzheimer's disease, amyloid, biomarker, blood‐based biomarkers, clinical implementation, clinical practice, cognitive impairment, disease‐modifying treatment, patient journey, primary care, secondary care
1. INTRODUCTION
Recent estimates from the US Health and Retirement Study suggest that approximately 22% of individuals aged 65 and older have mild cognitive impairment (MCI) and up to 10% have dementia. 1 Alzheimer's disease (AD) is the most common cause of dementia, accounting for approximately 60%–80% of dementia cases. 2 AD is characterized by the accumulation of extracellular plaques comprised of abnormal amyloid β (Aβ) proteins, neurofibrillary tangles consisting of abnormally hyperphosphorylated tau protein, and neuronal degeneration in brain regions critical for cognitive function. 3 AD pathology manifests decades before the onset of cognitive symptoms. 4 As the disease progresses to the symptomatic phase, patients first develop MCI before transitioning to AD dementia. 2
Despite the high prevalence of MCI and dementia, up to 92% of patients with MCI remain undiagnosed or misdiagnosed in primary care settings. 5 This diagnostic challenge is further compounded by delayed diagnoses in dementia cases, 6 in part due to limited cognitive screening in primary care and the absence of time‐ and cost‐effective diagnostic tools. Moreover, the scarcity of dementia specialists in the United States exacerbates the situation, with many patients failing to access specialty care, 7 and those who are referred experiencing prolonged wait times (e.g., approximately 56 months following a referral based on a brief cognitive assessment). 8 Consequently, because the majority of AD neuroimaging or cerebrospinal fluid (CSF) biomarker testing takes place in specialty care settings (i.e., secondary or tertiary care), only a small fraction of patients with cognitive impairment receive such testing. 9
The advent of disease‐modifying treatments (DMTs) that slow the clinical progression of AD by acting on the underlying pathophysiological mechanisms is expected to trigger a surge in patients seeking a determination of eligibility for these therapies. 10 Lecanemab was the first DMT to receive traditional approval from the US Food & Drug Administration (FDA), followed by Donanemab and additional drugs are in development. These new treatments act by lowering brain amyloid and are indicated for patients with early symptomatic AD, encompassing MCI and mild dementia due to AD. 11 , 12 Because these therapies target aggregated soluble and insoluble forms of Aß, biomarker confirmation of amyloid pathology is required before initiation. As these DMTs continue to gain momentum in clinical practice, the need for biomarker testing to facilitate timely diagnosis of AD is expected to increase by orders of magnitude. 13 , 14
Traditional biomarker modalities for confirming AD pathology include positron emission tomography (PET) with an amyloid‐binding radiotracer or a lumbar puncture to collect CSF to measure concentrations of Aß and tau. 9 PET and CSF biomarkers have a number of shortcomings including high cost, limited accessibility, and perceived invasiveness, making them unsuitable for widespread use across care settings for diagnosing AD in the general population. 9 , 14 , 15 Recent advancements in blood‐based biomarker (BBM) tests for AD offer a promising alternative. These tests are less costly, more accessible, more acceptable to patients, and more practical for serial collection to monitor disease progression. Furthermore, of the currently available biomarker modalities, BBMs may be the only class capable of meeting the demand for diagnosis that DMTs are likely to herald. 9 , 13 , 14 , 15 , 16 Here, we present recommendations from The Global CEO Initiative on Alzheimer's Disease (CEOi) BBM Workgroup on implementing BBM for clinical use in symptomatic AD. These recommendations aim to facilitate the uptake of BBM tests in clinical practice to support a more efficient diagnosis and accelerate access to novel treatments for eligible patients.
2. METHODS
CEOi, under the auspices of UsAgainstAlzheimer's, launched the BBM Workgroup in 2022 to prepare stakeholders for the widespread adoption of BBM tests in clinical practice. This initiative aimed to enable a simpler, more timely, and more accurate diagnostic experience for patients with symptomatic AD in the United States. 17 Additionally, the integration of BBMs into clinical practice will accelerate the AD diagnostic process and potential access to DMTs for eligible patients. The focus on symptomatic patients stemmed from several considerations: current DMTs are only approved for those with cognitive impairment and amyloid pathology; the prognostic value of AD BBM testing in asymptomatic populations is not well understood; and ethical considerations regarding the diagnosis of AD pathology in cognitively unimpaired individuals require further investigation.
The BBM Workgroup established two primary objectives: defining minimum acceptable performance standards for BBM tests and providing implementation recommendations for clinical practice. Minimum performance standards and clinical implementation pathways were established for two anticipated uses of BBM tests: triaging and confirming amyloid pathology. For triaging, a negative BBM test result identifies individuals unlikely to have amyloid pathology, prompting further evaluation of non‐AD causes of cognitive impairment. A positive result suggests an increased likelihood of amyloid pathology, necessitating further testing with a more accurate test. For BBM tests used to confirm amyloid pathology, a positive test result identifies amyloid pathology without the need for a second test. 9 , 13 , 15
RESEARCH IN CONTEXT
Systematic review: Experts in biomarker testing and clinical management of symptomatic Alzheimer's disease (AD) convened to discuss the adoption of AD blood‐based biomarkers (BBMs) in primary and secondary care settings, physician decision pathways to identify appropriate individuals for BBM tests, and interpretation of test results. Input from healthcare, industry, academia, government, and patient advocacy sectors was included in the drafting of consensus recommendations for clinical implementation of AD BBMs.
Interpretation: Two implementation pathways for AD BBMs are recommended to facilitate a more efficient and accurate diagnostic journey for patients with symptomatic AD: one for current use for triaging and another for future use to confirm amyloid pathology.
Future directions: Integrating BBMs into clinical practice will facilitate early detection of pathology and timely intervention. Multistakeholder engagement is required to create and widely disseminate training resources for healthcare professionals and patient educational content that will facilitate the clinical use of BBMs.
The minimum performance recommendations for AD BBM tests have been previously published. 18 It is critical that BBM tests achieve sufficient performance for their intended contexts of use. These recommendations serve to support clinicians in determining whether a BBM test is acceptable for triaging or amyloid confirmation and guide manufacturers in validating these tests.
Within the Workgroup, a dedicated workstream was established to develop clinical implementation recommendations. Coleaders of the workstream, who are AD biomarker experts with extensive expertise and publications on biomarker validation (M.M.M. and C.U.), recruited a core team of diverse experts in biomarker testing and clinical management of symptomatic AD (M.A., J.W.A., A.J., P.J.L., A.R., J.T., and D.W.). The coleaders and core team served voluntarily and without financial compensation. CEOi provided administrative support, including coordinating meetings, taking notes, and slide deck creation.
The BBM Workgroup invited stakeholders from academia, industry, private foundations, and patient advocacy groups to a kick‐off meeting. During this meeting, the coleaders outlined the group's goal of developing clinical implementation recommendations for AD BBM tests and gathered feedback on issues to consider. Following the kick‐off meeting, the core team met weekly for several months to review current literature and discuss the following topics: barriers and facilitators to implementing AD BBM tests in primary and secondary care, the patient journey, physician decision pathways for identifying appropriate candidates for AD BBM tests and DMTs, and the interpretation of AD BBM test results. After extensive deliberation, two clinical implementation pathways were drafted by the core team. The coleaders then presented these pathways to BBM Workgroup members for feedback.
The consensus recommendations encompass two clinical implementation pathways: one for BBM tests used in triage and another for confirming amyloid pathology. The integration of BBM tests for triaging reflects a present‐day scenario, where BBM tests that have reached sufficient accuracy can be used as a preliminary tool before confirming amyloid pathology with a confirmatory biomarker test. The second pathway describes an emerging scenario in which a BBM test reaches the minimum acceptable performance to confirm amyloid pathology without a subsequent test. 18
Several limitations to implementing these pathways were identified, including a lack of consistent recommendations for assessing cognitive impairment, the need for education on BBM test performance characteristics, insufficient evidence on BBM performance in diverse populations, potential implications of future direct‐to‐consumer tests, and ethical considerations such as patients' rights to decide whether to undergo BBM testing, access to test results, and confidentiality. A thorough discussion of these considerations can be found in our companion article. 19
3. CLINICAL IMPLEMENTATION PATHWAY FOR AD BBM TESTING AS A TRIAGING TOOL FOR ASSESSING DMT ELIGIBILITY IN SYMPTOMATIC PATIENTS
The recommended implementation pathway for use of an AD BBM test as a triaging tool is detailed in Sections 3.1–3.7 and illustrated in Figure 1. For a simplified version of this pathway, refer to Figure 2. In a triaging scenario, a negative BBM test result would indicate individuals unlikely to have detectable brain amyloid pathology. This outcome would prompt healthcare professionals (HCPs) to focus on evaluating non‐AD‐related causes of cognitive impairment, which may streamline the diagnosis of other causes of cognitive impairment. A positive result would suggest a higher likelihood of amyloid pathology, prompting referral to secondary care for further assessment and consideration for a second, more accurate test (i.e., amyloid PET or CSF) for amyloid confirmation. The minimum acceptable performance criteria for a BBM test for triage have been previously reported by the BBM Workgroup. 18 In primary care settings, BBM tests for triage are recommended for patients typically aged ≥55 years, with no recommended age cutoff in secondary care settings.
FIGURE 1.
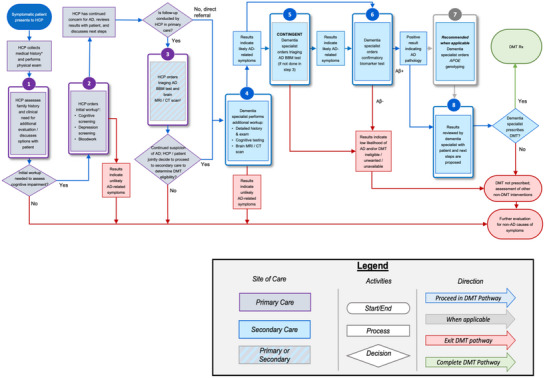
The integration of AD BBM tests as a triaging tool to support the determination of eligibility for DMT. The pathway follows a symptomatic patient with cognitive concerns, starting in a primary care setting (purple boxes) and through secondary care (blue boxes) to determine eligibility for a DMT. Determining APOE ε4 status should occur after a confirmatory CSF/PET test (gray outline). Patients can exit the DMT pathway at various steps when AD is not deemed the likely cause of cognitive impairment (red line). * Medical history includes history of present illness, past medical history, family history, social history, medications, allergies, and review of systems. † Steps 1 and 2 can occur during the same visit. ‡ Step 3 can occur in primary or secondary care depending on how comfortable the primary care HCP is interpreting and disclosing BBM and/or neuroimaging results, the availability of structural brain neuroimaging, and associated wait times. Aβ, amyloid beta; AD, Alzheimer's disease; APOE, apolipoprotein E; BBM, blood‐based biomarker; CSF, cerebrospinal fluid; DMT, disease‐modifying therapy; HCP, healthcare professional; PET, positron emission tomography; Rx, medical prescription.
FIGURE 2.
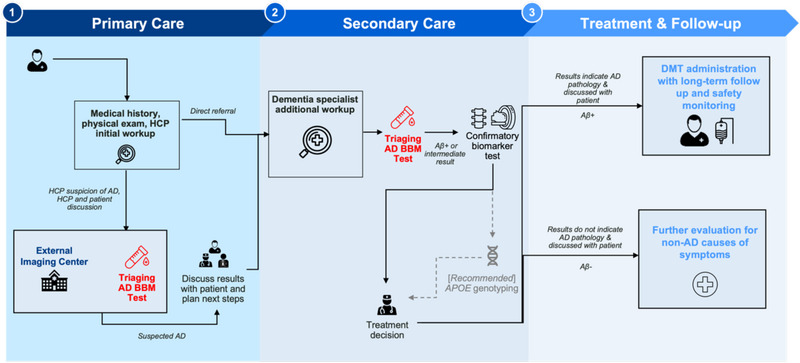
This figure is a simplified version of the pathway presented in Figure 1 illustrating where AD BBM tests (red text) can be integrated as triaging tools to support the determination of eligibility for DMT. Determining APOE ε4 status should occur after a confirmatory CSF/PET test (dotted line). Aβ, amyloid beta; AD, Alzheimer's disease; APOE, apolipoprotein E; BBM, blood‐based biomarker; CSF, cerebrospinal fluid; DMT, disease‐modifying therapy; HCP, healthcare professional; PET, positron emission tomography; Rx, medical prescription.
Importantly, BBM test results must be interpreted in the context of all clinical data, including information resulting from a thorough medical history and a brief cognitive assessment at a minimum. In addition, because some medical conditions (e.g., obesity or chronic kidney disease) and some medications (e.g., secubitril/valsartan) may influence the concentration and interpretation of biomarkers, assessing for these comorbidities is critical to ensure accurate interpretation of results. 20 , 21
3.1. Patient presents with cognitive symptoms
The pathway begins with a patient presenting with cognitive concerns in a primary care setting (Figure 1). The HCP collects the patient's medical history (history of cognitive concerns, sleep and mood changes, past medical history, family history, social history [e.g., alcohol/drug use], medications, and review of systems) and performs a physical examination. The exact assessment may vary based on the differing levels of dementia training received by HCPs in this setting (i.e., primary care providers, advanced practice providers, registered nurses, medical assistants, and community health workers). Regardless of the perceived level of risk for AD as the cause of cognitive impairment, the HCP should consider other potential etiologies of cognitive impairment and whether the patient's medications or chronic conditions could cause or contribute to the cognitive symptoms. 22
3.2. Determining the need for initial workup and determining the level of concern for AD
The HCP should assess whether the patient has objective cognitive symptoms that warrant further evaluation (Figure 1, Step 1). If no objective cognitive symptoms are present and/or the patient declines further workup, no additional follow‐up should be conducted. It may be suggested that the patient be reassessed in 6 to 12 months if the cognitive symptoms persist. If the patient has objective cognitive symptoms and is amenable to further testing, an initial workup should be initiated. The initial workup may occur during the same visit or at a follow‐up visit.
The initial workup should include cognitive screening, depression and/or neurobehavioral screening, and routine bloodwork to evaluate for potential treatable causes of cognitive impairment (e.g., vitamin B12 deficiency, thyroid abnormalities) (Figure 1, Step 2). 23 Upon reviewing the results of the initial workup, the HCP should determine the patient's level of risk for AD in accordance with local clinical and practice standards. If AD is suspected as the cause of cognitive impairment, the HCP should discuss the results with the patient and care partner, ensuring their interest in continued workup before proceeding. Conversely, if the patient's cognitive impairment is not thought to be caused by AD, the patient should be evaluated for non‐AD causes of cognitive impairment.
3.3. Conducting a BBM test in primary or secondary care
The next step is to conduct a BBM test in either primary or secondary care (Figure 1, Step 3). This choice is driven by how comfortable the primary care HCP is in interpreting and disclosing BBM test results and associated wait times for specialty care. If the HCP is comfortable interpreting a BBM test, an AD BBM test for triaging can be ordered and conducted in primary care. Structural neuroimaging tests, such as brain magnetic resonance imaging (MRI) or computed tomography (CT) scans, can also be ordered concurrently or sequentially if these resources are readily available. BBM tests are anticipated to become more accessible than structural imaging modalities, potentially leading to quicker availability of BBM test results. Consequently, some patients may choose to forego neuroimaging at this stage.
Following BBM testing, the HCP should evaluate the level of concern for AD (higher or lower), based on the patient's age and clinical symptoms. For instance, patients aged ≥65 years with a typical AD amnestic clinical syndrome, due to nonreversible or biological causes, including clear objective evidence of cognitive impairment would be categorized as having a higher concern for AD. For patients with a higher concern for AD and a positive BBM test result, the HCP, patient, and care partner can jointly decide whether to proceed to secondary care for additional evaluations to confirm amyloid pathology and assess eligibility for DMTs. If a patient of high concern for AD has a negative BBM test result, the HCP should discuss a referral to secondary care for additional evaluation, including consideration of non‐AD dementias.
For patients with a lower concern for AD and a positive BBM test result, the interpretation of the results depends on the rationale used to determine the low risk of AD, such as the patient's age and symptoms. The HCP should consider potential causes of false positivity, such as lower pretest probability of AD or the presence of comorbidities and associated medications that may impact BBM levels. 20 , 21 Patients who fall into this category may undergo concurrent evaluation for non‐AD causes of cognitive impairment in primary care while being referred to secondary care for further assessment of AD DMT eligibility. In contrast, patients with a lower concern for AD and a negative BBM test result may be deemed DMT‐ineligible, and concurrent evaluation for non‐AD causes is recommended. Importantly, the BBM test used at this step to triage patients suspected of amyloid pathology should have a high negative predictive value (NPV; defined as the likelihood that a patient with a negative BBM test will also receive a negative result when tested with a validated reference standard) so there is high confidence that individuals with a negative test are amyloid negative. 18 If the concern for AD increases after additional consideration, a repeat BBM test can be performed after 6 to 12 months. If the HCP is not comfortable interpreting and disclosing BBM test and/or neuroimaging results, a direct referral to secondary care is recommended.
3.4. Secondary care evaluation
As described in Section 3.3, symptomatic patients may be referred to secondary care under two scenarios: first, if concern for AD persists after BBM testing with or without MRI/CT scans in primary care, and second, if the patient is directly referred to a dementia specialist without undergoing BBM testing. In secondary care, the dementia specialist should first perform additional workup such as a more detailed patient history and examination, cognitive testing, and neuroimaging testing (i.e., MRI/CT scans if not previously conducted in primary care) (Figure 1, Step 4). If the results indicate that cognitive symptoms are likely not related to AD, the patient may undergo further evaluation for non‐AD causes. If results indicate AD‐related cognitive symptoms, the dementia specialist can perform a triage AD BBM test if not previously done in primary care (Figure 1, Step 5).
Following a positive BBM test (performed either in primary or secondary care), the dementia specialist may speak with the patient about undergoing a confirmatory biomarker test to confirm the presence of amyloid pathology (Figure 1, Step 6). If the BBM test is negative, further evaluation should focus on identifying non‐AD causes of cognitive symptoms. For patients with biomarker‐confirmed amyloid pathology who meet eligibility criteria for DMTs, 12 the dementia specialist may discuss determining apolipoprotein E (APOE) ε4 status (see Section 3.5) to inform a more thorough discussion of the risks and benefits associated with DMTs (see Section 3.6).
FIGURE 3.
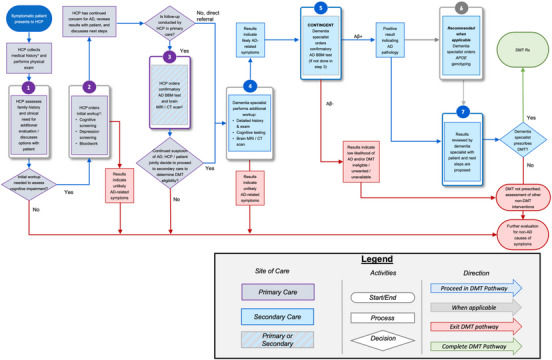
The integration of AD BBM tests as a confirmatory tool to detect the presence or absence of amyloid pathology to support the determination of eligibility for DMT. The pathway follows a symptomatic patient with cognitive concerns, starting in a primary care setting (purple boxes) through secondary care (blue boxes) to determine eligibility for a DMT. Determining APOE ε4 status should occur after a confirmatory BBM test (gray outline). Patient can exit the DMT pathway at various steps when AD is not deemed the likely cause of cognitive impairment (red line). * Medical history includes history of present illness, past medical history, family history, social history, medications, allergies, and review of systems. † Steps 1 and 2 can occur during the same visit. ‡ Step 3 can occur in primary or secondary care depending on how comfortable primary care HCP is interpreting and disclosing BBM and/or neuroimaging results, the availability of structural brain neuroimaging, and associated wait times. Aβ, amyloid beta; AD, Alzheimer's disease; APOE, apolipoprotein E; BBM, blood‐based biomarker; CSF, cerebrospinal fluid; DMT, disease‐modifying therapy; HCP, healthcare professional; PET, positron emission tomography; Rx, medical prescription.
3.5. Determining APOE ε4 status for patients eligible for DMTs
After confirming amyloid pathology with biomarker testing, determining APOE ε4 status is recommended for patients eligible for DMTs (Figure 1, Step 7; see Eisai Inc. Highlights of Prescribing Information). 12 APOE ε4 homozygotes may have an increased risk of side effects, including amyloid‐related imaging abnormalities (ARIA) associated with currently available DMTs. Importantly, if APOE ε4 status is determined, the result should be returned to the patient by the dementia specialist and supported with genetic counseling. If APOE ε4 status is not assessed, the patient should be informed that it cannot be determined whether they may be at higher risk for ARIA.
3.6. Discussing risks and benefits of DMTs with patients and their care partners
The dementia specialist should review the risks and benefits of DMTs with eligible patients and their care partners (in the context of the patient's APOE ε4 status if available) and address treatment logistics monitoring (Figure 1, Step 8). For patients who identify with a minoritized group, it should be noted that clinical trials of lecanemab included approximately 20% of non‐White individuals. 24
3.7. DMT prescription
If the patient is eligible for a DMT and the patient and care partner provide informed consent, a DMT may be prescribed (for further details on eligibility criteria see Eisai Inc. Highlights of Prescribing Information.). 12
4. CLINICAL IMPLEMENTATION PATHWAY FOR AD BBM TESTING TO CONFIRM AMYLOID PATHOLOGY
The recommended implementation pathway for an AD BBM test to confirm the presence of amyloid pathology is illustrated in Figure 3. For a simplified version of this pathway, refer to Figure 4. In the primary care setting, a BBM test that meets the minimum acceptable performance for use as a confirmatory test is intended for patients typically aged ≥65 years, when AD is suspected as the etiology of cognitive impairment following an assessment of the patient's medical conditions and clinical presentation. 18 This age guideline considers the lower likelihood of amyloid pathology in younger patients and the limited research on BBM tests in this demographic. In secondary care, where confirmatory BBM testing would be conducted following a comprehensive evaluation by a dementia specialist, no age cutoff is recommended.
FIGURE 4.
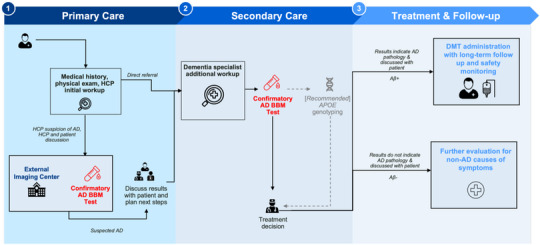
This figure is a simplified version of the pathway presented in Figure 3 illustrating where AD BBMs tests (red text) can be integrated as confirmatory tools to support the determination of eligibility for DMT. Determining APOE ε4 status should occur after a confirmatory BBM test (dotted line). Aβ, amyloid beta; AD, Alzheimer's disease; APOE, apolipoprotein E; BBM, blood‐based biomarker; CSF, cerebrospinal fluid; DMT, disease‐modifying therapy; HCP, healthcare professional; PET, positron emission tomography; Rx, medical prescription.
In this pathway, a positive BBM test result identifies amyloid pathology without the need for a second test (Figure 3). When the BBM test is conducted in primary care (Figure 1, Step 3), patients with positive results may be directly referred to secondary care for further assessment of DMT eligibility (Figure 3, Steps 4–6). Patients with a negative result would be deemed DMT‐ineligible and concurrently evaluated for non‐AD causes of cognitive symptoms. In secondary care, following a comprehensive workup, a positive BBM test result prompts the determination of APOE ε4 status where applicable (Figure 3, Steps 4–6), followed by a thorough discussion on the risks and benefits of DMTs for patients who meet eligibility criteria (Figure 3, Step 7). Conversely, a negative result prompts evaluation for non‐AD causes of symptoms, with appropriate interventions considered. Eligible patients providing informed consent alongside their care partner may be prescribed DMTs.
5. CONCLUSIONS
Confirmation of amyloid pathology is required before initiating DMTs for AD. BBM tests offer a promising solution to address the anticipated surge in demand for AD biomarker testing that will accompany the introduction of DMTs in clinical practice. These tests may alleviate the burden in specialty care settings by triaging patients before confirmatory biomarker testing. Moreover, as BBM tests continue to advance, they are expected to attain a level of accuracy that could eliminate the need for a second biomarker test in some cases. To prepare stakeholders for the widespread adoption of BBMs in clinical practice, CEOi convened a BBM Workgroup to provide recommendations for two clinical implementational pathways: one for current triage use and another to prepare for BBMs tests that have reached sufficient performance to confirm amyloid pathology. Integrating BBMs into clinical practice will accelerate access to DMTs for eligible patients.
CONFLICT OF INTEREST STATEMENT
M.M.M. has consulted, or served on advisory boards, for Biogen, Eisai, LabCorp, Eli Lilly, Merck, Roche, Siemens Healthineers, and Sunbird Bio and receives grant support from the National Institute of Health, Department of Defense, and Alzheimer's Association. A.J. is an employee to ALZpath, Inc, and equity holder. P‐J. L. has consulted for Eli Lilly and reports research support from Biogen, Eisai, Eli Lilly, Genentech, and Incyte (to the institution). L.V. is a principal investigator for clinical trials sponsored by Biogen. O.H. has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, O.H. has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Bristol Meyer Squibb, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. A.S.K. received payments through organizational affiliations for grants, contracts, and consulting fees, honoraria, meeting support, travel support, in‐kind research/professional support over the last 36 months from the Alzheimer's Association, Acadia Pharmaceuticals, Alzheon, Biogen, Clinical Trials Alzheimer's Disease Conference, Davos Alzheimer's Consortium, Digicare Realized, Eisai, Eli Lilly, Embic, Relz Plc, High Lantern Group, International Neurodegenerative Disorders Research Center, and Serdi Publishing. S.E.S. has analyzed plasma biomarker data provided by C2N Diagnostics to Washington University; no personal or research funding was provided by C2N Diagnostics to S.E.S. S.E.S. has served on scientific advisory boards for Eisai. S.E.S. has an unpaid position on the Board of the Greater Missouri Alzheimer's Association. J.F.M. is a stockholder of Eli Lilly and Company. S.B. is an employee and stock owner at Hoffman–La Roche. E.S. is an employee and shareholder of Biogen. J.B.B. and M.M. are employees and shareholders of C2N Diagnostics. F.F.O. receives research support from FAPESP—The State of São Paulo Research Foundation. S.M. serves on the board of directors of Senscio Systems, Inc. and the scientific advisory board of AiCure Technologies, ALZPath and Boston Millennia Partners., and has received consulting and/or speaker fees from Biogen, C2N, Eisai, Novartis, Novo Nordisk, and Roche/Genentech. M.W.W. has served on Advisory Boards for Acumen Pharmaceutical, Alzheon, Inc., Cerecin, Merck Sharp & Dohme Corp., and NC Registry for Brain Health. M.W.W. also serves on the USC ACTC grant which receives funding from Eisai. M.W.W. has provided consulting to Boxer Captial, LLC, Cerecin, Inc., Clario, Dementia Society of Japan, Dolby Family Ventures, Eisai, Guidepoint, Health and Wellness Partners, Indiana University, LCN Consulting, MEDA Corp., Merck Sharp & Dohme Corp., NC Registry for Brain Health, Prova Education, T3D Therapeutics, University of Southern California (USC), and WebMD. M.W.W. holds stock options with Alzeca, Alzheon, Inc., ALZPath, Inc., and Anven. M.W.W. received support for research from the following funding sources: National Institutes of Health (NIH)/NINDS/National Institute on Aging (NIA), Department of Defense (DOD), California Department of Public Health (CDPH), University of Michigan, Siemens, Biogen, Hillblom Foundation, Alzheimer's Association, Johnson & Johnson, Kevin and Connie Shanahan, GE, VUmc, Australian Catholic University (HBI‐BHR), The Stroke Foundation, and the Veterans Administration. D.R.J., R.B., and Y.H.H. are employees of Eisai Inc. S. C. B. is an employee and minor shareholder of Eli Lilly and Company and has a patent for a method for the detection of neurological disease. M.A., J.W.A., A.R., J.T., D.W., J.W., J.R.D., D.H., K.A.P., E.S., G.V., D.Y., M.N.S., Z.M., and C.U‐M. declare no competing interests. Author disclosures are available in the Supporting Information.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Gates Ventures, Eisai, and Eli Lilly for providing financial support for administrative and medical writing services.
Mielke MM, Anderson M, Ashford JW, et al. Recommendations for clinical implementation of blood‐based biomarkers for Alzheimer's disease. Alzheimer's Dement. 2024;20:8216–8224. 10.1002/alz.14184
Contributor Information
Michelle M. Mielke, Email: mmielke@wakehealth.edu.
Chinedu T. Udeh‐Momoh, Email: cmomoh@wakehealth.edu.
REFERENCES
- 1. Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 health and retirement study harmonized cognitive assessment protocol project. JAMA Neurol. 2022;79:1242‐1249. doi: 10.1001/jamaneurol.2022.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19:1598‐1695. doi: 10.1002/alz.13016 [DOI] [PubMed] [Google Scholar]
- 3. Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021;27:954‐963. doi: 10.1038/s41591-021-01382-x [DOI] [PubMed] [Google Scholar]
- 4. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357‐367. doi: 10.1016/S1474-4422(13)70044-9 [DOI] [PubMed] [Google Scholar]
- 5. Mattke S, Jun H, Chen E, Liu Y, Becker A, Wallick C. Expected and diagnosed rates of mild cognitive impairment and dementia in the U.S. Medicare population: observational analysis. Alzheimers Res Ther. 2023;15:128. doi: 10.1186/s13195-023-01272-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gamble LD, Matthews FE, Jones IR, et al. Characteristics of people living with undiagnosed dementia: findings from the CFAS Wales study. BMC Geriatrics. 2022;22:409. doi: 10.1186/s12877-022-03086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drabo EF, Barthold D, Joyce G, Ferido P, Chang Chui H, Zissimopoulos J. Longitudinal analysis of dementia diagnosis and specialty care among racially diverse Medicare beneficiaries. Alzheimers Dement. 2019;15:1402‐1411. doi: 10.1016/j.jalz.2019.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mattke S, Hanson M. Expected wait times for access to a disease‐modifying Alzheimer's treatment in the United States. Alzheimers Dement. 2022;18:1071‐1074. doi: 10.1002/alz.12470 [DOI] [PubMed] [Google Scholar]
- 9. Hampel H, Hu Y, Cummings J, et al. Blood‐based biomarkers for Alzheimer's disease: current state and future use in a transformed global healthcare landscape. Neuron. 2023;111:2781‐2799. doi: 10.1016/j.neuron.2023.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cummings J, Fox N. Defining disease modifying therapy for Alzheimer's disease. J Prev Alzheimers Dis. 2017;4:109‐115. doi: 10.14283/jpad.2017.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384:1691‐1704. doi: 10.1056/NEJMoa2100708 [DOI] [PubMed] [Google Scholar]
- 12. Eisai Inc . Highlights of Prescribing Information. Eisai Inc. and Biogen; 2023. https://www.leqembi.com/‐/media/Files/Leqembi/Prescribing‐Information.pdf [Google Scholar]
- 13. Hansson O, Blennow K, Zetterberg H, Dage J. Blood biomarkers for Alzheimer's disease in clinical practice and trials. Nat Aging. 2023;3:506‐519. doi: 10.1038/s43587-023-00403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schindler SE, Atri A. The role of cerebrospinal fluid and other biomarker modalities in the Alzheimer's disease diagnostic revolution. Nat Aging. 2023;3:460‐462. doi: 10.1038/s43587-023-00400-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;18:2669‐2686. doi: 10.1002/alz.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hampel H, O'Bryant SE, Molinuevo JL, et al. Blood‐based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol. 2018;14:639‐652. doi: 10.1038/s41582-018-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. UsAgainstAlzheimer's . The Global CEO Initiative on Alzheimer's Disease. UsAgainstAlzheimer's; https://www.usagainstalzheimers.org/our‐enterprise/CEOi [Google Scholar]
- 18. Schindler SE, Galasko D, Pereira AC, et al. Acceptable performance of blood biomarker tests of amyloid pathology—recommendations from the Global CEO Initiative on Alzheimer's Disease. Nat Rev Neurol. 2024;20:426‐439. doi: 10.1038/s41582-024-00977-5 [DOI] [PubMed] [Google Scholar]
- 19. Mielke MM, Anderson M, Ashford JW, et al. Considerations for widespread implementation of blood‐based biomarkers of Alzheimer's disease. Alzheimer's Dement. 2024;1‐7 in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022;28:1398‐1405. doi: 10.1038/s41591-022-01822-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brum WS, Docherty KF, Ashton NJ, et al. Effect of neprilysin inhibition on Alzheimer disease plasma biomarkers: a secondary analysis of a randomized clinical trial. JAMA Neurol. 2024;81:197‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment. Neurology. 2018;90:126‐135. doi: 10.1212/wnl.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knopman DS, DeKosky ST, Cummings JL, et al, Practice parameter: diagnosis of dementia (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143‐1153. doi: 10.1212/wnl.56.9.1143 [DOI] [PubMed] [Google Scholar]
- 24. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9‐21. doi: 10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


