Abstract
1. Monochain (OS2) and multichain (taipoxin) neurotoxic phospholipases A2 (PLA2), purified from taipan snake venom, both inhibited ACh release at a concentration of 20 nM (90% inhibition in 2 h) at an identified synapse from buccal ganglion of Aplysia californica. 2. The Na+ current was unchanged upon application of either OS2 or taipoxin. Conversely, presynaptic K+ currents (IA and IK) were increased by taipoxin but not by OS2. In addition, OS2 induced a significant decrease of the presynaptic Ca2+ current (30%) while taipoxin increased this latter current by 20-30%. 3. Bee venom PLA2, another monochain neurotoxic PLA2, also inhibited ACh release while non-toxic enzymatically active PLA2s like OS1 (also purified from taipan snake venom) or porcine pancreatic PLA2 elicited a much weaker inhibition of ACh release, suggesting a specific action of neurotoxic PLA2s versus non-toxic PLA2s on ACh release. 4. Using iodinated OS2, specific high affinity binding sites with molecular masses of 140 and 18 kDa have been identified on Aplysia ganglia. The maximal binding capacities were 55 and 300-400 fmol (mg protein)-1 for membrane preparations from whole and buccal ganglia, respectively. These binding sites are of high affinity for neurotoxic PLA2s (Kd values, 100-800 pM) and of very low affinity for non-toxic PLA2s (Kd values in the micromolar range), thus indicating that these binding sites are presumably involved in the blockade of ACh release by neurotoxic PLA2s.
Full text
PDF

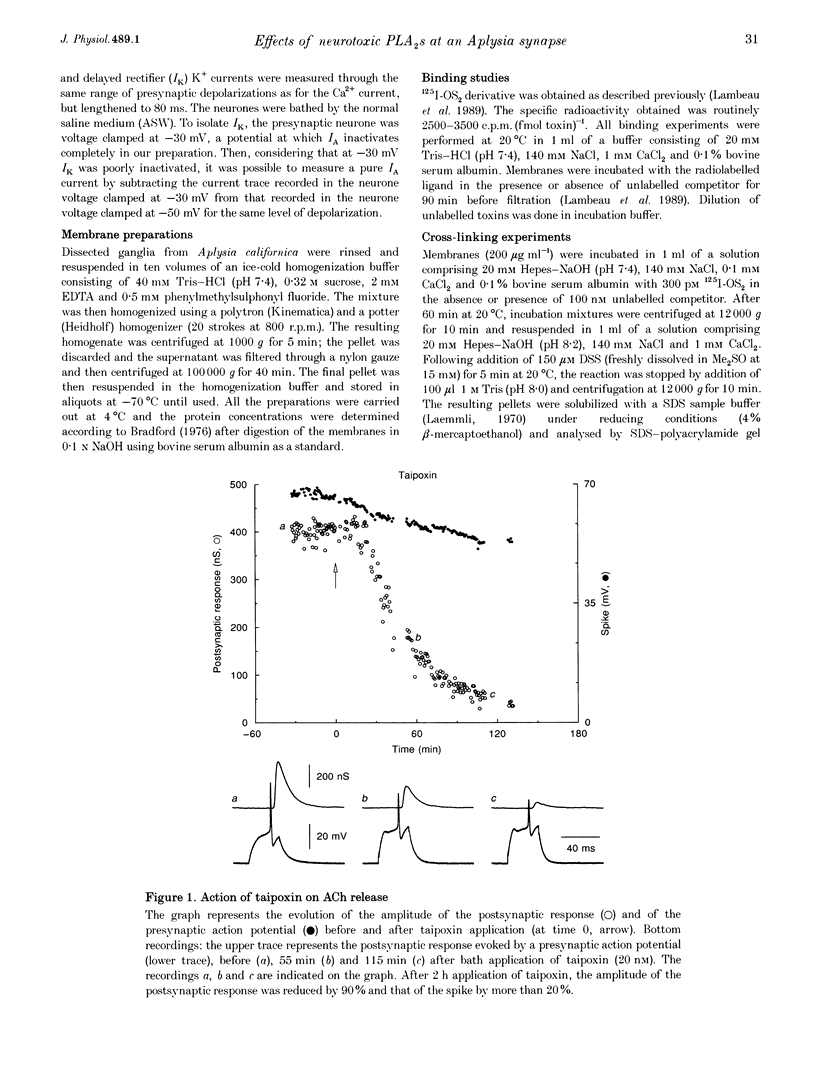
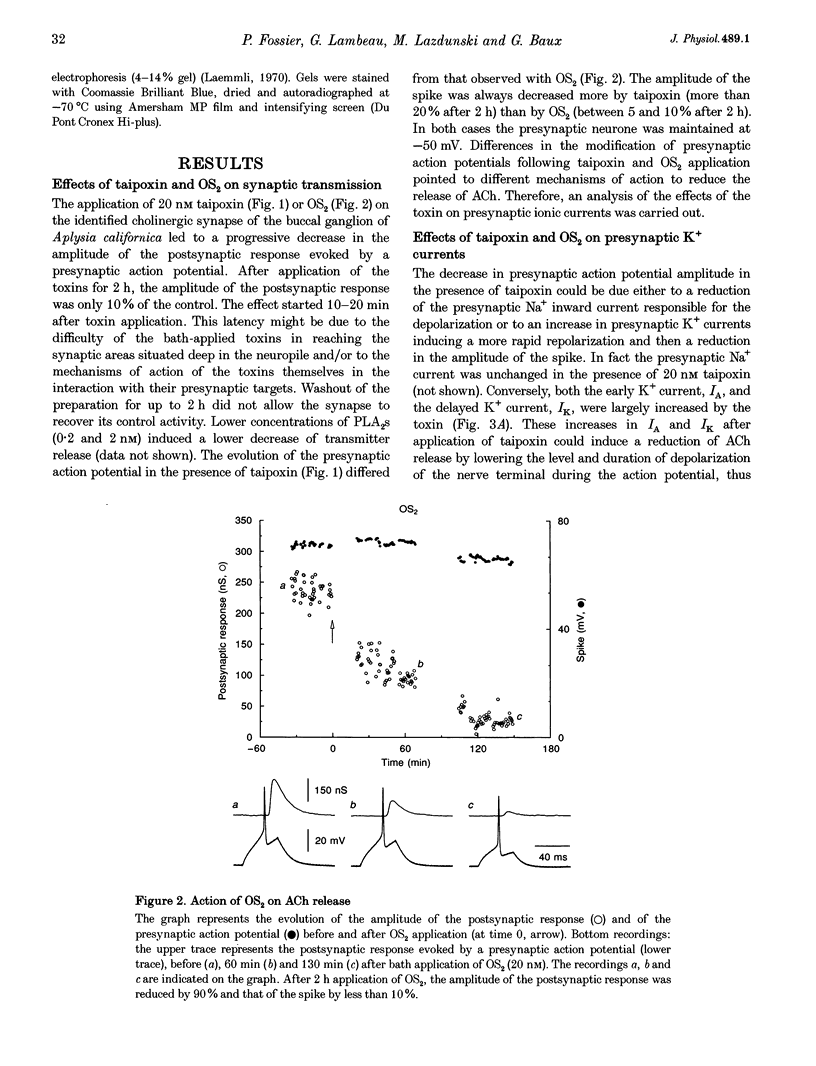








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asaoka Y., Oka M., Yoshida K., Sasaki Y., Nishizuka Y. Role of lysophosphatidylcholine in T-lymphocyte activation: involvement of phospholipase A2 in signal transduction through protein kinase C. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6447–6451. doi: 10.1073/pnas.89.14.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baux G., Fossier P., Tauc L. Histamine and FLRFamide regulate acetylcholine release at an identified synapse in Aplysia in opposite ways. J Physiol. 1990 Oct;429:147–168. doi: 10.1113/jphysiol.1990.sp018249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baux G., Fossier P., Trudeau L. E., Tauc L. Transmitter release and calcium currents at an Aplysia buccal ganglion synapse--II. Modulation by presynaptic receptors. Neuroscience. 1993 Mar;53(2):581–593. doi: 10.1016/0306-4522(93)90223-3. [DOI] [PubMed] [Google Scholar]
- Baux G., Tauc L. Presynaptic actions of curare and atropine on quantal acetylcholine release at a central synapse of Aplysia. J Physiol. 1987 Jul;388:665–680. doi: 10.1113/jphysiol.1987.sp016637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Penner R. The actions of presynaptic snake toxins on membrane currents of mouse motor nerve terminals. J Physiol. 1987 May;386:455–463. doi: 10.1113/jphysiol.1987.sp016544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohlman J., Eaker D., Karlsoon E., Thesleff S. Taipoxin, an extremely potent presynaptic neurotoxin from the venom of the australian snake taipan (Oxyuranus s. scutellatus). Isolation, characterization, quaternary structure and pharmacological properties. Eur J Biochem. 1976 Sep 15;68(2):457–469. doi: 10.1111/j.1432-1033.1976.tb10833.x. [DOI] [PubMed] [Google Scholar]
- Fossier P., Baux G., Tauc L. Activation of protein kinase C by presynaptic FLRFamide receptors facilitates transmitter release at an aplysia cholinergic synapse. Neuron. 1990 Oct;5(4):479–486. doi: 10.1016/0896-6273(90)90087-v. [DOI] [PubMed] [Google Scholar]
- Fossier P., Baux G., Tauc L. N- and P-type Ca2+ channels are involved in acetylcholine release at a neuroneuronal synapse: only the N-type channel is the target of neuromodulators. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4771–4775. doi: 10.1073/pnas.91.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Valtorta F., Czernik A. J., Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993 Feb 5;259(5096):780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Guillemare E., Honoré E., Pradier L., Lesage F., Schweitz H., Attali B., Barhanin J., Lazdunski M. Effects of the level of mRNA expression on biophysical properties, sensitivity to neurotoxins, and regulation of the brain delayed-rectifier K+ channels Kv1.2. Biochemistry. 1992 Dec 15;31(49):12463–12468. doi: 10.1021/bi00164a024. [DOI] [PubMed] [Google Scholar]
- Honoré E., Barhanin J., Attali B., Lesage F., Lazdunski M. External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1937–1941. doi: 10.1073/pnas.91.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini R. M., Evans H. J. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon. 1989;27(6):613–635. doi: 10.1016/0041-0101(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Lambeau G., Barhanin J., Schweitz H., Qar J., Lazdunski M. Identification and properties of very high affinity brain membrane-binding sites for a neurotoxic phospholipase from the taipan venom. J Biol Chem. 1989 Jul 5;264(19):11503–11510. [PubMed] [Google Scholar]
- Lambeau G., Schmid-Alliana A., Lazdunski M., Barhanin J. Identification and purification of a very high affinity binding protein for toxic phospholipases A2 in skeletal muscle. J Biol Chem. 1990 Jun 5;265(16):9526–9532. [PubMed] [Google Scholar]
- Magazanik L. G., Gotgilf I. M., Slavnova T. I., Miroshnikov A. I., Apsalon U. R. Effects of phospholipase A2 from cobra and bee venom on the presynaptic membrane. Toxicon. 1979;17(5):477–488. doi: 10.1016/0041-0101(79)90281-2. [DOI] [PubMed] [Google Scholar]
- Mollier P., Brochier G., Morot Gaudry-Talarmain Y. The action of notexin from tiger snake venom (Notechis scutatus scutatus) on acetylcholine release and compartmentation in synaptosomes from electric organ of Torpedo marmorata. Toxicon. 1990;28(9):1039–1052. doi: 10.1016/0041-0101(90)90142-t. [DOI] [PubMed] [Google Scholar]
- Müller M., Szewczyk A., De Weille J. R., Lazdunski M. ATP-sensitive K+ channels in insulinoma cells are activated by nonesterified fatty acids. Biochemistry. 1992 May 19;31(19):4656–4661. doi: 10.1021/bi00134a017. [DOI] [PubMed] [Google Scholar]
- Ordway R. W., Singer J. J., Walsh J. V., Jr Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991 Mar;14(3):96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Othman I. B., Spokes J. W., Dolly J. O. Preparation of neurotoxic 3H-beta-bungarotoxin: demonstration of saturable binding to brain synapses and its inhibition by toxin I. Eur J Biochem. 1982 Nov;128(1):267–276. doi: 10.1111/j.1432-1033.1982.tb06961.x. [DOI] [PubMed] [Google Scholar]
- Petersen M., Penner R., Pierau F. K., Dreyer F. Beta-bungarotoxin inhibits a non-inactivating potassium current in guinea pig dorsal root ganglion neurones. Neurosci Lett. 1986 Jul 11;68(1):141–145. doi: 10.1016/0304-3940(86)90244-2. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Scheller R. H. Mechanisms of vesicle docking and fusion: insights from the nervous system. Curr Opin Cell Biol. 1994 Aug;6(4):555–560. doi: 10.1016/0955-0674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Piomelli D. Arachidonic acid in cell signaling. Curr Opin Cell Biol. 1993 Apr;5(2):274–280. doi: 10.1016/0955-0674(93)90116-8. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Greengard P. Lipoxygenase metabolites of arachidonic acid in neuronal transmembrane signalling. Trends Pharmacol Sci. 1990 Sep;11(9):367–373. doi: 10.1016/0165-6147(90)90182-8. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Volterra A., Dale N., Siegelbaum S. A., Kandel E. R., Schwartz J. H., Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987 Jul 2;328(6125):38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Possani L. D., Mochca-Morales J., Amezcua J., Martin B. M., Prestipino G., Nobile M. Anionic currents of chick sensory neurons are affected by a phospholipase A2 purified from the venom of the taipan snake. Biochim Biophys Acta. 1992 Apr 7;1134(3):210–216. doi: 10.1016/0167-4889(92)90178-e. [DOI] [PubMed] [Google Scholar]
- Rehm H., Betz H. Binding of beta-bungarotoxin to synaptic membrane fractions of chick brain. J Biol Chem. 1982 Sep 10;257(17):10015–10022. [PubMed] [Google Scholar]
- Rehm H., Lazdunski M. Existence of different populations of the dendrotoxin I binding protein associated with neuronal K+ channels. Biochem Biophys Res Commun. 1988 May 31;153(1):231–240. doi: 10.1016/s0006-291x(88)81213-0. [DOI] [PubMed] [Google Scholar]
- Rehm H. Molecular aspects of neuronal voltage-dependent K+ channels. Eur J Biochem. 1991 Dec 18;202(3):701–713. doi: 10.1111/j.1432-1033.1991.tb16425.x. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994 Nov 3;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rowan E. G., Harvey A. L. Potassium channel blocking actions of beta-bungarotoxin and related toxins on mouse and frog motor nerve terminals. Br J Pharmacol. 1988 Jul;94(3):839–847. doi: 10.1111/j.1476-5381.1988.tb11595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T., Asaoka Y., Oka M., Yoshida K., Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: its possible implications. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. L., Coffield J. A., Bakry N. Inhibition of vacuolar adenosine triphosphatase antagonizes the effects of clostridial neurotoxins but not phospholipase A2 neurotoxins. J Pharmacol Exp Ther. 1994 Apr;269(1):256–262. [PubMed] [Google Scholar]
- Südhof T. C., De Camilli P., Niemann H., Jahn R. Membrane fusion machinery: insights from synaptic proteins. Cell. 1993 Oct 8;75(1):1–4. [PubMed] [Google Scholar]
- Ueno E., Rosenberg P. Inhibition of phosphorylation of synapsin I and other synaptosomal proteins by beta-bungarotoxin, a phospholipase A2 neurotoxin. J Neurochem. 1992 Dec;59(6):2030–2039. doi: 10.1111/j.1471-4159.1992.tb10091.x. [DOI] [PubMed] [Google Scholar]




