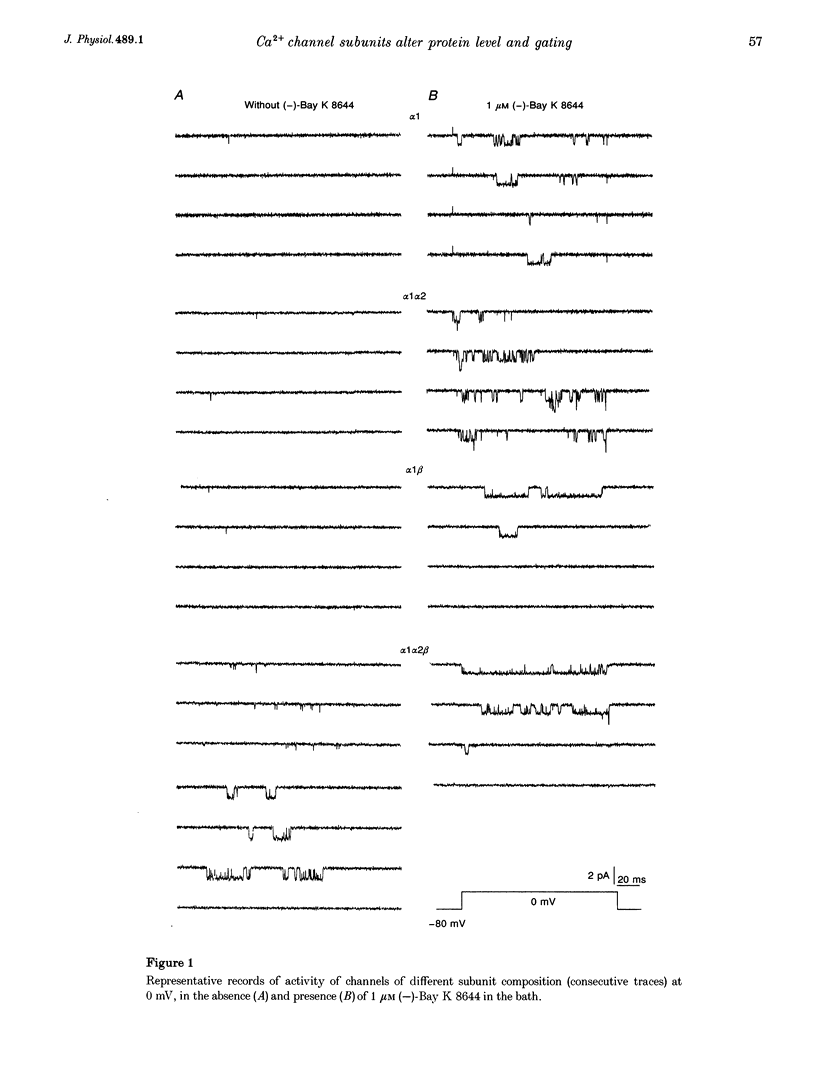
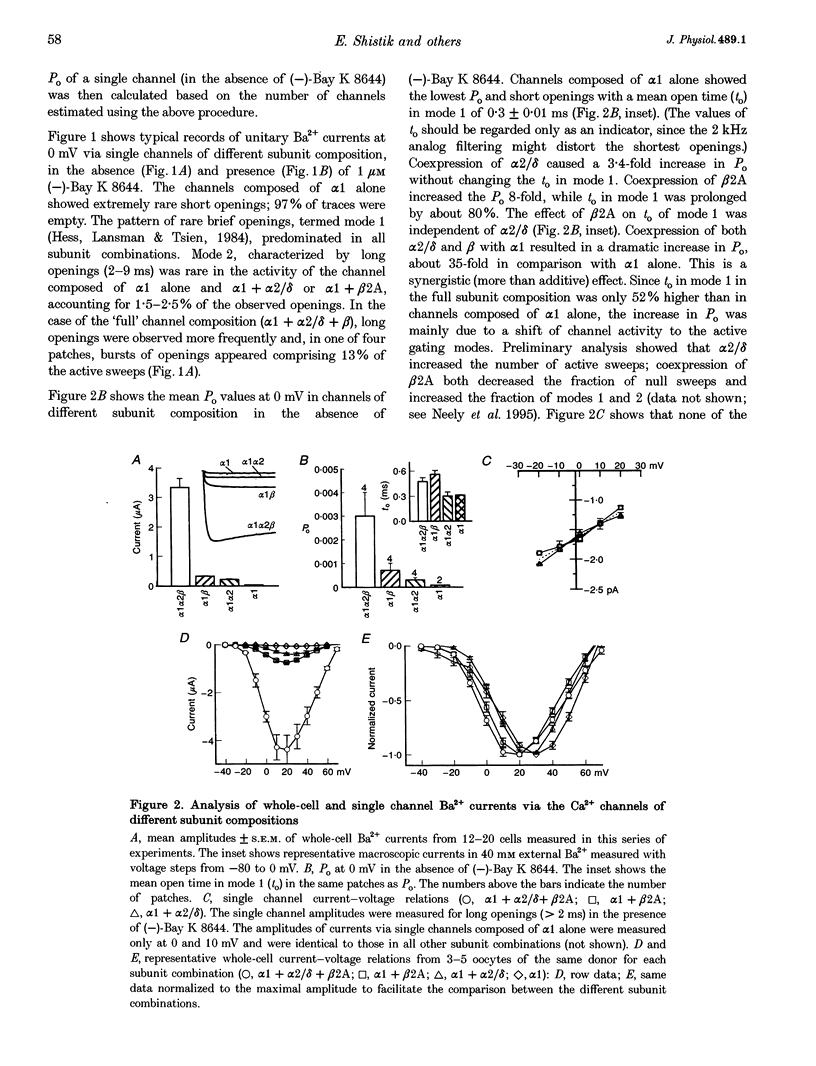
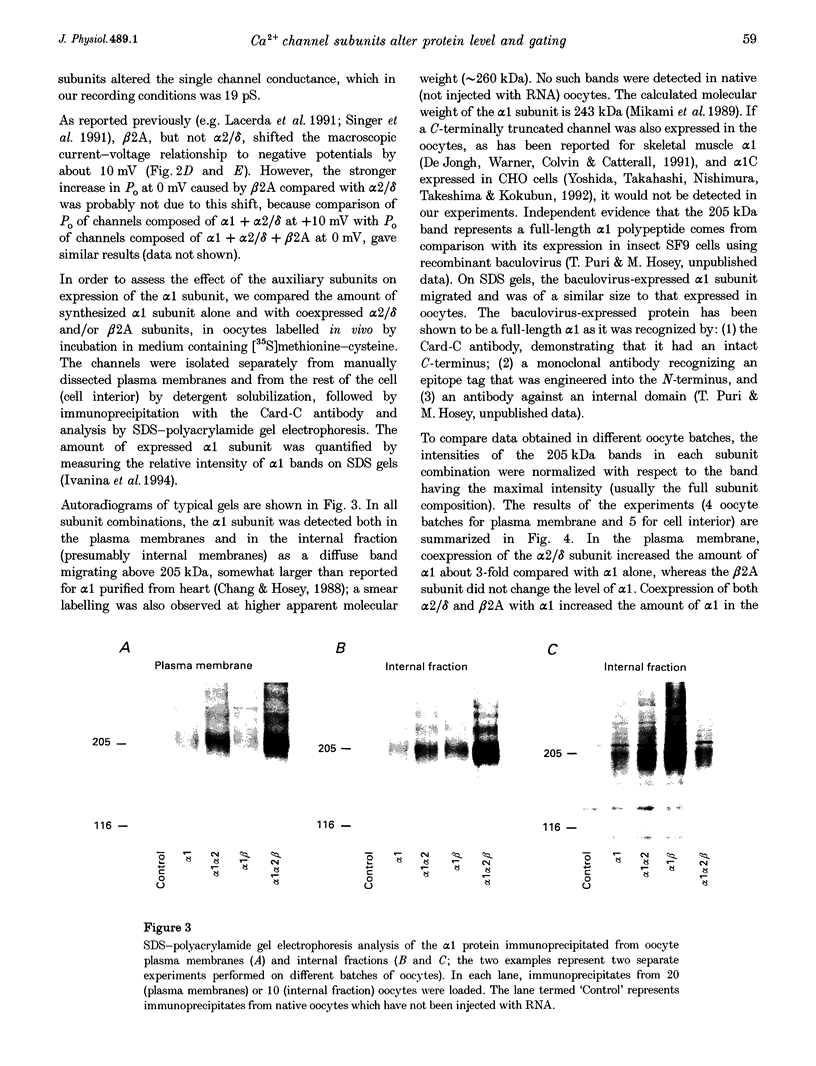
Abstract
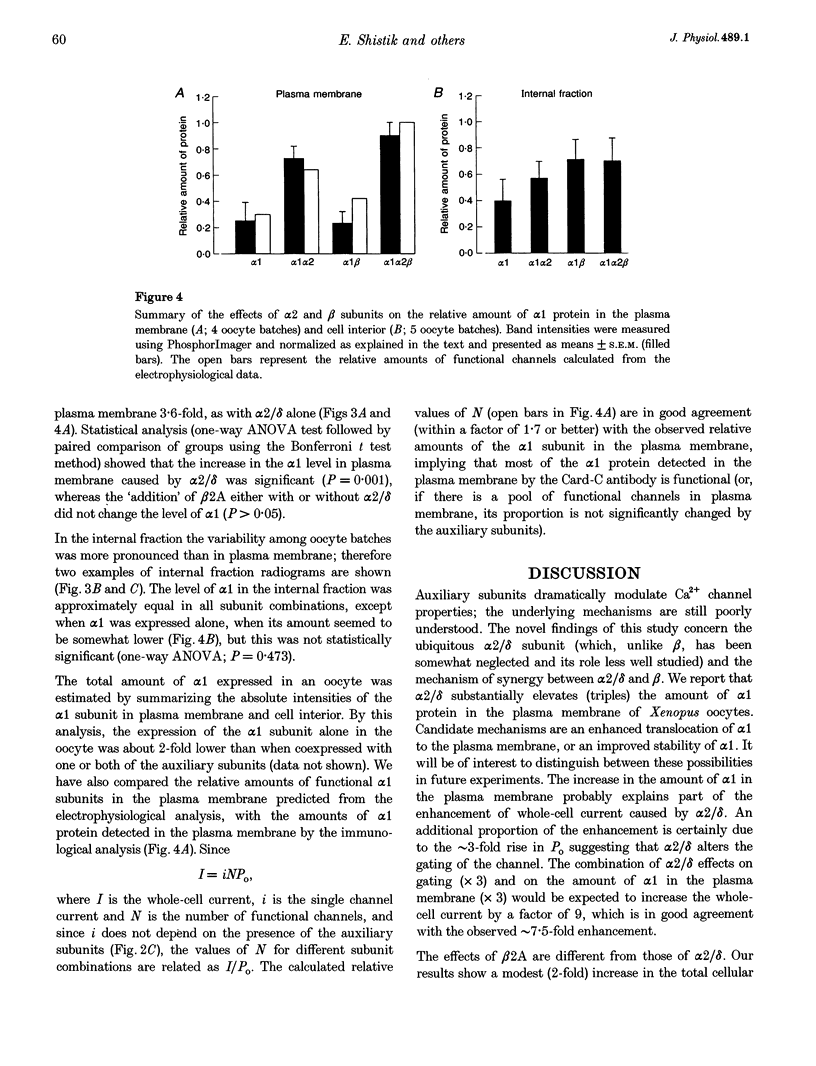
1. A combined biochemical and electrophysiological approach was used to determine the mechanism by which the auxiliary subunits of Ca2+ channel enhance the macroscopic Ca2+ currents. Xenopus oocytes were injected with RNA of the main pore-forming subunit (cardiac: alpha 1C), and various combinations of RNAs of the auxiliary subunits (alpha 2/delta and beta 2A). 2. The single channel open probability (Po; measured at 0 mV) was increased approximately 3-, approximately 8- and approximately 35-fold by alpha 2/delta, beta 2A and alpha 2/delta+beta 2A, respectively. The whole-cell Ca2+ channel current was increased approximately 8- to 10-fold by either alpha 2/delta or beta 2A, and synergistically > 100-fold by alpha 2/delta+beta 2A. The amount of 35S-labelled alpha 1 protein in the plasma membrane was not changed by coexpression of beta 2A, but was tripled by coexpression of alpha 2/delta (either with or without beta). 3. We conclude that the increase in macroscopic current by alpha 2/delta is equally due to changes in amount of alpha 1 in the plasma membrane and an increase in Po, whereas all of the effect of beta 2A is due to an increase in Po. The synergy between alpha 2/delta and beta in increasing the macroscopic current is due mainly to synergistic changes in channel gating.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang F. C., Hosey M. M. Dihydropyridine and phenylalkylamine receptors associated with cardiac and skeletal muscle calcium channels are structurally different. J Biol Chem. 1988 Dec 15;263(35):18929–18937. [PubMed] [Google Scholar]
- De Jongh K. S., Warner C., Colvin A. A., Catterall W. A. Characterization of the two size forms of the alpha 1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Biel M., Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- Hullin R., Singer-Lahat D., Freichel M., Biel M., Dascal N., Hofmann F., Flockerzi V. Calcium channel beta subunit heterogeneity: functional expression of cloned cDNA from heart, aorta and brain. EMBO J. 1992 Mar;11(3):885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom L. L., De Jongh K. S., Catterall W. A. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994 Jun;12(6):1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- Ivanina T., Perets T., Thornhill W. B., Levin G., Dascal N., Lotan I. Phosphorylation by protein kinase A of RCK1 K+ channels expressed in Xenopus oocytes. Biochemistry. 1994 Jul 26;33(29):8786–8792. doi: 10.1021/bi00195a021. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Kim H. S., Ruth P., Perez-Reyes E., Flockerzi V., Hofmann F., Birnbaumer L., Brown A. M. Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991 Aug 8;352(6335):527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Mori Y., Friedrich T., Kim M. S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991 Apr 4;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Neely A., Olcese R., Baldelli P., Wei X., Birnbaumer L., Stefani E. Dual activation of the cardiac Ca2+ channel alpha 1C-subunit and its modulation by the beta-subunit. Am J Physiol. 1995 Mar;268(3 Pt 1):C732–C740. doi: 10.1152/ajpcell.1995.268.3.C732. [DOI] [PubMed] [Google Scholar]
- Neely A., Wei X., Olcese R., Birnbaumer L., Stefani E. Potentiation by the beta subunit of the ratio of the ionic current to the charge movement in the cardiac calcium channel. Science. 1993 Oct 22;262(5133):575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Takeshima H., Hofmann F., Flockerzi V., Imoto K. Requirement of the calcium channel beta subunit for functional conformation. FEBS Lett. 1993 Jun 21;324(3):283–286. doi: 10.1016/0014-5793(93)80135-h. [DOI] [PubMed] [Google Scholar]
- Singer D., Biel M., Lotan I., Flockerzi V., Hofmann F., Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991 Sep 27;253(5027):1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Varadi G., Lory P., Schultz D., Varadi M., Schwartz A. Acceleration of activation and inactivation by the beta subunit of the skeletal muscle calcium channel. Nature. 1991 Jul 11;352(6331):159–162. doi: 10.1038/352159a0. [DOI] [PubMed] [Google Scholar]
- Wakamori M., Mikala G., Schwartz A., Yatani A. Single-channel analysis of a cloned human heart L-type Ca2+ channel alpha 1 subunit and the effects of a cardiac beta subunit. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1170–1176. doi: 10.1006/bbrc.1993.2374. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Takahashi M., Nishimura S., Takeshima H., Kokubun S. Cyclic AMP-dependent phosphorylation and regulation of the cardiac dihydropyridine-sensitive Ca channel. FEBS Lett. 1992 Sep 14;309(3):343–349. doi: 10.1016/0014-5793(92)80804-p. [DOI] [PubMed] [Google Scholar]