Summary
Neurodegenerative diseases (NDs) are a group of neurological disorders characterized by the progressive dysfunction of neurons and glial cells, leading to their structural and functional degradation in the central and/or peripheral nervous system. Historically, research on NDs has primarily focused on the brain, brain stem, or spinal cord associated with disease-related symptoms, often overlooking the role of the cerebellum. However, an increasing body of clinical and biological evidence suggests a significant connection between the cerebellum and NDs. In several NDs, cerebellar pathology and biochemical changes may start in the early disease stages. This article provides a comprehensive update on the involvement of the cerebellum in the clinical features and pathogenesis of multiple NDs, suggesting that the cerebellum is involved in the onset and progression of NDs through various mechanisms, including specific neurodegeneration, neuroinflammation, abnormal mitochondrial function, and altered metabolism. Additionally, this review highlights the significant therapeutic potential of cerebellum-related treatments for NDs.
Subject areas: Neuroscience, Clinical neuroscience
Graphical abstract
Neuroscience; Clinical neuroscience
Introduction
Neurodegenerative diseases (NDs) are characterized by the progressive dysfunction of neuronal structure and function in the nervous system, with the highest incidence among the elderly. Patients with NDs exhibit high mortality and morbidity rates, and they are currently incurable.1
The cerebellum, situated beneath the cerebral hemispheres, consists of two hemispheres and a central vermis. At the cellular level, the cerebellum is rich in granule cells (GCs), Purkinje cells (PCs), and various types of glial cells. Traditionally, the cerebellum has been considered a structure controlling movement, such as motion, gait, posture, and balance. However, increasing research in recent years has highlighted the cerebellum’s significant non-motor functions, including cognitive, behavioral, and emotional processing.2 Cerebellar lobules VI, VII, Crus I, and Crus II have been shown to be associated with cognition and emotion.3,4 The cerebellum and cerebrum are connected via the cerebello-thalamo-cortical (CTC) circuit, with the functional connectivity (FC) networks of the cerebral cortex mapped to distinct cerebellar regions.5 In NDs, the misfolding and abnormal aggregation of pathogenic proteins, along with their formation and propagation, inevitably impact the cerebellum as well.6
In this review, we summarize the relationship between various NDs and the cerebellum, including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), Niemann-Pick C (NPC) disease, Huntington’s disease (HD), and frontotemporal dementia (FTD), with a focus on the pathological and biochemical changes in the cerebellum associated with these diseases. The cerebellum is implicated in ND (Table 1), so treatments targeting the cerebellum would be valuable in treating these diseases.
Table 1.
Clinical evidence linking cerebellum to neurodegenerative diseases
| Disease | Changes and functions of cerebellum | Reference |
|---|---|---|
| Alzheimer’s disease | Cerebellar atrophy | Toniolo et al.7; Chen et al.8 |
| Impairment of cortico-cerebellar functional connections | Tang et al.9 | |
| Aβ and phosphorylated Tau deposits | Sepulveda-Falla et al.10 | |
| Purkinje cell loss | Sepulveda-Falla et al.11 | |
| Activation of microglia and astrocytes | Singh-Bains et al.12 | |
| Parkinson’s disease | Cerebellar atrophy | Ma et al.13; Kerestes et al.14 |
| Early cerebellar functional connectivity changes, associated with cognitive impairment | Dan et al.15 | |
| Associated with rest tremor and gait disorders | Piccinin et al.16; Maiti et al.17 | |
| α-synuclein | Seidel et al.18 | |
| Purkinje cell injury | Hartstone et al.19 | |
| Dopaminergic transmission markers' expression levels decrease | Hurley et al.20 | |
| Amyotrophic Lateral Sclerosis | Cerebellar atrophy | Gellersen et al.21 |
| Associated with motor and cognitive impairment | Consonni et al.22 | |
| Insoluble and ubiquitinated p62 positive aggregates | Al-Sarraj et al.23 | |
| FUS RNA-binding protein expression | Tateishi et al.24 | |
| Purkinje cell loss | Tan et al.25 | |
| Activation of microglia and astrocytes | Sala et al.26 | |
| Niemann-Pick C disease | Cerebellar atrophy | Bowman et al.27 |
| Lysosome damage | Chung et al.28 | |
| Purkinje cell loss | Sarna et al.29 | |
| Huntington’s disease | Cerebellar atrophy | Ruocco et al.30 |
| The progression of HD is positively correlated with the degree of cerebellar atrophy. | Ruocco et al.31 | |
| Associated with motor dysfunction and psychiatric symptoms | Rees et al.32 | |
| Purkinje cell loss | Singh-Bains et al.33 | |
| A significant loss of the presynaptic marker synaptic vesicle protein 2A | Delva et al.34 | |
| Frontotemporal Dementia | Cerebellar atrophy | Guo et al.5 |
| The highest DPR load | Quaegebeur et al.35 | |
| Associated with behavioral and cognitive disorders. | Chen et al.36 | |
| Associated with disease progression | van Blitterswijk et al.37 |
Cerebellum and NDs
AD and cerebellum
AD is predominantly recognized for its debilitating impact on memory and cognitive functions.38 While past research has mainly focused on the cerebrum and the hippocampus, emerging studies reveal the cerebellum’s role in AD. The changes in cerebellar gray matter volume in AD patients are related to disease progression.7 Our team’s previous research reveals electrophysiological alterations in the cerebellum of AD-related APP/PS1 mice before any pathological changes.39 In the early stages of AD, the cerebellum may have already been affected by amyloid β-protein (Aβ) toxicity, subsequently impacting motor functions.40 Changes in cerebellar structure and function may be related to disease progression and could aid in the early diagnosis of AD.
Clinical symptoms and cerebellum
Cognitive and psychiatric disorders in AD patients are linked to the cerebellum.41 Cerebellar degeneration correlates with memory impairments in patients with mild cognitive impairment (MCI).42 The cerebellum has a higher predictive value for MCI than changes in the cerebral cortex.43,44 Significant morphological changes in PCs of the anterior lobe in AD patients may affect cognitive functions.45 Recent research has found that genes related to intelligence and cognitive functions are expressed in the cerebellum.46,47 The cerebellum may regulate cognitive functions in the early stages of AD.48
AD patients carrying the APOE4 variant exhibit a local reduction in cerebellar volume and thinner cortex.49 The cerebellum may influence motor dysfunction symptoms of AD. Most patients with the PS1 E280A variant present symptoms of cerebellar ataxia.10 AD-related TgCRND8 mice exhibit significant deficits in motor coordination and balance.50 Five-month-old APP/PS1 mice begin to show rotarod and balance beam performance impairments.51 Twelve-month-old APP/PS1 mice display evident motor dysfunctions.52 PS1-FAD mice exhibit mild ataxia before Aβ deposition in the cerebellum.11
In asymptomatic preclinical AD and MCI patients, increased cerebellar FC may represent functional compensation.53 Increased cerebellar activation in MCI patients may compensate for defects in the brain-cerebellar circuit.54 The cerebellum’s reserve and compensatory mechanisms could have neuroprotective value.55 As the disease progresses to later stages, cortico-cerebellar FC is significantly disrupted in AD patients, accompanied by a marked decline in cognitive function.9,56 The disruption of cerebellar FC may be associated with pathological changes in AD. Cerebrospinal fluid phosphorylated Tau (p-Tau) and Aβ42 disrupt synapses and cortical networks, leading to cognitive impairment.57 Additionally, Aβ influences brain-cerebellar FC.58 In AD, the cerebellum is associated with cognitive and motor disorders, and significant changes may occur early in the cerebellum. The cerebellum’s compensatory mechanisms could help alleviate clinical symptoms in the early stages.
Aβ and Tau changes in AD cerebellum
AD patients have Aβ and p-Tau deposits in the cerebellum.10,11 Aβ plaques begin to appear in the cerebellum ten years before the onset of autosomal dominant AD.59 The cerebellar Aβ42 correlates with disease progression in AD patients.60 In APP/PS1 mice, soluble Aβ42 levels at two months of age are about half that of the cerebral cortex, and at eight months, soluble Aβ42 levels are 40% higher than in the cerebral cortex.61 Numerous studies demonstrated that APP expression level in the cerebellum of AD mice is 1.1 times that of the cerebral cortex and 1.6 times that of the hippocampus, with molecular layer Aβ plaques appearing and increasing with age.52,62 In patients with AD, fragmentation of the Golgi apparatus in cerebellar PCs is associated with abnormal protein aggregation and synaptic dysfunction.63 Typical AD pathological changes can be found in the cerebellum of patients and animal models, correlating with the disease progression.
Neurodegeneration in AD cerebellum
The cerebellum contains various cell types and exhibits distinct atrophy in AD patients.8 The correlation between Aβ42 deposition and PC damage has been observed in AD patients.60 AD patients have shown a loss of PCs and a significant decrease in dendritic branching density.11,64 Additionally, AD patients exhibit defects in cerebellar calcium-binding proteins and neurotrophic receptors.65 However, one study showed that the total volume of the cerebellum decreased by 12.7% in AD without changes in the number of PCs.66 The cause of this phenomenon may be related to patients at different stages of the disease. The proliferation of astrocytes and activation of microglia have been observed in the cerebellum of AD patients.12 The PC loss in APP/PS1 mice cerebellum occurred as early as five months of age.51 In the AD cerebellum, loss of PCs and GCs, activation of microglia, and proliferation of astrocytes often occur.
Mitochondrial and oxidative stress in AD cerebellum
Redox imbalance is shown in the cerebellum during the preclinical and MCI stages of AD patients.67 Mitochondrial function in the cerebellum of AD patients is abnormal.11 Concurrently, an abnormal accumulation of reactive oxygen species (ROS) in the cerebellum of AD patients impairs cognitive function.67 Our previous research has revealed that mitochondrial dysfunction and oxidative stress can mutually exacerbate each other in the brains of AD, a process that may also occur in the cerebellum.68 Mitochondrial abnormalities appear in the cerebellum of 18-month-old APP/PS1 mice.52 Genes related to mitochondrial dysfunction significantly change in the cerebellum of 7-week-old 5xFAD mice.69 Additionally, NADPH oxidase and oxidative stress are activated in the cerebellum of TgCRND8 mice.50 The early AD cerebellum exhibits mitochondrial dysfunction and increased oxidative stress.
Metabolic disturbance and neurotransmitter changes
Brain metabolic dysregulation is related to AD. Metabolic increase in the cerebellum is observed during the transition from MCI to AD.70 High metabolism is found in AD cerebellar regions.71 In the cerebellum of APP/PS1 mice, extensive metabolic changes have been observed, mainly the dysregulation of energy and amino acid metabolism.72 Furthermore, in the 3xTg-AD mice cerebellum, proteins related to energy metabolism are altered.73 In AD mice, there is dysregulation of cholesterol metabolism in the cerebellum, characterized by elevated levels of the cholesterol precursor desmosterol and cholesterol metabolites, which may be associated with the Seladin-1/Dhcr24 gene.74 Dysregulation of noradrenergic modulation in the cerebellum of 2-month-old TgCRND8 mice suggests early neurotransmitter changes.50 Cholinergic dysfunction is one of the hallmark features of AD. The cerebellum may directly impact the cholinergic function of AD patients.75 In APP/PS1 mice, abnormal expression of Ly6/uPAR proteins in the cerebellum leads to dysfunction of the cholinergic system.76 In AD, the cerebellum exhibits various metabolic dysregulations and neurotransmitter imbalances.
AD cerebellum displays a number of pathological and biochemical changes (Figure 1). In the early stages, the cerebellum may play a compensatory role, slowing the progression of clinical symptoms.54,55 The AD cerebellum exhibits typical Aβ and Tau pathology, relating to cognitive and behavioral changes. Changes in mitochondria, oxidative stress, and metabolism in the AD cerebellum are significant and may occur early in the disease.11,67,70 Furthermore, loss of PCs, reduction in GCs, activation of microglia, and proliferation of astrocytes are observed in the AD cerebellum. The expression levels of APP in the cerebellum and the cerebrum are comparable during the same period, but Aβ plaques are sparser in the cerebellum.77 Cerebellum’s unique cell types and cytokines may provide the intrinsic mechanisms of delayed AD pathological changes. It seems that structural and functional changes occur in the AD cerebellum, and its FC with other brain regions also changes.
Figure 1.
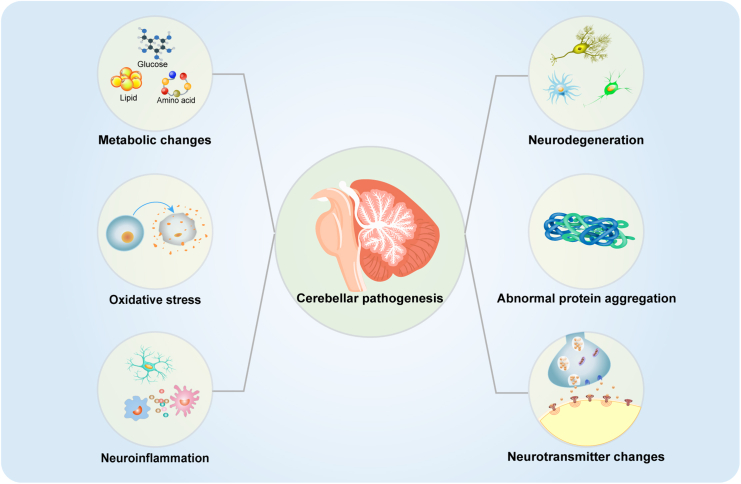
Major pathological and biochemical changes in the cerebellum in NDs
This figure summarizes the reported cerebellum-related pathological and biochemical changes in NDs. Neurodegeneration, metabolic disorders, neuroinflammation, oxidative stress and abnormal protein aggregation, are related to the occurrence and development of NDs.
PD and cerebellum
PD Patients exhibit clinical symptoms, including bradykinesia, rigidity, tremors, gait disturbances, cognitive impairments, and psychiatric symptoms. Pathophysiological changes in PD involve abnormalities in multiple brain regions. The cerebellum contains dopamine receptors and receives dopaminergic projections, with the pathway between the cerebellum and the ventral tegmental area of the midbrain transmitting dopamine to the prefrontal cortex.78 There are anatomical connections between the cerebellum and the basal ganglia.79 In PD, α-synuclein and dopaminergic dysfunction are present in the cerebellum.80 PD patients exhibit notable cerebellar gray matter atrophy, aiding in disease diagnosis.13 Cerebellar changes in PD patients may relate to clinical symptoms and disease staging, gradually decreasing in volume as the disease progresses.14 Different cerebellar subregions in PD patient subtypes exhibit varied change patterns, facilitating subtype differentiation.81 Increased activation of the cerebellum in PD patients may reflect a compensatory mechanism.82 In the early stages of PD, the basal ganglia-cerebellar connection compensates for deficits in the nigrostriatal-basal ganglia-cortical circuit, but the cerebellar circuit function weakens as the disease progresses to later stages.83 Overall, cerebellar abnormalities may broadly impact the progression of PD.
Psychiatric symptoms and cerebellum
The cerebellum of PD patients affects cognitive function.84 PD patients show early cerebellar FC changes, potentially related to cognitive deficits.15 In patients with PD, cerebellar vermis FC is related to cognitive function.85 Moreover, cerebellar changes can be used to distinguish PD patients with or without MCI.86 Increased FC between the caudate and cerebellum helps alleviate cognitive impairments in PD patients.87 The cerebellum may be involved in depression and anxiety. The cerebellum is crucial to the mechanism of depression in PD.88 Changes in cerebellar FC are significantly related to anxiety in PD patients.89 Depression is inversely associated with right cerebellar IX volume, and anxiety is inversely associated with right lobule VIII volume.13 Cerebellar FC aids in diagnosing PD patients with hallucinations.90 Collectively, the cerebellum is related to the emergence of psychiatric symptoms in PD, including cognition, anxiety, depression, and hallucinations.
Motor impairment and cerebellum
Cerebellar dysfunction may be associated with motor dysfunctions, including rest tremors and gait instability. In PD patients, the cerebellum is implicated in the mechanism of rest tremors.16 Moreover, cerebellar lobule IV correlates with the severity of tremors in PD patients.91 Dopamine-resistant tremors may be related to the cerebellum.92 Increased FC between the left cerebellar dentate nucleus and other brain areas is observed in PD patients with motor dysfunctions.93 Increased FC among cerebellar structures in PD may have compensatory effects for restoring motor functions.94 Cerebellar vermis dysfunction is related to gait disturbances.17 FC in the cerebellar motor areas correlates with the severity of freezing of gait in PD.95 Clearly, the cerebellum’s involvement is linked to the occurrence and severity of motor dysfunctions in PD.
Neurotransmitter changes in PD cerebellum
The cerebellum contains high levels of dopamine and widely distributed dopamine receptors.96 Aldose reductase, associated with dopamine synthesis, shows a significantly reduced level in the PD cerebellum.97 Dopaminergic transmission expression levels are decreased in the PD cerebellum.20 Early compensatory cholinergic upregulation occurs in the cerebellum of PD patients.98 Additionally, the cerebellar neurotransmitter systems in PD are related to cognitive functions.78 The cerebellar noradrenergic system may relate to cognitive, emotional, essential tremor, and motor in PD.99 Gamma-Aminobutyric Acid (GABA) level in the cerebellum are related to cognitive decline in PD patients.100 In PD rats, an imbalance between excitatory and inhibitory amino acids in the cerebellum is associated with redox imbalance and a significant increase in TNF-α.101 Various neurotransmitter disturbances occur in the PD cerebellum, correlating with clinical symptoms.
Neurodegeneration in PD cerebellum
Intracellular aggregation of α-synuclein is a hallmark pathological change in PD. Patients with α-synucleinopathy exhibit cerebellar connectivity disorders.102 Neurons and oligodendrocytes in the PD cerebellum are affected by α-synuclein.18 A significant loss of PCs is observed in essential tremor.19 Changes in the cerebellar climbing fiber-PC synaptic connections correlate positively with the severity of essential tremor.103 GABA neurons and oligodendrocytes in the PD mouse cerebellum are enriched with PD-related genes.104 It looks like various cells in the PD cerebellum undergo morphological and functional changes.
Metabolic changes and PD cerebellum
The cerebellum exhibits hypermetabolism in PD monkey models.105 Similarly, PD patients with dysphagia also show increased cerebellar metabolism.106 Cerebellar metabolism increases before the first fall in PD patients.107 Disease progression in PD patients is associated with increased metabolism of the cerebellum.108 PD patients exhibit changes in oxygen metabolism in the cerebellum.109 During the cognitive impairment stage of PD, regional cerebral glucose metabolism in the cerebellum increases.110 Cerebellar inosine increases, while tyrosine and pantothenate decrease in PD dementia patients.111 Metabolic changes in the cerebellum contribute to the early diagnosis and disease course evaluation of PD.
The cerebellum in PD undergoes significant atrophic changes. The compensatory function of the cerebellum can delay clinical symptoms in the early stages, but this compensatory ability may be lost in the later stages. Cerebellar abnormalities are related to motor and psychiatric symptoms. The PD cerebellum is also affected by α-synuclein. Neurotransmitter changes and metabolic alterations occur in the PD cerebellum, correlating with disease progression. In summary, studies on PD patients and animal models indicate that the cerebellum is affected in PD and has a broad impact on disease progression (Figure 1). Exploring cerebellar changes in PD can deepen our understanding of the disease and aid in its treatment and symptom improvement.
ALS and cerebellum
The characteristic pathological changes in ALS include glutamate excitotoxicity, protein misfolding and abnormal aggregation, inflammation, apoptosis, mitochondrial dysfunction, and oxidative stress.112 Over the past decades, pathogenic mutations in several genes, including C9orf72 on chromosome 9, superoxide dismutase 1 (SOD1), TAR DNA-binding protein (TDP-43), FUS RNA-binding protein (FUS), and several others, have been identified in ALS.113 Autopsy results of patients with FUS mutations show FUS expression and neurodegenerative changes in the cerebellum.24 SOD1 mutations are the second most common mutation in ALS, with some patients exhibiting cerebellar ataxia symptoms.114 GFP-PR28 transgenic mice partially mimic the pathological features of ALS, showing a reduction in PCs and cerebellar inflammation.115
Cerebellar atrophy and compensation
The cerebellar volume reduction and significant atrophy are observed in ALS patients.21 A GWAS meta-analysis also identifies the cerebellum as a functionally implicated organ in ALS.116 Symptoms in ALS patients may be related to changes in different cerebellar subregions. The involvement of various cerebellar areas may be associated with motor or cognitive impairments in ALS.22 The motor symptoms in ALS patients are related to atrophy in the lower lobules.117 The anterior lobules I-V of the cerebellum are implicated in sporadic ALS patients, while the posterior lobe and vermis are affected in carriers of the C9orf72 mutation.118 The cerebellum may play compensatory roles in ALS patients. The cerebellum in ALS patients may mitigate clinical symptoms in the early stages, but this compensation may be depleted as the disease progresses.119 The cerebellum is affected in ALS and the alterations of the cerebellum correlate with the changes of clinical symptoms.
Protein aggregates in ALS cerebellum
The repeated amplification of hexanucleotide in C9orf72 transforms into dipeptide repeat proteins (DPRs), forming insoluble and ubiquitinated p62 positive aggregates that are highly expressed in the cerebellum of ALS patients.23 The cerebellum of C9-ALS may initially be affected by DPRs.120 The ALS cerebellum expresses p62 positive and ubiquitinated aggregates, but there are no TDP-43 positive inclusions.121 RAN translation produces five types of repeat dipeptide proteins (GP, GA, GR, PR, PA), constituting the main components of TDP-43-negative and p62-positive inclusions.122 And inclusions of GP and GA are abundant in the cerebellum.123
Neurodegeneration in ALS cerebellum
Significant loss of PCs in the vermis of ALS patients, along with ATXN2 repeat expansions, has been observed.25 Overexpression of SOD1 leads to PC degeneration.124 GFP-PR28 transgenic mice, MATR3 S85C knock-in mice, and Tbk1-NKO mice partially mimic ALS neuropathological features, with significant reductions and morphological abnormalities in PCs.115,121 ALS patients exhibit increased reactive astrocytes and activated microglia.26 Moreover, microglia activation in the cerebellum of SOD1 mutation patients has been reported.125 Loss of PCs, along with increased microglia and astrocytes, occurs in the ALS cerebellum.
Neuroinflammation and oxidative stress in ALS cerebellum
Activation of microglia and neuroinflammation have been detected in the cerebellum of SOD1 mutation patients.125 In ALS Wobbler mice, abnormal protein aggregation in the cerebellum leads to elevated expression of IL-1β and TNF-α, an increase in microglial and astrocytic cells, ultimately resulting in motor deficits.126 Moreover, inflammatory changes are also found in the cerebellum of GFP-PR28 mice.115 ALS patients exhibit oxidative stress significant inflammation and increased oxidative stress in the cerebellum.127
The ALS cerebellum is affected by the typical pathology of ALS, exhibiting structural and functional abnormalities (Figure 1). Changes in the ALS cerebellum relate to clinical symptoms, and the cerebellum may have compensatory functions, which could be depleted in the later stages. Inflammation is evident in the ALS cerebellum. The contribution of the cerebellum to ALS requires careful consideration, and further study of this largely overlooked neuroanatomical area is warranted.
NPC disease and cerebellum
The cerebellum is severely affected in the early stages of NPC disease.128 Loss of cerebellar volume is related to the severity of clinical symptoms in NPC patients.27 In Npc1 mice, increased oxidative stress in the cerebellum causes lysosomal membrane damage and alterations in permeability, leading to leakage of lysosomal contents and ultimately resulting in cerebellar degeneration.28 In Npc1 mice, defects in Sonic hedgehog signaling in the cerebellum lead to proliferative deficits in GCs and abnormalities in cerebellar morphology.129
Neurodegeneration in NPC cerebellum
PC deaths occur in the cerebellum of NPC patients, increasing in number as the disease progresses.29 PCs are particularly sensitive to NPC1 deficiency and undergo degeneration early in the disease, while early activation of microglia may precede PC degeneration.130 In the cerebellum of Npc1 mice, the activation and interactions of microglial cells promote the degeneration of PCs.131 The death of cerebellar PCs is associated with increased levels of caspase 1, caspase 3, NPC2, LipA, apoE, apoD, GFAP, and TNF-α.132 Microglial activation precedes neuronal dysfunction in presymptomatic 3-week-old Npc1 mice.133 There is a significant early increase in astrocytes in the cerebellum of Npc1 mice.134 Additionally, proliferative defects in cerebellar GCs and impaired differentiation of cerebellar glomeruli are reported in Npc1 mice.129 In the cerebellum of Npc1 mice, elevated levels of cathepsins, cytochrome c, and Bax2 play a role in neuronal degeneration.135
Lipid metabolism disorders and NPC cerebellum
NPC1 disease is characterized by neuronal lipid storage in the cerebellum. Elevated levels of the gangliosides GM2 and GM3 in the cerebellar posterior lobules of NPC1 disease are associated with lipid alterations and cell death.136 In Npc1 mice, a significant increase in GM2 in deep cerebellar nuclei (DCN) neurons and the absence of the lipid raft marker Flot2 expression lead to cellular dysfunction.137 In the cerebellum of Npc1 mice, cholesterol imbalance affects the endocannabinoid (eCB) system, and defects in eCB signaling can promote disease progression.138 Obviously, significant lipid metabolism disorders occur in the cerebellum in NPC disease.
Inflammation and oxidative stress in NPC cerebellum
Microglia and astrocytes are activated in the cerebellum in NPC disease. Abnormal interferon expression is detected in the cerebellum of presymptomatic Npc1 mice.139 Presymptomatic Npc2 mice cerebellum also exhibited neuroinflammation.140 Presymptomatic Npc1 mice showed abnormal oxidative stress in the cerebellum.139 In Npc1 mice, oxidative stress in the cerebellum is a major stimulus activating apoptosis.141 ROS in the cerebellum of Npc1 mice damage the lysosomal membrane, ultimately leading to apoptosis.28 Oxidative stress and inflammation in the cerebellum of NPC disease can aggravate disease progression.
The primary symptom of NPC disease is progressive cerebellar ataxia. Loss of PCs and GCs and early activation of microglia occur in the NPC cerebellum. NPC cerebellum exhibits lipid metabolism disorders, oxidative stress, and neuroinflammation (Figure 1).
HD and cerebellum
Mutant huntingtin protein is significantly overexpressed in the HD cerebellum.142 A study suggests that the pathological process of HD may be characterized by multifocal onset, with cerebellar damage potentially being an early event.143 Both gray and white matter volumes are reduced in the cerebellum of HD patients, showing significant atrophy.30 Cerebellar involvement is an early event in HD.144 Furthermore, the progression of HD is positively correlated with the degree of cerebellar atrophy.31 HD patients exhibit widespread motor and cognitive impairments. Cerebellar changes are associated with motor dysfunction and psychiatric symptoms.32 Moreover, the posterior superior lobe of the cerebellum in HD is related to emotional symptoms.145 HD patients exhibit cerebellar atrophy, and cerebellar changes correlate with clinical symptoms.
Cellular and synaptic changes in HD cerebellum
In the HD cerebellum, continuous loss of PCs and neurons is observed.146 A significant loss of PCs in HD patients with motor symptoms is noted, whereas this change is not observed in patients primarily exhibiting emotional changes.33 Cerebellar PC dysfunction and death in HD mice are associated with ataxia symptoms.147 Furthermore, expression of cyclin D1 in the granular layer of HD mice cerebellum is increased, along with upregulation of cell cycle regulatory factors Cbx2, Cbx4, and Cbx8.148 A significant loss of the presynaptic marker synaptic vesicle protein 2A is observed in HD patients' cerebellum.34 There are significant cellular and synaptic alterations in the HD cerebellum.
Metabolic changes in HD cerebellum
Hypermetabolism in the cerebellum of HD patients may compensate for motor disorders.149 In the cerebellum of HD mice, about 11% of metabolites show significant changes.150 Significant differences in metabolites, mainly affecting amino acid metabolism, are observed in the cerebellum of the HD transgenic sheep model (OVT73).151 Dysregulation of the urea cycle in the cerebellum of the HD OVT73 sheep model and HD patients results in elevated urea and ammonia levels, causing neurological damage.152 The demand for fatty acids is reduced in the cerebellum of HD model mice.153 The cerebellum of HD mice exhibits changes in substance metabolism, including amino acids, fatty acids, and urea.
Cerebellar atrophy in HD occurs early in the disease and is related to disease progression and clinical symptoms. Multiple pathological and biochemical changes are also present in the cerebellum of HD (Figure 1). Extensive metabolic and cellular changes are also evident in the HD cerebellum. It seems that the HD cerebellum undergoes multifaceted changes.
FTD and cerebellum
FTD exists in familial and sporadic forms, with C9orf72 mutation being the most common cause. Loss of C9orf72 expression, formation of DPRs, and RNA foci all contribute to FTD. The cerebellum in C9orf72 shows the highest DPR load.35 The size of hexanucleotide repeat expansions in the cerebellum of C9orf72-repeat-associated FTD (C9-FTD) correlates with disease duration and severity.37 Presymptomatic C9-FTD patients also display evident gray matter atrophy in the cerebellum.154 FTD features focal cerebellar atrophy strongly connected intrinsically to atrophy regions within the cerebral cortex.5 Different subtypes of FTD involve specific cerebellar lobule alterations rather than global cerebellar atrophy.155
In FTD, cerebellar changes are related to behavioral disruptions and cognitive impairments.36 Cerebellar integrity in C9-FTD patients is associated with attention, language, and executive functions.156 Abnormalities in cerebello-cortical circuits in C9-FTD play a crucial role in cognitive and behavioral changes.157 The psychiatric symptoms in C9-FTD patients are associated with cerebellar atrophy.158 The degree of psychiatric disorders in C9-FTD correlates with cerebellar degeneration.159 Cortico-cerebellar networks are related to cognitive and psychiatric dysfunctions in behavioral variant FTD (bvFTD).160 In bvFTD, cerebellar output pathways are related to episodic memory, while input pathways are associated with memory, visuospatial skills, and emotion.161 Psychiatric symptoms in carriers of C9orf72 and GRN mutations are related to cerebellar atrophy.162 FTD cerebellum exhibits significant atrophy related to clinical manifestations and disease progression.
Cerebellum is the target for ND treatment
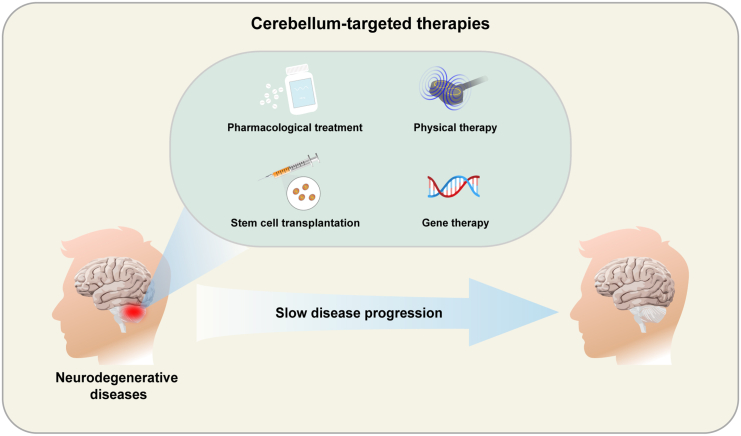
The cerebellum is interconnected with the cortex, frontal lobes, temporal lobes, and parietal cortex regions through multiple closed-loop circuits, participating in the regulation of movement, cognition, and emotion. Transcranial Magnetic Stimulation (TMS) and Transcranial Direct Current Stimulation (tDCS) both contribute to alleviating symptoms of cerebral dysregulation.163 There are two main hypotheses regarding the effects of TMS on the cerebellum. First, TMS ameliorates abnormal activation in the cerebellar cortex, alters the activity of PCs, and diminishes their inhibitory impact on the CTC pathway. Second, TMS can enhance cerebellar plasticity, increase long-term potentiation effects in the cerebellar cortex, and promote FC between brain regions.164 Cerebellum-related therapeutic approaches can facilitate the treatment of NDs and improve clinical symptoms (Figure 2).
Figure 2.
Potential cerebellum-related therapeutic modalities in NDs
Pharmacological treatment, stem cell transplantation, physical therapy, and gene therapy provide effective treatments for cerebellum-related NDs and help improve clinical symptoms.
AD treatment
Cholinergic dysfunction is a hallmark of AD. The cholinergic system in the cerebellum plays a vital role in the normal functioning of the cerebellum.165 Cerebellar magnetic stimulation may effectively modulate central cholinergic activity in AD patients by activating the CTC pathway.75 A study showed that 5 Hz repetitive TMS of the cerebellum is a promising treatment method for AD patients.166 Currently, there is limited research on cerebellum-related treatments for AD patients. Cerebellar therapeutic approaches exhibit significant potential in treating AD, which could help in delaying cognitive and motor dysfunctions in AD patients.
PD treatment
Neurostimulation therapies hold potential value for improving gait dysfunction in patients with PD. Transcranial Electrical Stimulation on the cerebellum can improve gait disturbances in PD patients.167 Transcranial Alternating Current Stimulation of the cerebellum can reduce resting tremors in PD patients.168 The tDCS of the cerebellum is a potential intervention for enhancing motor learning in PD.169 Cerebellar tDCS treatment in PD patients significantly improves gait speed.170 A single higher-intensity cerebellar tDCS treatment in PD patients can significantly improve balance disorders, but not enhance motor abilities, suggesting that multiple treatments may be necessary to improve motor dysfunction.171 Five consecutive days of cerebellar tDCS application can improve Levodopa-induced motor disorders in PD patients.172 In conclusion, the long-term application of cerebellar tDCS therapy can enhance motor function in PD. Furthermore, cerebellar Theta-Burst Stimulation can improve Levodopa-induced Dyskinesias in PD patients, accompanied by a reduction in serum BDNF levels.173 Cerebellar rTMS treatment has improved tremors in PD patients, with no serious adverse events reported.164
Exercise in PD patients can enhance cerebellar activation, thereby improving motor symptoms.174 Skilled aerobic exercise can increase cerebellar regional cerebral blood flow, thereby improving motor dysfunction.175 Magnetic and electrical stimulation of the cerebellum in PD can serve as reliable treatment methods to slow disease progression and improve patient symptoms. Physical training can also play a therapeutic role in PD through improvements to the cerebellum.
ALS treatment
Injecting IGF-1 into the DCN of ALS mice reduces ALS neuropathology, and significantly extends the lifespan of ALS mice.176 Sodium selenite mitigates motor deficits in ALS by inducing mitochondrial autophagy in the cerebellum.177 Cerebellum-related therapeutic approaches may hold significant potential value for ALS. Cerebellar magnetic and electrical stimulation could be valuable in improving ALS symptoms. However, current studies on cerebellum-related treatments are limited, and future research should be expanded into cerebellar involvement in ALS.
NPC treatment
Cerebellar transplantation of bone marrow-derived mesenchymal stem cells (BM-MSCs) alleviates inflammatory responses in the cerebellum of NPC mice.178 Injection of BM-MSCs into the cerebellum of ASM-KO mice significantly slows down the loss of PCs.179 Additionally, BM-MSC transplantation into the cerebellum of NPC mice upregulates neurotransmitter receptors, potentially aiding in synapse formation.180 Human umbilical cord blood-derived mesenchymal stem cells can inhibit inflammation and apoptotic signaling in the cerebellum, reducing the loss of PCs.181 Transplantation of adipose tissue-derived stem cells into the cerebellum of NPC mice alleviates inflammatory responses, rescues PCs, and promotes synapse formation, thereby restoring motor coordination.182
It is reported Miglustat, the only drug used to treat NPC disease,183 shows neuroprotective effects on the cerebellum.27 Intraperitoneal treatment with GSH ethyl ester improves oxidative stress and mitochondrial function in the cerebellum of Npc1 mice, thereby restoring PC activity and alleviating motor dysfunction.184 The cerebellum is a primary affected area in NPC, warranting further therapeutic approaches targeting on cerebellum.
Conclusions
NDs pose a significant threat to human health, causing considerable social and economic burdens, and currently remain incurable. In the context of NDs, apart from the primary lesion site, the cerebellum often receives insufficient attention. It is believed that the cerebellum plays a critical role in NDs. First, the cerebellum is affected in NDs and is not an unaffected region. Cerebellar atrophy occurs in these diseases and correlates with disease severity. In some conditions, cerebellar atrophy is evident early on, aiding in early diagnosis. Different subtypes of NDs may exhibit distinct cerebellar changes, facilitating differential diagnosis. The involvement of specific cerebellar regions may lead to particular symptoms. Second, the cerebellum may serve a reserve and compensatory role. It might compensate for clinical symptoms of NDs, slowing disease progression. However, this compensatory ability may be lost in the later stages. Lastly, the cerebellum in NDs undergoes various pathological and biochemical changes, primarily on cerebellar neuron degeneration, neuroinflammation, mitochondrial dysfunction, metabolic disorders, and neurotransmitter changes. These pathological and biochemical changes contribute to the onset and progression of NDs. We have also summarized therapeutic approaches related to the cerebellum in NDs. Cerebellar magnetic and electrical stimulation, physical therapy, stem cell therapy, and pharmacological treatments may contribute to the management of these diseases. In summary, the cerebellum holds significant value for the early diagnosis, treatment, and prevention of disease progression in NDs.
Future directions
In previous research related to NDs, the cerebellum has largely been overlooked. The functional contributions and molecular mechanisms of the cerebellum in NDs remain largely unknown. The neural circuits and functions associated with the cerebellum are still unclear. Further research is needed to understand the pathological changes in the cerebellum during NDs, explore the specific mechanisms of interaction between the cerebellum and other regions of the nervous system, and determine the extent to which cerebellar changes affect clinical presentations. The specificity of cerebellar cells and structures may have unique roles. For example, the cerebellum shows a later appearance of Aβ compared to the cerebral cortex, which may relate to endogenous and exogenous factors. Endogenous factors might include differences in cerebellar cell types, structures, and gene expression, while exogenous factors could involve lymphatic and vascular structures. Further research is required to elucidate the role of cerebellar tissue and structure in the onset and progression of NDs. There is less research on cerebellum-related treatments for AD, ALS, and HD, although several studies have been reported on PD and NPC diseases. Magnetic and electrical stimulation of the cerebellum and stem cell therapy hold great potential for NDs. It is necessary to investigate the value of cerebellum-related therapeutic approaches for NDs further. Cerebellum-related therapeutic approaches may offer a promising and safe option for treating these diseases.
Acknowledgments
This work was supported by funding from the National Natural Science Foundation of China (32220103006 and 82271524), the Science and Technology project of Sichuan Province (2022ZDZX0023), the Key Research and Development Program of Sichuan (2021YFS0382), and the Intramural Research Programs of National Institute on Aging, NIH (ZIA AG000944, AG000928).
Author contributions
G.L. conceived the review and drafted the manuscript. C.Y., X.W., X.C., and H.C. helped revise this manuscript; W.L. designed this review concept and helped edit and revise the manuscript. All the authors read and approved the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- 1.Erkkinen M.G., Kim M.O., Geschwind M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018;10 doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolph S., Badura A., Lutzu S., Pathak S.S., Thieme A., Verpeut J.L., Wagner M.J., Yang Y.M., Fioravante D. Cognitive-Affective Functions of the Cerebellum. J. Neurosci. 2023;43:7554–7564. doi: 10.1523/jneurosci.1451-23.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argyropoulos G.P.D., van Dun K., Adamaszek M., Leggio M., Manto M., Masciullo M., Molinari M., Stoodley C.J., Van Overwalle F., Ivry R.B., Schmahmann J.D. The Cerebellar Cognitive Affective/Schmahmann Syndrome: a Task Force Paper. Cerebellum. 2020;19:102–125. doi: 10.1007/s12311-019-01068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Reilly J.X., Beckmann C.F., Tomassini V., Ramnani N., Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo C.C., Tan R., Hodges J.R., Hu X., Sami S., Hornberger M. Network-selective vulnerability of the human cerebellum to Alzheimer's disease and frontotemporal dementia. Brain. 2016;139:1527–1538. doi: 10.1093/brain/aww003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jucker M., Walker L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toniolo S., Serra L., Olivito G., Marra C., Bozzali M., Cercignani M. Patterns of Cerebellar Gray Matter Atrophy Across Alzheimer's Disease Progression. Front. Cell. Neurosci. 2018;12:430. doi: 10.3389/fncel.2018.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Spina S., Callahan P., Grinberg L.T., Seeley W.W., Rosen H.J., Kramer J.H., Miller B.L., Rankin K.P. Pathology-specific patterns of cerebellar atrophy in neurodegenerative disorders. Alzheimers Dement. 2024;20:1771–1783. doi: 10.1002/alz.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang F., Zhu D., Ma W., Yao Q., Li Q., Shi J. Differences Changes in Cerebellar Functional Connectivity Between Mild Cognitive Impairment and Alzheimer's Disease: A Seed-Based Approach. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.645171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sepulveda-Falla D., Matschke J., Bernreuther C., Hagel C., Puig B., Villegas A., Garcia G., Zea J., Gomez-Mancilla B., Ferrer I., et al. Deposition of hyperphosphorylated tau in cerebellum of PS1 E280A Alzheimer's disease. Brain Pathol. 2011;21:452–463. doi: 10.1111/j.1750-3639.2010.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sepulveda-Falla D., Barrera-Ocampo A., Hagel C., Korwitz A., Vinueza-Veloz M.F., Zhou K., Schonewille M., Zhou H., Velazquez-Perez L., Rodriguez-Labrada R., et al. Familial Alzheimer's disease-associated presenilin-1 alters cerebellar activity and calcium homeostasis. J. Clin. Invest. 2014;124:1552–1567. doi: 10.1172/jci66407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh-Bains M.K., Linke V., Austria M.D., Tan A.Y., Scotter E.L., Mehrabi N.F., Faull R.L., Dragunow M. Altered microglia and neurovasculature in the Alzheimer's disease cerebellum. Neurobiol. Dis. 2019;132 doi: 10.1016/j.nbd.2019.104589. [DOI] [PubMed] [Google Scholar]
- 13.Ma X., Su W., Li S., Li C., Wang R., Chen M., Chen H. Cerebellar atrophy in different subtypes of Parkinson's disease. J. Neurol. Sci. 2018;392:105–112. doi: 10.1016/j.jns.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Kerestes R., Laansma M.A., Owens-Walton C., Perry A., van Heese E.M., Al-Bachari S., Anderson T.J., Assogna F., Aventurato Í.K., van Balkom T.D., et al. Cerebellar Volume and Disease Staging in Parkinson's Disease: An ENIGMA-PD Study. Mov. Disord. 2023;38:2269–2281. doi: 10.1002/mds.29611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dan X., Hu Y., Sun J., Gao L., Zhou Y., Ma J., Doyon J., Wu T., Chan P. Altered Cerebellar Resting-State Functional Connectivity in Early-Stage Parkinson's Disease Patients With Cognitive Impairment. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.678013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccinin C.C., Campos L.S., Guimarães R.P., Piovesana L.G., Dos Santos M.C.A., Azevedo P.C., Campos B.M., de Rezende T.J.R., Amato-Filho A., Cendes F., D'Abreu A. Differential Pattern of Cerebellar Atrophy in Tremor-Predominant and Akinetic/Rigidity-Predominant Parkinson's Disease. Cerebellum. 2017;16:623–628. doi: 10.1007/s12311-016-0834-5. [DOI] [PubMed] [Google Scholar]
- 17.Maiti B., Rawson K.S., Tanenbaum A.B., Koller J.M., Snyder A.Z., Campbell M.C., Earhart G.M., Perlmutter J.S. Functional Connectivity of Vermis Correlates with Future Gait Impairments in Parkinson's Disease. Mov. Disord. 2021;36:2559–2568. doi: 10.1002/mds.28684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seidel K., Bouzrou M., Heidemann N., Krüger R., Schöls L., den Dunnen W.F.A., Korf H.W., Rüb U. Involvement of the cerebellum in Parkinson disease and dementia with Lewy bodies. Ann. Neurol. 2017;81:898–903. doi: 10.1002/ana.24937. [DOI] [PubMed] [Google Scholar]
- 19.Hartstone W.G., Brown M.H., Kelly G.C., Tate W.J., Kuo S.H., Dwork A.J., Louis E.D., Faust P.L. Dentate Nucleus Neuronal Density: A Postmortem Study of Essential Tremor Versus Control Brains. Mov. Disord. 2021;36:995–999. doi: 10.1002/mds.28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurley M.J., Mash D.C., Jenner P. Markers for dopaminergic neurotransmission in the cerebellum in normal individuals and patients with Parkinson's disease examined by RT-PCR. Eur. J. Neurosci. 2003;18:2668–2672. doi: 10.1046/j.1460-9568.2003.02963.x. [DOI] [PubMed] [Google Scholar]
- 21.Gellersen H.M., Guo C.C., O'Callaghan C., Tan R.H., Sami S., Hornberger M. Cerebellar atrophy in neurodegeneration-a meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2017;88:780–788. doi: 10.1136/jnnp-2017-315607. [DOI] [PubMed] [Google Scholar]
- 22.Consonni M., Dalla Bella E., Nigri A., Pinardi C., Demichelis G., Porcu L., Gellera C., Pensato V., Cappa S.F., Bruzzone M.G., et al. Cognitive Syndromes and C9orf72 Mutation Are Not Related to Cerebellar Degeneration in Amyotrophic Lateral Sclerosis. Front. Neurosci. 2019;13:440. doi: 10.3389/fnins.2019.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Sarraj S., King A., Troakes C., Smith B., Maekawa S., Bodi I., Rogelj B., Al-Chalabi A., Hortobágyi T., Shaw C.E. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122:691–702. doi: 10.1007/s00401-011-0911-2. [DOI] [PubMed] [Google Scholar]
- 24.Tateishi T., Hokonohara T., Yamasaki R., Miura S., Kikuchi H., Iwaki A., Tashiro H., Furuya H., Nagara Y., Ohyagi Y., et al. Multiple system degeneration with basophilic inclusions in Japanese ALS patients with FUS mutation. Acta Neuropathol. 2010;119:355–364. doi: 10.1007/s00401-009-0621-1. [DOI] [PubMed] [Google Scholar]
- 25.Tan R.H., Kril J.J., McGinley C., Hassani M., Masuda-Suzukake M., Hasegawa M., Mito R., Kiernan M.C., Halliday G.M. Cerebellar neuronal loss in amyotrophic lateral sclerosis cases with ATXN2 intermediate repeat expansions. Ann. Neurol. 2016;79:295–305. doi: 10.1002/ana.24565. [DOI] [PubMed] [Google Scholar]
- 26.Sala A., Iaccarino L., Fania P., Vanoli E.G., Fallanca F., Pagnini C., Cerami C., Calvo A., Canosa A., Pagani M., et al. Testing the diagnostic accuracy of [18F]FDG-PET in discriminating spinal- and bulbar-onset amyotrophic lateral sclerosis. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:1117–1131. doi: 10.1007/s00259-018-4246-2. [DOI] [PubMed] [Google Scholar]
- 27.Bowman E.A., Walterfang M., Abel L., Desmond P., Fahey M., Velakoulis D. Longitudinal changes in cerebellar and subcortical volumes in adult-onset Niemann-Pick disease type C patients treated with miglustat. J. Neurol. 2015;262:2106–2114. doi: 10.1007/s00415-015-7819-z. [DOI] [PubMed] [Google Scholar]
- 28.Chung C., Puthanveetil P., Ory D.S., Lieberman A.P. Genetic and pharmacological evidence implicates cathepsins in Niemann-Pick C cerebellar degeneration. Hum. Mol. Genet. 2016;25:1434–1446. doi: 10.1093/hmg/ddw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarna J.R., Larouche M., Marzban H., Sillitoe R.V., Rancourt D.E., Hawkes R. Patterned Purkinje cell degeneration in mouse models of Niemann-Pick type C disease. J. Comp. Neurol. 2003;456:279–291. doi: 10.1002/cne.10522. [DOI] [PubMed] [Google Scholar]
- 30.Ruocco H.H., Bonilha L., Li L.M., Lopes-Cendes I., Cendes F. Longitudinal analysis of regional grey matter loss in Huntington disease: effects of the length of the expanded CAG repeat. J. Neurol. Neurosurg. Psychiatry. 2008;79:130–135. doi: 10.1136/jnnp.2007.116244. [DOI] [PubMed] [Google Scholar]
- 31.Ruocco H.H., Lopes-Cendes I., Li L.M., Santos-Silva M., Cendes F. Striatal and extrastriatal atrophy in Huntington's disease and its relationship with length of the CAG repeat. Braz. J. Med. Biol. Res. 2006;39:1129–1136. doi: 10.1590/s0100-879x2006000800016. [DOI] [PubMed] [Google Scholar]
- 32.Rees E.M., Farmer R., Cole J.H., Haider S., Durr A., Landwehrmeyer B., Scahill R.I., Tabrizi S.J., Hobbs N.Z. Cerebellar abnormalities in Huntington's disease: a role in motor and psychiatric impairment? Mov. Disord. 2014;29:1648–1654. doi: 10.1002/mds.25984. [DOI] [PubMed] [Google Scholar]
- 33.Singh-Bains M.K., Mehrabi N.F., Sehji T., Austria M.D.R., Tan A.Y.S., Tippett L.J., Dragunow M., Waldvogel H.J., Faull R.L.M. Cerebellar degeneration correlates with motor symptoms in Huntington disease. Ann. Neurol. 2019;85:396–405. doi: 10.1002/ana.25413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delva A., Michiels L., Koole M., Van Laere K., Vandenberghe W. Synaptic Damage and Its Clinical Correlates in People With Early Huntington Disease: A PET Study. Neurology. 2022;98:e83–e94. doi: 10.1212/wnl.0000000000012969. [DOI] [PubMed] [Google Scholar]
- 35.Quaegebeur A., Glaria I., Lashley T., Isaacs A.M. Soluble and insoluble dipeptide repeat protein measurements in C9orf72-frontotemporal dementia brains show regional differential solubility and correlation of poly-GR with clinical severity. Acta Neuropathol. Commun. 2020;8:184. doi: 10.1186/s40478-020-01036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y., Kumfor F., Landin-Romero R., Irish M., Piguet O. The Cerebellum in Frontotemporal Dementia: a Meta-Analysis of Neuroimaging Studies. Neuropsychol. Rev. 2019;29:450–464. doi: 10.1007/s11065-019-09414-7. [DOI] [PubMed] [Google Scholar]
- 37.van Blitterswijk M., DeJesus-Hernandez M., Niemantsverdriet E., Murray M.E., Heckman M.G., Diehl N.N., Brown P.H., Baker M.C., Finch N.A., Bauer P.O., et al. Association between repeat sizes and clinical and pathological characteristics in carriers of C9ORF72 repeat expansions (Xpansize-72): a cross-sectional cohort study. Lancet Neurol. 2013;12:978–988. doi: 10.1016/s1474-4422(13)70210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.S L. Benefits of physical exercise on Alzheimer's disease: an epigenetic view. Ageing Neur Dis. 2023;3:6. doi: 10.20517/and.2022.37. [DOI] [Google Scholar]
- 39.Yu H., Wang M., Yang Q., Xu X., Zhang R., Chen X., Le W. The electrophysiological and neuropathological profiles of cerebellum in APP(swe)/PS1(ΔE9) mice: A hypothesis on the role of cerebellum in Alzheimer's disease. Alzheimers Dement. 2023;19:2365–2375. doi: 10.1002/alz.12853. [DOI] [PubMed] [Google Scholar]
- 40.Hoxha E., Lippiello P., Zurlo F., Balbo I., Santamaria R., Tempia F., Miniaci M.C. The Emerging Role of Altered Cerebellar Synaptic Processing in Alzheimer's Disease. Front. Aging Neurosci. 2018;10:396. doi: 10.3389/fnagi.2018.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs H.I.L., Hopkins D.A., Mayrhofer H.C., Bruner E., van Leeuwen F.W., Raaijmakers W., Schmahmann J.D. The cerebellum in Alzheimer's disease: evaluating its role in cognitive decline. Brain. 2018;141:37–47. doi: 10.1093/brain/awx194. [DOI] [PubMed] [Google Scholar]
- 42.Pagen L.H.G., van de Ven V.G., Gronenschild E.H.B.M., Priovoulos N., Verhey F.R.J., Jacobs H.I.L. Contributions of Cerebro-Cerebellar Default Mode Connectivity Patterns to Memory Performance in Mild Cognitive Impairment. J. Alzheimers Dis. 2020;75:633–647. doi: 10.3233/jad-191127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruchhage M.M.K., Correia S., Malloy P., Salloway S., Deoni S. Machine Learning Classification Identifies Cerebellar Contributions to Early and Moderate Cognitive Decline in Alzheimer's Disease. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.524024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H.J., Cheong E.N., Jo S., Lee S., Shim W.H., Kwon M., Kim J.S., Kim B.J., Lee J.H. The cerebellum could serve as a potential imaging biomarker of dementia conversion in patients with amyloid-negative amnestic mild cognitive impairment. Eur. J. Neurol. 2021;28:1520–1527. doi: 10.1111/ene.14770. [DOI] [PubMed] [Google Scholar]
- 45.Mavroudis I., Petridis F., Kazis D., Njau S.N., Costa V., Baloyannis S.J. Purkinje Cells Pathology in Alzheimer's Disease. Am. J. Alzheimers Dis. Other Demen. 2019;34:439–449. doi: 10.1177/1533317519859200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savage J.E., Jansen P.R., Stringer S., Watanabe K., Bryois J., de Leeuw C.A., Nagel M., Awasthi S., Barr P.B., Coleman J.R.I., et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 2018;50:912–919. doi: 10.1038/s41588-018-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam M., Trampush J.W., Yu J., Knowles E., Davies G., Liewald D.C., Starr J.M., Djurovic S., Melle I., Sundet K., et al. Large-Scale Cognitive GWAS Meta-Analysis Reveals Tissue-Specific Neural Expression and Potential Nootropic Drug Targets. Cell Rep. 2017;21:2597–2613. doi: 10.1016/j.celrep.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin C.Y., Chen C.H., Tom S.E., Kuo S.H., Alzheimer’s Disease Neuroimaging Initiative Cerebellar Volume Is Associated with Cognitive Decline in Mild Cognitive Impairment: Results from ADNI. Cerebellum. 2020;19:217–225. doi: 10.1007/s12311-019-01099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Dieu Uwisengeyimana J., Wang Y., Nguchu B.A., Wang X., Qiu B. Effects of apolipoprotein E4 genotype on cerebro-cerebellar connectivity, brain atrophy, and cognition in patients with Alzheimer's disease. J. Neurol. Sci. 2022;442 doi: 10.1016/j.jns.2022.120435. [DOI] [PubMed] [Google Scholar]
- 50.Russo R., Cattaneo F., Lippiello P., Cristiano C., Zurlo F., Castaldo M., Irace C., Borsello T., Santamaria R., Ammendola R., et al. Motor coordination and synaptic plasticity deficits are associated with increased cerebellar activity of NADPH oxidase, CAMKII, and PKC at preplaque stage in the TgCRND8 mouse model of Alzheimer's disease. Neurobiol. Aging. 2018;68:123–133. doi: 10.1016/j.neurobiolaging.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 51.Chaudhari K., Wang L., Kruse J., Winters A., Sumien N., Shetty R., Prah J., Liu R., Shi J., Forster M., Yang S.H. Early loss of cerebellar Purkinje cells in human and a transgenic mouse model of Alzheimer's disease. Neurol. Res. 2021;43:570–581. doi: 10.1080/01616412.2021.1893566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lomoio S., López-González I., Aso E., Carmona M., Torrejón-Escribano B., Scherini E., Ferrer I. Cerebellar amyloid-β plaques: disturbed cortical circuitry in AβPP/PS1 transgenic mice as a model of familial Alzheimer's disease. J Alzheimers Dis. 2012;31:285–300. doi: 10.3233/jad-2012-112198. [DOI] [PubMed] [Google Scholar]
- 53.Skouras S., Falcon C., Tucholka A., Rami L., Sanchez-Valle R., Lladó A., Gispert J.D., Molinuevo J.L. Mechanisms of functional compensation, delineated by eigenvector centrality mapping, across the pathophysiological continuum of Alzheimer's disease. Neuroimage. Clin. 2019;22 doi: 10.1016/j.nicl.2019.101777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang C., Gao X., Liu N., Sun H., Gong Q., Yao L., Lui S. Convergent and distinct neural structural and functional patterns of mild cognitive impairment: a multimodal meta-analysis. Cereb. Cortex. 2023;33:8876–8889. doi: 10.1093/cercor/bhad167. [DOI] [PubMed] [Google Scholar]
- 55.Gelfo F., Serra L., Petrosini L. New prospects on cerebellar reserve: Remarks on neuroprotective effects of experience in animals and humans. Front. Syst. Neurosci. 2022;16 doi: 10.3389/fnsys.2022.1088587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang D.W., Wang S.M., Um Y.H., Na H.R., Kim N.Y., Lee C.U., Lim H.K. Distinctive Association of the Functional Connectivity of the Posterior Cingulate Cortex on Memory Performances in Early and Late Amnestic Mild Cognitive Impairment Patients. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.696735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Canuet L., Pusil S., López M.E., Bajo R., Pineda-Pardo J.Á., Cuesta P., Gálvez G., Gaztelu J.M., Lourido D., García-Ribas G., Maestú F. Network Disruption and Cerebrospinal Fluid Amyloid-Beta and Phospho-Tau Levels in Mild Cognitive Impairment. J. Neurosci. 2015;35:10325–10330. doi: 10.1523/jneurosci.0704-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steininger S.C., Liu X., Gietl A., Wyss M., Schreiner S., Gruber E., Treyer V., Kälin A., Leh S., Buck A., et al. Cortical Amyloid Beta in Cognitively Normal Elderly Adults is Associated with Decreased Network Efficiency within the Cerebro-Cerebellar System. Front. Aging Neurosci. 2014;6:52. doi: 10.3389/fnagi.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghisays V., Lopera F., Goradia D.D., Protas H.D., Malek-Ahmadi M.H., Chen Y., Devadas V., Luo J., Lee W., Baena A., et al. PET evidence of preclinical cerebellar amyloid plaque deposition in autosomal dominant Alzheimer's disease-causing Presenilin-1 E280A mutation carriers. Neuroimage. Clin. 2021;31 doi: 10.1016/j.nicl.2021.102749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez G., Magaki S.D., Williams C.K., Paganini-Hill A., Vinters H.V. Characterization of cerebellar amyloid-β deposits in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2024;83:72–78. doi: 10.1093/jnen/nlad107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoxha E., Boda E., Montarolo F., Parolisi R., Tempia F. Excitability and synaptic alterations in the cerebellum of APP/PS1 mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuwabara Y., Ishizeki M., Watamura N., Toba J., Yoshii A., Inoue T., Ohshima T. Impairments of long-term depression induction and motor coordination precede Aβ accumulation in the cerebellum of APPswe/PS1dE9 double transgenic mice. J. Neurochem. 2014;130:432–443. doi: 10.1111/jnc.12728. [DOI] [PubMed] [Google Scholar]
- 63.Baloyannis S.J. Golgi apparatus and protein trafficking in Alzheimer's disease. J. Alzheimers Dis. 2014;42:S153–S162. doi: 10.3233/jad-132660. [DOI] [PubMed] [Google Scholar]
- 64.Mavroudis I.A., Fotiou D.F., Adipepe L.F., Manani M.G., Njau S.D., Psaroulis D., Costa V.G., Baloyannis S.J. Morphological changes of the human purkinje cells and deposition of neuritic plaques and neurofibrillary tangles on the cerebellar cortex of Alzheimer's disease. Am. J. Alzheimers Dis. Other Demen. 2010;25:585–591. doi: 10.1177/1533317510382892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miguel J.C., Perez S.E., Malek-Ahmadi M., Mufson E.J. Cerebellar Calcium-Binding Protein and Neurotrophin Receptor Defects in Down Syndrome and Alzheimer's Disease. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.645334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersen K., Andersen B.B., Pakkenberg B. Stereological quantification of the cerebellum in patients with Alzheimer's disease. Neurobiol. Aging. 2012;33:197.e11–197.e20. doi: 10.1016/j.neurobiolaging.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 67.Smith M.A., Zhu X., Tabaton M., Liu G., McKeel D.W., Jr., Cohen M.L., Wang X., Siedlak S.L., Dwyer B.E., Hayashi T., et al. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J. Alzheimers Dis. 2010;19:363–372. doi: 10.3233/jad-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu G., Yang C., Wang X., Chen X., Wang Y., Le W. Oxygen metabolism abnormality and Alzheimer's disease: An update. Redox Biol. 2023;68 doi: 10.1016/j.redox.2023.102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim K.H., Moon M., Yu S.B., Mook-Jung I., Kim J.I. RNA-Seq analysis of frontal cortex and cerebellum from 5XFAD mice at early stage of disease pathology. J. Alzheimers Dis. 2012;29:793–808. doi: 10.3233/jad-2012-111793. [DOI] [PubMed] [Google Scholar]
- 70.Blazhenets G., Ma Y., Sörensen A., Rücker G., Schiller F., Eidelberg D., Frings L., Meyer P.T., Alzheimer’s Disease Neuroimaging Initiative Principal Components Analysis of Brain Metabolism Predicts Development of Alzheimer Dementia. J. Nucl. Med. 2019;60:837–843. doi: 10.2967/jnumed.118.219097. [DOI] [PubMed] [Google Scholar]
- 71.Gupta V., Booth S., Ko J.H. Hypermetabolic Cerebellar Connectome in Alzheimer's Disease. Brain Connect. 2023;13:356–366. doi: 10.1089/brain.2020.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang K.L., Wong L.R., Pee H.N., Yang S., Ho P.C.L. Reverting Metabolic Dysfunction in Cortex and Cerebellum of APP/PS1 Mice, a Model for Alzheimer's Disease by Pioglitazone, a Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) Agonist. Mol. Neurobiol. 2019;56:7267–7283. doi: 10.1007/s12035-019-1586-2. [DOI] [PubMed] [Google Scholar]
- 73.Ciavardelli D., Silvestri E., Del Viscovo A., Bomba M., De Gregorio D., Moreno M., Di Ilio C., Goglia F., Canzoniero L.M.T., Sensi S.L. Alterations of brain and cerebellar proteomes linked to Aβ and tau pathology in a female triple-transgenic murine model of Alzheimer's disease. Cell Death Dis. 2010;1 doi: 10.1038/cddis.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vanmierlo T., Bloks V.W., van Vark-van der Zee L.C., Rutten K., Kerksiek A., Friedrichs S., Sijbrands E., Steinbusch H.W., Kuipers F., Lütjohann D., Mulder M. Alterations in brain cholesterol metabolism in the APPSLxPS1mut mouse, a model for Alzheimer's disease. J. Alzheimers Dis. 2010;19:117–127. doi: 10.3233/jad-2010-1209. [DOI] [PubMed] [Google Scholar]
- 75.Di Lorenzo F., Martorana A., Ponzo V., Bonnì S., D'Angelo E., Caltagirone C., Koch G. Cerebellar theta burst stimulation modulates short latency afferent inhibition in Alzheimer's disease patients. Front. Aging Neurosci. 2013;5 doi: 10.3389/fnagi.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bychkov M.L., Isaev A.B., Andreev-Andrievskiy A.A., Petrov K., Paramonov A.S., Kirpichnikov M.P., Lyukmanova E.N. Aβ1-42 Accumulation Accompanies Changed Expression of Ly6/uPAR Proteins, Dysregulation of the Cholinergic System, and Degeneration of Astrocytes in the Cerebellum of Mouse Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241914852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaunt J.R., Zainolabidin N., Yip A.K.K., Tan J.M., Low A.Y.T., Chen A.I., Ch’ng T.H. Cytokine enrichment in deep cerebellar nuclei is contributed by multiple glial populations and linked to reduced amyloid plaque pathology. J. Neuroinflammation. 2023;20:269. doi: 10.1186/s12974-023-02913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X., Zhang Y. A review of the neurotransmitter system associated with cognitive function of the cerebellum in Parkinson's disease. Neural Regen. Res. 2024;19:324–330. doi: 10.4103/1673-5374.379042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li T., Le W., Jankovic J. Linking the cerebellum to Parkinson disease: an update. Nat. Rev. Neurol. 2023;19:645–654. doi: 10.1038/s41582-023-00874-3. [DOI] [PubMed] [Google Scholar]
- 80.Wu T., Hallett M. The cerebellum in Parkinson's disease. Brain. 2013;136:696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song C., Shen Q., Tan C., Li J., Zhou F., Wang T., Zhang L., Wang M., Liu Y., Yuan J., et al. Distinct changes in global brain synchronization in different motor subtypes of Parkinson's disease. Front. Neurosci. 2023;17 doi: 10.3389/fnins.2023.1170225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arioli M., Cattaneo Z., Rusconi M.L., Blandini F., Tettamanti M. Action and emotion perception in Parkinson's disease: A neuroimaging meta-analysis. Neuroimage. Clin. 2022;35 doi: 10.1016/j.nicl.2022.103031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O'Callaghan C., Hornberger M., Balsters J.H., Halliday G.M., Lewis S.J.G., Shine J.M. Cerebellar atrophy in Parkinson's disease and its implication for network connectivity. Brain. 2016;139:845–855. doi: 10.1093/brain/awv399. [DOI] [PubMed] [Google Scholar]
- 84.Sako W., Abe T., Matsumoto Y., Nakamura K., Haji S., Osaki Y., Harada M., Izumi Y. The Cerebellum Is a Common Key for Visuospatial Execution and Attention in Parkinson's Disease. Diagnostics. 2021;11:1042. doi: 10.3390/diagnostics11061042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maiti B., Koller J.M., Snyder A.Z., Tanenbaum A.B., Norris S.A., Campbell M.C., Perlmutter J.S. Cognitive correlates of cerebellar resting-state functional connectivity in Parkinson disease. Neurology. 2020;94:e384–e396. doi: 10.1212/wnl.0000000000008754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L., Ji B., Zhao T., Cui X., Chen J., Wang Z. The structural changes of gray matter in Parkinson disease patients with mild cognitive impairments. PLoS One. 2022;17 doi: 10.1371/journal.pone.0269787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shen B., Pan Y., Jiang X., Wu Z., Zhu J., Dong J., Zhang W., Xu P., Dai Y., Gao Y., et al. Altered putamen and cerebellum connectivity among different subtypes of Parkinson's disease. CNS Neurosci. Ther. 2020;26:207–214. doi: 10.1111/cns.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su D., Cui Y., Liu Z., Chen H., Fang J., Ma H., Zhou J., Feng T. Altered Brain Activity in Depression of Parkinson's Disease: A Meta-Analysis and Validation Study. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.806054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y., Zhang S., Yang H., Zhang X., He S., Wang J., Li J. Altered cerebellum functional network on newly diagnosed drug-naïve Parkinson's disease patients with anxiety. Transl. Neurosci. 2021;12:415–424. doi: 10.1515/tnsci-2020-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qu L., Liu C., Cao Y., Shi J., Yin K., Liu W. Differences and Changes in Cerebellar Functional Connectivity of Parkinson's Patients with Visual Hallucinations. Brain Sci. 2023;13:1458. doi: 10.3390/brainsci13101458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez A.M., Trujillo P., Hernandez A.B., Lin Y.C., Kang H., Landman B.A., Englot D.J., Dawant B.M., Konrad P.E., Claassen D.O. Structural Correlates of the Sensorimotor Cerebellum in Parkinson's Disease and Essential Tremor. Mov. Disord. 2020;35:1181–1188. doi: 10.1002/mds.28044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dirkx M.F., Zach H., van Nuland A., Bloem B.R., Toni I., Helmich R.C. Cerebral differences between dopamine-resistant and dopamine-responsive Parkinson's tremor. Brain. 2019;142:3144–3157. doi: 10.1093/brain/awz261. [DOI] [PubMed] [Google Scholar]
- 93.Zhang H., Wang L., Gan C., Cao X., Ji M., Sun H., Yuan Y., Zhang K. Altered functional connectivity of cerebellar dentate nucleus in peak-dose dyskinesia in Parkinson's disease. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.943179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaut O., Mielacher C., Hurlemann R., Wüllner U. Resting-state fMRI reveals increased functional connectivity in the cerebellum but decreased functional connectivity of the caudate nucleus in Parkinson's disease. Neurol. Res. 2020;42:62–67. doi: 10.1080/01616412.2019.1709141. [DOI] [PubMed] [Google Scholar]
- 95.Bharti K., Suppa A., Pietracupa S., Upadhyay N., Giannì C., Leodori G., Di Biasio F., Modugno N., Petsas N., Grillea G., et al. Abnormal Cerebellar Connectivity Patterns in Patients with Parkinson's Disease and Freezing of Gait. Cerebellum. 2019;18:298–308. doi: 10.1007/s12311-018-0988-4. [DOI] [PubMed] [Google Scholar]
- 96.Locke T.M., Soden M.E., Miller S.M., Hunker A., Knakal C., Licholai J.A., Dhillon K.S., Keene C.D., Zweifel L.S., Carlson E.S. Dopamine D(1) Receptor-Positive Neurons in the Lateral Nucleus of the Cerebellum Contribute to Cognitive Behavior. Biol. Psychiatry. 2018;84:401–412. doi: 10.1016/j.biopsych.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yeung P.K.K., Lai A.K.W., Son H.J., Zhang X., Hwang O., Chung S.S.M., Chung S.K. Aldose reductase deficiency leads to oxidative stress-induced dopaminergic neuronal loss and autophagic abnormality in an animal model of Parkinson's disease. Neurobiol. Aging. 2017;50:119–133. doi: 10.1016/j.neurobiolaging.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 98.van der Zee S., Kanel P., Gerritsen M.J., Boertien J.M., Slomp A.C., Müller M.L., Bohnen N.I., Spikman J.M., van Laar T. Altered Cholinergic Innervation in De Novo Parkinson's Disease with and Without Cognitive Impairment. Mov. Disord. 2022;37:713–723. doi: 10.1002/mds.28913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirano S., Sugiyama A., Arai K. Noradrenergic Pathway to the Cerebellum: the Study Must Go On. Cerebellum. 2023;22:1052–1054. doi: 10.1007/s12311-022-01479-0. [DOI] [PubMed] [Google Scholar]
- 100.Piras F., Vecchio D., Assogna F., Pellicano C., Ciullo V., Banaj N., Edden R.A.E., Pontieri F.E., Piras F., Spalletta G. Cerebellar GABA Levels and Cognitive Interference in Parkinson's disease and Healthy Comparators. J. Pers. Med. 2020;11:16. doi: 10.3390/jpm11010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khadrawy Y.A., Mourad I.M., Mohammed H.S., Noor N.A., Aboul Ezz H.S. Cerebellar neurochemical and histopathological changes in rat model of Parkinson's disease induced by intrastriatal injection of rotenone. Gen. Physiol. Biophys. 2017;36:99–108. doi: 10.4149/gpb_2016031. [DOI] [PubMed] [Google Scholar]
- 102.Tang S., Wang Y., Liu Y., Chau S.W., Chan J.W., Chu W.C., Abrigo J.M., Mok V.C., Wing Y.K. Large-scale network dysfunction in α-Synucleinopathy: A meta-analysis of resting-state functional connectivity. EBioMedicine. 2022;77 doi: 10.1016/j.ebiom.2022.103915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu Y.C., Louis E.D., Gionco J., Pan M.K., Faust P.L., Kuo S.H. Increased Climbing Fiber Lateral Crossings on Purkinje Cell Dendrites in the Cerebellar Hemisphere in Essential Tremor. Mov. Disord. 2021;36:1440–1445. doi: 10.1002/mds.28502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhong J., Tang G., Zhu J., Wu W., Li G., Lin X., Liang L., Chai C., Zeng Y., Wang F., et al. Single-cell brain atlas of Parkinson's disease mouse model. J Genet Genomics. 2021;48:277–288. doi: 10.1016/j.jgg.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Molinet-Dronda F., Blesa J., Del Rey N.L.G., Juri C., Collantes M., Pineda-Pardo J.A., Trigo-Damas I., Iglesias E., Hernández L.F., Rodríguez-Rojas R., et al. Cerebral metabolic pattern associated with progressive parkinsonism in non-human primates reveals early cortical hypometabolism. Neurobiol. Dis. 2022;167 doi: 10.1016/j.nbd.2022.105669. [DOI] [PubMed] [Google Scholar]
- 106.Oh J.Y., An E.J., Lee Y., Kim S.M., Cheon M., Kim J.Y. Association of dysphagia with altered brain glucose metabolism in Parkinson's disease. CNS Neurosci. Ther. 2023;29:2498–2507. doi: 10.1111/cns.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Isaias I.U., Brumberg J., Pozzi N.G., Palmisano C., Canessa A., Marotta G., Volkmann J., Pezzoli G. Brain metabolic alterations herald falls in patients with Parkinson's disease. Ann. Clin. Transl. Neurol. 2020;7:579–583. doi: 10.1002/acn3.51013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Riou A., Houvenaghel J.F., Dondaine T., Drapier S., Sauleau P., Drapier D., Duprez J., Guillery M., Le Jeune F., Verin M., Robert G. Functional Role of the Cerebellum in Parkinson Disease: A PET Study. Neurology. 2021;96:e2874–e2884. doi: 10.1212/wnl.0000000000012036. [DOI] [PubMed] [Google Scholar]
- 109.Yan S., Lu J., Li Y., Cho J., Zhang S., Zhu W., Wang Y. Spatiotemporal patterns of brain iron-oxygen metabolism in patients with Parkinson's disease. Eur. Radiol. 2024;34:3074–3083. doi: 10.1007/s00330-023-10283-1. [DOI] [PubMed] [Google Scholar]
- 110.Blum D., la Fougère C., Pilotto A., Maetzler W., Berg D., Reimold M., Liepelt-Scarfone I. Hypermetabolism in the cerebellum and brainstem and cortical hypometabolism are independently associated with cognitive impairment in Parkinson's disease. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:2387–2395. doi: 10.1007/s00259-018-4085-1. [DOI] [PubMed] [Google Scholar]
- 111.Scholefield M., Church S.J., Taylor G., Knight D., Unwin R.D., Cooper G.J.S. Multi-regional alterations in glucose and purine metabolic pathways in the Parkinson's disease dementia brain. NPJ Parkinsons Dis. 2023;9:66. doi: 10.1038/s41531-023-00488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu X., Shen D., Gao Y., Zhou Q., Ni Y., Meng H., Shi H., Le W., Chen S., Chen S. A perspective on therapies for amyotrophic lateral sclerosis: can disease progression be curbed? Transl. Neurodegener. 2021;10:29. doi: 10.1186/s40035-021-00250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lall D., Baloh R.H. Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia. J. Clin. Invest. 2017;127:3250–3258. doi: 10.1172/jci90607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sequeira M., Godinho F., Lourenço J. Amyotrophic Lateral Sclerosis with SOD1 Mutation Presenting with Progressive Cerebellar Ataxia. Cerebellum. 2023;23:1702–1704. doi: 10.1007/s12311-023-01643-0. [DOI] [PubMed] [Google Scholar]
- 115.Hao Z., Liu L., Tao Z., Wang R., Ren H., Sun H., Lin Z., Zhang Z., Mu C., Zhou J., Wang G. Motor dysfunction and neurodegeneration in a C9orf72 mouse line expressing poly-PR. Nat. Commun. 2019;10:2906. doi: 10.1038/s41467-019-10956-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan S., Liu X., Liu T., Zhao Z., Dai Y., Wang Y.Y., Jia P., Liu F. Causal Inference of Genetic Variants and Genes in Amyotrophic Lateral Sclerosis. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.917142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tan R.H., Devenney E., Dobson-Stone C., Kwok J.B., Hodges J.R., Kiernan M.C., Halliday G.M., Hornberger M. Cerebellar integrity in the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bede P., Chipika R.H., Christidi F., Hengeveld J.C., Karavasilis E., Argyropoulos G.D., Lope J., Li Hi Shing S., Velonakis G., Dupuis L., et al. Genotype-associated cerebellar profiles in ALS: focal cerebellar pathology and cerebro-cerebellar connectivity alterations. J. Neurol. Neurosurg. Psychiatry. 2021;92:1197–1205. doi: 10.1136/jnnp-2021-326854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qiu T., Zhang Y., Tang X., Liu X., Wang Y., Zhou C., Luo C., Zhang J. Precentral degeneration and cerebellar compensation in amyotrophic lateral sclerosis: A multimodal MRI analysis. Hum. Brain Mapp. 2019;40:3464–3474. doi: 10.1002/hbm.24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schönecker S., Neuhofer C., Otto M., Ludolph A., Kassubek J., Landwehrmeyer B., Anderl-Straub S., Semler E., Diehl-Schmid J., Prix C., et al. Atrophy in the Thalamus But Not Cerebellum Is Specific for C9orf72 FTD and ALS Patients - An Atlas-Based Volumetric MRI Study. Front. Aging Neurosci. 2018;10:45. doi: 10.3389/fnagi.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duan W., Guo M., Yi L., Zhang J., Bi Y., Liu Y., Li Y., Li Z., Ma Y., Zhang G., et al. Deletion of Tbk1 disrupts autophagy and reproduces behavioral and locomotor symptoms of FTD-ALS in mice. Aging (Albany NY) 2019;11:2457–2476. doi: 10.18632/aging.101936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gendron T.F., van Blitterswijk M., Bieniek K.F., Daughrity L.M., Jiang J., Rush B.K., Pedraza O., Lucas J.A., Murray M.E., Desaro P., et al. Cerebellar c9RAN proteins associate with clinical and neuropathological characteristics of C9ORF72 repeat expansion carriers. Acta Neuropathol. 2015;130:559–573. doi: 10.1007/s00401-015-1474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gendron T.F., Bieniek K.F., Zhang Y.J., Jansen-West K., Ash P.E.A., Caulfield T., Daughrity L., Dunmore J.H., Castanedes-Casey M., Chew J., et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013;126:829–844. doi: 10.1007/s00401-013-1192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Afshar P., Ashtari N., Jiao X., Rahimi-Balaei M., Zhang X., Yaganeh B., Del Bigio M.R., Kong J., Marzban H. Overexpression of Human SOD1 Leads to Discrete Defects in the Cerebellar Architecture in the Mouse. Front. Neuroanat. 2017;11:22. doi: 10.3389/fnana.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tondo G., Iaccarino L., Cerami C., Vanoli G.E., Presotto L., Masiello V., Coliva A., Salvi F., Bartolomei I., Mosca L., et al. (11) C-PK11195 PET-based molecular study of microglia activation in SOD1 amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2020;7:1513–1523. doi: 10.1002/acn3.51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saberi D., Ott B., Dahlke C., Matschke V., Schmitt-John T., Theiss C. The Spatiotemporal Pattern of Degeneration in the Cerebellum of the Wobbler Mouse. J. Neuropathol. Exp. Neurol. 2016;75:347–357. doi: 10.1093/jnen/nlw005. [DOI] [PubMed] [Google Scholar]
- 127.Kim S.H., Engelhardt J.I., Henkel J.S., Siklós L., Soós J., Goodman C., Appel S.H. Widespread increased expression of the DNA repair enzyme PARP in brain in ALS. Neurology. 2004;62:319–322. doi: 10.1212/01.wnl.0000103291.04985.dc. [DOI] [PubMed] [Google Scholar]
- 128.Walterfang M., Fahey M., Desmond P., Wood A., Seal M.L., Steward C., Adamson C., Kokkinos C., Fietz M., Velakoulis D. White and gray matter alterations in adults with Niemann-Pick disease type C: a cross-sectional study. Neurology. 2010;75:49–56. doi: 10.1212/WNL.0b013e3181e6210e. [DOI] [PubMed] [Google Scholar]
- 129.Lucarelli M., Camuso S., Di Pietro C., Bruno F., La Rosa P., Marazziti D., Fiorenza M.T., Canterini S. Reduced Cerebellar BDNF Availability Affects Postnatal Differentiation and Maturation of Granule Cells in a Mouse Model of Cholesterol Dyshomeostasis. Mol. Neurobiol. 2023;60:5395–5410. doi: 10.1007/s12035-023-03435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boyle B.R., Melli S.E., Altreche R.S., Padron Z.M., Yousufzai F.A.K., Kim S., Vasquez M.D., Carone D.M., Carone B.R., Soto I. NPC1 deficiency impairs cerebellar postnatal development of microglia and climbing fiber refinement in a mouse model of Niemann-Pick disease type C. Development. 2020;147 doi: 10.1242/dev.189019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kavetsky L., Green K.K., Boyle B.R., Yousufzai F.A.K., Padron Z.M., Melli S.E., Kuhnel V.L., Jackson H.M., Blanco R.E., Howell G.R., Soto I. Increased interactions and engulfment of dendrites by microglia precede Purkinje cell degeneration in a mouse model of Niemann Pick Type-C. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-51246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li H., Repa J.J., Valasek M.A., Beltroy E.P., Turley S.D., German D.C., Dietschy J.M. Molecular, anatomical, and biochemical events associated with neurodegeneration in mice with Niemann-Pick type C disease. J. Neuropathol. Exp. Neurol. 2005;64:323–333. doi: 10.1093/jnen/64.4.323. [DOI] [PubMed] [Google Scholar]
- 133.Cougnoux A., Yerger J.C., Fellmeth M., Serra-Vinardell J., Martin K., Navid F., Iben J., Wassif C.A., Cawley N.X., Porter F.D. Single Cell Transcriptome Analysis of Niemann-Pick Disease, Type C1 Cerebella. Int. J. Mol. Sci. 2020;21:5368. doi: 10.3390/ijms21155368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Santiago-Mujica E., Flunkert S., Rabl R., Neddens J., Loeffler T., Hutter-Paier B. Hepatic and neuronal phenotype of NPC1(-/-) mice. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Amritraj A., Peake K., Kodam A., Salio C., Merighi A., Vance J.E., Kar S. Increased activity and altered subcellular distribution of lysosomal enzymes determine neuronal vulnerability in Niemann-Pick type C1-deficient mice. Am. J. Pathol. 2009;175:2540–2556. doi: 10.2353/ajpath.2009.081096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tobias F., Pathmasiri K.C., Cologna S.M. Mass spectrometry imaging reveals ganglioside and ceramide localization patterns during cerebellar degeneration in the Npc1(-/-) mouse model. Anal. Bioanal. Chem. 2019;411:5659–5668. doi: 10.1007/s00216-019-01989-7. [DOI] [PubMed] [Google Scholar]
- 137.Chen T.I., Hsu P.C., Lee N.C., Liu Y.H., Wang H.C., Lu Y.H., Chien Y.H., Hwu W.L. Loss of Flot2 expression in deep cerebellar nuclei neurons of mice with Niemann-Pick disease type C. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e18082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oddi S., Caporali P., Dragotto J., Totaro A., Maiolati M., Scipioni L., Angelucci C.B., Orsini C., Canterini S., Rapino C., et al. The endocannabinoid system is affected by cholesterol dyshomeostasis: Insights from a murine model of Niemann Pick type C disease. Neurobiol. Dis. 2019;130 doi: 10.1016/j.nbd.2019.104531. [DOI] [PubMed] [Google Scholar]
- 139.Tolan A.J., Sanchez K.L., Shin S.D., White J.B., Currais A., Soriano-Castell D., Wilson C.G., Maher P., Soriano S. Differential Interferon Signaling Regulation and Oxidative Stress Responses in the Cerebral Cortex and Cerebellum Could Account for the Spatiotemporal Pattern of Neurodegeneration in Niemann-Pick Disease Type C. Genes. 2024;15 doi: 10.3390/genes15010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rasmussen C.L.M., Thomsen L.B., Heegaard C.W., Moos T., Burkhart A. The Npc2(Gt(LST105)BygNya) mouse signifies pathological changes comparable to human Niemann-Pick type C2 disease. Mol. Cell. Neurosci. 2023;126 doi: 10.1016/j.mcn.2023.103880. [DOI] [PubMed] [Google Scholar]
- 141.Klein A., Maldonado C., Vargas L.M., Gonzalez M., Robledo F., Perez de Arce K., Muñoz F.J., Hetz C., Alvarez A.R., Zanlungo S. Oxidative stress activates the c-Abl/p73 proapoptotic pathway in Niemann-Pick type C neurons. Neurobiol. Dis. 2011;41:209–218. doi: 10.1016/j.nbd.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 142.Franich N.R., Basso M., André E.A., Ochaba J., Kumar A., Thein S., Fote G., Kachemov M., Lau A.L., Yeung S.Y., et al. Striatal Mutant Huntingtin Protein Levels Decline with Age in Homozygous Huntington's Disease Knock-In Mouse Models. J. Huntingtons Dis. 2018;7:137–150. doi: 10.3233/jhd-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rüb U., Hoche F., Brunt E.R., Heinsen H., Seidel K., Del Turco D., Paulson H.L., Bohl J., von Gall C., Vonsattel J.P., et al. Degeneration of the cerebellum in Huntington's disease (HD): possible relevance for the clinical picture and potential gateway to pathological mechanisms of the disease process. Brain Pathol. 2013;23:165–177. doi: 10.1111/j.1750-3639.2012.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Franklin G.L., Camargo C.H.F., Meira A.T., Lima N.S.C., Teive H.A.G. The Role of the Cerebellum in Huntington's Disease: a Systematic Review. Cerebellum. 2021;20:254–265. doi: 10.1007/s12311-020-01198-4. [DOI] [PubMed] [Google Scholar]
- 145.de Azevedo P.C., Guimarães R.P., Piccinin C.C., Piovesana L.G., Campos L.S., Zuiani J.R., Tamashiro E.M., Pinheiro G., Amato-Filho A.C., Cendes F., et al. Cerebellar Gray Matter Alterations in Huntington Disease: A Voxel-Based Morphometry Study. Cerebellum. 2017;16:923–928. doi: 10.1007/s12311-017-0865-6. [DOI] [PubMed] [Google Scholar]
- 146.D'Egidio F., Castelli V., Lombardozzi G., Ammannito F., Cimini A., d'Angelo M. Therapeutic advances in neural regeneration for Huntington's disease. Neural Regen. Res. 2024;19:1991–1997. doi: 10.4103/1673-5374.390969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Egorova P.A., Gavrilova A.V., Bezprozvanny I.B. Ataxic Symptoms in Huntington's Disease Transgenic Mouse Model Are Alleviated by Chlorzoxazone. Front. Neurosci. 2020;14:279. doi: 10.3389/fnins.2020.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bauer S., Chen C.Y., Jonson M., Kaczmarczyk L., Magadi S.S., Jackson W.S. Cerebellar granule neurons induce Cyclin D1 before the onset of motor symptoms in Huntington's disease mice. Acta Neuropathol. Commun. 2023;11:17. doi: 10.1186/s40478-022-01500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gaura V., Lavisse S., Payoux P., Goldman S., Verny C., Krystkowiak P., Damier P., Supiot F., Bachoud-Levi A.C., Remy P. Association Between Motor Symptoms and Brain Metabolism in Early Huntington Disease. JAMA Neurol. 2017;74:1088–1096. doi: 10.1001/jamaneurol.2017.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carroll J.B., Deik A., Fossale E., Weston R.M., Guide J.R., Arjomand J., Kwak S., Clish C.B., MacDonald M.E. HdhQ111 Mice Exhibit Tissue Specific Metabolite Profiles that Include Striatal Lipid Accumulation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Handley R.R., Reid S.J., Patassini S., Rudiger S.R., Obolonkin V., McLaughlan C.J., Jacobsen J.C., Gusella J.F., MacDonald M.E., Waldvogel H.J., et al. Metabolic disruption identified in the Huntington's disease transgenic sheep model. Sci. Rep. 2016;6 doi: 10.1038/srep20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Handley R.R., Reid S.J., Brauning R., Maclean P., Mears E.R., Fourie I., Patassini S., Cooper G.J.S., Rudiger S.R., McLaughlan C.J., et al. Brain urea increase is an early Huntington's disease pathogenic event observed in a prodromal transgenic sheep model and HD cases. Proc. Natl. Acad. Sci. USA. 2017;114:E11293–E11302. doi: 10.1073/pnas.1711243115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Polyzos A.A., Lee D.Y., Datta R., Hauser M., Budworth H., Holt A., Mihalik S., Goldschmidt P., Frankel K., Trego K., et al. Metabolic Reprogramming in Astrocytes Distinguishes Region-Specific Neuronal Susceptibility in Huntington Mice. Cell Metab. 2019;29:1258–1273.e11. doi: 10.1016/j.cmet.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cash D.M., Bocchetta M., Thomas D.L., Dick K.M., van Swieten J.C., Borroni B., Galimberti D., Masellis M., Tartaglia M.C., Rowe J.B., et al. Patterns of gray matter atrophy in genetic frontotemporal dementia: results from the GENFI study. Neurobiol. Aging. 2018;62:191–196. doi: 10.1016/j.neurobiolaging.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McKenna M.C., Chipika R.H., Li Hi Shing S., Christidi F., Lope J., Doherty M.A., Hengeveld J.C., Vajda A., McLaughlin R.L., Hardiman O., et al. Infratentorial pathology in frontotemporal dementia: cerebellar grey and white matter alterations in FTD phenotypes. J. Neurol. 2021;268:4687–4697. doi: 10.1007/s00415-021-10575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen Y., Landin-Romero R., Kumfor F., Irish M., Dobson-Stone C., Kwok J.B., Halliday G.M., Hodges J.R., Piguet O. Cerebellar integrity and contributions to cognition in C9orf72-mediated frontotemporal dementia. Cortex. 2022;149:73–84. doi: 10.1016/j.cortex.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 157.Bussy A., Levy J.P., Best T., Patel R., Cupo L., Van Langenhove T., Nielsen J.E., Pijnenburg Y., Waldö M.L., Remes A.M., et al. Cerebellar and subcortical atrophy contribute to psychiatric symptoms in frontotemporal dementia. Hum. Brain Mapp. 2023;44:2684–2700. doi: 10.1002/hbm.26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Devenney E.M., Landin-Romero R., Irish M., Hornberger M., Mioshi E., Halliday G.M., Kiernan M.C., Hodges J.R. The neural correlates and clinical characteristics of psychosis in the frontotemporal dementia continuum and the C9orf72 expansion. Neuroimage. Clin. 2017;13:439–445. doi: 10.1016/j.nicl.2016.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hornberger M. Assessment of psychiatric changes in C9ORF72 frontotemporal dementia. Alzheimers Res. Ther. 2012;4:49. doi: 10.1186/alzrt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tan R.H., Devenney E., Kiernan M.C., Halliday G.M., Hodges J.R., Hornberger M. Terra incognita-cerebellar contributions to neuropsychiatric and cognitive dysfunction in behavioral variant frontotemporal dementia. Front. Aging Neurosci. 2015;7:121. doi: 10.3389/fnagi.2015.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen Y., Landin-Romero R., Kumfor F., Irish M., Hodges J.R., Piguet O. Cerebellar structural connectivity and contributions to cognition in frontotemporal dementias. Cortex. 2020;129:57–67. doi: 10.1016/j.cortex.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 162.Sellami L., Bocchetta M., Masellis M., Cash D.M., Dick K.M., van Swieten J., Borroni B., Galimberti D., Tartaglia M.C., Rowe J.B., et al. Distinct Neuroanatomical Correlates of Neuropsychiatric Symptoms in the Three Main Forms of Genetic Frontotemporal Dementia in the GENFI Cohort. J. Alzheimers Dis. 2018;65:147–163. doi: 10.3233/jad-180053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Manto M., Argyropoulos G.P.D., Bocci T., Celnik P.A., Corben L.A., Guidetti M., Koch G., Priori A., Rothwell J.C., Sadnicka A., et al. Consensus Paper: Novel Directions and Next Steps of Non-invasive Brain Stimulation of the Cerebellum in Health and Disease. Cerebellum. 2022;21:1092–1122. doi: 10.1007/s12311-021-01344-6. [DOI] [PubMed] [Google Scholar]
- 164.Xia Y., Wang M., Zhu Y. The Effect of Cerebellar rTMS on Modulating Motor Dysfunction in Neurological Disorders: a Systematic Review. Cerebellum. 2023;22:954–972. doi: 10.1007/s12311-022-01465-6. [DOI] [PubMed] [Google Scholar]
- 165.Zhang C., Zhou P., Yuan T. The cholinergic system in the cerebellum: from structure to function. Rev. Neurosci. 2016;27:769–776. doi: 10.1515/revneuro-2016-0008. [DOI] [PubMed] [Google Scholar]
- 166.Yao Q., Tang F., Wang Y., Yan Y., Dong L., Wang T., Zhu D., Tian M., Lin X., Shi J. Effect of cerebellum stimulation on cognitive recovery in patients with Alzheimer disease: A randomized clinical trial. Brain Stimul. 2022;15:910–920. doi: 10.1016/j.brs.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 167.Nojima I., Horiba M., Sahashi K., Koganemaru S., Murakami S., Aoyama K., Matsukawa N., Ono Y., Mima T., Ueki Y. Gait-combined closed-loop brain stimulation can improve walking dynamics in Parkinsonian gait disturbances: a randomised-control trial. J. Neurol. Neurosurg. Psychiatry. 2023;94:938–944. doi: 10.1136/jnnp-2022-329966. [DOI] [PubMed] [Google Scholar]
- 168.Rahimi S., Towhidkhah F., Baghdadi G., Forogh B., Saadat P., Soleimani G., Habibi S.A. Modeling of cerebellar transcranial electrical stimulation effects on hand tremor in Parkinson's disease. Front. Aging Neurosci. 2023;15 doi: 10.3389/fnagi.2023.1187157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.de Albuquerque L.L., Pantovic M., Clingo M.G., Fischer K.M., Jalene S., Landers M.R., Mari Z., Poston B. Long-Term Application of Cerebellar Transcranial Direct Current Stimulation Does Not Improve Motor Learning in Parkinson's Disease. Cerebellum. 2022;21:333–349. doi: 10.1007/s12311-021-01297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wong P.L., Yang Y.R., Huang S.F., Fuh J.L., Chiang H.L., Wang R.Y. Transcranial Direct Current Stimulation on Different Targets to Modulate Cortical Activity and Dual-Task Walking in Individuals With Parkinson's Disease: A Double Blinded Randomized Controlled Trial. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.807151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.de Albuquerque L.L., Pantovic M., Clingo M., Fischer K., Jalene S., Landers M., Mari Z., Poston B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson's Disease: A Pilot Study. Biomedicines. 2023;11 doi: 10.3390/biomedicines11082219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ferrucci R., Cortese F., Bianchi M., Pittera D., Turrone R., Bocci T., Borroni B., Vergari M., Cogiamanian F., Ardolino G., et al. Cerebellar and Motor Cortical Transcranial Stimulation Decrease Levodopa-Induced Dyskinesias in Parkinson's Disease. Cerebellum. 2016;15:43–47. doi: 10.1007/s12311-015-0737-x. [DOI] [PubMed] [Google Scholar]
- 173.Sanna A., Follesa P., Puligheddu M., Cannas A., Serra M., Pisu M.G., Dagostino S., Solla P., Tacconi P., Marrosu F. Cerebellar continuous theta burst stimulation reduces levodopa-induced dyskinesias and decreases serum BDNF levels. Neurosci. Lett. 2020;716 doi: 10.1016/j.neulet.2019.134653. [DOI] [PubMed] [Google Scholar]
- 174.Li J., Guo J., Sun W., Mei J., Wang Y., Zhang L., Zhang J., Gao J., Su K., Lv Z., et al. Effects of Exercise on Parkinson's Disease: A Meta-Analysis of Brain Imaging Studies. Front. Hum. Neurosci. 2022;16 doi: 10.3389/fnhum.2022.796712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Z., Guo Y., Myers K.G., Heintz R., Holschneider D.P. Recruitment of the prefrontal cortex and cerebellum in Parkinsonian rats following skilled aerobic exercise. Neurobiol. Dis. 2015;77:71–87. doi: 10.1016/j.nbd.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dodge J.C., Haidet A.M., Yang W., Passini M.A., Hester M., Clarke J., Roskelley E.M., Treleaven C.M., Rizo L., Martin H., et al. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol. Ther. 2008;16:1056–1064. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oleinik N., Albayram O., Kassir M.F., Atilgan F.C., Walton C., Karakaya E., Kurtz J., Alekseyenko A., Alsudani H., Sheridan M., et al. Alterations of lipid-mediated mitophagy result in aging-dependent sensorimotor defects. Aging Cell. 2023;22 doi: 10.1111/acel.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bae J.S., Furuya S., Ahn S.J., Yi S.J., Hirabayashi Y., Jin H.K. Neuroglial activation in Niemann-Pick Type C mice is suppressed by intracerebral transplantation of bone marrow-derived mesenchymal stem cells. Neurosci. Lett. 2005;381:234–236. doi: 10.1016/j.neulet.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 179.Jin H.K., Carter J.E., Huntley G.W., Schuchman E.H. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase-deficient mice delays the onset of neurological abnormalities and extends their life span. J. Clin. Invest. 2002;109:1183–1191. doi: 10.1172/jci14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bae J.S., Han H.S., Youn D.H., Carter J.E., Modo M., Schuchman E.H., Jin H.K. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cell. 2007;25:1307–1316. doi: 10.1634/stemcells.2006-0561. [DOI] [PubMed] [Google Scholar]
- 181.Seo Y., Yang S.R., Jee M.K., Joo E.K., Roh K.H., Seo M.S., Han T.H., Lee S.Y., Ryu P.D., Jung J.W., et al. Human umbilical cord blood-derived mesenchymal stem cells protect against neuronal cell death and ameliorate motor deficits in Niemann Pick type C1 mice. Cell Transplant. 2011;20:1033–1047. doi: 10.3727/096368910x545086. [DOI] [PubMed] [Google Scholar]
- 182.Bae J.S., Carter J.E., Jin H.K. Adipose tissue-derived stem cells rescue Purkinje neurons and alleviate inflammatory responses in Niemann-Pick disease type C mice. Cell Tissue Res. 2010;340:357–369. doi: 10.1007/s00441-010-0942-3. [DOI] [PubMed] [Google Scholar]
- 183.Solomon B.I., Smith A.C., Sinaii N., Farhat N., King M.C., Machielse L., Porter F.D. Association of Miglustat With Swallowing Outcomes in Niemann-Pick Disease, Type C1. JAMA Neurol. 2020;77:1564–1568. doi: 10.1001/jamaneurol.2020.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Torres S., Matías N., Baulies A., Nuñez S., Alarcon-Vila C., Martinez L., Nuño N., Fernandez A., Caballeria J., Levade T., et al. Mitochondrial GSH replenishment as a potential therapeutic approach for Niemann Pick type C disease. Redox Biol. 2017;11:60–72. doi: 10.1016/j.redox.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]