Abstract
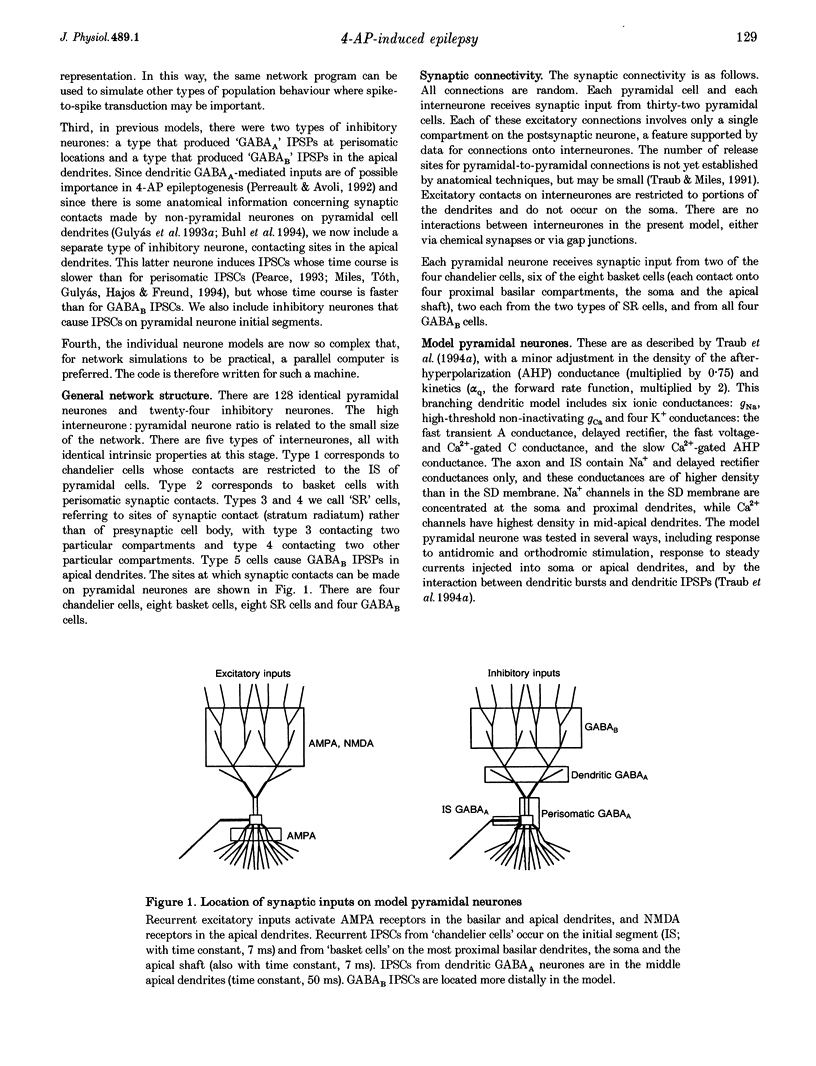
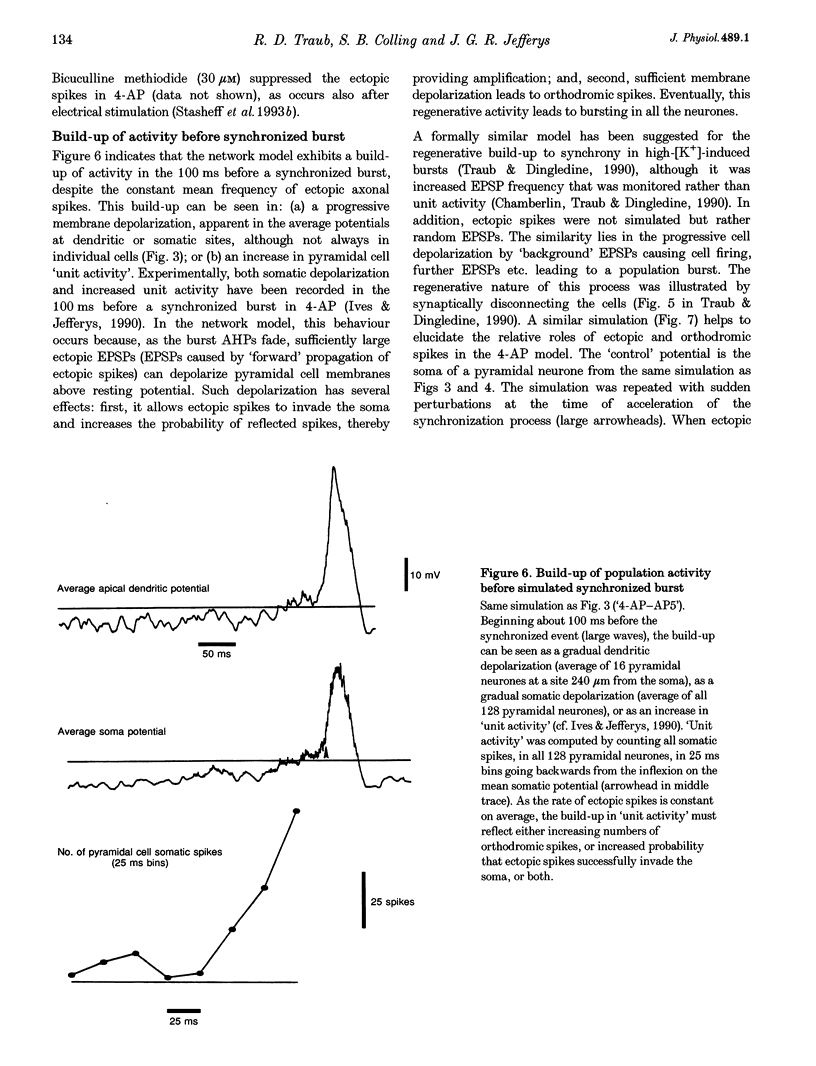
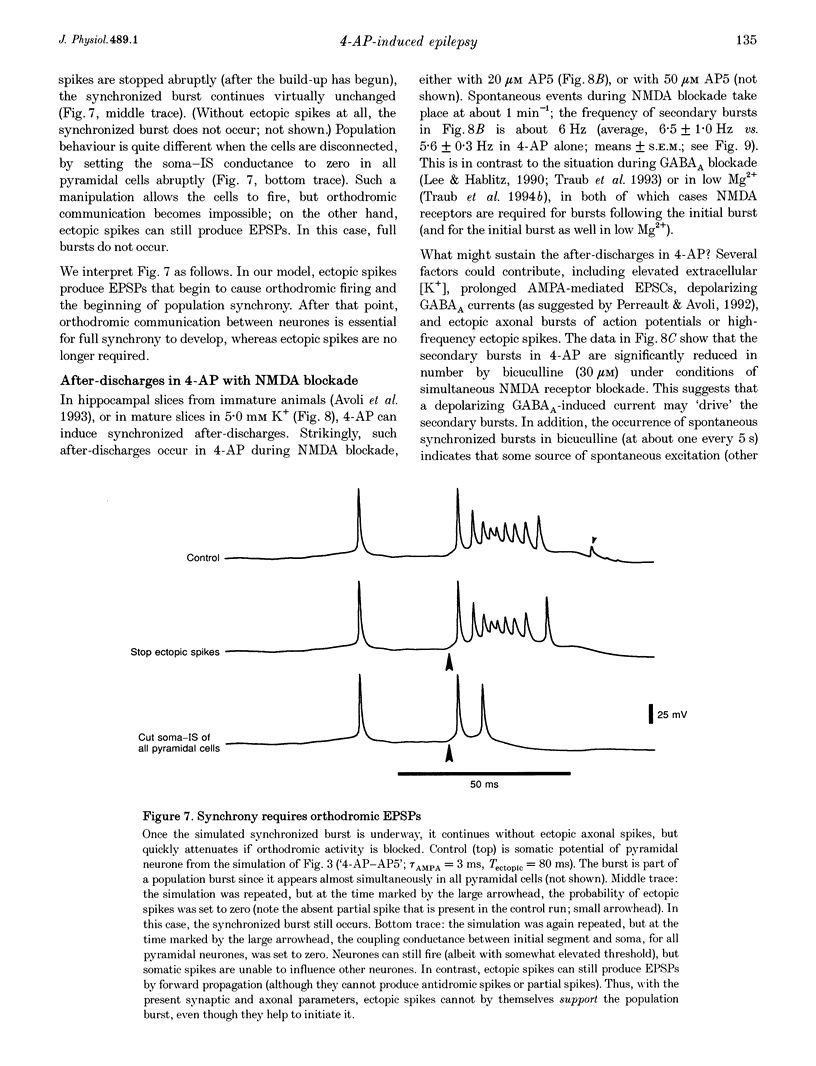
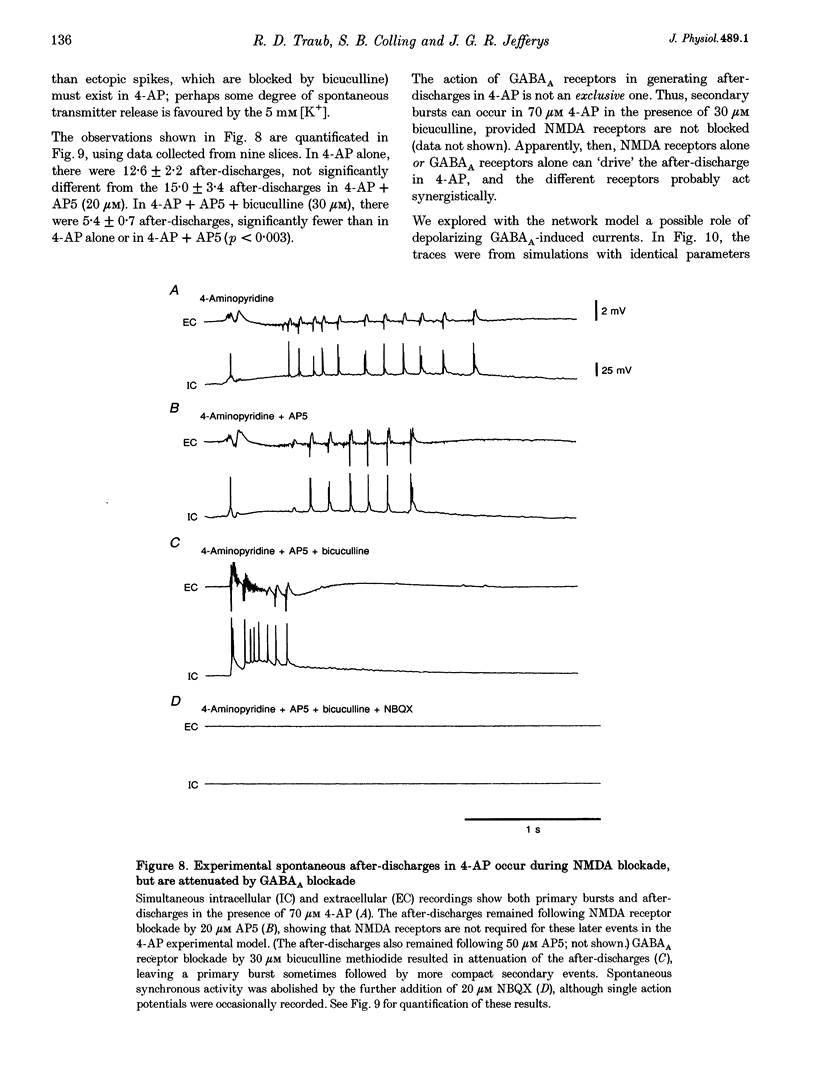
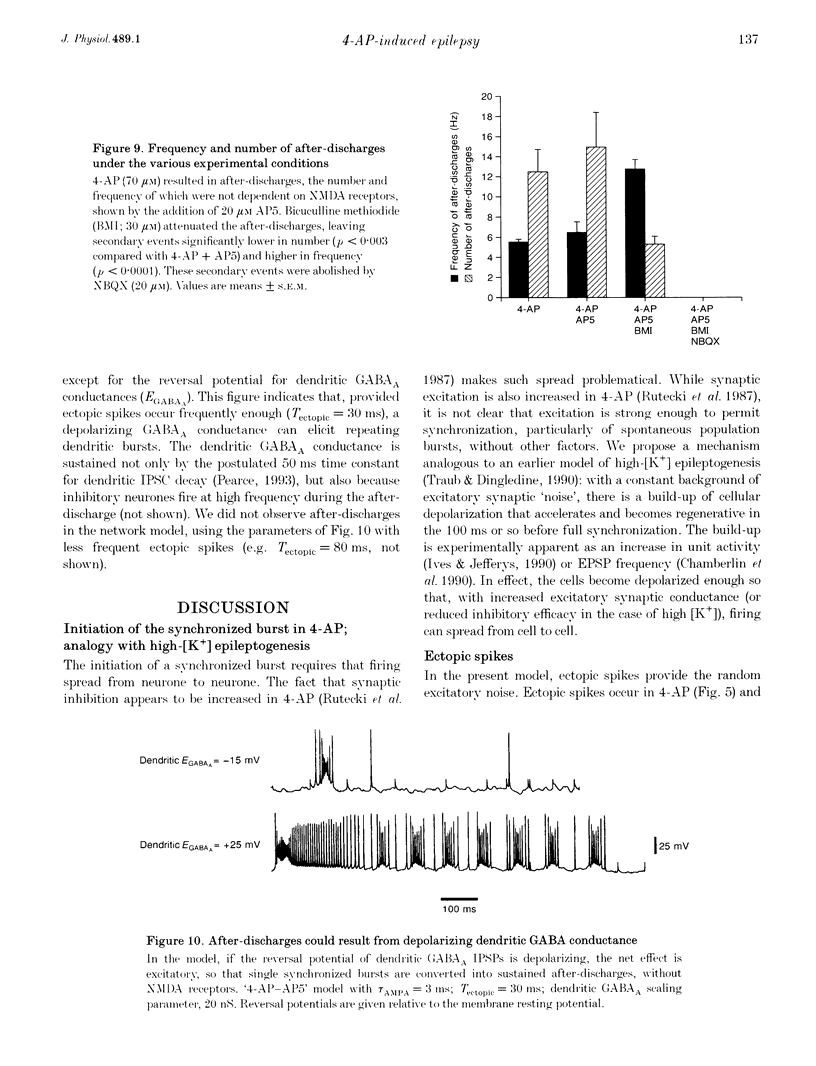
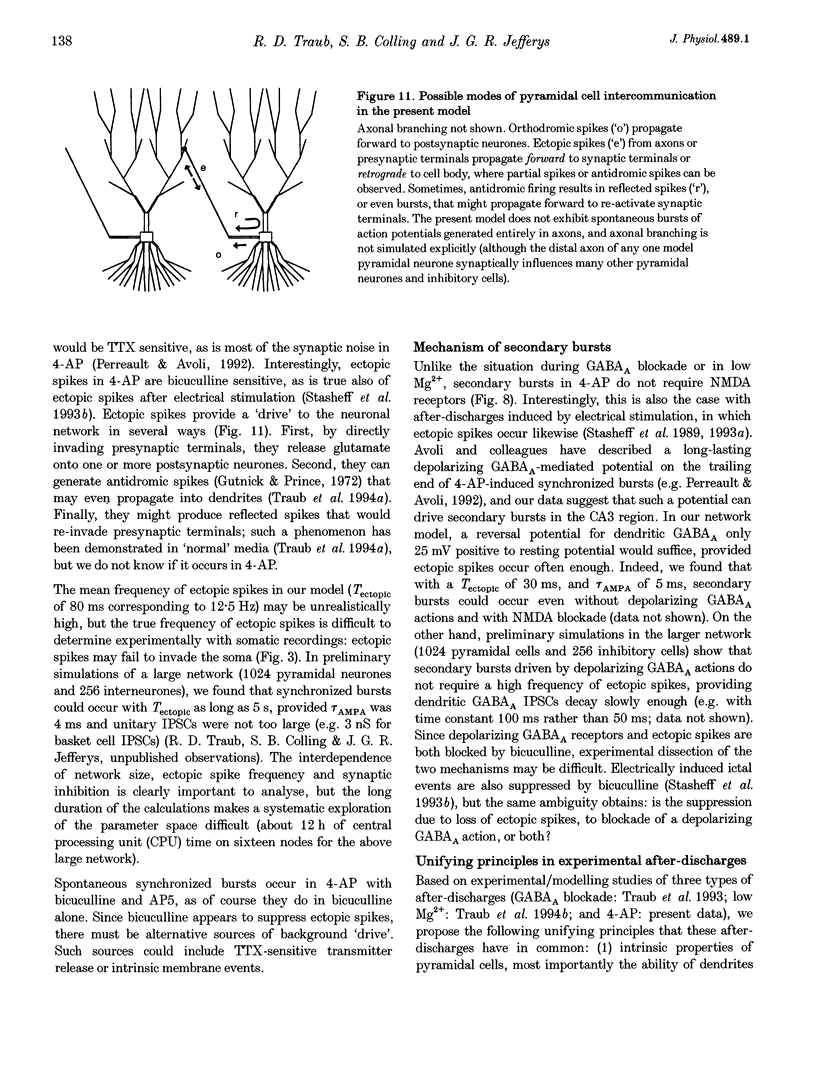
1. We constructed a model of the in vitro rodent CA3 region with 128 pyramidal neurones and twenty-four inhibitory neurones. The model was used to analyse synchronized firing induced in the rat hippocampal slice by 4-aminopyridine (4-AP), a problem simultaneously studied in experiments in rat hippocampal slices. N-methyl-D-aspartate (NMDA) receptors were blocked. 2. Consistent with a known action of 4-AP, unitary EPSCs were assumed to be large and prolonged. With augmented EPSCs, spontaneous synchronized bursts occurred in the model if random ectopic axonal spikes were present. We observed probable antidromic spikes and miniature spikes experimentally. 3. Consistent with experiment, model synchronized bursts were preceded by a period of about 100 ms of increased unit activity and cell depolarization. In the model, this was caused in part by EPSPs consequent to ectopic axonal spikes. 4. After widespread firing had begun, full-blown synchrony in the model required orthodromic EPSPs. A single synchronized burst, once initiated, could proceed without further ectopic activity. 5. A depolarizing change in reversal potential for dendritic GABAA favoured the occurrence of synchronized after-discharges in the model. Consistent with this, bicuculline was found to block after-discharges in slices bathed in 4-AP (70 microM) during NMDA blockade. 6. These data indicate that, even with synaptic inhibition present, ectopic spikes can 'set the stage' for synchronized activity by depolarizing pyramidal cell dendrites, but that recurrent orthodromic EPSPs are required for expression of this synchrony. When synaptic inhibition is present, EPSCs may need to be larger than usual for synchrony to take place. Secondary bursts in 4-AP appear to be driven in part by a depolarizing GABAA-mediated current.
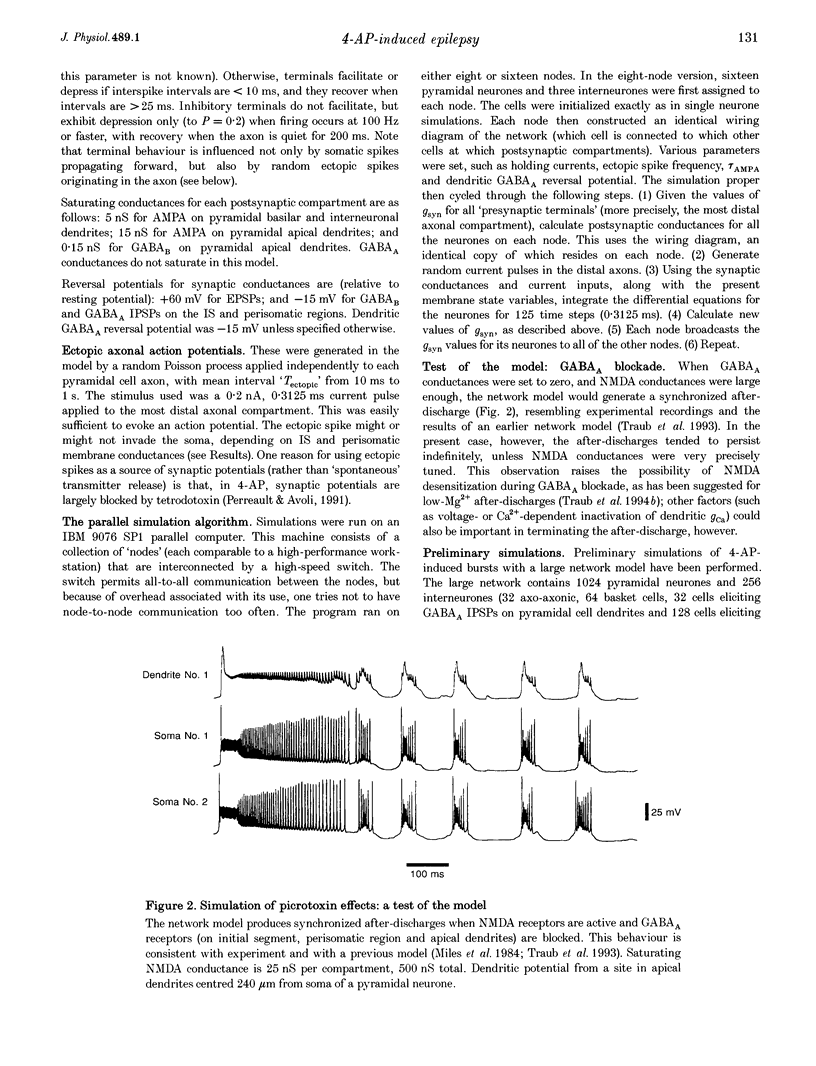
Full text
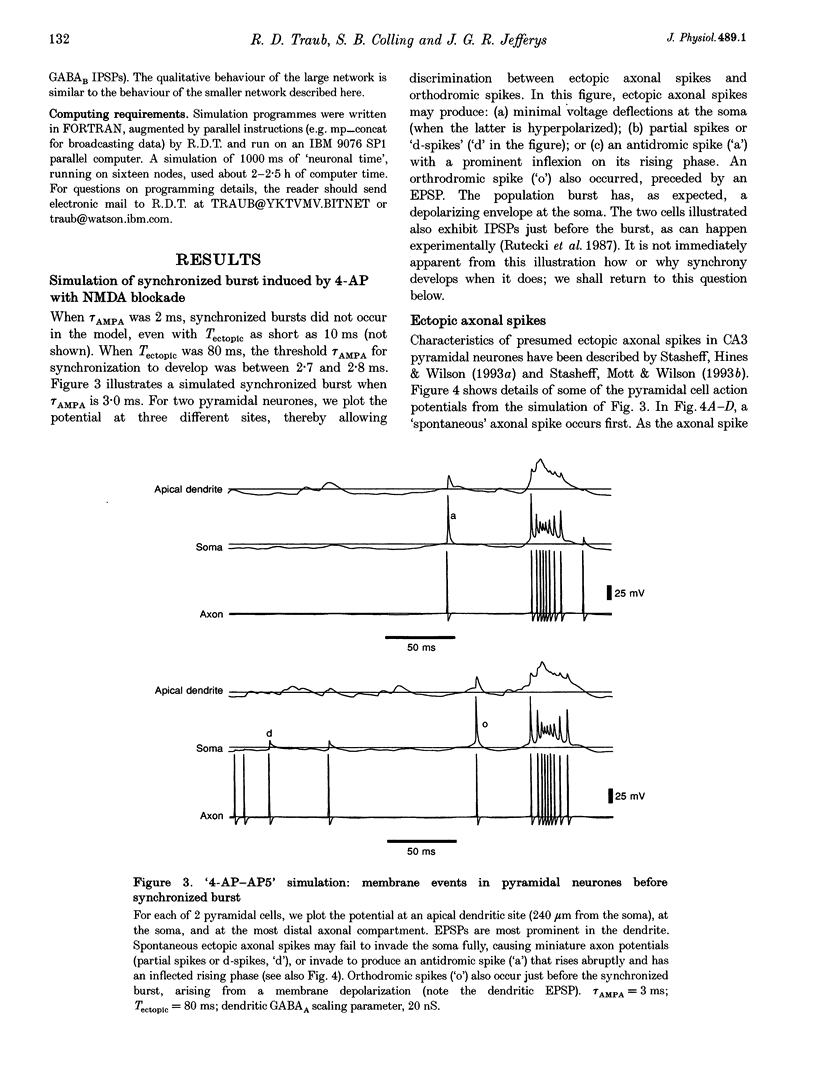
PDF


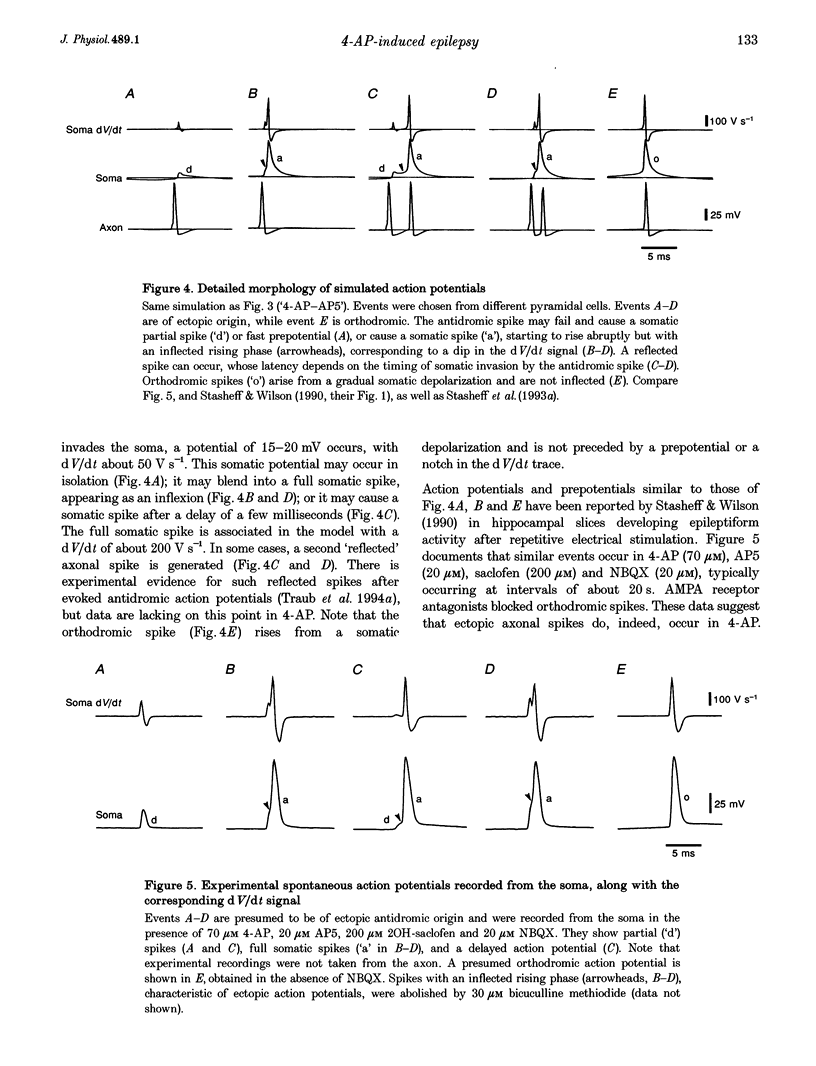


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avoli M., Psarropoulou C., Tancredi V., Fueta Y. On the synchronous activity induced by 4-aminopyridine in the CA3 subfield of juvenile rat hippocampus. J Neurophysiol. 1993 Sep;70(3):1018–1029. doi: 10.1152/jn.1993.70.3.1018. [DOI] [PubMed] [Google Scholar]
- Buckle P. J., Haas H. L. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol. 1982 May;326:109–122. doi: 10.1113/jphysiol.1982.sp014180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl E. H., Halasy K., Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994 Apr 28;368(6474):823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Chamberlin N. L., Traub R. D., Dingledine R. Role of EPSPs in initiation of spontaneous synchronized burst firing in rat hippocampal neurons bathed in high potassium. J Neurophysiol. 1990 Sep;64(3):1000–1008. doi: 10.1152/jn.1990.64.3.1000. [DOI] [PubMed] [Google Scholar]
- Flores-Hernández J., Galarraga E., Pineda J. C., Bargas J. Patterns of excitatory and inhibitory synaptic transmission in the rat neostriatum as revealed by 4-AP. J Neurophysiol. 1994 Nov;72(5):2246–2256. doi: 10.1152/jn.1994.72.5.2246. [DOI] [PubMed] [Google Scholar]
- Gulyás A. I., Miles R., Hájos N., Freund T. F. Precision and variability in postsynaptic target selection of inhibitory cells in the hippocampal CA3 region. Eur J Neurosci. 1993 Dec 1;5(12):1729–1751. doi: 10.1111/j.1460-9568.1993.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Gulyás A. I., Miles R., Sík A., Tóth K., Tamamaki N., Freund T. F. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993 Dec 16;366(6456):683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Gutnick M. J., Prince D. A. Thalamocortical relay neurons: antidromic invasion of spikes from a cortical epileptogenic focus. Science. 1972 Apr 28;176(4033):424–426. doi: 10.1126/science.176.4033.424. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Jefferys J. G. Low-calcium field burst discharges of CA1 pyramidal neurones in rat hippocampal slices. J Physiol. 1984 Sep;354:185–201. doi: 10.1113/jphysiol.1984.sp015371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives A. E., Jefferys J. G. Synchronization of epileptiform bursts induced by 4-aminopyridine in the in vitro hippocampal slice preparation. Neurosci Lett. 1990 May 4;112(2-3):239–245. doi: 10.1016/0304-3940(90)90210-z. [DOI] [PubMed] [Google Scholar]
- Kocsis J. D., Ruiz J. A., Waxman S. G. Maturation of mammalian myelinated fibers: changes in action-potential characteristics following 4-aminopyridine application. J Neurophysiol. 1983 Aug;50(2):449–463. doi: 10.1152/jn.1983.50.2.449. [DOI] [PubMed] [Google Scholar]
- Lee W. L., Hablitz J. J. Effect of APV and ketamine on epileptiform activity in the CA1 and CA3 regions of the hippocampus. Epilepsy Res. 1990 Jul;6(2):87–94. doi: 10.1016/0920-1211(90)90082-7. [DOI] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol. 1990 Sep;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987 Jul;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K., Traub R. D. Synchronized afterdischarges in the hippocampus: contribution of local synaptic interactions. Neuroscience. 1984 Aug;12(4):1179–1189. doi: 10.1016/0306-4522(84)90012-5. [DOI] [PubMed] [Google Scholar]
- Otis T. S., De Koninck Y., Mody I. Characterization of synaptically elicited GABAB responses using patch-clamp recordings in rat hippocampal slices. J Physiol. 1993 Apr;463:391–407. doi: 10.1113/jphysiol.1993.sp019600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce R. A. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993 Feb;10(2):189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Perreault P., Avoli M. 4-aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci. 1992 Jan;12(1):104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault P., Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991 Apr;65(4):771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- Rutecki P. A., Lebeda F. J., Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 1987 Jun;57(6):1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- Stasheff S. F., Anderson W. W., Clark S., Wilson W. A. NMDA antagonists differentiate epileptogenesis from seizure expression in an in vitro model. Science. 1989 Aug 11;245(4918):648–651. doi: 10.1126/science.2569762. [DOI] [PubMed] [Google Scholar]
- Stasheff S. F., Hines M., Wilson W. A. Axon terminal hyperexcitability associated with epileptogenesis in vitro. I. Origin of ectopic spikes. J Neurophysiol. 1993 Sep;70(3):961–975. doi: 10.1152/jn.1993.70.3.961. [DOI] [PubMed] [Google Scholar]
- Stasheff S. F., Mott D. D., Wilson W. A. Axon terminal hyperexcitability associated with epileptogenesis in vitro. II. Pharmacological regulation by NMDA and GABAA receptors. J Neurophysiol. 1993 Sep;70(3):976–984. doi: 10.1152/jn.1993.70.3.976. [DOI] [PubMed] [Google Scholar]
- Stasheff S. F., Wilson W. A. Increased ectopic action potential generation accompanies epileptogenesis in vitro. Neurosci Lett. 1990 Mar 26;111(1-2):144–150. doi: 10.1016/0304-3940(90)90359-h. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988 Nov 24;336(6197):379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Dingledine R. Model of synchronized epileptiform bursts induced by high potassium in CA3 region of rat hippocampal slice. Role of spontaneous EPSPs in initiation. J Neurophysiol. 1990 Sep;64(3):1009–1018. doi: 10.1152/jn.1990.64.3.1009. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Jefferys J. G., Miles R., Whittington M. A., Tóth K. A branching dendritic model of a rodent CA3 pyramidal neurone. J Physiol. 1994 Nov 15;481(Pt 1):79–95. doi: 10.1113/jphysiol.1994.sp020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R. D., Jefferys J. G., Whittington M. A. Enhanced NMDA conductance can account for epileptiform activity induced by low Mg2+ in the rat hippocampal slice. J Physiol. 1994 Aug 1;478(Pt 3):379–393. doi: 10.1113/jphysiol.1994.sp020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R. D., Miles R., Jefferys J. G. Synaptic and intrinsic conductances shape picrotoxin-induced synchronized after-discharges in the guinea-pig hippocampal slice. J Physiol. 1993 Feb;461:525–547. doi: 10.1113/jphysiol.1993.sp019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington M. A., Traub R. D., Jefferys J. G. Erosion of inhibition contributes to the progression of low magnesium bursts in rat hippocampal slices. J Physiol. 1995 Aug 1;486(Pt 3):723–734. doi: 10.1113/jphysiol.1995.sp020848. [DOI] [PMC free article] [PubMed] [Google Scholar]



