Abstract
Glutathione (GSH) is a master antioxidant which primarily protects cells from oxidative stress. Clinical studies have found significant depletion of GSH from the hippocampus in patients with mild cognitive impairment (MCI), a transitional stage before conversion to Alzheimer’s disease (AD). Significant depletion of GSH is considered an early diagnostic biomarker of AD. Postmortem studies have confirmed significant GSH depletion in hippocampal tissue in MCI patients. The stability of GSH in different microenvironments is essential to validate GSH as a reliable biomarker for AD. Accordingly, we have conducted longitudinal monitoring of GSH from various brain regions (frontal cortex (FC), parietal cortex (PC), occipital cortex (OC), and cerebellum (CER)) from healthy subjects using MEshcher-GArwood Point RESolved Spectroscopy (MEGA-PRESS) pulse sequence on a 3T scanner. Additionally, in vitro magnetic resonance spectroscopy (MRS) assessments were conducted longitudinally using the same study protocol involving GSH supplement in a physiologically relevant phosphate buffer solution (PBS). We report that GSH within the brain microenvironment of a healthy person remains stable over time. GSH, however, is susceptible to oxidation over time in a phosphate buffer environment. The stability of GSH in a longitudinal study in the brains of healthy individuals supports the consideration of GSH as a candidate for stable biomarker for AD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11064-024-04265-y.
Keywords: Antioxidant, Glutathione, Longitudinal, MEGA-PRESS, Microenvironment, Biomarker, Stability validation
Introduction
Glutathione (GSH) is a tripeptide (L-γ-glutamyl-L-cysteinyl-glycine) which plays a profound role for antioxidant defence in various cells (e.g. neuron) against the damaging effect from free radicals [1]. Studies have revealed GSH reactivity towards radicals in the following order: •OOCH3 < •OOCHCH2 < •OOCCl3 < •OOH < •OCH3 < •OH [2]. The sulfhydryl group (− SH) in cysteine, known for its high reactivity, attracts reactive oxygen species (ROS) - and free radicals [3]. GSH has a diverse distribution pattern in various anatomical regions of human as follows: liver (5 to 10 mM) [4], kidney (2 to 5 mM) [5], brain (2 to 3 mM) [6], blood (around 1 mM) [7, 8], and lung epithelial cells (0.42 mM) [9]. Ongoing research from various laboratories attests to the growing importance of the GSH molecule and its substantial potential clinical applications [10, 11]. A postmortem study involving bipolar disorder, major depressive disorder and schizophrenia have shown a significant reduction in total GSH levels compared to nonpsychiatric groups [12]. Several autopsy studies found that the GSH levels were significantly decreased in Alzheimer’s disease (AD) brain compared to healthy cognitively normal subject (CN) [13, 14]. Autopsy studies on MCI brain samples from the hippocampal (HP) region also showed a significant reduction of GSH levels [13–15]. MEscher GArwood-Point RESolved Spectroscopy (MEGA-PRESS) pulse sequence is now widely used for non-invasive detection of GSH using magnetic resonance spectroscopy (MRS) [16–18].
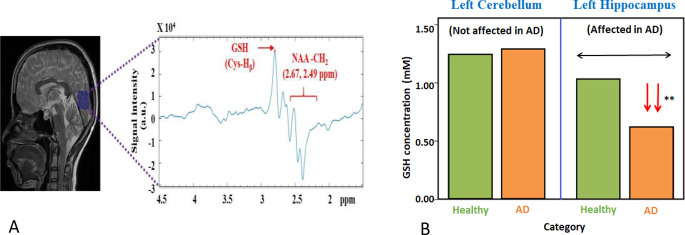
A representative spectrum of GSH from a CN participant using MEGA-PRESS is shown in Fig. 1A and absolute concentration of GSH both from cerebellum and hippocampal regions from CN and AD patients [19] are presented in Fig. 1B.
Fig. 1.
A: Illustration of MRS spectra from the single voxel (shown as blue box) from the occipital region of a healthy young participant. Data was acquired using MEGA-PRESS sequence. (B) Comparative of GSH concentration from left cerebellum and left hippocampus for healthy old and AD patients. Statistically significant depletion of GSH is found exclusively in the hippocampal region of AD patients compared to healthy elderly participants, whereas the GSH level in the cerebellum is not affected in AD or in healthy old participants [19]
Various cross-sectional studies have indicated the depletion of GSH concentration in distinct anatomical regions (e.g. frontal cortex, hippocampal) and is considered an early diagnostic biomarker of AD [19, 20]. It is essential to monitor longitudinally the brain GSH level for stability of GSH level in healthy subjects to establish GSH as a reliable and a stable biomarker. Accordingly, we have undertaken longitudinal studies to monitor GSH levels from various brain regions over time involving healthy participants. For comparative purposes, the GSH supplement (in a neutral pH and phosphate buffer) was investigated over time. This is the first longitudinal study to test the stability of GSH peaks in humans.
Methods
Study Design
This study design was aimed to test the stability of GSH over time from various brain region in a healthy control subject. For comparative analysis, a similar approach was taken to test the GSH stability in PBS medium in a physiologically relevant PBS buffer solution at three-time points. The GSH data acquisition protocol and signal processing protocol were kept the same in both in human study and in the PBS medium.
Phantom Study with GSH supplement. The phantom (labelled A) consists of 500 mg of GSH supplements in a 1 L phosphate buffer, with pH adjusted to 7.2. MRS data was collected from the GSH in PBS solution over time (three-time points over a period of 8 days). The name of the GSH supplement is anonymized to avoid biased reporting. We have also recorded a GSH sample in liquid spray form and a time dependent study outcome is presented in Figure S3, supplementary materials.
Human Study: Eight healthy young volunteers (N = 8, M/F = 3/5, mean age: 23.87 ± 4.29) participated in this longitudinal study. All the participants have completed a minimum of 15 years of education. Participants were on a regular diet and regular nourished individuals and had no contraindications (e.g. metallic implants or claustrophobia) for magnetic resonance imaging (MRI). According to the study plan, MRS recordings were obtained at three times points over a sixteen-day span to track changes in GSH levels in frontal cortex (FC), parietal cortex (PC), occipital cortex (OC) and cerebellum (CER) regions. All studies were conducted as per approved protocol at NBRC.
Data Acquisition
Proton (1H) MRI/MRS data were collected from both human subjects and phantom containing GSH sample using a 3T MRI scanner (Prisma) equipped with a 64-channel 1H head coil at NBRC. First, scout images were obtained in transverse, sagittal, and coronal planes, and these MRI images served as visual guide for placing measurement voxels in different brain regions. Two dimensional T2-weighted MRI images were acquired in all three planes with the following settings: repetition time (TR) = 6450 ms and echo time (TE) = 40 ms, and each plane’s scan time was 1 minute and 19 s. The MRS data for quantifying GSH in both phantom and healthy participants were acquired using the MEGA-PRESS sequence [21], with the following parameters: ON = 4.40 ppm, OFF = 5.00 ppm, TE = 120 ms and TR = 2500 ms, voxel size = 25 × 25 × 25 mm, and an average of 32 acquisitions as per our earlier protocol [3, 22, 23]. The VAPOR pulse sequence was utilized to suppress the water signal during acquisition. Automatic shimming and 3D shimming was applied resulting in a water peak linewidth of ≤ 15 Hz for all four anatomical regions. In this study, voxel placement in vivo MRI and MRS experiments as well as GSH phantom were conducted by one of the members to maintain consistency in voxel positioning. Voxel placement for in vivo measurements was guided by anatomical references, and rigorous shimming (ensuring full width half maximum (FWHM) values ≤ 15 Hz for human experiments and ≤ 3 Hz for phantom experiments) was achieved during data acquisition. Each participant was requested not to move head during experiment and participant head was strapped to minimize any head movement. The MRS raw data was saved in (.rda) format, and MRI images were saved in DICOM formats for further processing.
Data Analysis
MATLAB-based MRS signal processing package KALPANA [22] was utilized to process the MEGA-PRESS data and quantification [22]. The MEGA-PRESS data quality was further assessed by calculating the signal-to-noise ratio (SNR) for each individual spectrum. This calculation was performed in the frequency domain within the 0.5–6.0 ppm range using the method as elaborated earlier [22, 24]. The absolute GSH concentration of human brain was calculated from external reference GSH phantom as described in our earlier work [20].
Results
GSH Level (in vitro) Monitoring in GSH Supplement Longitudinally
MRS experiment was performed using the GSH supplement (labelled as A) over eight-day time span. We have also studied the standard laboratory GSH compound (Sigma Aldrich labelled as B, in supplementary Figure S1 & S2). The longitudinal data points (GSH peak area) for phantoms containing GSH (A) were fitted with a linear model to indicate the decay of GSH peak (Fig. 2).
Fig. 2.
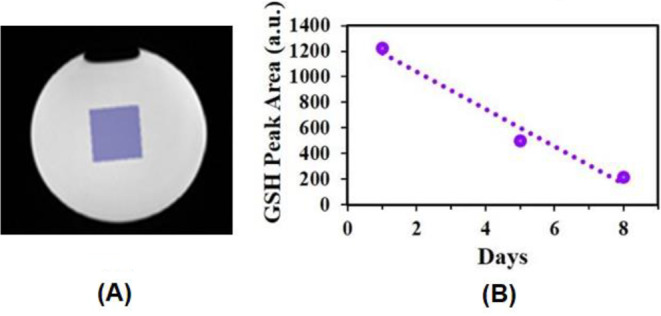
(A) Voxel (25 × 25 × 25 mm3) was placed on the phantom to detect GSH in solution. (B) Longitudinal monitoring of GSH levels from GSH supplement in the phantom. Three time points MRS recordings were acquired from the GSH solution in the phantom over the time course of 8 days
Discussion
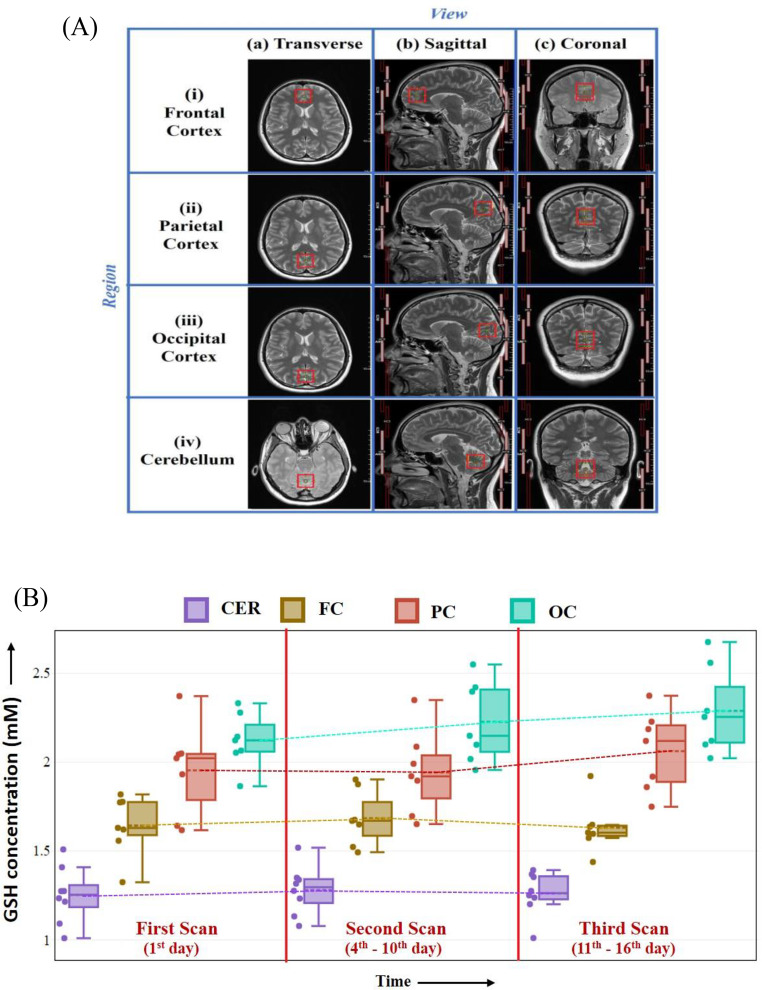
The study presents regional in vivo GSH distributions of brain GSH levels in healthy young participants as well as consistency of GSH levels over time. The quantitative distribution of GSH levels exhibited a consistent pattern across brain regions, (OC > PC > FC > CER) (Fig. 3(A)) from healthy young participants. These results agree with the previous cross-sectional study which reported GSH distribution pattern as PC > FC > hippocampus ~ CER from the healthy young brain [18]. This is the first longitudinal study to test the stability of GSH peak over time.
Fig. 3.
MRS Voxel (25 × 25 × 25 mm³) placement and acquired GSH data from different brain regions. (A) Transverse, sagittal, and coronal representations of voxel positioned in anatomical regions: frontal cortex (FC), parietal cortex (PC), occipital cortex (OC), and cerebellum (CER) from healthy young participants. (B) Plot of brain GSH concentration from four anatomical regions in three time points
Relevance of GSH Levels in Brain Disorders
Neurodegenerative diseases are characterized by the progressive degeneration of neurons in the central nervous system. Although, the exact cause of these diseases is not identified, emerging research has highlighted that oxidative stress plays a critical role in their pathogenesis [3, 25]. It is observed that significant depletion of GSH levels are associated with the conversion of HC to MCI, and, further, significant depletion of GSH level occurs for the conversion of MCI to AD [19]. Notably, GSH levels in the cerebellum of the same AD patients does not show any depletion (Fig. 1B) compared to HC [19]. Cingulate cortex (CC) containing anterior (ACC) and posterior (PCC) regions are an important part of the default mode network. These ACC, and PPC anatomical regions showed significant depletion of GSH in the transition of HC to MCI/AD [22]. GSH depletion from substantia nigra was also negatively correlated to motor function performance in PD patients [3].
In addition to AD [19, 20] and PD [3, 26], alteration in the GSH levels is also suggested to be linked with the development of psychiatric disorders. The GSH in the dorsolateral prefrontal cortex is depleted significantly in schizophrenia patients [27].
GSH levels measured from a single precentral gyrus voxel was found to be lower (31%) in amyotrophic lateral sclerosis (ALS) patients compared to HC (p =.005) [28]. In a postmortem study, the association between deficiency of GSH antioxidant defense in specific brain regions (temporal cortex and cerebellum) with autism was suggested [29]. The study suggested that perturbation in GSH homeostasis can lead to oxidative stress and immune malfunction which can further cause autism related neurodevelopmental conditions [29]. A similar study used frozen post-mortem samples of cerebellum and Brodmann area 22 (temporal cortex) [30]. Based on clinical research involving AD and MCI patients, it is now indicated that GSH depletion occurs prior to amyloid Aβ plaque formation and tau phosphorylation in AD [31]. Indeed, animal model studies involving transgenic mice indicated hippocampal depletion of GSH precedes the elevation of Aβ42/Aβ40 ratio and the aggregation of the tau protein [32, 33].
Microenvironment and Glutathione Oxidation
Normal brain is associated with the following: changes in antioxidant content, blood flow and metabolism, a gradual decline in several cognitive functions, changes in brain volume, the amount of white matter (WM), and an increase in the cerebral iron content [34–36]. All these changes occur to a different extent in the healthy aging process. The master antioxidant, GSH, is a thiol-containing tripeptide and a reservoir pool of the amino acid cysteine and is important for optimal neuronal functioning [37]. The highly reactive sulfhydryl group (− SH) of cysteine (Cys) acts like an “antenna” to attract reactive oxygen species (ROS) and free radicals [38].
In our case, GSH in phosphate buffered solution environment, cysteine S-H is directly getting exposed to oxygen and is prone to oxidation (Fig. 2(B)). In the healthy human brain, the cysteine S-H is in protected microenvironment (Fig. 3(B)). In which brain homeostasis is maintained. Thus, cysteine S-H groups are not exposed to oxygen and free radical. Hence, the GSH peak area from cysteine b-H remain, stable over time in those healthy persons.
One study has reported oxidation of GSH in aqueous medium involving a standard laboratory GSH sample (in vitro) like (Fig. 2B) over time [39].
Proposed Platform for Longitudinal Brain GSH Monitoring and Quantitation
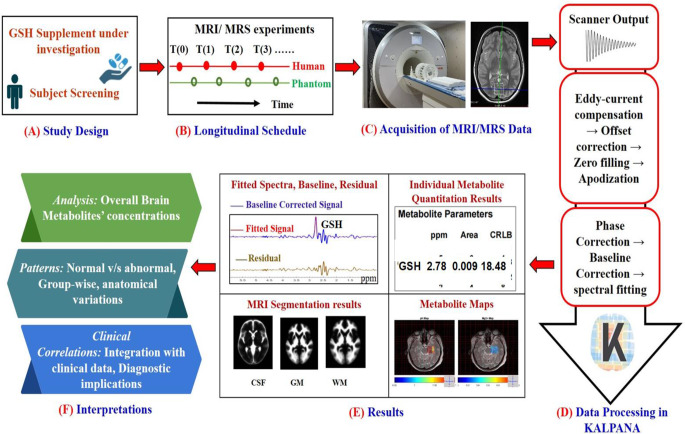
To monitor the GSH level in healthy/patient or in a clinical trial for brain GSH enrichment through GSH supplements or its other agents, a comprehensive general scheme is required (Fig. 4). The initial step involves the formulation of a study design (step A) aimed to evaluate brain GSH levels within a targeted population under study. A set longitudinal time schedule (step B) is established, outlining the timing and frequency of MRI/MRS experiments to monitor changes in brain GSH longitudinally. In step C, specialized sequence (MEGA-PRESS) is employed to acquire MRS data longitudinally from the study participants. For the processing of MRS data (step D), the KALPANA [22] tool can be involved for processing and quantitation. Following MRS data processing, various results (step E) derived from the KALPANA are presented. This includes processed MRS spectra, quantitative information about GSH levels in the brain, MRI segmentation results and metabolic maps on the anatomical region. Using the presented results, important interpretations are made (step F). Utilizing the MEGA-PRESS MRS sequence enables the simultaneous quantification of GSH and other proton metabolites [22]. Consequently, this comprehensive design offers a versatile framework for monitoring not only GSH but also other crucial brain metabolites.
Fig. 4.
Proposed platform for longitudinal monitoring of GSH. (A) Study design for the proposed platform for longitudinal monitoring of GSH. (B) Longitudinal schedule, outlining specific time points for monitoring GSH levels using a specialized pulse sequence, such as MEGA-PRESS. The scheme involves to addresses to monitor GSH levels using specialized pulse sequence (like MEGA-PRESS) at various time points. (C) MRI scanner equipped suitable head coil for MRS data collection from human participants. (D) The raw data can be analyzed in an appropriate toolbox (e.g. KALPANA is utilized for MRS data processing, step (E)) Tissue segmentation (CSF: Cerebrospinal fluid, GM: grey matter, WM: white matter) as well as absolute quantitation of GSH using KALPANA. (F) Interference from the study
Conclusion
GSH has become an important diagnostic biomarker in various brain disorders, and the reliability and stability of GSH is very useful to assess the microenvironment of the anatomical brain regions. It also indicates that in a specific disease, only particular anatomical region brain microenvironment is affected. The GSH level is depleted in the hippocampus in AD, however, the cerebellum GSH of that same AD patient remains unchanged. Similarly in the healthy brain there is no alternation of GSH level in various anatomical regions. Presently, GSH detection is limited to a single voxel mode. In the future, multivoxel MRS would be very helpful in the clinical trial involving various GSH enriching supplements (e.g., gamma-glutamylcysteine).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Professor Pravat K Mandal (Principal Investigator) thanks Indo-Australia strategic funding (BT/Indo-Aus/10/31/2016/PKM) and dementia science program (Principal investigator and Co-coordinator (PKM), Department of Biotechnology, Govt of India). We appreciate Ms. Gayathri Kumaran (Intern, NINS lab) for her contribution in upgrading Siemens data processing pipeline in KALPANA toolbox. Thanks to Ms. Anushka Mandal for proofreading the manuscript.
Author Contributions
P.K.M. (Principal Investigator) conceptualized the research idea, involved in experimental design, brain imaging data generation and quality of data monitoring, and wrote the manuscript. Y.A was involved in experimental design, brain imaging data acquisition, analysis of data, figure preparation and involved in writing the paper. A.S was involved in experimental design and data collection and manuscript writing. J.C.M. provided GSH supplements, manuscript writing and was involved in manuscript discussions.
Data Availability
Data will be shared on request.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dringen R, Gutterer JM, Hirrlinger J (2000) Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267:4912–4916 [DOI] [PubMed] [Google Scholar]
- 2.Higashi Y, Aratake T, Shimizu T, Shimizu S, Saito M (2021) Protective role of glutathione in the Hippocampus after Brain Ischemia. Int J Mol Sci 22 [DOI] [PMC free article] [PubMed]
- 3.Shukla D, Goel A, Mandal PK, Joon S, Punjabi K, Arora Y, Kumar R, Mehta VS, Singh P, Maroon JC, Bansal R, Sandal K, Roy RG, Samkaria A, Sharma S, Sandhilya S, Gaur S, Parvathi S, Joshi M (2023) Glutathione Depletion and Concomitant Elevation of Susceptibility in Patients with Parkinson’s Disease: State-of-the-Art MR Spectroscopy and Neuropsychological Study. ACS Chem Neurosci [DOI] [PMC free article] [PubMed]
- 4.Commandeur JN, Stijntjes GJ, Vermeulen NP (1995) Enzymes and transport systems involved in the formation and disposition of glutathione S-conjugates. Role in bioactivation and detoxication mechanisms of xenobiotics. Pharmacol Rev 47:271–330 [PubMed] [Google Scholar]
- 5.Lash LH (2005) Role of glutathione transport processes in kidney function. Toxicol Appl Pharmacol 204:329–342 [DOI] [PubMed] [Google Scholar]
- 6.Sun X, Shih AY, Johannssen HC, Erb H, Li P, Murphy TH (2006) Two-photon imaging of glutathione levels in intact brain indicates enhanced redox buffering in developing neurons and cells at the cerebrospinal fluid and blood-brain interface. J Biol Chem 281:17420–17431 [DOI] [PubMed] [Google Scholar]
- 7.Michelet F, Gueguen R, Leroy P, Wellman M, Nicolas A, Siest G (1995) Blood and plasma glutathione measured in healthy subjects by HPLC: relation to sex, aging, biological variables, and life habits. Clin Chem 41:1509–1517 [PubMed] [Google Scholar]
- 8.Richie JP Jr., Skowronski L, Abraham P, Leutzinger Y (1996) Blood glutathione concentrations in a large-scale human study. Clin Chem 42:64–70 [PubMed] [Google Scholar]
- 9.Cantin AM, North SL, Hubbard RC, Crystal RG (1987) Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 63:152–157 [DOI] [PubMed]
- 10.Dwivedi D, Megha K, Mishra R, Mandal PK (2020) Glutathione in brain: overview of its conformations, functions, biochemical characteristics, quantitation and potential therapeutic role in Brain disorders. Neurochem Res 45:1461–1480 [DOI] [PubMed] [Google Scholar]
- 11.Aoyama K (2021) Glutathione in the brain. Int J Mol Sci 22 [DOI] [PMC free article] [PubMed]
- 12.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT (2011) Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14:123–130 [DOI] [PubMed] [Google Scholar]
- 13.Gu M, Owen AD, Toffa SE, Cooper JM, Dexter DT, Jenner P, Marsden CD, Schapira AH (1998) Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J Neurol Sci 158:24–29 [DOI] [PubMed] [Google Scholar]
- 14.Ansari MA, Scheff SW (2010) Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol 69:155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sultana R, Piroddi M, Galli F, Butterfield DA (2008) Protein levels and activity of some antioxidant enzymes in hippocampus of subjects with amnestic mild cognitive impairment. Neurochem Res 33:2540–2546 [DOI] [PubMed] [Google Scholar]
- 16.Mandal PK (2007) Magnetic resonance spectroscopy (MRS) and its application in Alzheimer’s disease. Concepts Magn Reson Part A: Educational J 30:40–64 [Google Scholar]
- 17.Terpstra M, Henry PG, Gruetter R (2003) Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn Reson Med 50:19–23 [DOI] [PubMed] [Google Scholar]
- 18.Mandal PK, Tripathi M, Sugunan S (2012) Brain oxidative stress: detection and mapping of anti-oxidant marker ‘Glutathione’in different brain regions of healthy male/female, MCI and Alzheimer patients using non-invasive magnetic resonance spectroscopy. Biochem Biophys Res Commun 417:43–48 [DOI] [PubMed] [Google Scholar]
- 19.Mandal PK, Saharan S, Tripathi M, Murari G (2015) Brain glutathione levels–a novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biol Psychiatry 78:702–710 [DOI] [PubMed] [Google Scholar]
- 20.Mandal PK, Goel A, Bush AI, Punjabi K, Joon S, Mishra R, Tripathi M, Garg A, Kumar NK, Sharma P, Shukla D, Ayton SJ, Fazlollahi A, Maroon JC, Dwivedi D, Samkaria A, Sandal K, Megha K, Shandilya S (2022) Hippocampal glutathione depletion with enhanced iron level in patients with mild cognitive impairment and Alzheimer’s disease compared with healthy elderly participants. Brain Commun 4:fcac215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter (1998) Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine: An International Journal devoted to the development and application of magnetic. Reson Vivo 11:266–272 [DOI] [PubMed]
- 22.Shukla D, Mandal PK, Tripathi M, Vishwakarma G, Mishra R, Sandal K (2020) Quantitation of in vivo brain glutathione conformers in cingulate cortex among age-matched control, MCI, and AD patients using MEGA‐PRESS. Hum Brain Mapp 41:194–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla D, Mandal PK, Ersland L, Grüner ER, Tripathi M, Raghunathan P, Sharma A, Chaithya G, Punjabi K, Splaine C (2018) A multi-center study on human brain glutathione conformation using magnetic resonance spectroscopy. J Alzheimers Dis 66:517–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallat SG (1989) A theory for multiresolution signal decomposition: the wavelet representation. IEEE Trans Pattern Anal Mach Intell 11:674–693 [Google Scholar]
- 25.Rekatsina M, Paladini A, Piroli A, Zis P, Pergolizzi JV, Varrassi G (2020) Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv Therapy 37:113–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce RK, Owen A, Daniel S, Jenner P, Marsden CD (1997) Alterations in the distribution of glutathione in the substantia nigra in Parkinson’s disease. J Neural Transm (Vienna) 104:661–677 [DOI] [PubMed] [Google Scholar]
- 27.Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, Takanashi J, Matsuda T, Shimizu E, Ikehira H, Iyo M, Hashimoto K (2008) Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS ONE 3:e1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiduschat N, Mao X, Hupf J, Armstrong N, Kang G, Lange DJ, Mitsumoto H, Shungu DC (2014) Motor cortex glutathione deficit in ALS measured in vivo with the J-editing technique. Neurosci Lett 570:102–107 [DOI] [PubMed] [Google Scholar]
- 29.Chauhan A, Audhya T, Chauhan V (2012) Brain region-specific glutathione redox imbalance in autism. Neurochem Res 37:1681–1689 [DOI] [PubMed] [Google Scholar]
- 30.Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, James SJ (2012) Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry 2:e134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy RG, Mandal PK, Maroon JC (2023) Oxidative stress occurs prior to amyloid Aβ plaque formation and tau phosphorylation in Alzheimer’s Disease. Role of Glutathione and Metal Ions. ACS Chemical Neuroscience [DOI] [PMC free article] [PubMed]
- 32.Pontrello CG, McWhirt JM, Glabe CG, Brewer GJ (2022) Age-related oxidative redox and metabolic changes precede Intraneuronal amyloid-beta Accumulation and Plaque Deposition in a transgenic Alzheimer’s Disease Mouse Model. J Alzheimers Dis 90:1501–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashim KNB, Matsuba Y, Takahashi M, Kamano N, Tooyama I, Saido TC, Hashimoto S (2024) Loss of glutathione elevates the Aβ42/Aβ40 ratio and the aggregation of the tau protein. FEBS Letters [DOI] [PubMed]
- 34.Peters R (2006) Ageing and the brain. Postgrad Med J 82:84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deelchand DK, McCarten JR, Hemmy LS, Auerbach EJ, Eberly LE, Marjanska M (2020) Changes in the intracellular microenvironment in the aging human brain. Neurobiol Aging 95:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921 [DOI] [PubMed] [Google Scholar]
- 37.Paul BD, Sbodio JI, Snyder SH (2018) Cysteine metabolism in neuronal Redox Homeostasis. Trends Pharmacol Sci 39:513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandal PK, Dwivedi D, Joon S, Goel A, Ahasan Z, Maroon JC, Singh P, Saxena R, Roy RG (2023) Quantitation of brain and blood glutathione and Iron in healthy age groups using Biophysical and in vivo MR Spectroscopy: potential clinical application. ACS Chem Neurosci 14:2375–2384 [DOI] [PubMed] [Google Scholar]
- 39.Brix MK, Dwyer GE, Craven AR, Grüner R, Noeske R, Ersland L (2019) MEGA-PRESS and PRESS measure oxidation of glutathione in a phantom. Magn Reson Imaging 60:32–37 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared on request.