Abstract
1. The activity of the human endothelial cell L-arginine transporter (system y+) has been correlated with cGMP production (index of nitric oxide) and prostacyclin (PGI2) release in umbilical vein endothelial cells cultured from normal or gestational diabetic pregnancies. 2. In non-diabetic and diabetic cells, transport of L-arginine was Na+ and pH independent, inhibited by other cationic L-arginine analogues and unaffected by neutral amino acids. 3. Diabetes was associated with an increased Vmax for saturable L-arginine transport (4.6 +/- 0.13 vs. 9.9 +/- 0.5 pmol (microgram protein)-1 min-1, P < 0.01), but had no effect on initial rates of transport for L-serine, L-citrulline, L-leucine or 2-deoxyglucose. 4. In non-diabetic and diabetic cells, elevated K+ resulted in a concentration-dependent inhibition in the initial rates of transport for L-arginine and the membrane potential-sensitive probe tetra[3H]phenylphosphonium (TPP+). 5. When resting membrane potential was measured using the whole-cell patch voltage clamp technique, diabetic cells were hyperpolarized (-78 +/- 0.3 mV) compared with non-diabetic cells (-70 +/- 0.04 mV, P < 0.04). Accumulation of [3H]TPP+ was also increased in diabetic compared with non-diabetic cells. 6. Basal intracellular cGMP levels were elevated 2.5-fold in diabetic cells, and L-NAME (100 microM), an inhibitor of nitric oxide synthase, abolished basal cGMP accumulation in non-diabetic and diabetic cells. 7. Histamine (10 microM) had no effect on L-arginine transport but evoked significant increases in cGMP in non-diabetic and diabetic cells, which were completely inhibited by L-NAME but unaffected by superoxide dismutase. 8. Basal and histamine-stimulated PGI2 release was decreased markedly in diabetic cells. 9. Our findings demonstrate that gestational diabetes is associated with phenotypic changes in fetal endothelial cells, which result in a membrane hyperpolarization, activation of the human endothelial cell L-arginine transporter (system y+), elevation of basal nitric oxide synthesis and decreased PGI2 production.
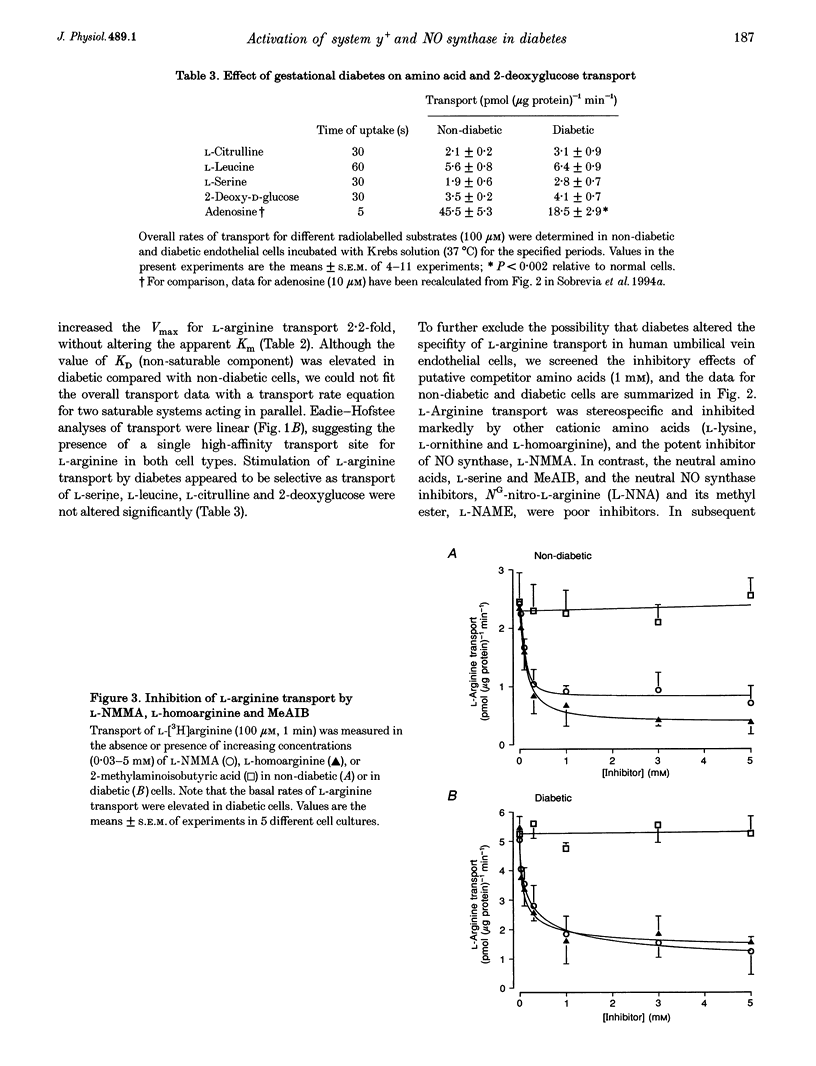
Full text
PDF


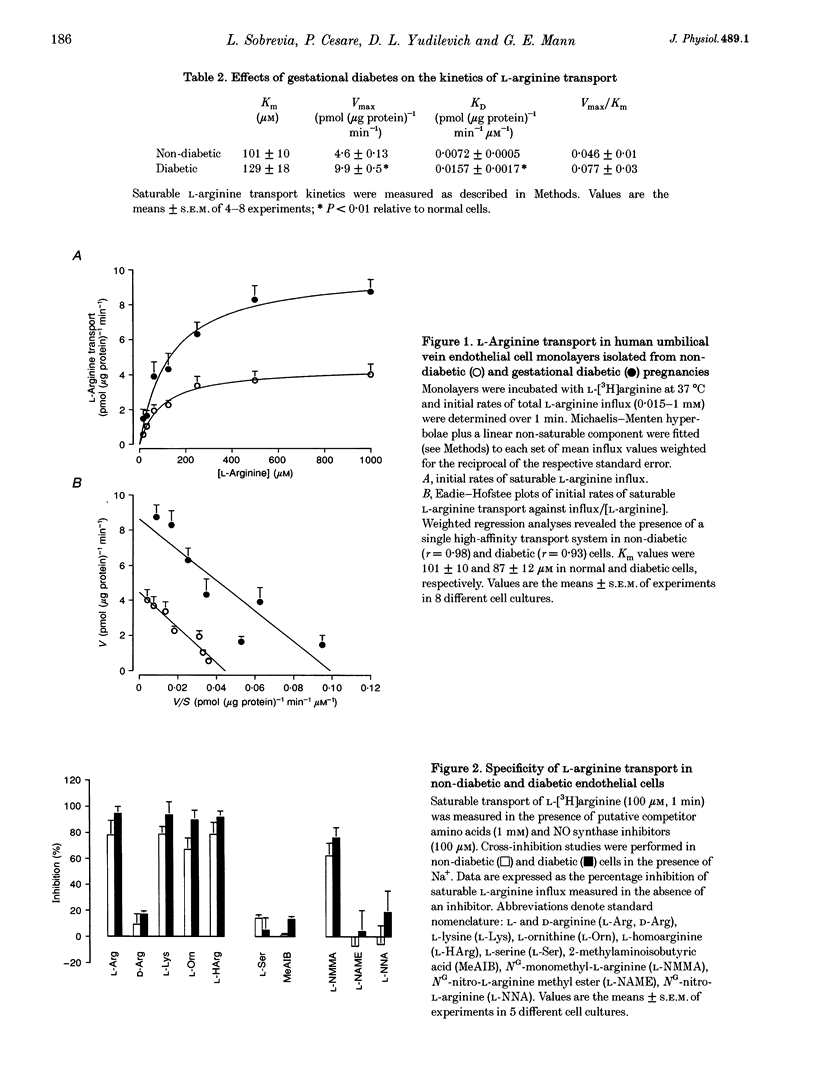
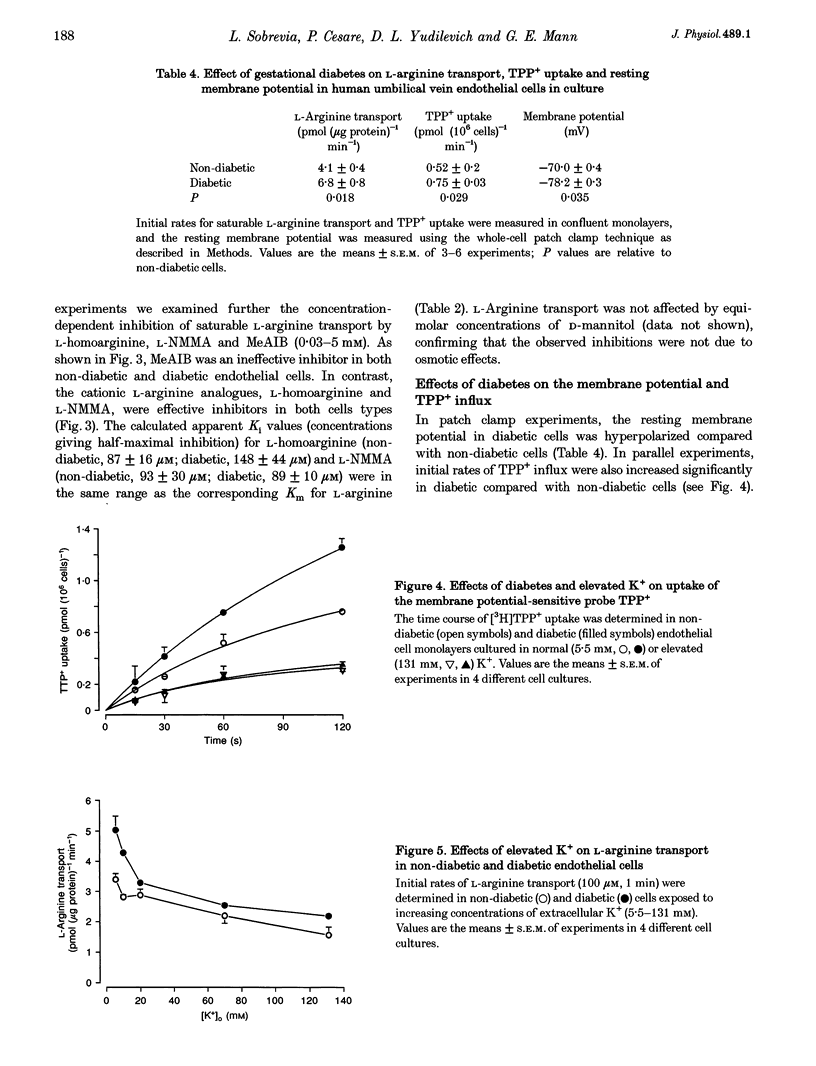



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
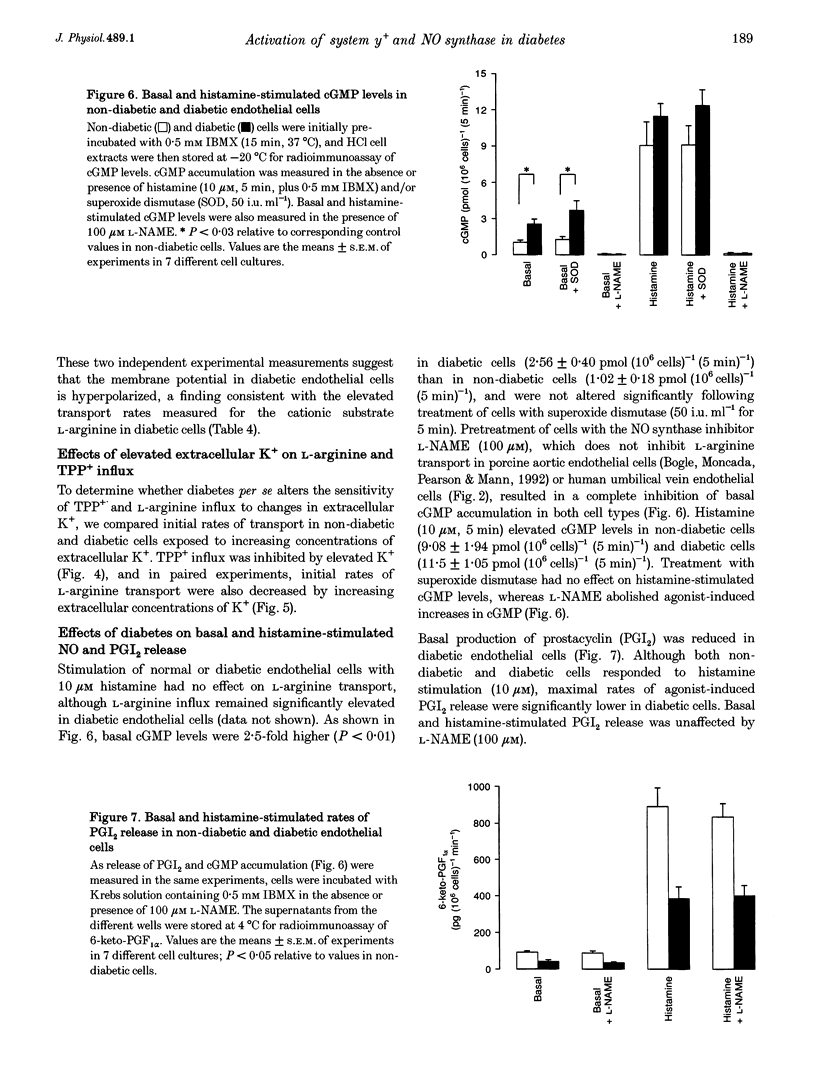
- Baydoun A. R., Mann G. E. Selective targeting of nitric oxide synthase inhibitors to system y+ in activated macrophages. Biochem Biophys Res Commun. 1994 Apr 29;200(2):726–731. doi: 10.1006/bbrc.1994.1511. [DOI] [PubMed] [Google Scholar]
- Bogle R. G., Coade S. B., Moncada S., Pearson J. D., Mann G. E. Bradykinin and ATP stimulate L-arginine uptake and nitric oxide release in vascular endothelial cells. Biochem Biophys Res Commun. 1991 Oct 31;180(2):926–932. doi: 10.1016/s0006-291x(05)81154-4. [DOI] [PubMed] [Google Scholar]
- Bogle R. G., Moncada S., Pearson J. D., Mann G. E. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-arginine transporter. Br J Pharmacol. 1992 Apr;105(4):768–770. doi: 10.1111/j.1476-5381.1992.tb09053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R., Tracey K. J., Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991 Feb;87(2):432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati O., Sala R., Astorri A., Rotoli B. M., Dall'Asta V., Gazzola G. C. Characterization of amino acid transport in human endothelial cells. Am J Physiol. 1993 Oct;265(4 Pt 1):C1006–C1014. doi: 10.1152/ajpcell.1993.265.4.C1006. [DOI] [PubMed] [Google Scholar]
- Calver A., Collier J., Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992 Dec;90(6):2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R., Angelo S., Chávez P. N-ethylmaleimide discriminates between two lysine transport systems in human erythrocytes. J Physiol. 1993 Aug;468:753–766. doi: 10.1113/jphysiol.1993.sp019799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R., Chavez P., Boyd C. A. Identification of a new transport system (y+L) in human erythrocytes that recognizes lysine and leucine with high affinity. J Physiol. 1992 Aug;454:491–501. doi: 10.1113/jphysiol.1992.sp019275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornhorst A., Beard R. W. Gestational diabetes: a challenge for the future. Diabet Med. 1993 Dec;10(10):897–905. doi: 10.1111/j.1464-5491.1993.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Elliott T. G., Cockcroft J. R., Groop P. H., Viberti G. C., Ritter J. M. Inhibition of nitric oxide synthesis in forearm vasculature of insulin-dependent diabetic patients: blunted vasoconstriction in patients with microalbuminuria. Clin Sci (Lond) 1993 Dec;85(6):687–693. doi: 10.1042/cs0850687. [DOI] [PubMed] [Google Scholar]
- Greene B., Pacitti A. J., Souba W. W. Characterization of L-arginine transport by pulmonary artery endothelial cells. Am J Physiol. 1993 Apr;264(4 Pt 1):L351–L356. doi: 10.1152/ajplung.1993.264.4.L351. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Kawasaki H., Abe K., Kanno M. Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1991 Oct;261(4 Pt 2):H1086–H1094. doi: 10.1152/ajpheart.1991.261.4.H1086. [DOI] [PubMed] [Google Scholar]
- Hecker M., Mülsch A., Bassenge E., Förstermann U., Busse R. Subcellular localization and characterization of nitric oxide synthase(s) in endothelial cells: physiological implications. Biochem J. 1994 Apr 1;299(Pt 1):247–252. doi: 10.1042/bj2990247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski B., Bauch H. J., Schneider H. P. Funktionelle Differenzierung des vaskulären Endothels bei Hochrisikoschwangerschaften. Z Geburtshilfe Perinatol. 1989 Jan-Feb;193(1):8–12. [PubMed] [Google Scholar]
- Kavanaugh M. P. Voltage dependence of facilitated arginine flux mediated by the system y+ basic amino acid transporter. Biochemistry. 1993 Jun 8;32(22):5781–5785. doi: 10.1021/bi00073a009. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994 Mar 1;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla A. G., D'Souza S. W., Glazier J. D., Mahendran D., Maresh M. J., Sibley C. P. Altered activity of the system A amino acid transporter in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women. J Clin Invest. 1994 Aug;94(2):689–695. doi: 10.1172/JCI117386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenstroer P., Pieper G. M. Regulation of spontaneous EDRF release in diabetic rat aorta by oxygen free radicals. Am J Physiol. 1992 Jul;263(1 Pt 2):H257–H265. doi: 10.1152/ajpheart.1992.263.1.H257. [DOI] [PubMed] [Google Scholar]
- Mann G. E., Pearson J. D., Sheriff C. J., Toothill V. J. Expression of amino acid transport systems in cultured human umbilical vein endothelial cells. J Physiol. 1989 Mar;410:325–339. doi: 10.1113/jphysiol.1989.sp017535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh G. E., Brennan G. M., Johnston G. D., McDermott B. J., McGrath L. T., Henry W. R., Andrews J. W., Hayes J. R. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992 Aug;35(8):771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- PEDERSEN J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954 Aug;16(4):330–342. doi: 10.1530/acta.0.0160330. [DOI] [PubMed] [Google Scholar]
- Poston L., Taylor P. D. Glaxo/MRS Young Investigator Prize. Endothelium-mediated vascular function in insulin-dependent diabetes mellitus. Clin Sci (Lond) 1995 Mar;88(3):245–255. doi: 10.1042/cs0880245. [DOI] [PubMed] [Google Scholar]
- Schilling W. P. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am J Physiol. 1989 Sep;257(3 Pt 2):H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- Smulders R. A., Stehouwer C. D., Olthof C. G., van Kamp G. J., Teerlink T., de Vries P. M., Donker A. J. Plasma endothelin levels and vascular effects of intravenous L-arginine infusion in subjects with uncomplicated insulin-dependent diabetes mellitus. Clin Sci (Lond) 1994 Jul;87(1):37–43. doi: 10.1042/cs0870037. [DOI] [PubMed] [Google Scholar]
- Tilton R. G., Chang K., Hasan K. S., Smith S. R., Petrash J. M., Misko T. P., Moore W. M., Currie M. G., Corbett J. A., McDaniel M. L. Prevention of diabetic vascular dysfunction by guanidines. Inhibition of nitric oxide synthase versus advanced glycation end-product formation. Diabetes. 1993 Feb;42(2):221–232. doi: 10.2337/diab.42.2.221. [DOI] [PubMed] [Google Scholar]
- Toothill V. J., Needham L., Gordon J. L., Pearson J. D. Desensitization of agonist-stimulated prostacyclin release in human umbilical vein endothelial cells. Eur J Pharmacol. 1988 Nov 22;157(2-3):189–196. doi: 10.1016/0014-2999(88)90382-2. [DOI] [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]


