Abstract
Background and Aim
Esophageal duplication cysts (EDCs) are rare congenital malformations, often discovered incidentally during endoscopy or on computed tomography (CT) scans. The role of endoscopic ultrasound (EUS) and CT scan in the diagnosis of these lesions and indications for surgical treatment are underreported. The aim of this study was to investigate these topics in a cohort of patients.
Materials and Methods
Between January 2001 and October 2020, 82 patients had a suspicion of esophageal duplication cyst on endoscopic ultrasound. Thirty four of these patients were referred for surgical enucleation of the lesion, but three patients were lost to follow-up. At the end, 31 patients, who underwent surgical treatment for their suspected EDC were included in this study. Clinical features, EUS findings, CT images, surgical treatment, and outcome were collected from hospital health records. CT images were re-evaluated by a chest radiologist. Type of surgery, surgical complications, and final histological diagnosis were reported.
Results and Conclusion
The patients referred for surgery were younger (p = 0.0001) and had larger lesions (> 2 cm; p = 0.005) than the patients who had non-operative follow-up. From thirty-one operated patients, eighteen (58%) had post-operative histological diagnosis of duplication cyst. On EUS the final histological diagnosis was correct in 58% (18/31) of all the operated cases and on CT scan 57% (17/30). CT scan misdiagnosed three of the EDCs but found two leiomyomas correctly. None of these patients developed malignancy. According to this study, neither EUS without fine-needle biopsy nor CT scan alone can differentiate EDCs from other mediastinal masses.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-024-08655-8.
Keywords: Mediastinal mass, Esophageal tumor, Endoscopic ultrasound, Computed tomography, Surgery
Introduction
Esophageal duplication cysts (EDCs) are rare congenital malformations, arising likely during the separation of trachea/lung buds and pharynx/esophagus at 1 month gestation [1]. The prevalence of these lesions is estimated at around 0.01%, and 80% of the cysts are diagnosed in childhood [2], but they also account for 6–15% of all mediastinal masses in adults [3]. EDCs are discovered as incidental findings in up to 60% of the patients, but some patients suffer from symptoms like dysphagia or pain [2]. On computed tomography (CT) scan EDCs may appear as fluid lesions, although they may look solid in up to 60% of the patients [4]. Endoscopic ultrasound (EUS) is referred to as the gold standard for the diagnosis of EDCs, since it can distinguish between solid and fluid lesions [5]. However, in up to 30% of the cases EDCs can have hypo- or mixed echoic appearance (i.e., containing mucous, debris, and calcium) and are sometimes indistinguishable from muscular layer, making differential diagnosis with leiomyoma, GIST or tumor/metastasis challenging [5].
Follow-up is usually recommended for asymptomatic lesions, even though the risk of developing adenocarcinoma is still debated [6, 7]. Treatment by surgery or endoscopic enucleation for patients with symptoms has been reported [8, 9]. Surgical treatment is recommended if there is a clear growth tendency or suspicion of malignancy [5]. It can be also considered in asymptomatic cases to prevent future complications [9, 10].
Since EDCs are rare, the literature is based on case reports and small case series. Thus, the primary aim of this study was to compare the role of EUS and CT scan in the diagnosis of these lesions. The secondary aims were to report the indications and outcome of surgery, and eventually the risk for malignant transformation.
Methods
Study Design
This is a retrospective observational cross-sectional study.
Patient Population
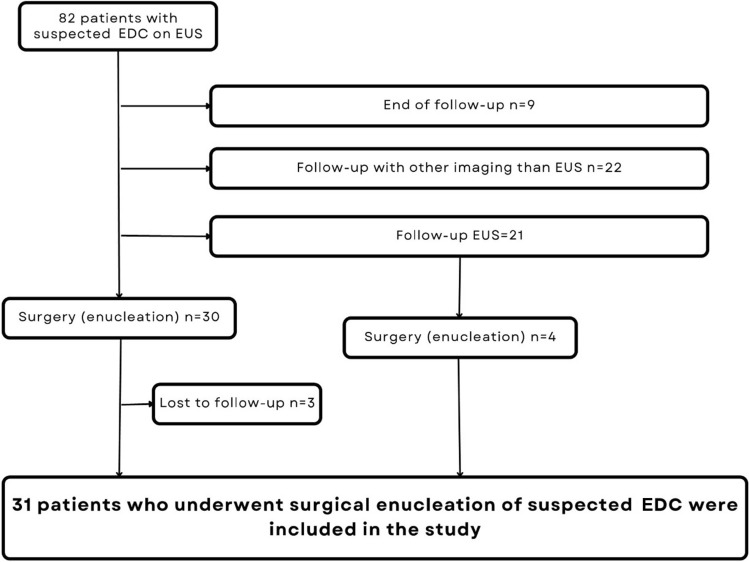
Figure 1 shows the patient inclusion process to the study.
Fig. 1.
The patient inclusion process
Between 2001 and 2020 we collected 82 patients referred for EUS due to suspicion of foregut cyst or mediastinal mass of unknown origin. These patients had been diagnosed with a mediastinal mass with an appearance of EDC on EUS and they were identified from EUS-registry of Helsinki University Hospital by ICD-code Q39.8. The registry covers the catchment area of HUH, which is almost 1.5 million inhabitants and accounts for 27% of the population in Finland. Sixty-one of the patients were referred for EUS after a CT scan and twenty-one after a gastroscopy.
Only patients, who underwent surgery and had histological assessment, were included in the further analysis (Fig. 1). In addition, the demographical differences of the patients who had non-surgical and surgical follow-up were reported (Table 1). Thirty-four patients (41%) were referred to operative treatment of the suspected EDC, but three were lost to follow-up (Fig. 1). Indications for surgery were presence of symptoms, size of suspected EDC ≥ 2 cm, inconclusive EUS diagnosis, significant growth of the suspected EDC during the follow-up. At the end, 31 (38%) patients were included (Fig. 1).
Table 1.
Baseline characteristics between surgery and non-surgery patients
| Operated | Not operated | p | |
|---|---|---|---|
| Number of patients | 31 | 48 | |
| Gender (male/female) | 15/16 | 25/23 | 0.8 |
| Median age (25–75th percentile at surgery) | 45 (36–57) | 59 (51–65) | 0.0001* |
| Occasional finding n (%) | 18 (58%) | 15 (31%) | 0.34 |
| Symptomatic | 13 (42%) | 33 (69%) | |
| Size | 0.005* | ||
| • ≥ 2 cm | 30 | 38 | |
| • < 2 cm | 0 | 10 | |
| • NA | 1 | 0 |
Data Collection
All baseline characteristics of the patients (sex, age, symptoms) were collected from health records and registered. The lesion was defined as an occasional finding when discovered during a procedure performed for a different indication. Gastroscopy finding was also reported. An experienced chest radiologist reviewed the patients’ CT scan images and reported systemically the features of the mediastinal lesions.
EUS Procedure
All the procedures were performed by the same experienced endoscopists with the patients in left side position. Topical anesthetic (i.e., Lidocaine spray) and conscious sedation were used (i.e., Midazolam 1–5 mg iv and Fentanyl 0–100 ug iv). A radial scope (Olympus GF, Type UE 160-AL5) was placed into the esophagus and advanced until the coeliac trunk was visualized. Afterward, the scope was withdrawn and all mediastinal structures including the suspected EDC were visualized. Once the lesion was identified the features of the suspected EDC were systematically evaluated and reported. A needle biopsy was not performed in any patient because of the risk of mediastinitis [11].
Surgery and Histology
Surgical reports of all patients were reviewed for indication, type of surgery, macroscopic findings, and immediate and delayed complications. Finally, histologic findings were also reported.
Outcomes and Follow-Up
The outcomes were surgery and the development of carcinoma. The follow-up started when the patient was referred for the first EUS and ended at the time of the last consultation before the 31st of December 2021.
Statistical Analysis
Data are presented as numbers and percentages when categorical and as median and 25–75th when continuous. Categorical variables were compared by using Fisher´s Exact Test (small number of subjects). Continuous variables were compared by using Mann–Whitney Test or Wilcoxon Test. A p-value < 0.05 was considered statistically significant. IBM SPSS and GraphPad were used for statistical analysis.
Results
Baseline Characteristics
Table 1 presents the baseline characteristics of surgery and non-surgery patients.
Thirty-one patients out of 82 with suspected EDC (male: 15, 48%, median age 45 years, 25–75th percentiles: 36–57 years) underwent surgery. In the surgery group the lesion was an incidental finding in 18 (58%), but symptoms were present in 13 patients (42%); 2 had fever (6%), 1 had cough (3%), 4 had dysphagia (13%), and 6 had pain (19%). Gastroscopy was performed at diagnosis in 10 (32%); a submucosal lesion was discovered in 9 (90%). The surgically treated patients were younger and had larger cysts compared to the patients who did not have the suspected EDC surgically enucleated but remained in imaging follow-up (Table 1). There was no significant difference in the presence of enlarged lymph nodes between these two groups.
Features of Suspected Mediastinal EDCs in EUS and CT Scan
On endoscopic ultrasound the majority of suspected EDCs were located in the central (68%) and distal (26%) parts of the esophagus. Sixteen (52%) were intramural lesions. Fifteen (48%) were anechoic, two (6%) hypoechoic, and 13 (42%) had mixed echogenicity. Enlarged lymph nodes (> 10 mm) were present in only two patients (6%). The median size of the lesions was 49.5 mm, the size of the suspected EDC varied from 20 to 92 mm.
Thirty patients had a CT scan of the thoracic area available for re-evaluation. All but one of the re-evaluated CT scans were contrast enhanced. The density of the suspected EDC was reported as Hounsfield Units (HU) and 23 (77%) lesions had a density ≥ 20HU (soft tissue density). Only 6 (20%) had the density 0–19 HU as a cystic lesion containing simple fluid (i.e., transudate). One lesion had a density of -20HU (fat tissue) in CT scan and turned out to be a hamartoma in post-operative histology.
Table 2 shows comparison of clinical and imaging findings in patients with histologically documented lesions.
Table 2.
Comparison of clinical and imaging findings in patients with histologically documented lesions
| Variables | Histology | |||
|---|---|---|---|---|
| EDC | non-EDC | |||
| EUS | CT | EUS | CT | |
| Number of patients | 18 | 18 | 13 | 12 |
| Gender (male/female) | 9/9 | 9/9 | 6/7 | 5/7 |
| Symptomatic | 3 | 3 | 7 | 6 |
| Localization in the oesophagus, n (%) | ||||
| • Proximal | 1 (6%) | 3 (17%) | 1 (8%) | 3 (25%) |
| • Middle | 13 (72%) | 12 (66%) | 8 (62%) | 5 (42%) |
| • Distal | 4 (22%) | 3 (17%) | 4 (30%) | 4 (33%) |
| Localization in the oesophageal wall, n (%) | ||||
| • Inside | 7 (39%) | 3 (17%) | 9 (69%) | 8 (67%) |
| • Outside | 11 (61%) | 15 (82%) | 4 (31%) | 4 (33%) |
| Size ≥ 2 cm | 18 | 18 | 12 | 2 |
| Size < 2 cm | 0 | 0 | 0 | 10 |
| Lymph nodes n | ||||
| > 10 mm | 1 | 2 | 1 | 1 |
| Echogenicity on EUS | ||||
| ● Anechoic | 8 (44%) | 7 (54%) | ||
| ● Hypoechoic | 2 (11%) | – | 0 | – |
| ● Mixed echogenicity | 7 (39%) | – | 6 (46%) | – |
| ● Echogenicity NA | 1 (5%) | – | 0 | – |
| Density on CT scan | ||||
| ● < 0 HU | – | 0 | – | 1 (8,3%) |
| ● 0–19 HU | – | 2 (11%) | – | 4 (33,3%) |
| ● ≥ 20 HU | – | 16 (89%) | – | 7 (58,3%) |
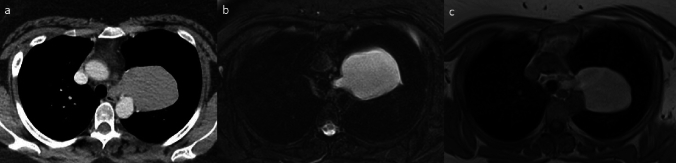
Patients are divided into two groups by histological diagnosis EDC and non-EDC. The histologically confirmed EDCs were all ≥ 2 cm, which is likely due to the inclusion criteria of the patients as larger cysts were referred to operation even when the appearance on EUS was consistent with EDC. Surprisingly majority as over 65% of the non-EDCs seemed intramural in both CT scan and EUS, which likely was partial reason for misdiagnosis. CT scan was performed on all 18 patients with EDC, interestingly showing that 16 (89%) as a majority of the duplication cysts had a density ≥ 20 HU and only two of the cysts (11%) density of cyst containing simple fluid. Figure 2 presents a CT scan and MRI image of a large histologically confirmed EDC.
Fig. 2.
Large esophageal duplication cyst (9.1 cm) on the left originating from the upper esophageal region protruding into the left upper lobe of the lung. a Contrast-enhanced computed tomography image shows a high-attenuation mass with a density of 82 Hounsfield Units (HU). b Axial T2-weighted fat-saturated (Blade) magnetic resonance image reveals a lesion of increased signal intensity owing to liquid content. c Axial non-contrast-enhanced T1-weighted fat-saturated (Vibe) magnetic resonance image shows a lesion of increased signal intensity due to the high proteinous content of the liquid inside the duplication cyst
Surgery
Overall, 34 patients were referred to operative treatment for the suspected EDC, but three dropped out. In total, 31 patients underwent surgery. The indication for surgery was size ≥ 2 cm in 30 (97%), presence of symptoms in 13 (42%), and/or inconclusive appearance in imaging.
Post-operative complications were reported in eight patients (26%); three developed thoracic pain, two had cough, one had gastro-esophageal reflux disease, one had a phoniatric problem, and one suffered from post-operative hematoma.
Supplementary Table 1 shows surgical techniques used for enucleation of the lesions.
Histology
A histologic report was available for all 31 patients before the end of the study (Table 3), confirming an EDC in 18 (58%), a leiomyoma in 7 (23%), Müllerian duct cyst in 2 (6%), a mesenchymal tumor in 2 (6%), a paratracheal vascular malformation in 1 (3%), and a hamartoma in one (3%).
Table 3.
Histological diagnosis compared to the diagnosis on EUS and CT scan
| Histological dg | EUS appearance | CT appearance | |
|---|---|---|---|
| 1 | Duplication cyst | Duplication cyst | Leiomyoma |
| 2 | Duplication cyst | Duplication cyst | Leiomyoma |
| 3 | Duplication cyst | Duplication cyst | Castleman’s disease |
| 4 | Duplication cyst | Duplication cyst | Duplication cyst |
| 5 | Duplication cyst | Duplication cyst | Duplication cyst |
| 6 | Duplication cyst | Duplication cyst | Duplication cyst |
| 7 | Duplication cyst | Duplication cyst | Duplication cyst |
| 8 | Duplication cyst | Duplication cyst | Duplication cyst |
| 9 | Duplication cyst | Duplication cyst | Duplication cyst |
| 10 | Duplication cyst | Duplication cyst | Duplication cyst |
| 11 | Duplication cyst | Duplication cyst | Duplication cyst |
| 12 | Duplication cyst | Duplication cyst | Duplication cyst |
| 13 | Duplication cyst | Duplication cyst | Duplication cyst |
| 14 | Duplication cyst | Duplication cyst | Duplication cyst |
| 15 | Duplication cyst | Duplication cyst | Duplication cyst |
| 16 | Duplication cyst | Duplication cyst | Duplication cyst |
| 17 | Duplication cyst | Duplication cyst | Duplication cyst |
| 18 | Duplication cyst | Duplication cyst | Duplication cyst |
| 19 | Müllerian duct cyst | Duplication cyst | Duplication cyst |
| 20 | Hamartoma | Duplication cyst | Duplication cyst |
| 21 | Leiomyoma | Duplication cyst | Duplication cyst |
| 22 | Müllerian duct cyst | Duplication cyst | Duplication cyst |
| 23 | Leiomyoma | Duplication cyst | Leiomyoma |
| 24 | Mesenchymal cyst | Duplication cyst | Duplication cyst |
| 25 | Leiomyoma | Duplication cyst | Duplication cyst |
| 26 | Leiomyoma | Duplication cyst | Duplication cyst |
| 27 | Mesenchymal cyst | Duplication cyst | Duplication cyst |
| 28 | Leiomyoma | Duplication cyst | Leiomyoma |
| 29 | Paratracheal vascular malformation | Duplication cyst | Pericardial cyst |
| 30 | Leiomyoma | Duplication cyst | Not available |
| 31 | Leiomyoma | Duplication cyst | GIST |
On EUS the diagnosis was correct in 58% (18/31) of the cases and on CT scan 57% (17/30). CT scan misdiagnosed three of the EDCs but found two leiomyomas correctly.
Imaging findings of histologically confirmed duplication cysts are presented in Table 3.
Interestingly, no malignancy was detected after a median follow-up of 3 years.
Discussion
At present, only one systematic review including case reports and case series of patients with EDC has been published [8]. According to our study, neither EUS nor CT scan was able to differentiate these lesions from other submucosal lesions of the esophagus (e.g., leiomyoma). There were no malignancies detected in the follow-up. Surgery may be an option in patients with symptoms and large or growing cysts.
Although EDCs might be detected in childhood [1], all our patients were adults. Of the patients, 58% were asymptomatic and EDC was an incidental finding [4]. This might be due to the widespread use of cross-sectional imaging in the population, similar to what happens for pancreatic cysts [12, 13]. However, symptoms caused by mechanical compression of the surrounding structures [14] were not uncommon [4, 15–17].
Chest CT scan has been the most used cross-sectional imaging for the detection of EDCs. In some studies CT is thought to be able to distinguish between fluid and solid lesions [4], while other studies highlight the difficulty of differentiating cyst containing high-protein content from solid lesion. MRI technique is superior to CT in distinguishing fluid from solid mass [14]. In our study we didn’t include the MRI scan results in the comparison as only six of the patients in the study population had an MRI scan performed before or after EUS.
Moreover, Fazel et al. reported a negative CT scan in about 23% of the patients with a large EDC [18]. In our study, the HU of the histologically confirmed EDCs was higher than simple fluid (≥ 20) in almost 90% of the patients, suggesting a dense protein content of the cyst comparable with previous reports. EDCs can look very much alike to bronchogenic cysts, but EDCs can be more oval / longer in form [1, 19].
EUS has been considered the tool of choice to investigate EDCs since it can distinguish between solid and cystic lesions [5]. However, in our study 42% of the patients with EUS diagnosis of an EDC had a different diagnosis, most commonly leiomyoma, at final histology. Other studies have also reported wrong preoperative diagnosis of mediastinal cysts that were leiomyomas, neurogenic tumors, lymph nodes or even malignant tumors at operation [18, 20]. The reliability of diagnostic EUS depends on the performing clinician and both anatomical variations of normal structures and localization of the lesion under inspection can affect visibility that is critical to evaluation. In our study the inclusion criteria of lesion size for operative treatment may have had an impact on the results. In addition, one criterion for surgical referral was an inconclusive appearance of the lesion on EUS. In our series, needle biopsy was not performed in any patients, since the question of whether to perform it on a lesion suspected of being an EDC is still controversial. One study reported a high risk of infection and another one a case of aortitis with pseudoaneurysm, needing surgery [11, 21]. EUS-needle (22G) aspiration with a prophylactic antibiotic administration could be useful in selected cases to overcome the possibility of misdiagnosis. Even without needle biopsy EUS is an invasive imaging method with a risk of complications and cause of discomfort to patients. The additional value of EUS to the diagnostic of EDCs remains unclear and further studies are required to compare other non-invasive imaging modalities in the diagnostics of EDCs.
There are case reports on a cancer transformation of EDCs [6, 7], but case series with a median follow-up of 321 days to 16.5 months did not show an increased cancer risk. In our series no cases of cancer were reported after a median follow-up of 3 years. Further studies with a longer follow-up are needed to elucidate this risk.
The main limitation of this study is the limited number of patients. Since lesions with other EUS diagnoses were excluded from this study from early on and other esophageal benign masses like leiomyomas are rarely surgically treated when the imaging diagnosis is clear, there was no control group to compare the results to or calculate the sensitivity and specificity of EUS and CT for diagnosis of ODC. Moreover, we do not have data on how many patients underwent surgery for esophageal duplication cysts during the time immaterial of the modality used for the diagnosis and this introduced a selection bias in the study. Due to the rarity of EDCs there are no large cohort studies on this subject and a clear follow-up strategy is lacking for incidentally found EDCs [21].
Conclusion
The differential diagnosis of EDCs is challenging in both EUS and CT scan as these lesions can resemble other submucosal lesions around the esophagus. Either EUS without fine need biopsy or CT scan alone are not able to differentiate EDCs from other mediastinal lesions. Surgical treatment is usually offered for symptomatic cases, for larger lesions or when the diagnosis is unclear. However, further prospective studies are needed to have clear guidelines on the indication for surgery and follow-up for these lesions especially when found incidentally. Malignancy development seems to be rare.
Supplementary Information
Below is the link to the electronic supplementary material.
Author's contribution
A.T, N.B-R, P.A designed the study. A.T, N. B-R and E.R collected data and performed statistical analysis. K.V reviewed CT images of the patients included in the study. All the authors took part to the discussion of the results. E.R and A.T drafted the paper. All the authors agreed with the final version of the paper.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). The authors have indicated they have no financial relationships relevant to this article to disclose.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors have indicated they have no financial relationships relevant or conflict of interest to this article to disclose.
Ethical approval
In Finland, no ethical committee approval for a retrospective register-based study is required, when the patients are not contacted. Abdominal Centre in Helsinki University approved the permission for the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nobuhara KK, Gorski YC, La Quaglia MP, Shamberger RC. Bronchogenic cysts and esophageal duplications: common origins and treatment. J Pediatr Surg. 1997;32:1408–1413. 10.1016/S0022-3468(97)90550-9. [DOI] [PubMed] [Google Scholar]
- 2.Whitaker JA, Deffenbaugh LD, Cooke AR. Esophageal duplication cyst: case report. Am J Gastroenterol. 1980;73:329–332. [PubMed] [Google Scholar]
- 3.Snyder ME, Luck SR, Hernandez R, Sherman JO, Raffensperger JG. Diagnostic dilemmas of mediastinal cysts. J Pediatr Surg. 1985;20:810–815. 10.1016/S0022-3468(85)80048-8. [DOI] [PubMed] [Google Scholar]
- 4.Wildi SM, Hoda RS, Fickling W et al. Diagnosis of benign cysts of the mediastinum: the role and risks of EUS and FNA. Gastrointest Endosc. 2003;58:362–368. [PubMed] [Google Scholar]
- 5.Liu R, Adler D. Duplication cysts: diagnosis, management, and the role of endoscopic ultrasound. Endosc Ultrasound. 2014;3:152. 10.4103/2303-9027.138783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miralles Lozano F, Gonzalez-Martínez B, Luna More S, Valencia Rodríguez A. Carcinoma arising in a calcified bronchogenic cyst. Respiration. 1981;42:135–137. 10.1159/000194417. [DOI] [PubMed] [Google Scholar]
- 7.Olsen JB, Clemmensen O, Andersen K. Adenocarcinoma arising in a foregut cyst of the mediastinum. Ann Thorac Surg. 1991;51:497–499. 10.1016/0003-4975(91)90881-p. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Urquijo M, Hinojosa-Gonzalez DE, Padilla-Armendariz DP et al. Esophageal duplication cysts in 97 adult patients: a systematic review. World J Surg. 2022;46:154–162. 10.1007/s00268-021-06325-8. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi T, Hashimoto T, Takeno S, Wada S, Tohara K, Uchida Y. Laparoscopic resection of esophageal duplication cyst in an adult. Dis Esophagus. 2003;16:148–150. 10.1046/j.1442-2050.2003.00314.x. [DOI] [PubMed] [Google Scholar]
- 10.Salo JA, Ala-Kulju KV. Congenital esophageal cysts in adults. Ann Thorac Surg. 1987;44:135–138. 10.1016/s0003-4975(10)62023-1. [DOI] [PubMed] [Google Scholar]
- 11.Diehl DL, Cheruvattath R, Facktor MA, Go BD. Infection after endoscopic ultrasound-guided aspiration of mediastinal cysts. Interact Cardiovasc Thorac Surg. 2010;10:338–340. 10.1510/icvts.2009.217067. [DOI] [PubMed] [Google Scholar]
- 12.Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG clinical guideline: diagnosis and management of pancreatic cysts. Am J Gastroenterol. 2018;113:464–479. 10.1038/ajg.2018.14. [DOI] [PubMed] [Google Scholar]
- 13.Robinson SM, Scott J, Oppong KW, White SA. What to do for the incidental pancreatic cystic lesion? Surg Oncol. 2014;23:117–125. 10.1016/j.suronc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi D, Garg T, Shah J, Sawhney H, Crowder BJ, Nagar A. Gastrointestinal duplication cysts: what a radiologist needs to know. Abdom Radiol (NY). 2022;47:13–27. 10.1007/s00261-021-03239-w. [DOI] [PubMed] [Google Scholar]
- 15.Bowton DL, Katz PO. Esophageal cyst as a cause of chronic cough. Chest. 1984;86:150–152. 10.1378/chest.86.1.150. [DOI] [PubMed] [Google Scholar]
- 16.Neo EL, Watson DI, Bessell JR. Acute ruptured esophageal duplication cyst. Dis Esophagus. 2004;17:109–111. 10.1111/j.1442-2050.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 17.Eloubeidi MA, Cohn M, Cerfolio RJ et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of foregut duplication cysts: the value of demonstrating detached ciliary tufts in cyst fluid. Cancer. 2004;102:253–258. 10.1002/cncr.20369. [DOI] [PubMed] [Google Scholar]
- 18.Fazel A, Moezardalan K, Varadarajulu S, Drananov P, Eloubeidi MA. The utility and the safety of EUS-guided FNA in the evaluation of duplication cysts. Gastrointest Endosc. 2005;62:575–580. 10.1016/j.gie.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Khoury T. Foregut duplication cysts: a report of two cases with emphasis on embryogenesis. World J Gastroenterol. 2011;17:130. 10.3748/wjg.v17.i1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribet ME, Copin MC, Gosselin B. Bronchogenic cysts of the mediastinum. J Thorac Cardiovasc Surg. 1995;109:1003–1010. 10.1016/S0022-5223(95)70327-6. [DOI] [PubMed] [Google Scholar]
- 21.Cevasco M, Menard MT, Bafford R, McNamee CJ. Acute infectious pseudoaneurysm of the descending thoracic aorta and review of infectious aortitis. Vasc Endovascular Surg. 2010;44:697–700. 10.1177/1538574410376449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.