Abstract
Purpose
Cancer outcome is dependent on multiple predetermining factors including cancer, type of cancer and its related factors. This study aims to investigate the association between COVID-19 & cancer/cancer types, focusing on risk of in-hospital mortality within 30 days of hospitalization of COVID-19 patients with cancer.
Materials and methods
We did a registry (National Clinical Registry for COVID-19) based retrospective observational study including 51,544 patients, of whom 976 were patients with cancer, admitted with COVID-19 between August 2020 and August 2023 across 42 hospitals of India.
Results
Out of 51,544 patients, 976 (1.8%) had cancer. Hematological malignancies made up 15.06% (147 cases), while solid cancers accounted for 29.5% (288 cases), with genitourinary (18.4%, 80 cases), gastrointestinal (15.2%, 49 cases), and lung cancers (10.1%, 34 cases) being the most common. Solid cancers had the highest in-hospital mortality rate at 25%. Survival analysis showed that cancer-related hazards were highest at admission but decreased to levels comparable with other morbidities within nine to ten days. For each cancer type, the hazard was significantly elevated compared to that of the cancer-free (Other Comorbidities and No Comorbiditiy) groups during the initial period of hospitalization. The use of Remdesivir, steroids, and anticoagulants reduced mortality risk, and prior COVID-19 vaccination was protective against mortality across all cancer types.
Conclusion
This study shows that both cancer in general and specific cancer types significantly increase the risk of severe outcomes among SARS-CoV-2-infected patients, especially immediately after hospitalization. The findings highlight the need for close monitoring and personalized interventions for COVID-19 patients with cancer for at least 10 days post-hospitalization, with a more specific high-risk period ranging from 7 to 18 days depending on the type of cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-024-05966-1.
Keywords: COVID-19, SARS-CoV-2, Cancer, Vaccine, ICU
Introduction
Since the onset of the COVID-19 pandemic in late 2019, when the SARS-CoV-2 virus first emerged in Wuhan, China, its impact has been both profound and widespread, affecting populations around the world (Li et al. 2020). It has been became evident that individuals with comorbidities faced particularly severe consequences upon infection (Huang et al. 2020; Hui and Zumla 2019; Turtle et al. 2024).
Among these comorbidities, cancer was flagged as a notable risk factor. Early studies from Wuhan, where the outbreak began, provided some of the first insights into how cancer patients were faring in the face of this new virus. In an initial investigation involving 1,524 cancer patients screened for COVID-19 in Wuhan, only 12 tested positive, representing a relatively low prevalence of 0.8%. However, the severity of the illness among those who were infected was concerning. The data revealed that patients with cancer, especially those with lung cancer or those undergoing active cancer treatment, were at a significantly higher risk of severe outcomes. Specifically, these patients were more likely to require admission to the intensive care unit (ICU) or face death due to complications from the virus. These findings from Wuhan were corroborated by subsequent studies conducted in the same city, which observed similar trends among hospitalized COVID-19 patients. The alignment of these results underscores the increased vulnerability of cancer patients to the severe effects of COVID-19, highlighting the need for targeted interventions and careful management for this high-risk group during the pandemic (Yu et al. 2020; Liang et al. 2020).
Later work has shed more light on the cancer related risks of the COVID-19 patients. A Hong Kong-based study found that COVID-19 patients with any cancer had nearly a fourfold higher risk of severe outcomes compared to cancer-free individuals, particularly for colorectal and gastrointestinal cancers. However, lung cancer patients were also reported to have increased risks of infection, pulmonary complications, and poorer survival outcomes (Zhou et al. 2023; Bungaro et al. 2022).
Certain groups of COVID-19 patients are more likely to experience severe illness or death. These include people who are older, men, and those with underlying conditions like high blood pressure, lung disease, diabetes, or active cancer undergoing chemotherapy (Lima et al. 2020; Nicola et al. 2020; Curigliano 2020).
Two large studies, CCC-19 and TERAVOLT, looked at how patients with cancer did with COVID-19. CCC-19 included patients with various cancers, most in remission or stable. Their overall death rate was 13%. TERAVOLT, focused on advanced lung cancer patients and documented higher death rate of 33% (Garassino et al. 2020; Kuderer et al. 2020).
Patients with cancer were found to be more susceptible to severe forms of COVID-19 and had an increased risk of mortality (Curigliano 2020). Several factors contributed to this elevated risk, including the type of cancer, the cancer treatment’s interaction with COVID-19, and the synergistic role of cancer in causing thrombotic complications alongside SARS-CoV-2 infection (Sharafeldin et al. 2021; Hachem, et al. 2020; Fernández-Cruz et al. 2022). In TERAVOLT, 6% of the hospitalized cancer population and none of the non-hospitalized counterpart had diagnosed coagulopathy (Garassino et al. 2020). This emphasizes the importance of risk assessment in COVID 19 cases among patients with cancer. Additional risk factors for increased mortality among patients with cancer included old age, smoking history, and multiple comorbidities (Orchard et al. 2020).
A case–control study conducted in the United States revealed that out of 16,570 COVID-19 patients, 11% had at least one common cancer, with hematologic malignancies and lung cancer patients being particularly at higher risk for COVID-19 infection (Wang et al. 2021). Similarly, a study from India reported that 34% of patients with solid organ malignancies and COVID-19 required hospitalization, and 15% of them succumbed to the disease, with increased mortality observed among patients with lung cancer as well (Roy et al. 2021). The mortality rate among patients with haematological malignancies was estimated to be around 28–34% (Wood et al. 2020; Vijenthira et al. 2020). Although the WHO has declared the end of COVID-19 as a public health emergency of international concern since 5 May 2023, the virus continues to cause fatalities and undergo mutations. The risk of new variants emerging and causing surges in cases and deaths remains high.
Given the potential for future threats and the sporadic surges of cases post pandemic, which have proven fatal mainly for patients with comorbidities and considering that in India, one in nine people are likely to develop cancer in their lifetime, this current study aims to enhance understanding and contribute to the knowledge base surrounding cancer and SARS-CoV-2 infection (Sathishkumar et al. 2022). The study findings will also be valuable for diseases of similar kind in future.
This research utilizes data from the National Clinical Registry for COVID-19 (NCRC) of India and aims to evaluate the following objectives:
To identify the frequency of different cancer types among hospitalized SARS-CoV-2 patients.
To analyse the distribution of demographic, clinical, laboratory, and outcome parameters between types of cancer patients and non-cancer patients.
To evaluate the hazard of all-cause mortality associated with various types of cancer compared to other comorbidities and no comorbidity within 30 days of hospitalization, while adjusting for other potential predictors.
To assess the impact of commonly administered COVID-19 drugs on patient outcomes.
To determine the effective duration of hospital or facility-based monitoring for various cancer types to maximize benefits of monitoring and decrease fatal outcome.
Methods
Study cohort
The National Clinical COVID-19 registry was built to collect information for laboratory-confirmed hospitalized COVID-19 patients and included 51,544 patients enrolled between 1st September 2020 and 31st August 2023 from 42 participating hospitals from fifteen different states across India (Supplementary Figure 2). The Governance Structure and the components and functions of the NCRC is detailed in (Supplementary Figure 3). The data flow and feedback loop of NCRC is presented in (Supplementary Figure 4). The cohort of patients were classified as No- cancer, Unspecified cancer, Haematological Cancer and Solid Cancer.
Study variables
The study collected information on socio-demographic characteristics, symptom complex prior to admission in COVID-19 facility, comorbidities of the patients, previous medication history, treatment received by the patients during hospital stay, complications developed during hospital stay, and outcome of the patients while leaving the facility.
Geographic distribution of patients
The patients were categorized into 6 geographic zones based on the location of their enrolling hospitals: North (Punjab, Haryana, Chandigarh, Uttar Pradesh), South (Karnataka, Telangana, Andhra Pradesh), East (West Bengal, Odisha), West (Rajasthan, Gujarat, Maharashtra), Central (Madhya Pradesh, Chhattisgarh) and Northeast. The distribution of registry patients in the 6 geographic zones was compared with zone wise geographic distribution of cancer patients across India reported by the Hospital Based Cancer Registry of India 2012–2020 to assess its comparability (Sathishkumar et al. 2022; Mathur 2020).
General definition
Age Category.
Infants – Less than or equal to one year
Children – Patients age less than or equal to 18 years.
Adults – Patients age greater than 18 years.
No Comorbidity – Patients with no reported morbidities.
Geographic zones as per state to which the peripheral institute was located.
Centre – Madya Pradesh, Chhattisgarh
East – Orissa, West Bengal
North – Punjab, Haryana, Uttar Pradesh.
North East – Meghalaya, Nagaland
South – Karnataka, Telangana
West – Rajasthan, Gujarat, Maharashtra
Cancer classification
Other comorbidity-Any other morbidity besides cancer.
Unspecified Cancer- Patients identified as having cancer, but where no information regarding the site or organs involved was provided, were classified under the category ‘unspecified cancer’.
Cancer of Genitourinary system- Included cancer of reproductive system (ovary, cervix, prostrate) and Urinary system (Urinary Bladder)
Cancer of Gastrointestinal system-Includes Cancer from Oesophagus to Rectum/Anal Canal, Pancreas
Cancer of Lungs-Cancer of structures below vocal cord, Larynx, Trachea, Lungs, Pleura
Cancer of Head and Neck/Brain -Includes cancer of Head, Orbit, Oropharynx, Nasopharynx, oral cavity, Base of oral cavity till vocal cord, brain
Cancer of Hepatobiliary System-Includes cancer of Hepatic and Biliary System
Cancer of Musculoskeletal system
Inclusion criteria
Hospitalized, COVID-19 confirmed patient of any age and gender admitted to the participating sites under the National Clinical Registry For COVID-19.
Statistical analysis
Frequencies of demographic, clinical, laboratory, and outcome parameters were presented as numbers with percentages for categorical variables and median with interquartile range for continuous variables. The solid cancer, haematological cancer, unspecified cancer and non-cancer groups were compared using chi-square tests/Fischer exact test as appropriate for categorical variables and kruskalll wallis test/Mann Whitney U test as appropriate for continuous variables.
Kaplan Meier survival curves were plotted for a duration of 30 days of hospital stay with respect to the factors/predictors of interest to visualize the levels and differences in survival over time in the presence of the risk factors. Univariate Cox proportional hazards (PH) models were fitted to obtain unadjusted hazard ratio associated with each factor of interest. Further, a multivariable Cox PH model was employed to obtain adjusted hazard ratios associated with the risk factors. Factors included in the multivariate model were- comorbidities (including different types of cancer), age, gender, presence of symptoms at admission, vaccination history, complications like coagulation and cardiovascular complication during the hospital stay, the need for ICU or organ support, as well as the administration of various drugs for managing COVID-19 (specifically, Remdesivir, Steroids, Anticoagulants, and Supplemental Oxygen), and immunological parameters like CRP and dimer. In addition, our model accounted for potential drug interactions between pairs and trios of drugs. Only significant interaction terms were retained in the final model. Comorbidity-subgroup that included different type of cancers were among the key risk factors of interest in the analysis. The fitted Cox PH models were tested for the proportional hazards (PH) assumption using Schoenfeld residuals test. The results concluded that the PH assumption was being violated for the comorbidity-subgroup (includes cancer type) risk factor, as was also indicated by the corresponding Kaplan Meier curves. Consequently, the PH assumption was relaxed for the comorbidity-subgroup risk factor by assuming that the effect of comorbidity-subgroup varies with time. This was accomplished by introducing a linear time interaction for the cancer-subgroup risk factor in the multivariable Cox survival model making it a non-proportional hazards model (Grambsch and Therneau 1994; Bellera et al. 2010). The Significance level was set at 5% threshold. All the analysis were performed using stata version 18.
Ethics approval
Ethics approval was obtained from the Central Ethics Committee on Human Research of ICMR and respective ethics committee from all participating institutes. The study variable was anonymized and waiver of consent was obtained.
Consent and patient data confidentiality
Since the data collected was anonymous and unlinked to the patient, and this posed no additional risk to the participating patient, the process was undertaken without seeking consent from the patient or their family members. Patient confidentiality was maintained while data analysis.
Results
Of 51,544 patients, 976 (1.8%) patients had reported having cancer. Haematological malignancies accounted for 15.06% (N = 147) of cases, while solid cancers comprised 29.5% (N = 288), with genitourinary (n = 80, 18.4%), gastrointestinal (n = 49,15.4%) and lung (n = 34,10.6%) cancers being the most prevalent among the solid cancers. Among haematological cancers, non-Hodkin’s lymphoma (n = 15, 3.4%), Lymphoma Unclassified (n = 9, 2%) were common lymphomas while chronic lymphocytic leukaemia (n = 24, 5.5%) was the most common leukaemia’s. Frequency of in-hospital mortality was more among solid cancers (69.9%), followed by leukaemia (21.3%). The requirement of ICU or Organ Support was highest among those with solid cancer (70.4%%) (Table 1).
Table 1.
Frequency Distribution of types of cancer among SARS-CoV-2 infected patients enrolled in NCRC (N = 976)
| Types of cancer | Number (%) | Death N = 102 |
ICU_Organ supporta N = 61 |
|||
|---|---|---|---|---|---|---|
| Solid Tumors, N = 288 | Total 435 |
Males 253 |
Females 182 |
72 (69.9%) |
43 (70.4%) |
|
| 1 | Cancer of Genitourinary system | N = 80 (18.4) | 45 (17.79) | 35(19.23) | 21 | 9 |
| 2 | Cancer of Gastrointestinal system | N = 67 (15.4) | 49 (19.37) | 18(9.89) | 14 | 10 |
| 3 | Cancer of Lungs | N = 46 (10.6) | 31 (12.25) | 15(8.24) | 16 | 10 |
| 4 | Cancer of Breast | N = 37 (8.5) | 3 (1.19) | 34(18.68) | 6 | 3 |
| 5 | Cancer of Head and Neck | N = 36 (8.3) | 17 (6.72) | 19(10.44) | 11 | 9 |
| 6 | Cancer of Hepatobiliary System | N = 13 (2.9) | 8 (3.16) | 5(2.75) | 3 | 2 |
| 7 | Cancer of Musculoskeletal system | N = 8 (1.8) | 4 (1.58) | 4(2.2) | – | – |
| 8 | Mesothelioma | N = 1 (0.2) | 1 (0.4) | 1 | – | |
| Hematological Tumor, N = 147 | ||||||
| Lymphoma, N = 42 | 9 (8.7%) | 2 (3.2%) | ||||
| 10 | Non-Hodgkin’s Lymphoma | N = 15 (3.4) | 10 (3.95) | 5(2.75) | 1 | |
| 11 | Lymphoma Unclassified | N = 9 (2.0) | 3 (1.19) | 6(3.3) | 2 | – |
| 12 | Mantle Cell Lymphoma | N = 4 (0.9) | 4 (1.58) | – | 3 | – |
| 13 | Diffuse Large B Cell Lymphoma | N = 4 (0.9) | 4 (1.58) | – | – | 1 |
| 14 | Follicular Lymphoma | N = 3 (0.7) | 3 (1.19) | – | 3 | – |
| 15 | Hodgkin’s Lymphoma | N = 5 (1.1) | 5 (1.98) | – | – | – |
| 17 | T Cell Lymphoma | N = 1 (0.2) | - | 1(0.55) | – | – |
| 18 | Waldenström’s Macroglobulinemia | N = 1 (0.3) | 1 (0.4) | – | – | – |
| Leukemia, N = 105 |
21 (21.3%) |
17(27.8%) | ||||
| 19 | Chronic lymphocytic leukemia | N = 24 (5.5) | 15 (5.93) | 9(4.95) | 9 | 4 |
| 20 | Acute Leukemia | N = 5 (1.1) | 4 (1.58) | 1(0.55) | – | 1 |
| 21 | Multiple Myeloma | N = 21 (4.8) | 12 (4.74) | 9(4.95) | 4 | 1 |
| 22 | Acute Myeloid Leukemia | N = 18 (4.1) | 9 (3.56) | 9(4.95) | 6 | 7 |
| 23 | Acute lymphoblastic leukemia | N = 3 (0.7) | 3 (1.19) | - | 1 | - |
| 24 | Acute lymphocytic leukemia | N = 21 (4.8) | 14 (5.53) | 7(3.85) | 2 | 1 |
| 25 | Chronic Myeloid leukemia | N = 4 (0.9) | 2 (0.79) | 2(1.1) | – | 2 |
| 26 | Leukemia unclassified | N = 6 (1.4) | 4 (1.58) | 2(1.1) | – | 1 |
| 27 | Myelodysplastic Syndrome | N = 2 (0.5) | 1 (0.4) | 1(0.55) | – | – |
| 28 | Chronic Myelomonocytic Leukemia | N = 1 (0.2) | 1 (0.4) | – | – | – |
Out of 976 cancer patients, definite classification was available for 435 patients
This considers ICU admission or requirement of ECMO or Renal Replacement Therapy or Vasopressor
Among all enrolled patients, 50,568 (98.1%) patients had no cancer. Demographic characteristic of non-Cancer vs Unspecified Cancer vs Haematological cancer vs Solid Cancer Cancer cohort is described in (Table 2.1) Within the entire cohort, the distribution of No cancer, Unspecified Cancer, Haematological Cancer and Solid Cancer had significantly different proportions by age (p < 0.001), Gender(p = 0.002), BMI Category (p < 0.001), Presence of symptoms(p < 0.001). (Table 2.1, Supplementary Table 1).
Table 2.1.
Study Cohort Epidemiological and Clinical Characteristics among cancer and no cancer cohort and Type of cancer
| Variable | No Cancer N = 50,568 |
Unspecified Cancer N = 541 |
Hematological N = 147 |
Solid Cancer N = 288 |
P Value |
|---|---|---|---|---|---|
| Age | 50 (36, 63) | 56 (44, 65) | 55 (34, 66) | 60 (51, 68) | < 0.001 |
| Age | |||||
| Infant, | 349 (0.69) | 2 (0.37) | - | – | < 0.001 |
| 1–18 | 1393 (2.7) | 35 (6.5) | 19 (12.9) | 5 (1.74) | |
| 19–39 | 12,951 (25.6) | 67 (12.4) | 25 (17.0) | 25 (8.7) | |
| 40–59 | 19,347 (38.3) | 215 (39.7) | 44 (29.9) | 110 (38.2) | |
| 60 & above | 16,528 (32.7) | 222 (41.0) | 59 (40.1) | 148 (51.4) | |
| Gender | |||||
| Male, | 32,161 (63.6) | 319 (58.9) | 95 (64.6) | 158 (54.8) | 0.002 |
| Female, | 18,407 (36.4) | 222 (41.1) | 52 (35.4) | 130 (45.1) | |
| BMI Category (N = 16,025) | |||||
| Underweight, n = 675 | 646 (3.1) | 24 (12.6) | 2 (11.1) | 3 (5.8) | < 0.001 |
| Normal Weight, 12,144 | 12,004 (58.2) | 100 (52.6) | 9 (50) | 31 (60.7) | |
| Over Weight /obese, n = 8054 | 7967 (38.6) | 66 (34.8) | 7 (38.9) | 17 (33.3) | |
| Vaccine (N = 46,393) | |||||
| Yes, n = 8762 | 8571 (18.8) | 111 (22.5) | 17 (13.9) | 63 (25.2) | 0.005 |
| No, n = 37,631 | 36,957 (81.17) | 382 (77.48) | 105 (86.07) | 187 (74.8) | |
| Symptomatic | |||||
| Yes | 41,680 (82.4) | 380 (70.2) | 131 (89.1) | 239 (82.9) | < 0.001 |
| No | 8888 (17.6) | 161 (29.7) | 16 (10.8) | 49 (17.01) | |
| Comorbidity other than cancer | |||||
| No_other_comorbidity, | 25,670 (50.8) | 290 (53.6) | 93 (63.3) | 163 (56.6) | 0.02 |
| 1_2_other comorbidity, | 20,301 (40.1) | 212 (39.2) | 45 (30.6) | 109 (37.8) | |
| 3_4_other comorbidity, | 4358 (8.6) | 38 (7) | 9 (6.1) | 16 (5.6) | |
| 5 or more-morbidity, | 235 (0.5) | 1 (0.2) | 0 |
aVaccination history before admission
Table 2.2 provides the distribution of maximum recorded inflammatory and Coagulation markers across different categories of cancer and no cancer. There was a significant difference in the distribution of maximum recorded value of major inflammatory markers and coagulation markers such as total leukocyte count (0.016), lactate dehydrogenase (0.04), procalcitonin (0.003), interleukin 6 (0.003), INR (< 0.001) and d-dimer (< 0.001) across non-cancer, unspecified cancer, haematological cancer and solid cancer.
Table 2.2.
Distribution of maximum recorded value of inflammatory markers and coagulation factors across patients from different categories of cancer and no cancer
| Laboratory Parameters | No Cancer N = 50,568 |
Unspecified Cancer N = 541 |
Hematological N = 147 |
Solid Cancer N = 288 |
P Value* |
|---|---|---|---|---|---|
| Inflammatory marker | |||||
| Total leukocyte Count (Cells /mm3) | 8890 (5980, 12,500) | 8900 (5600, 13,500) | 10,300(4000, 22,800) | 9200 (6500, 14,700) | 0.016 |
| CRP (mg/L) | 44.49(10.5, 124) | 56.08 (8.8, 102) | 61.4 (15.9, 147.4) | 73 (14.3, 125.505) | 0.1169 |
| LDH (IU/L) | 454 (291, 658) | 426 (286.5, 696) | 455.5 (352.3, 696) | 370.6 (265, 580) | 0.04 |
| Ferritin (ng/ml) | 452 (170.15, 698) | 489 (188.15, 1000) | 749.55(304.55, 1768.5) | 378 (168.3, 1019) | 0.15 |
| Procalcitonin (ng/ml) | 0.222 (0.1, 0.88) | 0.224 (0.1, 0.86) | 0.84 (0.41, 7.6) | 1.04 (0.25, 4.22) | 0.003 |
| IL 6_level (pg/ml) | 19.3 (6.2, 65.59) | 34.45 (10.34, 99.08) | 43.8 (11.2, 74) | 70 (29.8, 284.2) | 0.003 |
| Coagulation | |||||
| PT (seconds) | 13.8 (12.3, 15.6) | 14.6 (13.2, 16.7) | 14.7 (12.4, 17.9) | 13.4 (11.8, 16.35) | 0.41 |
| INR | 1.09 (1, 1.21) | 1.16 (1.02, 1.339) | 1.16 (1.08, 1.41) | 1.14 (1.02, 1.3) | < 0.001 |
| D_Dimer (mg/L) | 0.92 (0.36, 5.19) | 0.83 (0.27, 2.25) | 1.1 (0.46, 4.03) | 1.2 (0.57, 3.11) | < 0.001 |
*P value for Kruskal wallis test
There was significant difference in proportion of cardiovascular complications among no cancer [n = 562(1.1%)], unspecified cancer [n = 13(2.4%)], haematological cancer [n = 3(2%)], and solid cancer [n = 7(2.4%)], p (0.004). Also, there was significant distribution in requirement of remdesivir (p < 0.001), steroids (< 0.001), anticoagulant (< 0.001) and requirement of ICU organ support (0.04) across the groups. Proportion of death was highest among solid cancer [n = 72(25%)] while lowest among no cancer group [n = 5977(11.8%)], p < 0. 001 (Table 2.3).
Table 2.3.
Comparing thrombotic complications, COVID treatment modalities and outcomes, among cancer and Non-cancer control cohorts and among different type of cancer
| Treatment | No Cancer N = 50,568 |
Unspecified Cancer N = 541 |
Hematological N = 147 |
Solid Cancer N = 288 |
P Value |
|---|---|---|---|---|---|
| Thrombotic Complications | |||||
| Yes | 157 (0.31) | 1 (0.2) | 1 (0.68) | - | 0.58 |
| No | 50,411 (99.7) | 540 (99.8) | 146 (99.3) | 288 (100) | |
| Cardio Vascular Complication | |||||
| Yes | 562 (1.1) | 13 (2.4) | 3 (2) | 7 (2.4) | 0.004 |
| No | 50,006 (98.9) | 528 (97.6) | 144 (98) | 281 (97.6) | |
| Remdesivir | |||||
| Yes | 16,579 (32.79) | 76 (14.05) | 33 (22.45) | 60 (20.8) | < 0.001 |
| No | 33,989 (67.2) | 465 (85.9) | 114 (77.55) | 228 (79.17) | |
| Steroids | |||||
| Yes | 28,920 (57.19) | 255 (47.13) | 71 (48.3) | 105 (36.46) | < 0.001 |
| No | 21,648 (42.8) | 286 (52.87) | 76 (51.7) | 183 (63.54 | |
| Anticoagulants | |||||
| Yes | 28,916 (57.18) | 257 (47.5) | 68 (46.2) | 127 (44.10) | < 0.001 |
| No | 21,652 (42.8) | 284 (52.5) | 79 (53.7) | 161 (55.9) | |
| Supportive Oxygen Therapy | |||||
| Yes | 24,988 (49.4) | 259 (47.87) | 69 (46.9) | 129 (44.7) | 0.105 |
| No | 25,580 (50.6) | 282 (52.13) | 78 (53.06) | 159 (55.2) | |
| Invasive Mechanical Ventilation | |||||
| Yes | 1554 (3.1) | 24 (4.4) | 5 (3.4) | 7 (2.4) | 0.28 |
| No | 49,014 (96.9) | 517 (95.6) | 142 (96.6) | 281 (97.6) | |
| ICU-Organ support requireda | |||||
| Yes | 5291 (10.46) | 65 (12.01) | 19 (12.9) | 43 (14.9) | 0.040 |
| No | 45,277 (89.54) | 476 (87.99) | 128 (87.07) | 245 (85.07) | |
| Outcome | |||||
| Discharged to home | 40,823 (80.7) | 372 (68.8) | 98 (66.7) | 183 (63.5) | < 0.001 |
| Death | 5977 (11.8) | 105 (19.4) | 31 (21.09) | 72 (25) | |
| Transferred /LAMA | 3768 | 64 (11.8) | 18 (12.2) | 33 11.46) |
aICU-Organ support is a composite outcome taking into account the requirement of ICU admission during hospital stay, ECHMO Support, Vasopressor and Renal Replacement
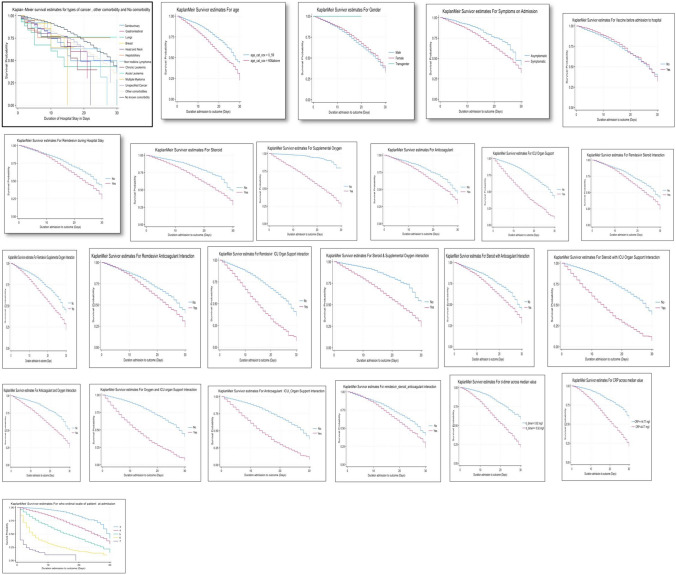
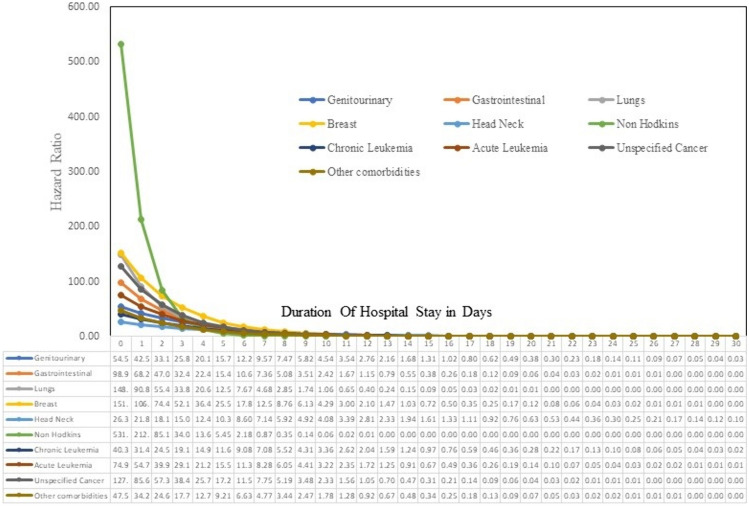
Kaplan Meier graph for survival analysis to represent survival over time for a duration of 30 days of hospital stay and Univariate Cox proportional hazards (PH) models were fitted to obtain unadjusted hazard ratio associated with each factor of interest. The factors those were statistically or clinically relevant were included in the multivariable Cox PH model. As per model all cancer types, genitourinary[aHR-54.5, p < 0.001], gastrointestinal[aHR-98.9, p < 0.001], lungs [aHR-148.9, p < 0.001], breast [aHR-151.9, p < 0.001], non hodkins lymphoma [aHR-5531.9, p < 0.001], chronic leukemia [aHR-40.3, p < 0.001], acute leukemia [aHR-74.9, p < 0.001], genitourinary[aHR-54.5, p < 0.001], genitourinary[aHR-54.5, p < 0.001], unspecified cancer [aHR-127.8, p < 0.001] had significant risk of in-hospital mortality at the time of admission (time zero). Adjusted hazard for all cancer types gradually declined and converged with time, and became comparable to the hazard associated with other comorbidities in around 10 days since admission. Also, the range of time in which adjusted hazard ratios with respect to the reference group No Comorbidity declined to one varied between 7 to 18 days for different cancer types, with non-Hodgkin’s lymphoma and head & neck brain cancer holding the extremes respectively (Table 3, and Figs. 1, 2).
Table 3.
Prognostic Factors: Multivariable cox proportional hazard model for in-hospital patients with cancer in (Adult)
| Variable | Unadjusted Hazard Ratio | Adjusted Hazard Ratio (Final Model) N = 35,224 | P value |
|---|---|---|---|
| Type of Comorbidity (HR At Time Zero) | |||
| Genitourinary | 2.43 (1.55, 3.83) | 54.5(19.19, 154.80) | < 0.001 |
| Gastrointestinal | 2.29 (1.35, 3.88) | 98.9 (22.4, 435.8) | < 0.001 |
| Lungs | 4.03(2.4, 6.6) | 148.9(46.51, 474.47) | < 0.001 |
| Breast | 2.01(0.9, 4.49) | 151.93(23.28, 991.43) | < 0.001 |
| Head Neck Brain | 3.3(1.8, 6.01) | 26.3(6., 101.7) | < 0.001 |
| Hepatobiliary | 1.86(0.6, 5.79) | 4 (0.99, 16.17) | 0.051 |
| Non-Hodkins Lymphoma | 2.72(1.3, 5.72) | 531.96(56.45, 5012.89) | < 0.001 |
| Chronic Leukemia | 2.89(1.5, 5.56) | 40.38(12.2, 133.38) | < 0.001 |
| Acute Leukemia | 2.2(1.04, 4.62) | 74.96(16.47, 340.78) | < 0.001 |
| Multiple Myeloma | 2.62(0.98, 6.9) | 1.56(0.21, 11.11) | 0.659 |
| Un Specified Cancer | 2.16(1.77, 2.64) | 127.8(81.19, 201.20) | < 0.001 |
| Other Comorbidities | 1.85(1.75, 1.96) | 47.5(41.0, 55.23) | < 0.001 |
| No Comorbidity | Ref | Ref | |
| Time Interaction for comorbidity type | |||
| Time Interaction for Genitourinary | 1.0(0.96, 1.03) | 0.78(0.71, 0.87) | < 0.001 |
| Time Interaction for Gastrointestinal | 0.99 (0.94, 1.04) | 0.69(0.56, 0.85) | 0.001 |
| Time Interaction for Lungs | 0.99 (0.94, 1.04) | 0.61(0.45, 0.82) | 0.002 |
| Time Interaction for Breast | 0.99 (0.91, 1.07) | 0.7(0.56, 0.87) | 0.002 |
| Time Interaction for Head Neck Brain | 1.02 (0.98, 1.07) | 0.83(0.72, 0.95) | 0.009 |
| Time Interaction for Non-Hodkins Lymphoma | 1.01 (0.97, 1.06) | 0.40 (0.2, 0.82) | 0.012 |
| Time Interaction for Chronic Leukemia | 0.99(0.94, 1.05) | 0.78(0.68, 0.91) | 0.001 |
| Time Interaction for Acute Leukemia | 0.93 (0.84, 1.03) | 0.73(0.56, 0.96) | 0.026 |
| Time Interaction for Un Specified Cancer | 0.98(0.96, 0.99) | 0.67(0.63, 0.71) | < 0.001 |
| Time Interaction for Other Comorbidities | 0.96 (0.95, 0.96) | 0.72(0.71, 0.73) | < 0.001 |
| Age Category | |||
| 60 years and above | |||
| Less than 60 years | 1.8 (1.7, 1.9) | 1.27(1.19, 1.35) | < 0.001 |
| Gender | |||
| Male | 1.11 (1.05, 1.17) | 1.1(1.03, 1.17) | 0.005 |
| Female | Ref | Ref | |
| Symptomatic on admission | |||
| Yes | 1.7 (1.5, 1.8) | 0.97(0.86, 1.08) | 0.534 |
| No | Ref | Ref | |
| WHO ordinal Scale on admission, n = 39,363 | |||
| 3 | Ref | Ref | |
| 4 | 4.05(3.7, 4.43) | 1.2(1.07, 1.35) | 0.002 |
| 5 | 9.77(8.88, 10.75) | 2.71(2.4, 3.06) | < 0.001 |
| 6 | 27.9(24.91, 31.24) | 4.45(3.86, 5.13) | < 0.001 |
| 7 | 89.66(73.1, 109.98) | 7.02(5.55, 8.88) | < 0.001 |
| Vaccine Taken | |||
| Yes | 0.76 (0.71, 0.83) | 0.6(0.54, 0.65) | < 0.001 |
| No | Ref | Ref | |
| Coagulation complication during hospital stay | |||
| Yes | 3.05 (2.4, 3.7) | 1.12(0.86, 1.46) | 0.395 |
| No | Ref | Ref | |
| Cardiac Complication during hospital stay | |||
| Yes | 4.6(4.1,5.1) | 1.89(1.66, 2.16) | < 0.001 |
| No | Ref | Ref | |
| Remdesivir | |||
| Yes | 1.16 (1.11, 1.23) | 0.36(0.23, 0.58) | < 0.001 |
| No | Ref | Ref | |
| Steroid | |||
| Yes | 1.86 (1.7, 1.9) | 0.87(0.61, 1.24) | 0.452 |
| No | Ref | Ref | |
| Anticoagulant | |||
| Yes | 1.3 (1.3, 1.4) | 0.94(0.68, 1.3) | 0.692 |
| No | Ref | Ref | |
| Supplemental Oxygen | |||
| Yes, | 11.69 (10.4, 13.0) | 8.47(6.86, 10.47) | < 0.001 |
| No | Ref | Ref | |
| Supplemental Oxygen with Remdesivir | |||
| Yes | 1.6 (1.58, 1.75) | 2.22(1.4, 3.53) | 0.001 |
| No | Ref | Ref | |
| Supplemental oxygen with Steroid | |||
| Yes | 3.02 (2.84, 3.2) | 1.07(0.75, 1.52) | 0.715 |
| No | Ref | Ref | |
| Supplemental oxygen with Anticoagulant | |||
| Yes | 2.42 (2.29, 2.5) | 0.83(0.59, 1.17) | 0.297 |
| No | Ref | Ref | |
| ICU and Organ Support | |||
| Yes | 3.8 (3.6, 4.04) | 2.38(1.6, 3.56) | < 0.001 |
| No | Ref | Ref | |
| ICU and Organ Support with Remdesivir | |||
| Yes | 2.83 (2.61, 3.07) | 1(0.87, 1.15) | 0.976 |
| No | Ref | Ref | |
| ICU and Organ Support with Steroid | |||
| Yes | 3.8(3.6, 4.1) | 1.31(1.09, 1.58) | 0.004 |
| No | Ref | Ref | |
| ICU and Organ Support with Anticoagulant | |||
| Yes | 3.6 (3.3, 3.8) | 0.97(0.82, 1.14) | 0.708 |
| No | Ref | Ref | |
| ICU and Organ Support with Oxygen | |||
| Yes | 4.6 (4.3, 4.9) | 0.63(0.42, 0.96) | 0.03 |
| No | Ref | Ref | |
| Remdesivir with Steroid | |||
| Yes | 1.24 (1.18, 1.31) | 1.01(0.76, 1.33) | 0.95 |
| No | Ref | Ref | |
| Steroids with Anticoagulant | |||
| Yes | 1.46 (1.38, 1.54) | 0.95(0.79, 1.15) | 0.593 |
| No | Ref | Ref | |
| Received all (Remdesivir, Steroid, Anticoagulant) | |||
| Yes | 1.21 (1.15, 1.28) | 1.07(0.88, 1.3) | 0.497 |
| No | Ref | Ref |
aIf value of a variable had frequency less than 10 they were not included in the model
bRemdesivir with anticoagulant was not having confidence interval so was excluded from the model
Fig. 1.
Kaplan Meir Survival curves for predictors of hospital mortality among cancer patients during 30 days of hospital stay
Fig. 2.
Line Graph representing Hazard Ratio of different type of cancers, unspecified cancer and other comorbidities compared to no comorbidity over a duration of 30 days during hospital stay when adjusted for predictors
Geographic distribution of cancer cases in registry was also compared with national information and it was observed that the distribution of cases across zones were comparable with highest recorded cases in both registry and national figure is from south zone [29.1% vs 24.6%]. Supplementary Fig. 1.
The adjusted hazard for patients having values of CRP and D-Dimer greater than the median value was [aHR-1.9, p < 0.001] & [aHR- 1.98, p < 0.001] respectively. (Supplementary Table 2).
Discussion
The research involved the analysis of data from the National Clinical Registry for Covid-19, which, included approximately 51,544 patients enrolled till 31st August 2023, who tested positive for the SARS-CoV-2 virus and were hospitalized across 42 hospitals in India. The primary focus of this study was to evaluate the hazard associated with all-cause mortality across various types of cancer when co-affected with COVID-19 within 30 days of hospital admission in comparison to other comorbidities and No Comorbidity. Additionally, the study aimed to compare the demographic and clinical characteristics of patients with different categories of cancer.
In this study, the most common cancers reported in males were gastrointestinal, followed by genitourinary and lung cancers. In females, breast cancer was most common, followed by genitourinary cancers. These findings are consistent with national cancer reports in India, which identify lung, mouth, oesophagus, stomach, and nasopharynx as the most common cancers in males, and genitourinary and breast cancers as the most common in females (Sathishkumar et al. 2022; Mathur 2020). The distribution of cancer cases across geographic zones in our registry showed heterogeneity, with the highest representation from North India, followed by Central and East India. While our findings align with national figures in terms of heterogeneous representation of cases, they differ from the magnitude and proportion of cancer cases reported by hospital-based cancer registries nationwide. These registries indicate the highest cancer burden in Central India, followed by the North East Zone. This discrepancy may be attributed to the design of our registry, whose initial mission was to focused on COVID-19 cases and considered designated COVID-19 hospitals. These hospitals might or might not have been to serve as the centres for cancer referrals (Mathur 2020).
There was significant difference in requirement of ICU and Organ Support across various categories of cancer with proportion of patients requiring support was highest among solid cancer (14.9%) while minimum among no form of cancer (10.46%). A study conducted in Chicago reported an ICU admission rate of 17.6%, figures that align with our findings. Also, there was no significant difference in distribution of requirement of supportive oxygen therapy and Invasive mechanical ventilation among Various categories of cancer. This observation contrasts with data from Brazil, which indicated a high usage of invasive mechanical ventilation (IMV) among cancer patients (Caruso et al. 2021). There has been ongoing debate about the relatively low rates of ICU admission and IMV use among cancer patients with COVID-19 (Mechanical Ventilation Rates and in Oncology Patients with COVID-19 – Consult QD. 2021). This could be attributed to the fact that many cancer patients in our study may have had advanced cancer and were primarily receiving palliative care when they contracted COVID-19, leading to a disproportionately low rate of ICU admission and IMV utilization. Additionally, it’s worth noting that the median age of our cancer cohort ranged between 56 to 60 years, which may have contributed to the lower proportion of ICU admissions and organ support, as older cancer patients with reduced life expectancy might have been triaged due to the global shortage of ICU beds during the peak of the pandemic (Azoulay et al. 2011).
In this study, there was no discernible difference in the occurrence of thrombotic complications between patients across various groups of cancer (p = 0.58). This is in contrast to other studies where there was a significant difference between occurrence of cardiovascular complication across types of cancer. The mechanism of synergy between COVID-19 infection and cancer leading to cardiovascular complication may be because of hyperinflammation, which negatively affects the cardiovascular system. The dysregulated immune response can promote cardiovascular and endothelial dysfunction manifesting as cardiovascular complication, but further studies are needed (Gupta et al. 2021).
The findings of this study revealed that the in-hospital mortality rate was among COVID-19 patients with cancer was 21.3%, with 208 out of 976 cancer patients succumbing to the illness. This mortality rate was lower than the estimate derived from a systematic review and meta-analysis of cohort studies involving hospitalized cancer patients with COVID-19, where the mortality rate was reported to be 30% (Desai et al. 2021). Another study conducted at Guy’s Cancer Centre and King’s College Hospital during the period from February 29 to July 31, 2020, reported a mortality rate similar to our cohort, at 24% (Sathishkumar et al. 2022). The in-hospital mortality among Solid and Haematological Cancer were (n = 72, 25%) & (n = 31, 21.09%) respectively. A cohort study of 2,515 adult cancer patients with COVID-19 published in JAMA found that haematological malignant neoplasms and lung cancer were associated with higher mortality rates, with an overall mortality rate of 38% (Várnai et al. 2022). Notably number of deaths were high in patients suffering from Genitourinary, gastrointestinal and lung cancers among solid cancers, non-Hodkin’s type of Lymphoma among Lymphomas, and Chronic lymphocytic leukaemia among Leukaemia’s, a pattern consistent with data previously reported from Tata Medical Centre in Kolkata, India, which also participated in the registry (Roy et al. 2021).
Adjusted hazard ratio models were employed to assess overall survival across various types of cancer compared to no reported morbidity, adjusting for age, gender, severity of disease at admission, cardiac complications, COVID-19 vaccination status, and commonly used COVID-19 treatments such as Remdesivir, steroids, and anticoagulants. Due to the non-proportionality of hazard ratios over time for different types of cancer, compared to those without comorbidities, adjustment for time interaction term for each type of cancer was also incorporated into the model. The model demonstrated that the risk of all-cause mortality for different types of cancer at day zero ranged from 531.8 to 25.74, with Non-Hodgkin’s Lymphoma and Head Neck brain Cancer malignancies exhibiting these extremes, respectively. Other cancers with significantly higher mortality are lungs and breast cancer. This is in line with previous studies that mention that lungs cancer has significantly high mortality when co-affected with COVID-19 (Lei et al. 2021).
The change in hazard ratio over time was also plotted, revealing that hazard ratios for all cancer types were significantly elevated immediately after admission but converged around and became similar to other comorbidities around days 10. This indicates that certain pathophysiology exclusive to cancer might play significant role in early mortality and hence should be dealt with aggressively. Also, this suggests that stringent facility-based management of cancer patients would be critical during this period. Additionally, the graph demonstrated that the time required for the hazard ratio to decline to one after hospitalization varied by cancer type: 17 days for genitourinary cancers, 13 days for gastrointestinal cancers, 11 days for lung cancer, 15 days for breast cancer, 18 days for head and neck cancers, 7 days for non-Hodgkin’s lymphoma, 15 days for chronic leukaemia, 14 days for acute leukaemia, and 13 days for unspecified cancers. Other comorbidities required 12 days. These findings indicate that extensive monitoring should be tailored considering the specific durations required for each cancer type following admission. Moreover, these results may be considered in the development of patient triaging protocols if needed during future hospitalizations.
The relatively high risk of mortality in the early days of hospitalization among cancer patients with COVID-19 may be attributed to their immunosuppressed state, which can make them more severely ill during the viremic phase, leading to more early deaths. Additionally, some reports suggest that receiving cancer treatment within 14 days of a COVID-19 diagnosis may pose a significant risk for developing severe complications. Many cancer patients, due to fear of contracting COVID-19, reported to hospitals only when necessary for cancer management. It may be possible that the relatively high mortality during the early days could be related to undergoing cancer treatment within 14 days of contracting COVID-19 (Cheng 2021; Sengar et al. 2022). The decreased risk of mortality observed in cancer patients beyond 14 days post-hospitalization may be attributed to surviving the initial viral load during the viremic phase and not experiencing immune-mediated complications, potentially due to their underlying immunosuppressed state (Bhaskar 2020). Above observation suggests that monitoring viral load at various time points, along with assessing the immune response, could be beneficial approach in optimizing treatment strategies for cancer patients. Further research is encouraged to validate these findings and refine clinical approaches.
In our investigation examining the impact of commonly used COVID-19 drugs, the addition of remdesivir and steroids to COVID-19 management regimen significantly reduced hazard of mortality as can be observed from reduction in Hazard Ratio in ICU patients. This finding contrasts with the Solidarity trial in terms of remdesivir, in which mortality was higher with remdesivir use among ventilated patients, although it was consistent with the findings of other studies (WHO Solidarity Trial Consortium 2022). The efficacy of remdesivir seemed to depend on various factors, including the need for oxygen or respiratory support and the timing of drug initiation, as evidenced by various trials. However, due to the lack of uniformity of available data for all patients, our data could not be further analysed in this regard. It is also worthwhile to mention here that Unadjusted hazard ratio associated with Remdesivir, indicates that the individuals who received the medication were at around 24% higher risk of dying as compared to those who did not. However, this result should not lead to the conclusion that the use of Remdesivir may have increased the chances of mortality because the administration of Remdesivir was not in random and, in practice, only individuals with severe infection or those at higher risk of severe outcome were prescribed Remdesivir. Also as discussed in previous studies age, male gender, severity of disease at admission, cardiac complication was significantly associated with higher risk of mortality within 30 days of hospital stay. While Covid-19 vaccination and commonly given COVID-19 drugs like remdesivir, steroids and anticoagulants reduce risk of mortality during hospital stay within 30 Days.
Additionally, cancer patients exhibited higher value of d-dimer with patients having solid cancer exhibiting higher median value, a biochemical proxy indicative of thrombotic complications and disease severity. This finding is similar with the outcomes of previous studies that reported elevated d-dimer levels among individuals with cancer (Ali et al. 2023). This observation contrast other studies that suggest patients with haematological cancers are at a higher risk of developing thrombotic complications when hospitalized with SARS-CoV-2 infection (Fernández-Cruz et al. 2022). Also in this study, we used the median CRP value of the cohort as a reference point (44.77 mg/L), and it was observed that patients with CRP levels exceeding 44.77 mg/dL were linked to an increased risk of overall mortality (model 2 Supplementary Table 1). This finding aligns with available data (Lentner et al. 2021; Rubio-Rivas, et al. 2022). Some studies have indicated that a CRP level of ≥ 40 mg/L is a good predictor of mortality. However, the optimal cutoff may vary depending on the methods and kits used in the assays. CRP has been acknowledged as a predictor of COVID-19 mortality in cancer patients (Lentner et al. 2021). Nevertheless, elevated CRP levels in cancer patients may be influenced by their underlying disease burden, suggesting that a higher cutoff may be necessary for predicting COVID-19 mortality in this cohort. Further research is needed to establish the optimal CRP cutoff for this population. A similar association was also noted with higher levels of d-dimer, value greater than 0.92,g/l (Ali et al. 2023).
Strength and limitation
To the best of our knowledge, this study represents one of the few multicentre investigations in India that comprehensively examines the characteristics of hospitalized SARS-CoV-2-infected patients in context of cancer. Also, it would be one of the few studies that considers many cancer types in a single study. Though the relatively small number of cancer patients in our study may not fully represent the magnitude and diversity of India’s cancer population, and may limit the generalizability of our findings. However, the results offer a foundational understanding of COVID-19’s impact on cancer patients in India and can serve as a catalyst for further research and development of broader healthcare strategies for managing different cancer in the context of COVID-19.
It is crucial to interpret the study’s findings in light of several inherent limitations. Firstly, although efforts were made to minimize heterogeneity, complete homogeneity in the data could not be achieved. Also, registry’s data run the risk of misclassification bias due to inadequate or faulty response, potentially impacting the accuracy of information like cancer types. Secondly, the study was not originally designed as a cancer registry, thus certain essential aspects of cancer patient care and evaluation remain unaddressed and not evaluated like duration of cancer, type of cancer treatment, duration since start of cancer treatment, duration between cancer treatment and COVID-19 diagnosis & stage of cancer. Further studies considering these parameters will add more understanding around the topic Thirdly, the study did not account for the timing of drug initiation during the construction of the multivariate model. Fourthly, the study did not explore higher-order drug interactions within the hazard model. Fifth, our findings offer a general overview of cancer cases in the context of COVID-19 but may not fully reflect detailed regional cancer burdens. Last, the study also could not assess the impact of the interval between COVID-19 vaccination and patient outcomes. Notably, the vaccines employed in India, namely Covaxin (BBV 152) and Covishield (ChAdOx1 nCoV-19), were known to offer protection against severe disease and mortality".
Conclusion
Our research findings indicate that the presence of any form of malignancy constitutes a distinct and autonomous risk element for severe outcomes in hospitalized COVID-19 patients. Consequently, individuals with malignancies should be regarded as a high-priority subgroup among SARS-CoV-2 patients, warranting specialized attention, particularly in settings where resources are constrained or during the process of patient prioritization. Additionally, stringent monitoring of patients should be intensive at hospital admission and should be continued till 10 to 18 days depending on the type of cancer. It would be prudent to tailor monitoring strategy differently for different type of cancer. Elevated level of c-reactive protein and d-dimer are significant predictors for in hospital mortality and should be considered in monitoring plan.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to every member of The National Clinical COVID-19 Registry team for their participation in this study.
Author contributions
SC, AT, PD, AM contributed to data collection and analysis and study design, and contributed to the ethical approval and data analysis and was a major contributor in writing the manuscript SB, SM, PSG, AC Co-led the contributed to data collection and reviewed the written manuscript before submission GK, AS, KJ, AB, PM, SB, AT, SG, SM, PB, SC, GRM, VD, VVR, NCRCT Contributed to the data collection and data analysis, and reviewed the written manuscript before submission. All authors read and approved the final manuscript.
Funding
This study was supported by Indian Council of Medical Research, New Delhi, India (No. VIR/COVID-19/28/2020/ECD-I).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Soumyadip Chatterji and Alka Turuk are Co-first authors.
References
- Ali A et al (2023) Elevation of D-dimer levels are associated with early need for mechanical ventilation support in patients with COVID-19. BMC Pulm Med 23:283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay E et al (2011) Intensive care of the cancer patient: recent achievements and remaining challenges. Ann Intens Care 1:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellera CA et al (2010) Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol 10:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar S et al (2020) Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: The REPROGRAM consortium position paper. Front Immunol. 10.3389/fimmu.2020.01648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungaro M, Passiglia F, Scagliotti GV (2022) COVID-19 and lung cancer: a comprehensive overview from outbreak to recovery. Biomedicines 10:776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso P et al (2021) Cancer-related characteristics associated with invasive mechanical ventilation or in-hospital mortality in patients with COVID-19 admitted to ICU: a cohort multicenter study. Front Oncol 11:746431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HWB (2021) Palliative care for cancer patients with severe COVID-19: the challenge of uncertainty. Support Care Cancer 29:1153–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curigliano G (2020) Cancer patients and risk of mortality for COVID-19. Cancer Cell 38:161–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A et al (2021) Mortality in hospitalized patients with cancer and coronavirus disease 2019: aA systematic review and meta-analysis of cohort studies. Cancer 127:1459–1468 [DOI] [PubMed] [Google Scholar]
- Fernández-Cruz A et al (2022) Higher mortality of hospitalized haematologic patients with COVID-19 compared to non-haematologic is driven by thrombotic complications and development of ARDS: an age-matched cohorts study. Clin Infect Pract 13:100137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garassino MC et al (2020) COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol 21:914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambsch PM, Therneau TM (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81(3):515–526 [Google Scholar]
- Gupta K et al (2021) Cancer patients and COVID-19: Mortality, serious complications, biomarkers, and ways forward. Cancer Treat Res Commun 26:100285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem RY et al (2020) 372 comparing the outcome of COVID-19 in cancer and non-cancer patients: an International Multicenter Study. Open Forum Infect Dis 7:S256
- Huang C et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DSC, Zumla A (2019) Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am 33:869–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer NM et al (2020) Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet Lond Engl 395:1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H et al (2021) Higher mortality in lung cancer patients with COVID-19? A systematic review and meta-analysis. Lung Cancer Amst Neth 157:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentner J et al (2021) C-reactive protein levels associated with COVID-19 outcomes in the United States. J Osteopath Med 121:869–873 [DOI] [PubMed] [Google Scholar]
- Li Q et al (2020) Early transmission dynamics in Wuhan, China, of Novel Coronavirus-infected Pneumonia. N Engl J Med 382:1199–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W et al (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21:335–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima NT, Buss PM, Paes-Sousa R (2020) COVID-19 pandemic: a health and humanitarian crisis. Cad Saude Publica 36:e00177020 [DOI] [PubMed] [Google Scholar]
- Mathur P et al (2020) Cancer Statistics, 2020: Report from national cancer registry programme, India. JCO Glob Oncol 1063–1075. 10.1200/GO.20.00122 [DOI] [PMC free article] [PubMed]
- Nicola M et al (2020) The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg Lond Engl 78:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard SG et al (2020) Cancer history and risk factors in healthy older people enrolling in the ASPREE clinical trial. Contemp Clin Trials 96:106095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanical Ventilation Rates in Oncology Patients with COVID-19 – Consult QD (2021). https://consultqd.clevelandclinic.org/mechanical-ventilation-rates-in-oncology-patients-with-covid-19/
- Roy S et al (2021) Outcome of COVID-19 in solid organ malignancies: experience from a tertiary cancer Center in Eastern India. JCO Glob Oncol 7:1374–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Rivas M et al (2022) WHO ordinal scale and inflammation risk categories in COVID-19. comparative study of the severity scales. J Gen Intern Med 37:1980–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar K, Chaturvedi M, Das P, Stephen S, Mathur P (2022) Cancer incidence estimates for 2022 & projection for 2025: Result from National Cancer Registry Programme, India. Indian J Med Res 156:598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengar M et al (2022) Outcomes of COVID-19 and risk factors in patients with cancer. Nat Cancer 3:547–551 [DOI] [PubMed] [Google Scholar]
- Sharafeldin N et al (2021) Outcomes of COVID-19 in patients with cancer: report from the National COVID Cohort Collaborative (N3C). J Clin Oncol Off J Am Soc Clin Oncol 39:2232–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle L et al (2024) Changes in hospital mortality in patients with cancer during the COVID-19 pandemic (ISARIC-CCP-UK): a prospective, multicentre cohort study. Lancet Oncol 25:636–648 [DOI] [PubMed] [Google Scholar]
- Várnai C et al (2022) Mortality among adults with cancer undergoing chemotherapy or immunotherapy and infected with COVID-19. JAMA Netw Open 5:e220130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijenthira A et al (2020) Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood 136:2881–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Berger NA, Xu R (2021) Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol 7:220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Solidarity Trial Consortium (2022) Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet Lond Engl 399:1941–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WA et al (2020) Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub. Blood Adv 4:5966–5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Ouyang W, Chua MLK, Xie C (2020) SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 6:1108–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J et al (2023) Clinical characteristics, risk factors and outcomes of cancer patients with COVID-19: a population-based study. Cancer Med 12:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.