Abstract
Contrast-induced acute kidney injury (CI-AKI) is one of the main causes of hospital-acquired renal failure, and still lacks of effective treatments. Previously, we demonstrated that αKlotho, which is an anti-aging protein that highly expresses in the kidney, has therapeutic activity in CI-AKI through promoting autophagy. However, the specific mechanism underlying αKlotho-mediated autophagy remains unclear. The RNA sequencing analysis of renal cortex revealed that the differentially expressed genes related to autophagy between αKlotho-treated CI-AKI mice and vehicle-treated CI-AKI mice were found to be associated with mitophagy and apoptosis. In the kidney of CI-AKI mice and HK-2 cells exposed to Iohexol, we revealed that αKlotho promoted mitophagy and decreased cell apoptosis. Mechanistically, αKlotho attenuated mitochondria damage, decreased mitochondrial ROS by upregulating BNIP3-mediated mitophagy. BNIP3 deletion abolished the beneficial effects of αKlotho both in vivo and in vitro. Moreover, we further demonstrated that αKlotho upregulated FoxO3 nuclear expression in Iohexol-treated HK-2 cells. Knockdown of FOXO3 gene inhibited αKlotho-promoted BNIP3-mediated mitophagy and subsequently increased the oxidative injury and cell apoptosis. Taken together, our results indicated a critical role of αKlotho in alleviating CI-AKI via mitophagy promotion involving the FoxO3-BNIP3 pathway.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-024-05473-z.
Keywords: Acute kidney injury, Contrast media, Klotho, Mitophagy, FoxO3
Introduction
Contrast-induced acute kidney injury (CI-AKI) is an iatrogenic renal dysfunction occurred after intravascular administration of contrast media (CM) for diagnostic or interventional procedures [1]. Researches have confirmed that CI-AKI is associated with short- and long-term adverse clinical outcomes including longer hospitalization, in-hospital morbidity, cardiovascular events, progression to chronic kidney disease (CKD) and increased mortality [2–4]. While there had been incremental advance in our understanding of pathogenesis of CI-AKI, treatment options remain rare. Interventions such as intravascular volume expansion, sodium bicarbonate and acetylcysteine, are often mentioned to prevent the adverse consequences of CI-AKI, but no benefits have been found in recent large-scale randomized controlled trials [5–7], which emphasize the need to further understand the pathogenesis of CI-AKI and develop new treatment and preventive measures.
Although the pathophysiological mechanisms of CI-AKI have not been fully clarified, reactive oxygen species (ROS) are proposed to play a major role in CI-AKI [8]. During the process of CI-AKI, administration of CM can result in excessive production of ROS or reducing the activity of antioxidant enzymes, increasing oxidative stress and impairing renal function. Moreover, medullary hypoxia leads to enhanced ROS generation, mitochondrial oxidative stress and mitochondrial dysfunction [9]. Overall, it can be seen that mitochondrial function and oxidative stress play important roles in the pathophysiology of CI-AKI. Therefore, strategies to reduce oxidative stress and protect mitochondrial function are potential targets for CI-AKI prevention and treatment. Mitophagy, which contributes to preserve mitochondrial homeostasis by clearing damaged mitochondria and excessive ROS, is a critical component of mitochondrial quality control mechanisms [10]. A growing number of studies have revealed that mitophagy protects against AKI [11]. In our previous study, we found that both PRKN-dependent and BNIP3-dependent mitophagy protected against CM-induced AKI through preventing apoptosis and reducing mitochondrial ROS [12, 13], indicating that upregulation of mitophagy may be the potential therapeutic target for the prevention and treatment of CI-AKI.
αKlotho is an antiaging protein that is predominantly expressed in tubular epithelial cells of kidney, and has been reported to have pleiotropic properties, such as anti-inflammation, antioxidant, anti-apoptosis and modulating autophagy [14, 15]. Over the past decade, multiple studies have found that αKlotho may have preventive and therapeutic effects in AKI induced by ischemia-reperfusion [16], LPS [17], cisplatin [18] and folic acid. Recently, we reported that αKlotho also has therapeutic activity in CI-AKI through limiting NLRP3 inflammasome-mediated pyroptosis, decreasing ROS and promoting autophagy [19]. However, the role of αKlotho in mediating mitophagy and the underlying mechanism remain unknown. FoxO3 is a stress-responsive transcription factor and has been shown to modulate stress response, energy metabolism, apoptosis, autophagy and cell differentiation [20]. During renal hypoxia, tubular deletion of FoxO3 leads to decreased BNIP3 protein levels, autophagic response and increased mitochondrial dysfunction and oxidative damage [21]. Moreover, Yamamoto et al. [22] reported that αKlotho inhibits FoxO3 phosphorylation and promotes its nuclear translocation, which subsequently binds to MnSOD promoter and promotes MnSOD expression, thereby inhibiting ROS formation and alleviating oxidative stress response. Meanwhile, αKlotho had been found to protect the renal oxidative stress injury induced by tacrolimus by enhancing FoxO3 mediated MnSOD expression [23]. Hence, we put forward a hypothesis that αKlotho may alleviate oxidative stress injury, reduce apoptosis and alleviate CI-AKI by regulating mitophagy mediated by FoxO3/BNIP3 pathway.
Materials and methods
Antibodies and reagents
The antibodies used in the study were from the following sources. Anti-LC3B (L7543) from Sigma-Aldrich, anti-cleaved Caspase-3 (9664), anti-FoxO3 (2497), anti-LaminB1 (13435), and anti-GAPDH (2118) were from Cell Signaling Technology; Anti-VDAC/voltage dependent anion channel (ab14734) was from Abcam. Anti-BNIP3 (sc-56167) was from Santa Cruz Biotechnology. Anti-MnSOD (24127-1-AP), anti-Bcl-2 (12789-1-AP), anti-Bax (50599-2-Ig) and anti-ACTB (60008-1-Ig) were from Proteintech; Anti-TUBA (AF0001) was from Beyotime. Reagents in the present study were from the following source. Iohexol was from GE healthcare; Recombined αKlotho protein was from R&D systems. Secondary antibodies for immunoblot analysis were from Beyotime. Fluorescence secondary antibodies were from Abcam, including Donkey Anti-Mouse IgG (Alexa Fluor® 488, ab150105; Alexa Fluor® 555, ab150110), and Donkey Anti-Rabbit IgG (Alexa Fluor® 488, ab150073). Hoechst (HY-15631) was from MedChemExpress. TUNEL assay kit (C1088) and mitochondrial extraction from kidney tissue kit (C3606) were from Beyotime. Mitochondrial extraction from cells kit (89784) was from ThermoFisher scientific. Mitophagy detection kit (MD01) was from Dojindo. MitoSOX™ Red mitochondrial superoxide indicator(M36008) was from ThermoFisher scientific.
Animal models
Male C57BL/6 mice (6–8 weeks old) were purchased from the Chinese Academy of Science (Shanghai, China). Bnip3−/− mice on C57BL/6 background were constructed at Shanghai Model Organisms Center, Inc. as previously describe [13]. All mice were housed in specific pathogen-free conditions. The CI-AKI mice models were constructed as described in our previously study by tail vein injection of Iohexol (10 mL/kg bodyweight), which is a widely used non-ionic contrast agent with low-osmolality in clinical practice [12, 13, 19]. After 30 min of Iohexol injection, the model mice received one bonus intraperitoneally injection of recombined mouse αKlotho protein (0.01 mg/kg bodyweight), while control mice injected with the same dose of saline at the same time. All mice were sacrificed at 24 h after αKlotho or saline administration. All animal experiments were approved by the Animal Care Committee at the Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, and performed according to the Animal Protocol Committee of Shanghai Jiao Tong University.
Cell culture, treatment and transfection
Human renal proximal tubular cells (HK-2 cells) were obtained from American Type Culture Collection (ATCC). HK-2 cells were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (ThermoFisher Scientific) at 37℃. HK-2 cells were treated with αKlotho (400pm) for 2 h before exposure to CM (20mgI/ml). BNIP3 siRNA, FOXO3 siRNA, scrambled siRNA and BNIP3 plasmid were transfected into HK-2 cells using Lipofectamine 3000 transfection reagent (ThermoFisher, Scientific, L3000–015) according to the manufacturer`s instructions.
Measurement of cell viability
HK-2 cells were cultured in 96-well culture plates and incubated with different stimulus. To detect cell viability, cells were treated with 10ul CCK-8 reagent at 37 ° C for 2 h. Absorbance was detected at 450 nm.
Analysis of renal function and morphological evaluation
Serum creatinine levels were detected by the automatic analyzer (Roche Diagnostics GmbH, Mannheim). For histological analysis, kidney tissues were fixed with 4% paraformaldehyde and embedded in paraffin. Then kidney sections were stained with hematoxylin and eosin (H&E) for evaluation of tubular injury. The tubule injury score was quantified by the percentage of damaged tubules: 0, no injury; 1, < 25%; 2, 25–49%; 3, 50–74%; 4, ≥ 75%.
RNA sequencing
Total RNA was extracted using the mRNA Isolation kit (ThermoFisher) from the renal cortex of CI-AKI mice and αKlotho-treated CI-AKI mice according to the manufacturer`s instructions. RNA quality was examined on Agilent 2100 Bioanalyzer (Agilent Technologies). According to the manufacturer’s protocols, the libraries were constructed by using TruSeq Stranded mRNA LT Sample Prep kit (Illumina). RNA sequencing was implemented on the Illumina platform (HiSeqTM 2500, Illumina, Shanghai OE Biotech. Co., Ltd.). The differentially expressed genes (DEGs) were identified by the following criteria: P < 0.05 and fold changes < 0.5 or > 2.
Real-time polymerase chain reaction (RT-PCR)
Total RNA (1 µg) from each sample were transcribed with using a Prime Script RT Reagent Kit with gDNA Eraser PrimeScript RT Master Mix (Vanzyme Biotech, China) to produce the cDNA sample. RT-PCR was performed by using SYBR Premix Ex Taq II (Takara Bio) on a LightCycler 480 system (Roche). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene served as an internal control to normalize the expression levels of the samples. The primer sequences were described in Supplementary Table S1.
Assessment of apoptosis
Kidney tubular cells and HK-2 cells apoptosis were evaluated by terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labelling (TUNEL) staining according to the manufacturer`s instructions and the amount of TUNEL positive cells was evaluated.
Transmission electron microscopy
Transmission electron microscopy analysis was performed as previously described [12, 19]. In brief, fresh kidney tissues were cut into approximately 1mm3 sections, immediately pre-fixed in 2% glutaraldehyde and then fixed in 1% osmium tetroxide. The samples were then embedded in epoxy resin and propylene oxide overnight and polymerized after dehydrated by a series of grades of ethanol. After the samples were cut into 70 nm thick sections and stained with lead citrate, the sections were detected with H-7650 transmission electron microscope (Hitachi H-7650).
Mitochondrial extraction from kidney tissue and cells
Mitochondrial extraction from kidney tissues and HK-2 cells were isolated as described in our previous study [24]. Briefly, renal tissues were digested with trypsin, treated with mitochondrial-isolation reagent and then homogenized in dounce tissue grinders. After centrifuged the lysed tissues at 600 g for 10 min, the supernatant was collected and centrifuged at 1100 g for 10 min to get the pellets which contain mitochondrial. For cells mitochondrial isolation, harvested cells suspension was treated with mitochondrial isolation reagent A and then homogenized in dounce tissue grinders. After that, lysed cells were incubated with mitochondrial isolation reagent C and centrifuged at 700 g. Then the supernatant was centrifuged at 12,000 g for 10 min. The isolated mitochondrial was stored and used for subsequent measures.
Immunoblot analysis
Kidney tissues and cells were lysed in RIPA buffer, and the protein concentrations were measured by BCA protein assay kit (ThermoFisher). Equal amounts of each protein sample were separated on SDS-polyacrylamide gels and transferred to PVDF membranes. After blocking, membranes were incubated with appropriate primary antibodies (the anti-MnSOD antibody was diluted 1:2000, and the other antibodies were diluted 1:1000) overnight at 4℃. Then the membranes were incubated with HRP-conjugated secondary antibodies diluted 1:3000 (Beyotime) for 1 h at room temperature. Finally, the immunoreactive bands were visualized with the chemiluminescence method. Quantification of immunoblot analysis was performed with image J.
Immunofluorescence and immunohistochemical staining
Immunofluorescence and immunohistochemical staining were performed as described previously. For immunohistochemical study, anti-BNIP3 (1:50) antibody and anti-8OHDG (1:100) were incubated overnight at 4 °C. For immunofluorescence staining, the slides were incubated with primary antibodies to LC3B (1:100), FoxO3 (1:100) at 4 °C overnight. Images were visualized using light microscopy or fluorescence microscopy (ZEISS, Axio Vert A1).
Detection of mitophagy in HK-2 cells
Mitophagy in HK-2 cells were detected by the mitophagy detection kit according to the manufacturer`s protocols. Briefly, cells were incubated with 100nmol/l Mtphagy Dye for 30 min. After washing twice with Hank`s solution, cells were treated with different stimulus. Subsequently, cells were incubated with 1µmol/l Lyso Dye for 30 min. After washing with Hank`s solution twice, the cells were observed using a fluorescence microscopy.
Measurement of mitochondrial ROS (mtROS) in HK-2 cells
MitoSOX red indicator was used to detect mtROS in HK-2 cells according to the manufacturer`s instructions. Briefly, HK-2 cells were incubated with MitoSOX (5 µM) and Hoechst (5 µg/ml) at 37℃ for 30 min, and then observed the positive staining using a fluorescence microscope.
Statistics
Statistical analysis was performed using GraphPad Prism 6. Data were shown as the mean ± standard error of the mean. The comparisons between two groups were analyzed by two-tailed unpaired Student’s t-test, and the multi group comparisons were analyzed by one-way ANOVA of Bonferroni posttest. A P value of less than 0.05 was considered statistically significant.
Results
αKlotho administration ameliorated CM-induced renal injury and decreased tubular cells apoptosis
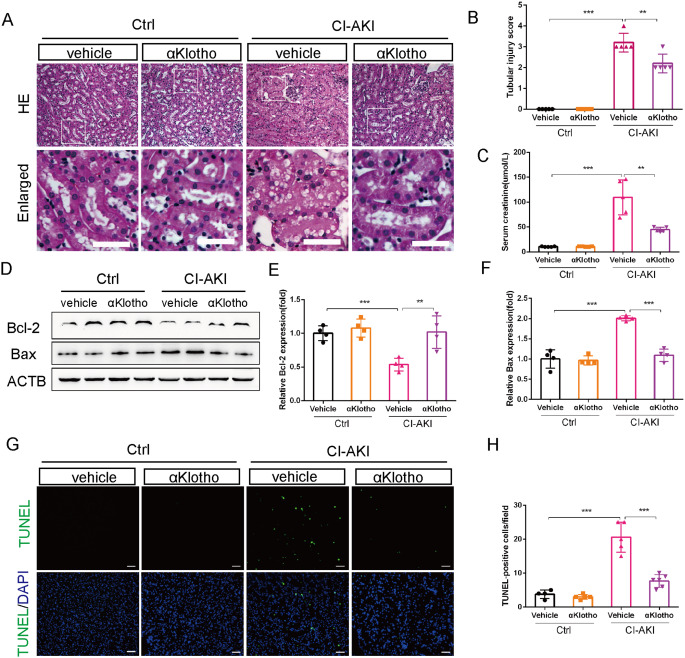
First, we explored the renal protective effect of αKlotho in CI-AKI by treating the CI-AKI mice with recombinant αKlotho protein. The results showed that one dose injection of αKlotho significantly ameliorated CM-induced renal injury. H&E staining showed that tubular epithelial cells dilatation and vacuolization were evident in the renal cortex of vehicle-treated CI-AKI mice while αKlotho treatment markedly reduced renal morphological injury and decreased tubular injury score (Fig. 1A and B). Furthermore, the level of serum creatinine significantly decreased after treating with αKlotho in CI-AKI mice (Fig. 1C). It is well known that CM-induced renal apoptosis plays a pathogenic role in CI-AKI [1]. Therefore, we detected the anti-apoptosis role of αKlotho in CI-AKI mice. Immunoblot analysis showed that αKlotho treatment significantly increased the expression level of Bcl-2 (anti-apoptosis protein) and decreased the expression level of Bax (pro-apoptosis protein) when compared with vehicle-treated CI-AKI mice (Fig. 1D-F). Furthermore, TUNEL staining in the kidney sections revealed that CM-induced renal tubular apoptosis cells were dramatically reduced by αKlotho treatment (Fig. 1G and H). Therefore, administration of αKlotho attenuated CM-induced renal damage and tubular cells apoptosis.
Fig. 1.
αKlotho administration ameliorated CM-induced renal injury and decreased tubular cells apoptosis in CI-AKI mice. (A and B) Representative histology images and pathological score of tubular injury by HE staining. Bottom panels are enlarged images of the boxed areas in the top panels. Scale bar, 50 μm. (C) Serum creatinine levels of different groups. (D, E and F) Immunoblot analysis of Bcl-2 and Bax in kidneys. (G and H) Representative images and quantification of TUNEL-positive cell by TUNEL staining in kidney tubules. Scale bar, 50 μm. Data were expressed as mean ± SEM. n = 4–5. **p < 0.01; ***p < 0.001
αKlotho enhanced renal mitophagy in CI-AKI mice
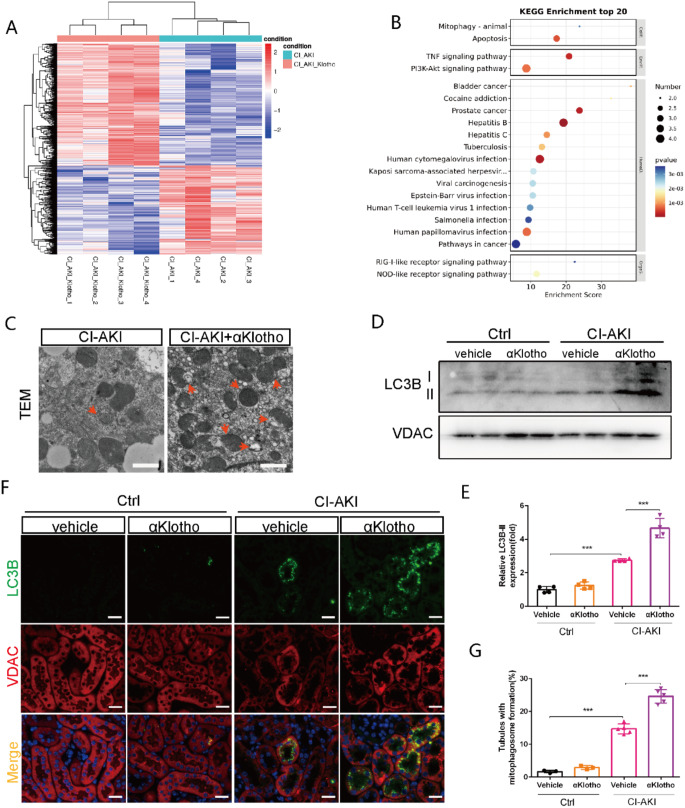
Next, we performed the analysis of RNA sequencing to identify the DEGs between αKlotho-treated CI-AKI mice and vehicle-treated CI-AKI mice. Based on the criteria of fold changes < 0.5 or > 2, 647 upregulated genes and 472 downregulated genes were identified in the renal cortex of αKlotho-treated CI-AKI mice comparing with vehicle-treated CI-AKI mice (Fig. 2A). Analysis of the Kyoto Encyclopedia of Genes and Genomes (KEGG) revealed that DEGs related to autophagy were significantly enriched in the pathways regulating mitophagy and apoptosis (Fig. 2B). Our previous study had demonstrated that mitophagy played a protective role in CI-AKI [12, 25]. Furthermore, we also examined the protective role of mitophagy in vitro by using Rapamycin (an autophagy activator) and UMI-77 (a mitophagy activator) in the present study. Results showed that pretreatment with Rapamycin or UMI-77 mitigated the Iohexol-induced decreased viability of HK-2 cells (Figure S1). Comprehensive above results indicated that αKlotho possibly attenuated contrast-induced apoptosis by regulating mitophagy, which may be an important but unexplored protective mechanism of αKlotho in CI-AKI. As shown in Fig. 2C, αKlotho-treated CI-AKI mice displayed renal mitophagy upregulation compared with vehicle-treated CI-AKI mice, as evidenced by markedly increased mitophagosome formation in renal tubular cells examined by transmission electron microscopy. Likewise, immunoblot analysis of mitochondrial fraction showed that renal mitochondrial LC3BII expression increased markedly in αKlotho-treated CI-AKI mice (Fig. 2D and E). Moreover, immunofluorescence analysis revealed that the colocalization of LC3B and VDAC, a mitochondrial outer membranous protein, was enhanced in renal tubular epithelial cells after αKlotho treatment (Fig. 2F and G). These results indicate that αKlotho participated in regulating mitophagy in CI-AKI.
Fig. 2.
αKlotho enhanced renal mitophagy in CI-AKI mice. (A). Heatmap of RNA-sequencing results comparing αKlotho-treated CI-AKI mice with vehicle-treated CI-AKI mice. (B) KEGG analysis in αKlotho-treated CI-AKI mice versus vehicle-treated CI-AKI mice. (C) Representative TEM images of mitophagosomes (red arrow) in renal tubular epithelial cells. Scale bar, 1 μm. (D and E) Immunoblot analysis of LC3B in the mitochondrial fraction of kidneys. (F and G) Representative images and quantification of co-staining of LC3B and VDAC in kidney tubules. Scale bar, 50 μm. Data were expressed as mean ± SEM. n = 4–5. **p < 0.01; ***p < 0.001
BNIP3 played a critical role in αKlotho-promoted mitophagy in CI-AKI mice
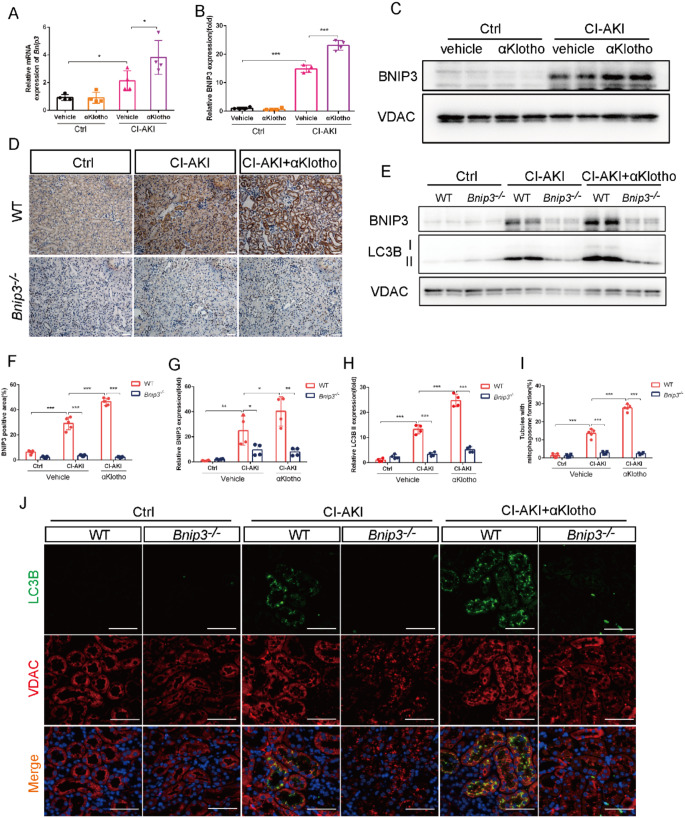
It has been reported that there are two major mechanisms for induction of mitophagy: PRKN-dependent pathway and receptor BNIP3-mediated pathway. Here, we examined that αKlotho treatment significantly increased renal expression of BNIP3 while PINK1 expression was not significantly changed in the kidney of αKlotho-treated CI-AKI mice (Figure S2). RT-PCR analysis showed that αKlotho further increased Bnip3 mRNA expression in the kidney of CI-AKI mice (Fig. 3A). Immunoblot analysis of mitochondrial proteins showed that αKlotho upregulated BNIP3 expression in the mitochondrial fraction of CI-AKI mice (Fig. 3B and C). To provide more evidence for the role of BNIP3 in αKlotho-mediated mitophagy, we introduced Bnip3 knockout (Bnip3-/-) mice into the present study. Immunohistochemical staining showed that αKlotho further increased the expression of BNIP3 in renal tubules of WT mice (Fig. 3D and F). However, BNIP3 deficiency abolished αKlotho-mediated mitophagy upregulation with dramatically lower LC3BII protein level and impaired mitophagosome formation visualized by co-staining LC3B and VDAC in αKlotho-treated CI-AKI mice (Fig. 3E and G-J). Taken together, these data indicated that αKlotho-mediated mitophagy promotion probably through upregulating BNIP3 in CI-AKI.
Fig. 3.
BNIP3 played a critical role in αKlotho-promoted mitophagy in CI-AKI mice. (A) Relative mRNA expressions of Bnip3 in the kidneys. (B and C) Immunoblot analysis of BNIP3 in the mitochondrial fraction of kidneys. (D and F) Representative images and quantification of BNIP3 by immunohistochemical staining in kidney tubules. Scale bar, 50 μm. (E, G and H) Immunoblot analysis of BNIP3 and LC3BII in the mitochondrial fraction of kidneys. (I and J) Representative images and quantification of co-staining of LC3B and VDAC in kidney tubules. Scale bar, 50 μm. Data were expressed as mean ± SEM. n = 4–5. *p < 0.05; **p < 0.01; ***p < 0.001
The protective effects of αKlotho against contrast media were abrogated in BNIP3 deficiency mice
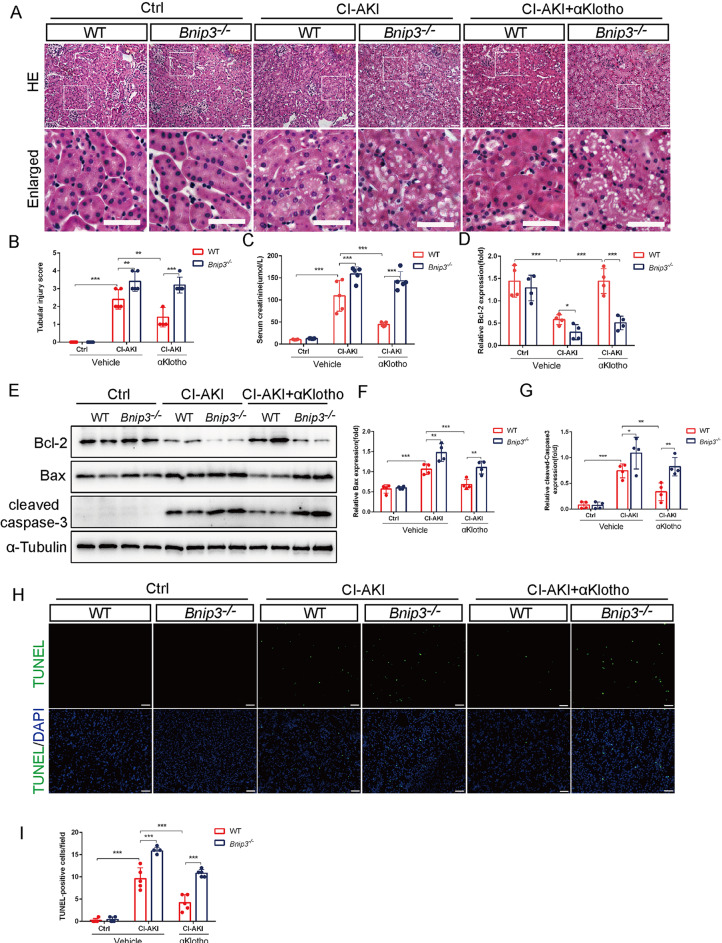
To further clarify the effect of BNIP3 in αKlotho-mediated mitophagy in CI-AKI, we evaluated kidney damage and assessed apoptosis of renal tubular epithelial cells in Bnip3-/- mice. H&E staining showed that the renal cortex of Bnip3-/- CI-AKI mice displayed severe tubular epithelial cells dilatation, intraepithelial vacuolar degeneration and higher renal tubular injury score compared with WT mice after αKlotho treatment (Fig. 4A and B). Furthermore, compared with WT mice, the serum creatinine level was significantly increased in Bnip3-/- CI-AKI mice even though they had been treated with αKlotho (Fig. 4C). Immunoblotting analysis revealed that BNIP3 deficiency reversed αKlotho-induced increase in Bcl-2 expression and reduction in Bax and cleaved caspase-3 levels (a key protease in the initiation and execution process of apoptosis) (Fig. 4D-G). TUNEL staining further confirmed that BNIP3 deficiency abolished αKlotho-induced reduction of renal tubular cells apoptosis (Fig. 4H and I). Therefore, these results suggested that αKlotho protected against CM-induced renal damage and tubular cells apoptosis by promoting BNIP3-mediated mitophagy.
Fig. 4.
The protective effects of αKlotho against contrast media were abrogated in BNIP3 deficiency mice. (A and B) Representative histology images and pathological score of tubular injury by HE staining. Bottom panels are enlarged images of the boxed areas in the top panels. Scale bar, 50 μm. (C) Serum creatinine levels of different groups. (D-G) Immunoblot analysis of Bcl-2, Bax and cleaved caspase-3 in kidneys. (H and I) Representative images and quantification of TUNEL-positive cell by TUNEL staining in kidney tubules. Scale bar, 50 μm. Data were expressed as mean ± SEM. n = 4–5. **p < 0.01; ***p < 0.001
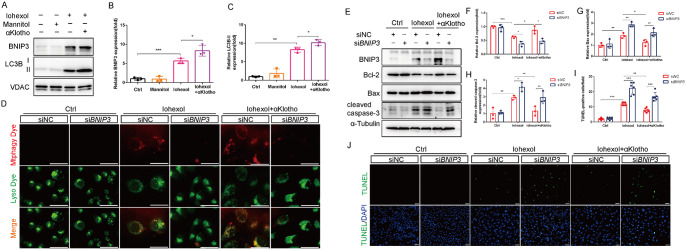
BNIP3 participated in αKlotho-mediated mitophagy and cytoprotection in HK-2 cells under contrast media injury
We also investigated whether BNIP3 participated in αKlotho-mediated mitophagy and cytoprotection in vitro. We established BNIP3-knockdown HK-2 cells using siRNA. Immunoblotting analysis demonstrated that αKlotho pretreatment significantly increased LC3BII and BNIP3 protein expressions in the mitochondrial fractions of HK-2 cells (Fig. 5A-C). To evaluate the occurrence of mitophagy in HK-2 cells, we treated cells with mitophagy dye (red) and lysosome dye (green), and mitophagy can be observed with the colocalization of mitophagy dye and lysosome dye. As shown in Fig. 5D, αKlotho further induced mitophagy in HK-2 cells exposured to Iohexol, whereas BNIP3-knockdown markedly abolished this effect. Finally, we examined apoptosis in BNIP3-knockdown HK-2 cells using immunoblotting analysis and TUNEL staining. Consistent with in vivo results, αKlotho treatment significantly mitigated Iohexol-induced HK-2 cells apoptosis evidenced by increased expression level of Bcl-2 and decreased expression levels of Bax and cleaved caspase-3 (Fig. 5E-H). Moreover, TUNEL staining revealed that αKlotho reduced the number of TUNEL-positive cells (Fig. 5I and J). However, BNIP3 deficiency largely reversed the αKlotho-mediated cytoprotection in HK-2 cells exposured to CM, as manifested by decreased expression level of Bcl-2, increased expression levels of Bax and cleaved caspase-3 (Fig. 5E-H), and increased TUNEL-positive cells (Fig. 5I and J) after silencing BNIP3. In addition, we introduced a BNIP3 overexpression plasmid into HK-2 cells. Immunoblotting analysis confirmed the successful overexpression of BNIP3 in HK-2 cells (Figure S3A). The results of cell viability assessed by CCK-8 assay showed that BNIP3 overexpression improved HK-2 cells viability after Iohexol treatment(Figure S3B), and TUNEL staining revealed that BNIP3 overexpression reduced the number of TUNEL-positive cells after exposured to Iohexol(Figure S3C). Overall, these results suggested that αKlotho enhanced BNIP3-mediated mitophagy and protected against CM-induced injury in vitro.
Fig. 5.
BNIP3 participated in αKlotho-mediated mitophagy in HK-2 cells under contrast media injury. (A-C) Immunoblot analysis of BNIP3 and LC3B in the mitochondrial fraction of HK-2 cells. (D) Representative images of mitophagy (yellow) by mitophagy dye (red) and lysosome dye (green) in HK-2 cells. Scale bar, 50 μm. (E-H) Immunoblot analysis of Bcl-2, Bax and cleaved caspase-3 in HK-2 cells. (I and J) Representative images and quantification of TUNEL staining in HK-2 cells. Scale bar, 50 μm. Data were expressed as mean ± SEM. N = 3. *p < 0.05; **p < 0.01; ***p < 0.001
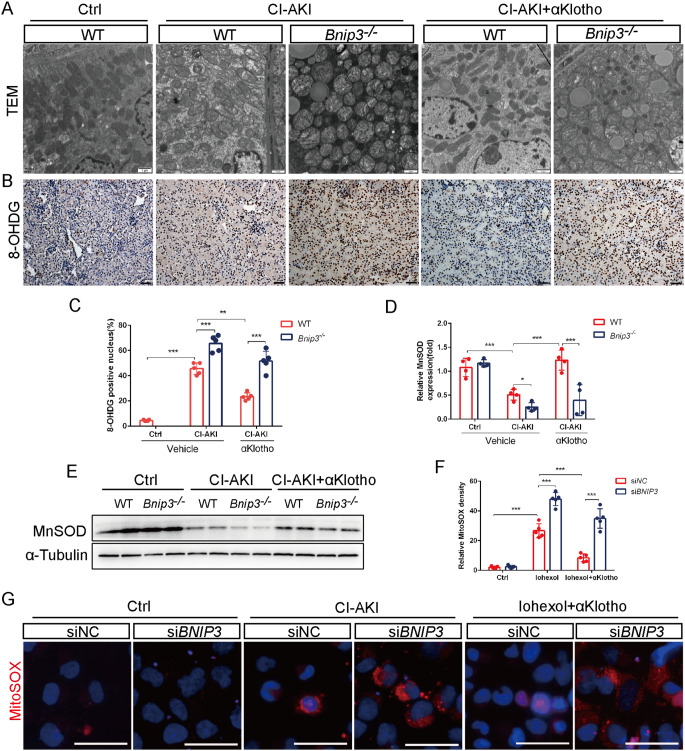
αKlotho relieved mitochondrial injury and reduced ROS generation via BNIP3-mediated mitophagy
Mitophagy induction is generally believed to play a protective role in pathological conditions by removing damaged mitochondria, decreasing ROS accumulation and thus reducing cell injury and death [11]. In the present study, we found that αKlotho obviously alleviated Iohexol-induced mitochondrial structural damage of renal tubular cells, including swelling, cristae rupture and vacuole of mitochondrial matrix (Fig. 6A). Nevertheless, Bnip3-/- CI-AKI mice kidneys displayed severer mitochondrial damage including heavier swelling and cristae rupture despite administrated with αKlotho (Fig. 6A). Next, we evaluated renal oxidative stress injury in CI-AKI kidneys and mitochondrial ROS level in HK-2 cells. Immunohistochemistry analysis of 8-Hydroxydeoxyguanosine (8-OHDG) showed that αKlotho markedly alleviated mitochondrial ROS-induced DNA oxidative damage by CM injury, which was aggravated in the kidneys of Bnip3-/- mice (Fig. 6B and C). Additionally, renal expression level of MnSOD, an important antioxidant enzyme which is located in mitochondria, was significantly decreased in the kidney of Bnip3-/- CI-AKI mice with αKlotho treatment when compared with WT mice (Fig. 6D and E). Then we directly evaluated mitochondrial ROS in HK-2 cells by MitoSOX staining. As shown in Fig. 6F and G, a significant reduction in the fluorescence intensity of MitoSOX was detected in αKlotho pretreated HK-2 cells whereas the MitoSOX signal significantly increased after BNIP3 silence. These results revealed that αKlotho protected renal mitochondria and reduced mitochondrial ROS level in CI-AKI, which were closely related to the promotion of BNIP3-mediated mitophagy.
Fig. 6.
αKlotho relieved mitochondrial injury and reduced ROS generation via BNIP3-mediated mitophagy. (A) Representative TEM images of mitochondrial morphology in renal tubular cells of WT and Bnip3−/− mice. Scale bar, 1 μm. (B and C) Representative images and quantification of 8-OHDG by immunohistochemical staining in kidney tubules. Scale bar, 50 μm. (D and E) Immunoblot analysis of MnSOD in kidneys. (F and G) Representative images and quantification of MitoSOX staining in HK-2 cells. Scale bar, 50 μm. Data were expressed as mean ± SEM. N = 4–5. **p < 0.01; ***p < 0.001
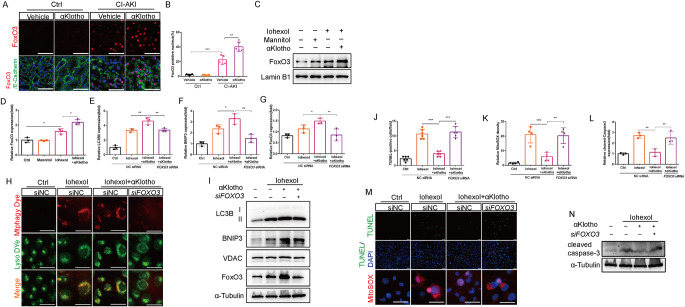
FoxO3 contributed to αKlotho-regulated BNIP3-mediated mitophagy and cytoprotection
Finally, we investigated the mechanism by which αKlotho upregulated BNIP3 protein expression. As shown in Fig. 2B, the KEGG analysis revealed that DEGs between αKlotho-treated CI-AKI and vehicle-treated CI-AKI mice enriched in PI3K/AKT signaling. FoxO3, which is a target transcription factor of PI3K/AKT pathway, has been reported to activate autophagy and reduce oxidative injury by increasing BNIP3 expression in the hypoxic kidney [21]. Here, we found that FoxO3 was activated in renal tubules and further activated by αKlotho in CI-AKI mice as evidenced by more positive nuclear expression of FoxO3 (Fig. 7A and B). Immunoblotting analysis also showed abundant nuclear FoxO3 expression in αKlotho-treated HK-2 cells (Fig. 7C and D). To further explore the role of FoxO3 in αKlotho-mediated mitophagy, we knocked down FOXO3 gene of HK-2 cells. The results showed that up-regulated mitochondrial BNIP3 and LC3BII expressions by αKlotho pretreatment were reversed by silencing FoxO3 expression (Fig. 7E, F and I). Furthermore, mitophagy dye staining showed that mitophagy dramatically inhibited in FOXO3-knockdown HK-2 cells which was pretreated with αKlotho and exposured to Iohexol (Fig. 7H). Finally, we explored the role of FoxO3 in αKlotho-mediated cytoprotection. Immunoblotting analysis revealed that silence of FoxO3 significantly increased cleaved caspase-3 expression (Fig. 7L and N) and TUNEL-positive cells in αKlotho-treated HK-2 cells (Fig. 7J and M). Moreover, knockdown of FOXO3 markedly increased mitochondrial ROS level in HK-2 cells pretreated with αKlotho (Fig. 7K and M). Overall, these findings indicated that αKlotho promoted BNIP3-mediated mitophagy by activation of FoxO3 and subsequently decreased the oxidative injury and cell apoptosis.
Fig. 7.
FoxO3 contributed to αKlotho-regulated BNIP3-mediated mitophagy and cytoprotection. (A and B) Representative images and quantification of nuclear FoxO3 by immunofluorescence in kidney tubules. Scale bar, 50 μm. (C and D) Immunoblot analysis of nuclear FoxO3 in HK-2 cells. (E-G, I) Immunoblot analysis of LC3B and BNIP3 in the mitochondrial fraction of HK-2 cells and FoxO3 in HK-2 cells. (H) Representative images of mitophagy (yellow) by mitophagy dye (red) and lysosome dye (green) in HK-2 cells. Scale bar, 50 μm. (J, K, M) Representative images and quantification of TUNEL staining and MitoSOX staining in HK-2 cells. Scale bar, 50 μm. (L and N) Immunoblot analysis of cleaved caspase-3 in HK-2 cells. Data were expressed as mean ± SEM. N = 3–5. *p < 0.05, **p < 0.01; ***p < 0.001
Discussion
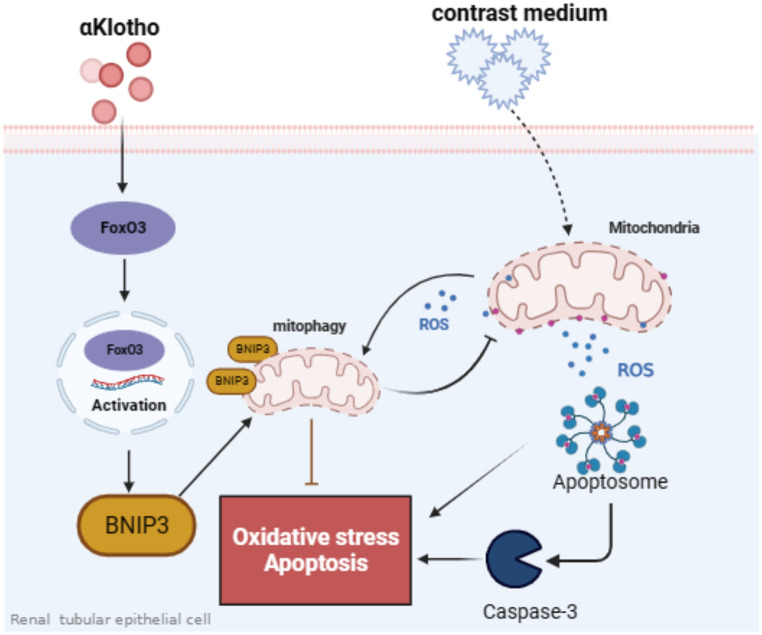
Mitophagy has been generally recognized as a potential therapeutic target of AKI [10, 13, 26]. In this study, we first identified that αKlotho enhanced BNIP3-mediated mitophagy in CI-AKI, both in vivo and in vitro. Then, we found that the protective role of αKlotho was abolished by Bnip3 gene knockout in CI-AKI as evidenced by deficiency of BNIP3 inhibiting mitophagy, aggravating mitochondrial damage, increasing mitochondrial ROS generation and cell apoptosis. Finally, we revealed that FoxO3 is closely associated with αKlotho-induced BNIP3 upregulation, contributing to mitophagy induction, mitochondrial ROS reduction and cell apoptosis decreasing. Hence, our data suggested a renoprotective mechanism of αKlotho by enhancing BNIP3-mediated mitophagy via upregulating FoxO3 in CI-AKI (Fig. 8).
Fig. 8.
Schematic representation of a plausible renoprotection mechanism of αKlotho by regulating FoxO3/BNIP3-mediated mitophagy in contrast-induced acute kidney injury. Contrast media (Iohexol) induced mitochondria damage, resulting in mitochondrial ROS production, oxidative stress, cell apoptosis and renal injury. αKlotho promoted BNIP3-mediated mitophagy by activating FoxO3 and thereby eliminating mitochondrial ROS, reducing cell apoptosis and attenuated contrast media-induced kidney injury
αKlotho is first recognized as an anti-aging protein and highly expresses in the kidney [27]. It has been reported that αKlotho expression decreased in many aging-related diseases such as kidney diseases [16, 28], Alzheimer’s disease [29], cardiovascular and cerebrovascular diseases [30]. The progression of these diseases and the poor prognosis of patients are related to the downregulation of αKlotho expression while overexpression of αKlotho has therapeutic effect with multifaceted functions including anti-apoptosis, anti-fibrosis, anti-inflammatory and anti-oxitative stress [31]. Recently, studies highlighted that αKlotho is an inducer of autophagy in AKI [28, 32]. Shi et al. [28] reported that autophagy was activated in kidney after acute ischemic injury and αKlotho expression was associated with autophagy activity. αKlotho-overexpression IRI-AKI mice displayed much milder renal injury and more autophagosomes and autolysosomes in the kidney whereas αKlotho deficiency mice showed severe kidney damage and less autophagic flux. Furthermore, αKlotho-induced autophagy activation and renoprotection effect was abolished by the autophagy inhibitor bafilomycin A1. In our previous study, we also found that αKlotho protected against CI-AKI via activating autophagy as evidence by the cytoprotection effect of αKlotho was blunted by 3-MA(an autophagy inhibitor). Obviously, enhancing autophagy activity is an important mechanism for αKlotho to protect kidney from injury. However, the role of mitophagy, a form of autophagy that selectively eliminates damaged mitochondria, in the renoprotection mechanism of αKlotho has not been reported yet. Lee et al. [33] demonstrated that αKlotho exerted a mitochondrial protective effect in diabetic kidney disease by inducing AMPK-PGC1α expression and another study found that αKlotho abolished renal fibrosis and significantly protected renal mitochondrial functions by preserving mass and diminishing the generation of ROS in an aging mouse model [34]. These results give us a hint that αKlotho may regulate mitophagy to maintain mitochondrial homeostasis. Basing on the results of RNA sequencing, we found that mitophagy was induced in the kidney under contrast media injury and αKlotho further promoted renal mitophagy activation both in vivo and in vitro, contributing to decreasing mitochondria damage and ROS generation. Inhibition of mitophagy by knockout of BNIP3 reversed these effects. To our knowledge, this is the first study to report the association between αKlotho and mitophagy in CI-AKI, further study is needed to explore the role of αKlotho on the regulation of mitophagy in different models of kidney diseases.
Mitochondria are intracellular organelles that play a key role in the cellular energy production, the regulation of cellular processes and the maintenance of intracellular homeostasis [10]. Mitochondrial dysfunction leads to the generation of ROS and cell apoptosis, both of which contribute to the pathogenesis of CI-AKI [9]. Maintaining mitochondrial homeostasis depends on multiple quality control mechanisms, among which mitophagy plays the critical role [10]. It is well-known that mitophagy is a highly selective quality control mechanism which can maintain the stability of mitochondrial homeostasis by eliminating excessive and damaged mitochondria via the autophagy pathway [10]. Indeed, the participation and control of mitophagy during AKI has received considerable attention in recent years. Accumulating studies had highlighted the protective role of mitophagy in AKI by removing damaged mitochondria and thereby reducing local inflammation and oxidative injury [12, 13, 35–37]. Fu and colleagues reported that BNIP3 overexpression reversed hypoxia-inducible factor 1α-induced mitophagy reduction and I/R-induced tubular cells apoptosis [35]. Deficiency of PINK1 or Parkin with cisplatin-induced AKI displayed more severe renal functional, tissue damage, and apoptosis [36]. Our previous study also demonstrated that activation of mitophagy alleviated renal damage, tubular cells apoptosis in CI-AKI and renal fibrosis in unilateral ureteral obstruction [12, 13, 24]. The present study further confirmed that inhibition of BNIP3-mediated mitophagy aggravated contrast media-induced mitochondria damage, oxidative stress, tubular cell apoptosis and kidney injury. Thus, activation of mitophagy could be a promising therapeutic approach to preventing and treating CI-AKI. Recent studies have reported some drugs can protect against AKI via regulating mitophagy. Farrerol, which is isolated from azaleas, has been reported to attenuate cisplatin-induced inflammation and renal fibrosis via activating PINK1/Parkin-mediated mitophagy [38]. Gao et al. [39]. found that polydatin, a natural polyphenol, protected against sepsis-induced mitochondrial dysfunction, renal NLRP3 infammasome activation and apoptosis by upregulation of Parkin-mediated mitophagy. In the present study, we demonstrated that the anti-aging protein αKlotho also played a protective role in CI-AKI via promoting mitophagy and thereby decreasing mitochondrial ROS production and apoptosis. It is well known that mitophagy is driven by two primary mechanisms including PINK1/Parkin pathway and receptor such as BNIP3 mediated mitophagy [10]. In this study, we focused on the association between αKlotho and mitophagy which is mediated by BNIP3 pathway. We found that αKlotho significantly upregulated BNIP3 expression both in the kidney of CI-AKI and HK-2 cells exposure to Iohexol. Particularly, Bnip3 gene knockout inhibited renal mitophagy and abolished the protective effect of αKlotho against CM-induced mitochondria damage, mitochondrial ROS generation, cell apoptosis and tubular injury. Thus, our results suggested a renoprotective mechanism of αKlotho by promoting mitophagy mediated by BNIP3 in CI-AKI.
FoxO3, a stress-responsive transcription factor, is considered to be an important regulator of BNIP3 during mitophagy induction. It has been found that hypoxia inhibited the degradation of FoxO3 in the kidney following I/R injury, leading to the accumulation and activation of FoxO3 in tubular cells [21]. Importantly, tubular deletion of FoxO3 resulted in aggravating renal structural and functional injury, inhibiting BNIP3 mediated autophagy and increasing oxidative stress damage [21]. Likewise, our data revealed that the nuclear FoxO3 expressions of tubular cells and HK-2 cells were increased by αKlotho after exposure to contrast media injury and downregulation of FoxO3 reduced BNIP3-mediated mitophagy and subsequently increased mitochondrial ROS generation and apoptosis. In addition, studies have shown that αKlotho inhibits the phosphorylation of FoxO3 and promotes its nuclear translocation [22, 40]. The nuclear FoxO3 then binds to the MnSOD promoter and promotes the expression of MnSOD, thereby reducing of ROS generation and decreasing oxidative damage [22]. Another research showed that αKlotho protected against tacrolimus-induced renal oxidative stress injury by enhancing the expression of MnSOD which was upregulated by FoxO3 [23]. Therefore, to investigate the mechanism of αKlotho regulating BNIP3 expression, we focused on transcription factor FoxO3. Our data showed that the nuclear expression of FoxO3 was increased after αKlotho treatment and silence FoxO3 expression in HK-2 cells blunted the protective role of αKlotho by inhibiting BNIP3-mediated mitophagy, indicating that FoxO3 is an mediator of the renoprotective effect of αKlotho-mediated BNIP3 upregulation and mitophagy induction in response to contrast media.
The present study has some limitations. The Bnip3-/- mice were global knockout mice. Tubular-specific knockout mice would be better to explore the role of BNIP3 in αKlotho-mediated mitophagy. In addition, we didn’t explore the mechanism of which FoxO3 regulates BNIP3 expression, although previous study suggested that FoxO3 increases BNIP3 expression by binding to the BNIP3 upstream promoter region [41].
In summary, our data demonstrated a renoprotective mechanism of αKlotho by promoting BNIP3-mediated mitophagy in CI-AKI. αKlotho prevented CM-induced tubular cells apoptosis and tissue damage by decreasing mitochondria damage and mitochondrial ROS generation via FoxO3-BNIP3-mitophagy axis. Modulation of αKlotho and mitophagy may be the promising targets for therapeutic intervention against CI-AKI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
X.Z. and Z.N. designed the study. X.Z., Q.L., Y.Y. carried out the experiments and analyzed the data. S.L., X. S, W.Z., H.C., J.L., J.W., K.Z., C.Q., M.Z., X.C., L.G. helped analyze the data. X.Z. and Z.N. wrote the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82070693, 81770666, 82200751, 82000633); Shanghai Sailing Program (22YF1423400, 20YF1424900); China Postdoctoral Science Foundation (2022M712112); Clinical Research Plan of SHDC (No. SHDC2020CR3029B).
Data availability
The RNA-seq datasets during the current study are available in the NCBI Sequence Read Archive database repository under accession code (PRJNA1036425). The additional data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethical approval
All animal experiments were approved by the Animal Care Committee at the Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, and performed according to the Animal Protocol Committee of Shanghai Jiao Tong University.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuying Zhu, Qisheng Lin and Yuanting Yang contributed equally to this work.
References
- 1.Mehran R, Dangas GD, Weisbord SD (2019) Contrast-Associated Acute kidney Injury. N Engl J Med 380(22):2146–2155 [DOI] [PubMed] [Google Scholar]
- 2.Weisbord SD, Palevsky PM, Kaufman JS, Wu H, Androsenko M, Ferguson RE, Parikh CR, Bhatt DL, Gallagher M, Investigators PT (2020) Contrast-Associated Acute kidney Injury and Serious adverse outcomes following angiography. J Am Coll Cardiol 75(11):1311–1320 [DOI] [PubMed] [Google Scholar]
- 3.Giacoppo D, Madhavan MV, Baber U, Warren J, Bansilal S, Witzenbichler B, Dangas GD, Kirtane AJ, Xu K, Kornowski R et al (2015) Impact of contrast-Induced Acute kidney Injury after Percutaneous Coronary intervention on short- and long-term outcomes: pooled analysis from the HORIZONS-AMI and ACUITY trials. Circ Cardiovasc Interv 8(8):e002475 [DOI] [PubMed] [Google Scholar]
- 4.James MT, Ghali WA, Tonelli M, Faris P, Knudtson ML, Pannu N, Klarenbach SW, Manns BJ, Hemmelgarn BR (2010) Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int 78(8):803–809 [DOI] [PubMed] [Google Scholar]
- 5.Timal RJ, Kooiman J, Sijpkens YWJ, de Vries JPM, Verberk-Jonkers I, Brulez HFH, van Buren M, van der Molen AJ, Cannegieter SC, Putter H et al (2020) Effect of no prehydration vs sodium bicarbonate Prehydration Prior to contrast-enhanced computed Tomography in the Prevention of Postcontrast Acute Kidney Injury in adults with chronic kidney disease: the Kompas Randomized Clinical Trial. JAMA Intern Med 180(4):533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass A, Thwin SS, Conner TA, Chertow GM, Bhatt DL, Shunk K et al (2018) Outcomes after angiography with sodium bicarbonate and Acetylcysteine. N Engl J Med 378(7):603–614 [DOI] [PubMed] [Google Scholar]
- 7.Nijssen EC, Rennenberg RJ, Nelemans PJ, Essers BA, Janssen MM, Vermeeren MA, Ommen VV, Wildberger JE (2017) Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet 389(10076):1312–1322 [DOI] [PubMed] [Google Scholar]
- 8.Heyman SN, Rosen S, Khamaisi M, Idee JM, Rosenberger C (2010) Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol 45(4):188–195 [DOI] [PubMed] [Google Scholar]
- 9.Kusirisin P, Chattipakorn SC, Chattipakorn N (2020) Contrast-induced nephropathy and oxidative stress: mechanistic insights for better interventional approaches. J Transl Med 18(1):400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang C, Cai J, Yin XM, Weinberg JM, Venkatachalam MA, Dong Z (2021) Mitochondrial quality control in kidney injury and repair. Nat Rev Nephrol 17(5):299–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su L, Zhang J, Gomez H, Kellum JA, Peng Z Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2022:1–14 [DOI] [PMC free article] [PubMed]
- 12.Lin Q, Li S, Jiang N, Shao X, Zhang M, Jin H, Zhang Z, Shen J, Zhou Y, Zhou W et al (2019) PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol 26:101254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Q, Li S, Jiang N, Jin H, Shao X, Zhu X, Wu J, Zhang M, Zhang Z, Shen J et al Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy 2020:1–16 [DOI] [PMC free article] [PubMed]
- 14.Hu MC, Moe OW (2012) Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol 8(7):423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neyra JA, Hu MC, Moe OW (2020) Klotho in Clinical Nephrology: diagnostic and therapeutic implications. Clin J Am Soc Nephrol 16(1):162–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW (2010) Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78(12):1240–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi F, Chen F, Li Y, Wei A, Cao W (2018) Klotho preservation by Rhein promotes toll-like receptor 4 proteolysis and attenuates lipopolysaccharide-induced acute kidney injury. J Mol Med (Berl) 96(9):915–927 [DOI] [PubMed] [Google Scholar]
- 18.Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, Hu MC (2014) Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 85(4):855–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu X, Li S, Lin Q, Shao X, Wu J, Zhang W, Cai H, Zhou W, Jiang N, Zhang Z et al (2021) alphaKlotho protein has therapeutic activity in contrast-induced acute kidney injury by limiting NLRP3 inflammasome-mediated pyroptosis and promoting autophagy. Pharmacol Res 167:105531 [DOI] [PubMed] [Google Scholar]
- 20.Webb AE, Brunet A (2014) FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci 39(4):159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Kang H, Zhang Q, D’Agati VD, Al-Awqati Q, Lin F (2019) FoxO3 activation in hypoxic tubules prevents chronic kidney disease. J Clin Invest 129(6):2374–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP et al (2005) Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280(45):38029–38034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim SW, Jin L, Luo K, Jin J, Shin YJ, Hong SY, Yang CW (2017) Klotho enhances FoxO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell Death Dis 8(8):e2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Lin Q, Shao X, Zhu X, Wu J, Wu B, Zhang M, Zhou W, Zhou Y, Jin H et al (2020) Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free Radic Biol Med 152:632–649 [DOI] [PubMed] [Google Scholar]
- 25.Lin Q, Li S, Jiang N, Jin H, Shao X, Zhu X, Wu J, Zhang M, Zhang Z, Shen J et al (2021) Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy 17(10):2975–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang C, Han H, Liu Z, Liu Y, Yin L, Cai J, He L, Liu Y, Chen G, Zhang Z et al (2019) Activation of BNIP3-mediated mitophagy protects against renal ischemia-reperfusion injury. Cell Death Dis 10(9):677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, Ravikumar P, Gillings N, Bian A, Sidhu SS et al (2016) Renal production, Uptake, and handling of circulating alphaKlotho. J Am Soc Nephrol 27(1):79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu MC (2016) alphaKlotho mitigates progression of AKI to CKD through activation of Autophagy. J Am Soc Nephrol 27(8):2331–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Zeng CY, Li XH, Yang TT, Kuang X, Du JR (2020) Klotho overexpression improves amyloid-beta clearance and cognition in the APP/PS1 mouse model of Alzheimer’s disease. Aging Cell 19(10):e13239 [DOI] [PMC free article] [PubMed]
- 30.Cai H, Zhu X, Lu J, Zhu M, Liu S, Zhan Y, Ni Z, Gu L, Zhang W, Mou S (2021) A decreased level of Soluble Klotho can predict Cardiovascular Death in no or mild abdominal aortic calcification hemodialysis patients. Front Med (Lausanne) 8:672000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu MC, Kuro-o M, Moe OW (2012) The emerging role of Klotho in clinical nephrology. Nephrol Dial Transpl 27(7):2650–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Zhang H, Hu J, Gu Y, Shen Z, Xu L, Jia X, Zhang X, Ding X (2017) Hydrogen-rich saline alleviates kidney fibrosis following AKI and retains Klotho expression. Front Pharmacol 8:499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Tsogbadrakh B, Yang S, Ryu H, Kang E, Kang M, Kang HG, Ahn C, Oh KH (2021) Klotho ameliorates diabetic nephropathy via LKB1-AMPK-PGC1alpha-mediated renal mitochondrial protection. Biochem Biophys Res Commun 534:1040–1046 [DOI] [PubMed] [Google Scholar]
- 34.Miao J, Liu J, Niu J, Zhang Y, Shen W, Luo C, Liu Y, Li C, Li H, Yang P et al (2019) Wnt/beta-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 18(5):e13004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu ZJ, Wang ZY, Xu L, Chen XH, Li XX, Liao WT, Ma HK, Jiang MD, Xu TT, Xu J et al (2020) HIF-1alpha-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol 36:101671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Tang C, Cai J, Chen G, Zhang D, Zhang Z, Dong Z (2018) PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis 9(11):1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Zhu J, Liu Z, Shu S, Fu Y, Liu Y, Cai J, Tang C, Liu Y, Yin X et al (2021) The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol 38:101767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma N, Wei Z, Hu J, Gu W, Ci X (2021) Farrerol ameliorated Cisplatin-Induced chronic kidney Disease through Mitophagy induction via Nrf2/PINK1 pathway. Front Pharmacol 12:768700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Y, Dai X, Li Y, Li G, Lin X, Ai C, Cao Y, Li T, Lin B (2020) Role of parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J Transl Med 18(1):114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afanas’ev I (2010) Reactive oxygen species and age-related genes p66shc, Sirtuin, FOX03 and Klotho in senescence. Oxid Med Cell Longev 3(2):77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu P, Kamboj A, Gibson SB, Anderson CM (2014) Poly(ADP-ribose) polymerase-1 causes mitochondrial damage and neuron death mediated by Bnip3. J Neurosci 34(48):15975–15987 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq datasets during the current study are available in the NCBI Sequence Read Archive database repository under accession code (PRJNA1036425). The additional data that support the findings of this study are available from the corresponding author upon reasonable request.