Abstract
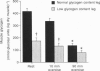
1. The influence of pre-exercise muscle glycogen content on ammonia production, adenine nucleotide breakdown and amino acid metabolism was investigated during prolonged exercise in six subjects having one leg with a normal and one leg with a low muscle glycogen content. One-leg knee-extensor exercise was performed for 90 min, at a workload of 60-65% of the maximal power output, first with one leg and then with the other. 2. During exercise ammonia was released in gradually increasing amounts and plateaued after 1 h exercise at a rate of approximately 80 mumol min-1. The total ammonia production was 9.1 +/- 0.4 and 9.5 +/- 1.4 mmol (kg dry muscle)-1 in the normal and low glycogen content leg, respectively. 3. Levels of muscle phosphocreatine (PC), total adenine nucleotides and inosine monophosphate (IMP) were similar at rest and after 90 min of exercise. 4. Only minor differences were observed between rest and exercise and between legs for the muscle concentrations of glutamine, alanine and the branched-chain amino acids. Muscle glutamate concentration decreased by 60-70% within the first 10 min of exercise. Glutamate consumption over 90 min quantitatively equalled ammonia production. Most of the glutamate was consumed within the first 10 min of exercise, while ammonia production gradually increased during exercise. Therefore deamination of glutamate cannot be the main source of ammonia production during the later stage of exercise. 5. It is concluded that during prolonged one-leg exercise at moderate intensity: (a) ammonia production is not affected by pre-exercise muscle glycogen content, (b) ammonia production exceeds by far the breakdown of adenine nucleotides to IMP and therefore has to be derived from alternative sources, and (c) deamination of amino acids is a likely source of ammonia production during prolonged exercise.
Full text
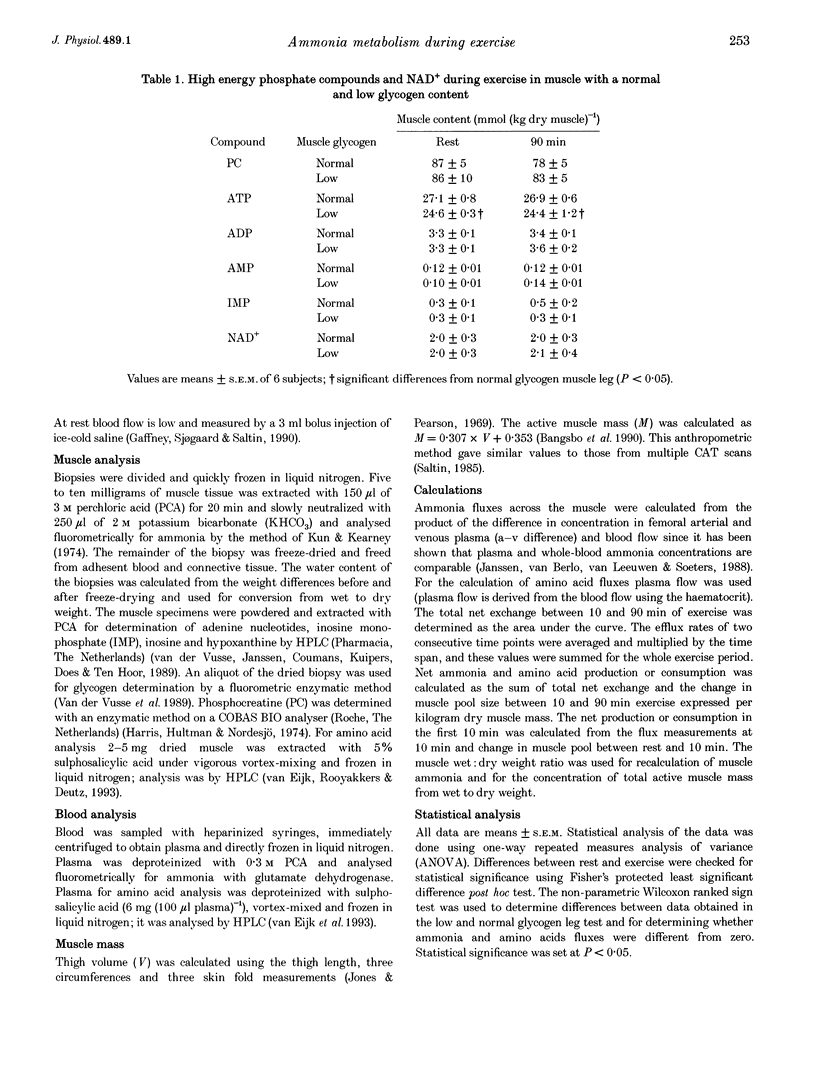
PDF

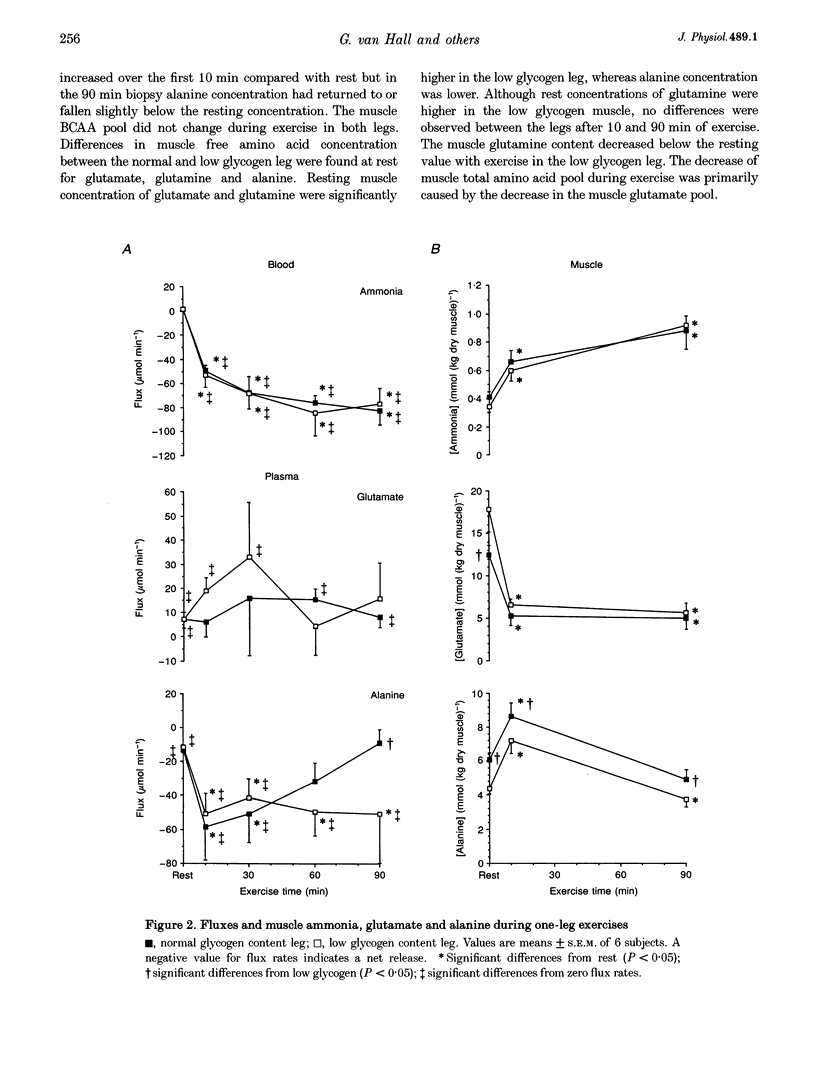
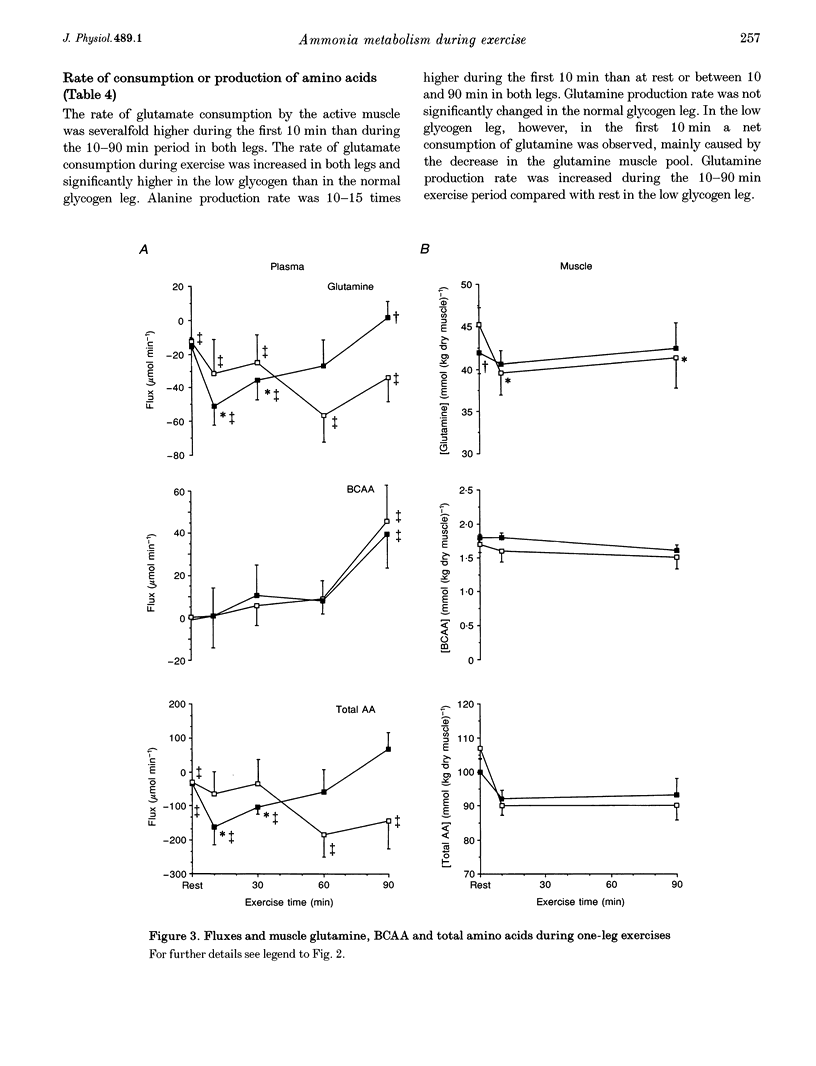
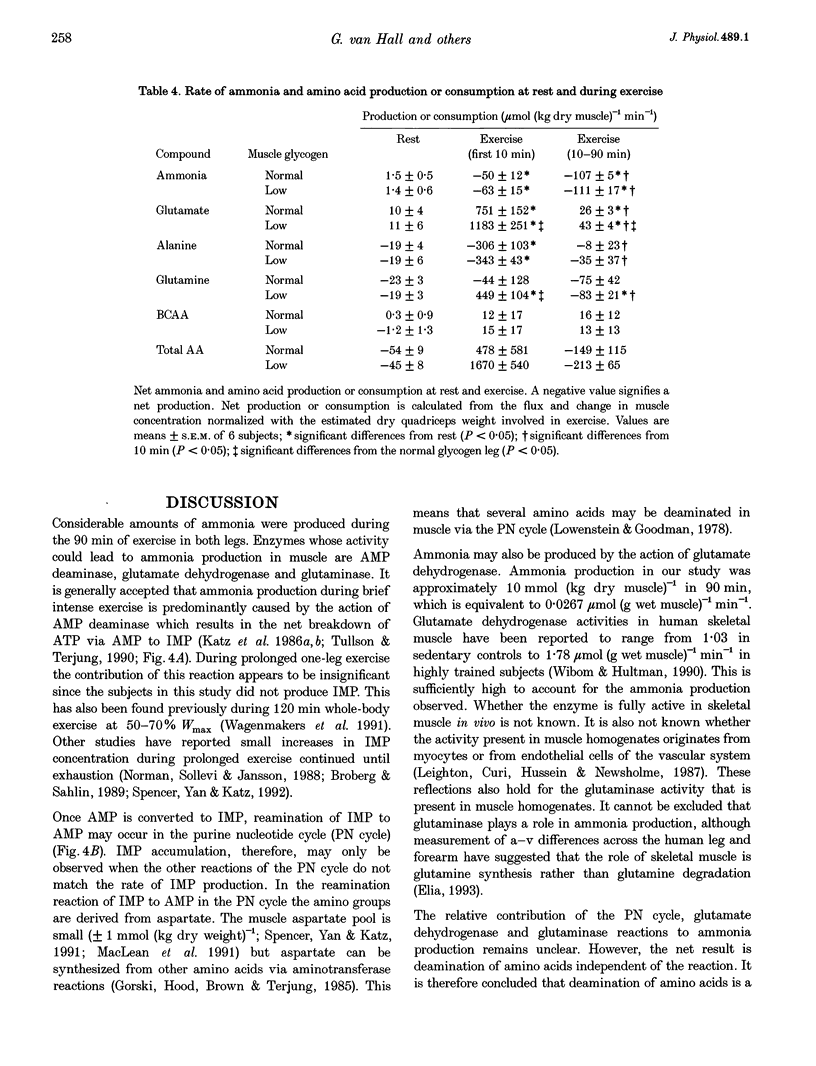



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg G., Felig P., Hagenfeldt L., Hendler R., Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974 Apr;53(4):1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985 Sep;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J., Gollnick P. D., Graham T. E., Juel C., Kiens B., Mizuno M., Saltin B. Anaerobic energy production and O2 deficit-debt relationship during exhaustive exercise in humans. J Physiol. 1990 Mar;422:539–559. doi: 10.1113/jphysiol.1990.sp018000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström J., Fürst P., Hultman E. Free amino acids in muscle tissue and plasma during exercise in man. Clin Physiol. 1985 Apr;5(2):155–160. doi: 10.1111/j.1475-097x.1985.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Broberg S., Sahlin K. Adenine nucleotide degradation in human skeletal muscle during prolonged exercise. J Appl Physiol (1985) 1989 Jul;67(1):116–122. doi: 10.1152/jappl.1989.67.1.116. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The metabolic fates of amino acids and the formation of glutamine in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3685–3693. [PubMed] [Google Scholar]
- Elia M. Glutamine metabolism in human adipose tissue in vivo. Clin Nutr. 1993 Feb;12(1):51–53. doi: 10.1016/0261-5614(93)90148-w. [DOI] [PubMed] [Google Scholar]
- Gaffney F. A., Sjøgaard G., Saltin B. Cardiovascular and metabolic responses to static contraction in man. Acta Physiol Scand. 1990 Mar;138(3):249–258. doi: 10.1111/j.1748-1716.1990.tb08844.x. [DOI] [PubMed] [Google Scholar]
- Gorski J., Hood D. A., Brown O. M., Terjung R. L. Incorporation of 15N-leucine amine into ATP of fast-twitch muscle following stimulation. Biochem Biophys Res Commun. 1985 May 16;128(3):1254–1260. doi: 10.1016/0006-291x(85)91075-7. [DOI] [PubMed] [Google Scholar]
- Graham T. E., Kiens B., Hargreaves M., Richter E. A. Influence of fatty acids on ammonia and amino acid flux from active human muscle. Am J Physiol. 1991 Aug;261(2 Pt 1):E168–E176. doi: 10.1152/ajpendo.1991.261.2.E168. [DOI] [PubMed] [Google Scholar]
- Graham T. E., Pedersen P. K., Saltin B. Muscle and blood ammonia and lactate responses to prolonged exercise with hyperoxia. J Appl Physiol (1985) 1987 Oct;63(4):1457–1462. doi: 10.1152/jappl.1987.63.4.1457. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Nordesjö L. O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974 Apr;33(2):109–120. [PubMed] [Google Scholar]
- Henriksson J. Effect of exercise on amino acid concentrations in skeletal muscle and plasma. J Exp Biol. 1991 Oct;160:149–165. doi: 10.1242/jeb.160.1.149. [DOI] [PubMed] [Google Scholar]
- Jones P. R., Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969 Oct;204(2):63P–66P. [PubMed] [Google Scholar]
- Kasperek G. J., Dohm G. L., Snider R. D. Activation of branched-chain keto acid dehydrogenase by exercise. Am J Physiol. 1985 Feb;248(2 Pt 2):R166–R171. doi: 10.1152/ajpregu.1985.248.2.R166. [DOI] [PubMed] [Google Scholar]
- Katz A., Broberg S., Sahlin K., Wahren J. Muscle ammonia and amino acid metabolism during dynamic exercise in man. Clin Physiol. 1986 Aug;6(4):365–379. doi: 10.1111/j.1475-097x.1986.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Katz A., Sahlin K., Henriksson J. Muscle ammonia metabolism during isometric contraction in humans. Am J Physiol. 1986 Jun;250(6 Pt 1):C834–C840. doi: 10.1152/ajpcell.1986.250.6.C834. [DOI] [PubMed] [Google Scholar]
- Leighton B., Curi R., Hussein A., Newsholme E. A. Maximum activities of some key enzymes of glycolysis, glutaminolysis, Krebs cycle and fatty acid utilization in bovine pulmonary endothelial cells. FEBS Lett. 1987 Dec 10;225(1-2):93–96. doi: 10.1016/0014-5793(87)81137-7. [DOI] [PubMed] [Google Scholar]
- Lowenstein J. M., Goodman M. N. The purine nucleotide cycle in skeletal muscle. Fed Proc. 1978 Jul;37(9):2308–2312. [PubMed] [Google Scholar]
- MacLean D. A., Spriet L. L., Hultman E., Graham T. E. Plasma and muscle amino acid and ammonia responses during prolonged exercise in humans. J Appl Physiol (1985) 1991 May;70(5):2095–2103. doi: 10.1152/jappl.1991.70.5.2095. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Dudley G. A., Terjung R. L. Ammonia and IMP in different skeletal muscle fibers after exercise in rats. J Appl Physiol Respir Environ Exerc Physiol. 1980 Dec;49(6):1037–1041. doi: 10.1152/jappl.1980.49.6.1037. [DOI] [PubMed] [Google Scholar]
- Norman B., Sollevi A., Jansson E. Increased IMP content in glycogen-depleted muscle fibres during submaximal exercise in man. Acta Physiol Scand. 1988 May;133(1):97–100. doi: 10.1111/j.1748-1716.1988.tb08385.x. [DOI] [PubMed] [Google Scholar]
- Norman B., Sollevi A., Kaijser L., Jansson E. ATP breakdown products in human skeletal muscle during prolonged exercise to exhaustion. Clin Physiol. 1987 Dec;7(6):503–510. doi: 10.1111/j.1475-097x.1987.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Katz A., Broberg S. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am J Physiol. 1990 Nov;259(5 Pt 1):C834–C841. doi: 10.1152/ajpcell.1990.259.5.C834. [DOI] [PubMed] [Google Scholar]
- Spencer M. K., Yan Z., Katz A. Carbohydrate supplementation attenuates IMP accumulation in human muscle during prolonged exercise. Am J Physiol. 1991 Jul;261(1 Pt 1):C71–C76. doi: 10.1152/ajpcell.1991.261.1.C71. [DOI] [PubMed] [Google Scholar]
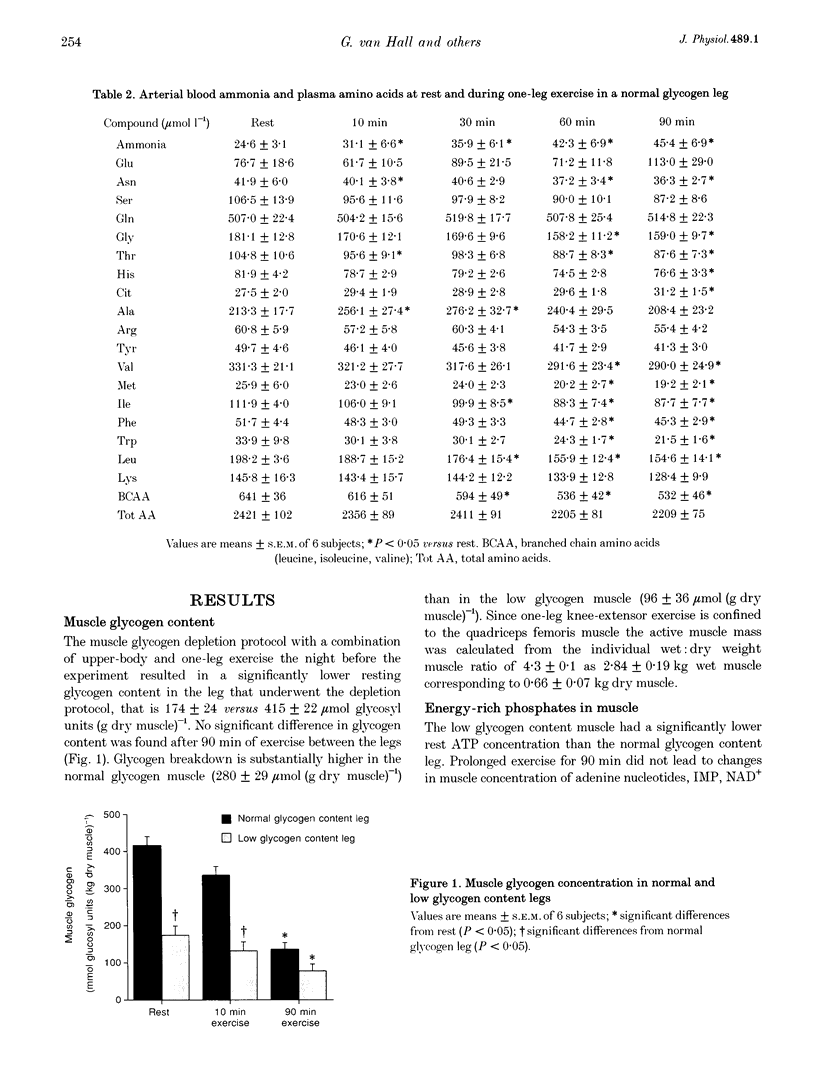
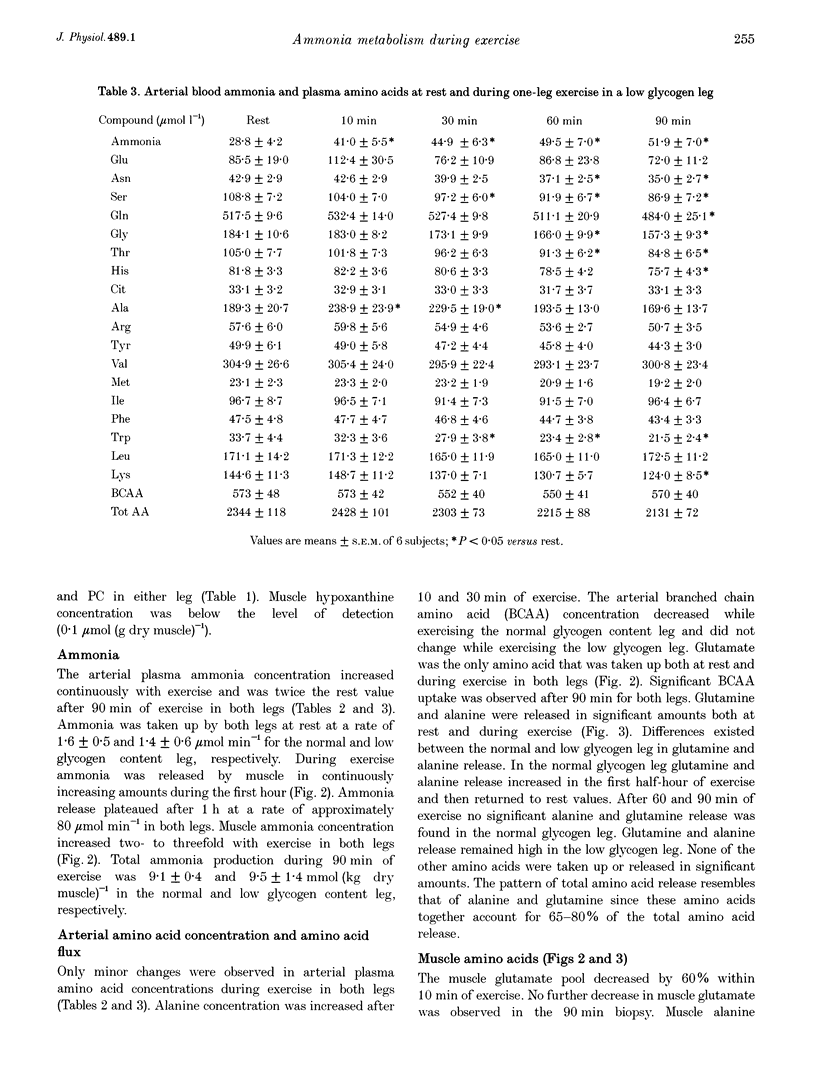
- Spencer M. K., Yan Z., Katz A. Effect of low glycogen on carbohydrate and energy metabolism in human muscle during exercise. Am J Physiol. 1992 Apr;262(4 Pt 1):C975–C979. doi: 10.1152/ajpcell.1992.262.4.C975. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Beckers E. J., Brouns F., Kuipers H., Soeters P. B., van der Vusse G. J., Saris W. H. Carbohydrate supplementation, glycogen depletion, and amino acid metabolism during exercise. Am J Physiol. 1991 Jun;260(6 Pt 1):E883–E890. doi: 10.1152/ajpendo.1991.260.6.E883. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Brookes J. H., Coakley J. H., Reilly T., Edwards R. H. Exercise-induced activation of the branched-chain 2-oxo acid dehydrogenase in human muscle. Eur J Appl Physiol Occup Physiol. 1989;59(3):159–167. doi: 10.1007/BF02386181. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Coakley J. H., Edwards R. H. Metabolism of branched-chain amino acids and ammonia during exercise: clues from McArdle's disease. Int J Sports Med. 1990 May;11 (Suppl 2):S101–S113. doi: 10.1055/s-2007-1024861. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Schepens J. T., Veerkamp J. H. Effect of starvation and exercise on actual and total activity of the branched-chain 2-oxo acid dehydrogenase complex in rat tissues. Biochem J. 1984 Nov 1;223(3):815–821. doi: 10.1042/bj2230815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibom R., Hultman E. ATP production rate in mitochondria isolated from microsamples of human muscle. Am J Physiol. 1990 Aug;259(2 Pt 1):E204–E209. doi: 10.1152/ajpendo.1990.259.2.E204. [DOI] [PubMed] [Google Scholar]
- van Eijk H. M., Rooyakkers D. R., Deutz N. E. Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2-3 microns Spherisorb ODS II column. J Chromatogr. 1993 Oct 22;620(1):143–148. doi: 10.1016/0378-4347(93)80062-9. [DOI] [PubMed] [Google Scholar]