Abstract
Synchronous interpersonal movements induce positive prosocial behaviors in adults and children. The processes that underlie this are debated. Here, we investigate the extent to which visual cues available during synchrony experience—particularly shared facial expressions and mutual eye contact—are necessary. Pairs of same-sex 4-year-olds (N = 216 children; 50% girls; 81% white) from the US were randomly assigned to synchronized versus asynchronized swinging experience. Access to visual information was experimentally manipulated by using a transparent versus translucent barrier between the children. The translucent barrier acted as a visual filter preventing children from monitoring facial cues while still enabling them to see whether the partner was swinging in synchrony. After the swinging experience, all pairs of children were administered the same tests of cooperation. The children administered synchronous movement performed better on the cooperation tasks, and there was no significant difference as a function of barrier transparency. This suggests that the positive effects of synchrony do not require visual resolution of the partner’s social-emotional facial cues. These findings advance our understanding about factors contributing to synchrony-induced cooperation between children.
Keywords: Interpersonal synchrony, Children, Cooperation, Prosocial, Music
Subject terms: Psychology, Human behaviour
Interpersonal behavioral synchrony consists of two or more individuals coordinating their movements in time. The human capacity for synchronizing behaviors is important for social coordination and cooperation in both evolutionarily old and more modern activities—group hunting, interactive art-making (music and dance), interpersonal communication (the timing of gestures and speech), and team activities (rowing, football). Interpersonal synchrony is a cross-cultural universal1, is implicated in parent-child bonding2, has neural correlates3,4, and contributes to human interactions at the macro-sociological level5. Understanding more about the emergence and consequences of interpersonal synchrony will help expand our knowledge about joint actions and human sociality.
Experimental studies have documented that the experience of interpersonal synchrony increases positive social-emotional attitudes and behaviors6–9. This includes helping behavior10, cooperation11–15, perceived similarity/closeness16, and compassion17. These effects have been reported in adults, older children, and in some cases, preschoolers11and infants18,19.
Little is known about the mechanisms by which synchrony experience induces these positive outcomes. At the theoretical level, a first step is to consider several possible components of the synchrony experience: (a) actual bodily movement of the self during the synchrony experience (as opposed to imagined movements); (b) visual experience of other’s movements (as opposed to perceptually isolated individuals moving synchronously); (c) live interaction between the individuals (as opposed to watching through a closed-circuit TV); (d) shared facial information, such as the facial expressions and mutual eye contact (as opposed to when such visual cues are blocked); and (e) self-initiated synchronization (as opposed to being put in synchrony by an external force). This is not an exhaustive list, but it points to several tractable experimental tests.
Based on the research findings to date, it appears that positive prosocial outcomes are induced by synchrony both when participants choose to move in sync with others10,14, and also when participants are randomly assigned to an experimental condition that causes interpersonal rhythmic synchrony (e.g., preschoolers being swung synchronously on a swing set11) and infants being bounced by the movements of an adult18. From these results, it can be inferred that agentive, self-generated synchrony is not a prerequisite for the synchrony-induced positive outcomes in children. There is something deeper about “being in sync with” the other that causes the effects. But what?
In the present study, we sought to further narrow the list of the “effective components” of interpersonal synchrony that induce the positive outcomes. We focused on a set of prominent visual social cues that commonly co-occur with the synchrony experience. When an infant bounces in sync with an adult or when two children swing together and subsequently show increased positive outcomes, this may be due to: (a) the synchronous movement experience itself, (b) increased opportunities to see the other person’s face, and/or (c) third-factor correlates of this experience—seeing positive cues from the synchronously moving person, such as joyful facial expressions, smiles, and mutual eye contact.
We modified a paradigm reported by Rabinowitch and Meltzoff11, in which they randomly assigned preschool children to a synchronous experience (or not) by moving them on a specially designed swing apparatus. In order to experimentally examine whether the behavioral cooperation on subsequent tasks required seeing the other person’s facial expressions during the synchronous movement, we modified the original apparatus by installing a channel that supported either a transparent or translucent barrier between the two children (depending on their experimental random assignment). Our expectation was that the previously reported effects of synchrony would persist with the insertion of the plastic see-through (transparent) barrier, because this was basically a conceptual replication of the original but using a see-through divider separating the children in space. The novel and more informative test was the new manipulation with the translucent barrier. The translucent barrier acted as a visual filter that prevented children from visually resolving the details of each other’s facial expressions and gaze. In this way we could have the peers swing synchronously (or asynchronously) but the block peers from seeing shared facial expressions that may co-occur with the movements (e.g., mutual gaze). The design of this study enables an initial assessment of whether seeing shared social-emotional visual cues during the synchronous experience is necessary for the synchrony-induced increase of children’s cooperation, or whether the experience of rhythmic, synchronous bodily movements between two individuals—even in the absence of visual facial expressions and mutual eye contact—is sufficient.
Following the Rabinowitch and Meltzoff procedures11, child cooperation was assessed by two standardized tasks—a cooperative give-and-take game and a cooperative button-push task, each tapping into a slightly different type of cooperativity (see below). Because Rabinowitch and Meltzoff noticed and measured children’s spontaneous signaling to each other during the tests of cooperation (as the children made efforts to coordinate their behavior), this too was assessed in the present study.
Methods
Participants
Participants were recruited from the University of Washington’s computerized participant pool and tested in a laboratory setting. Pre-established criteria for admission into the study were that the children were 4 years old, had no known developmental concerns or impairments according to parental report, and that the child participants (randomly paired for test) had not previously met each other according to parental report. The final analytic sample consisted of 108 same-sex dyads of 4-year-old children (N = 216 children, Mage = 53.33 months, SD = 2.79). Additional dyads were excluded due to avoidance of the swing apparatus or failure to engage in the experimental tasks by at least one of the participants (n = 6), or a technical failure of the cameras, experimenter headphones, or other equipment (n = 8). According to parental report of demographics, the sample was middle- to upper middle-class, with 81% identifying as White, 16.2% more than one race, 1.4% Asian, 0.5% African American, and 1% not disclosed; 6.2% of the participants identified as being of Hispanic ethnicity. All experimental protocols were approved by the University of Washington’s Human Subjects Institutional Review Board, Application #46757. The methods and procedures were performed in accordance with relevant regulations and in accordance with the Declaration of Helsinki. Prior to the start of testing, informed consent was obtained from all children’s parents or legal guardians.
The chid dyads (N = 108 dyads) were randomly assigned to one of three independent groups: synchrony transparent screen, synchrony translucent screen, and asynchrony translucent screen. There were 36 same-sex dyads in each group, half of which were girl dyads and half boy dyads. All children participated in the swinging episodes followed by the same, standardized assessments of behavioral cooperation (see below for details).
Apparatus for manipulating synchrony
We used Rabinowitch and Meltzoff’s11 apparatus to deliver the experience of synchrony to children in an experimentally controlled manner. Briefly, the apparatus consisted of a specially constructed swing set enabling the moving of the two children in a synchronous or asynchronous manner (see Rabinowitch and Meltzoff11, Fig. 1, p. 24). Black and white striped fabric was positioned on the side walls of the room in the children’s peripheral view to provide a visual reference of movement20.
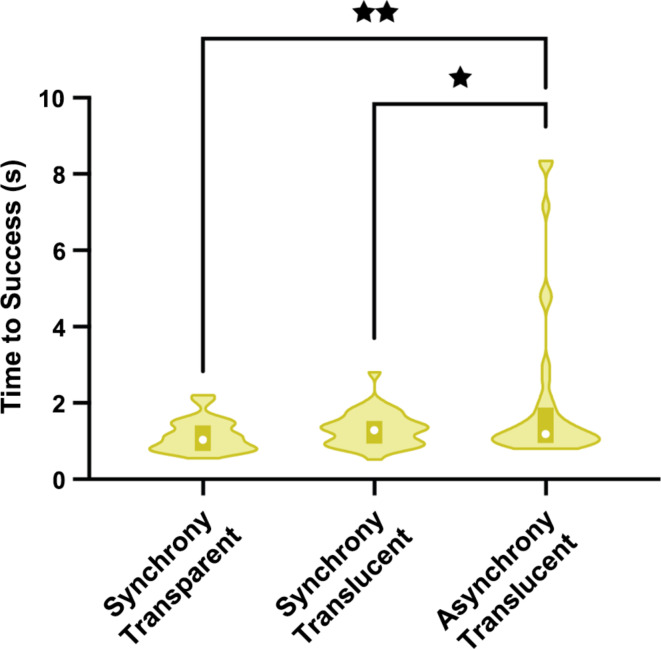
Fig. 1.
Cooperative give-and-take task: Time to success as a function of treatment group. Violin plots showing the duration (in seconds) of time to success as a function of treatment group. Higher values indicate it takes a longer time to succeed on this task, i.e., poorer performance. For each group, the plot spans the data range (lower extent corresponds to minimal value and upper extent to maximal value), with the white circle indicating the median and the bottom and top edges of the shaded rectangle indicating the first and third quartiles, respectively. * p < 0.05. ** p < 0.01.
The novelty of the present study was that a barrier was positioned between the swings, thus separating the two children. For two groups (synchronous and asynchronous) the barrier was translucent, allowing participants to see large blurry shapes and movement but eliminating fine visual details (e.g., facial expressions, mutual eye contact). The translucent barrier acted as a filter that created visual acuity worse than 20/2700 (Snellen equivalent) as measured by Teller Acuity Cards® (Vistech Consultants, Woodstock, Ill) with an adult viewer. For the third group (synchronous transparent), the barrier was transparent Plexiglass.
Two trained musicians, each with more than 10 years of training, pushed the swings according to a specified cycle time, which was transmitted via headphones to the musicians. This apparatus prevented children from hearing the audio cues. Using this same procedure, Rabinowitch and Meltzoff11 had validated that trained musicians were able to produce synchronized swinging, adhering to the predetermined cycle times with minimal errors in timing, mean error = 0.01 s and small standard deviations (see Rabinowitch and Meltzoff11 p. 25 for measurement details).
In the synchronous groups, the child peers swung in unison, moving at the same rate and in phase with each other, at a cycle time of either 2.0 s or 2.6 s determined by random assignment. In the asynchronous group, one child in the dyad (randomly selected) was swung at a cycle time of 2.0 s and the other at a 2.6 s cycle time. (In other words, in the asynchronous group the two children in a dyad were swung at different frequencies from each other.) The children were not given specific instructions on how to behave while swinging. With parental permission, a video camera recorded the session.
Apparatus for assessing cooperation
Cooperative give-and-take task
We used the Cooperative Give-and-Take task from Rabinowitch and Meltzoff11, which itself was adapted from Warneken et al21. The task was administered twice (see “Overall Procedure” below), each time using a different set of objects. Briefly, each trial required the children to pass objects from one to the other through a hole in the apparatus. One set of objects was four red tubes, and the other was four clear tubes containing chocolate M&M candy, hereafter called “food tubes” (used to assess possible effects of motivation on cooperation). Child participants were positioned at opposite sides of the device, so that one child (“Giver”) could access the hole from the bottom (a Plexiglass screen blocked direct access) and the other child (“Taker”) could access the hole from the top. Each child was given a black bucket (12.5 cm diameter). The children were told that the Giver had to sequentially pass the items through the hole and the Taker had to retrieve these objects as they were handed up through the hole and then place each in his/her own bucket. The children were asked to execute this task as quick as they could.
Cooperative button press task
We used the Cooperative Button-Press task from Rabinowitch and Meltzoff11. This task itself was an adaptation of a game described in Brownell et al.22, modified to become a computer-controlled procedure that allowed a quantitative measurement of children attempting to coordinate their behavior. The children were told that they would see an interesting display if they both pushed buttons in synchrony (see below for more detail). The task consisted of pressing two white Plexiglas (13 × 9 cm) button-panels, which triggered an image and sound on a computer screen directly above and behind the button-panels. Performance was evaluated as the number of attempts until success (success = simultaneously pressing a button with one’s partner). More specifically, the screen showed a digital movie of sequentially appearing boxes; and in order for the top of the box to open and a figure to pop up, both children had to depress their buttons simultaneously. This required the child peers to coordinate their actions to achieve the goal. Adopting Rabinowitch and Meltzoff’s criteria11, “simultaneity” was defined as ≤ 80 ms difference between the two participants’ button pushing, which was determined online by software (Inquisit 4). Simultaneous button-presses (hereafter “successful completion” of the task) triggered the figure popping-up, followed by a 3 s tune. A different brief tone was triggered in instances when a child pressed the button at a time that was not simultaneous with their partner (hereafter “non-simultaneous presses”). A camera, directly behind and above of the computer screen, recorded the behavior of the child peers.
Design
Using an independent groups design, pairs of same-sex children who had never before met were randomly assigned to one of three experimental groups, with n = 36 dyads per group: (a) Synchronous translucent group (synchronous movement experience with a translucent barrier), (b) Asynchronous translucent group (asynchronous movement experience with a translucent barrier), or (c) Synchronous transparent group (synchronous movement experience with a transparent barrier). The design thus called for N = 216 children. We did not choose to include a fourth group, which could have been children who experienced asynchronous movement with a transparent barrier. This was not conducted because: (a) of the heavy demand of recruiting same-age, same-sex peers who could come into the laboratory at the same time, and (b) there was no theoretical reason to think that performance would differ from what was originally reported by Rabinowitch and Meltzoff11. That study had demonstrated that there was less cooperation after the asynchronous experience when peers could see each other, and thus low cooperation would again be expected if we re-ran the asynchronous condition with the fully transparent screen. Given the practical difficulties of recruiting the dyads, we chose not to run this condition.
Overall procedure
There were three phases to the procedure that unfolded in the following order: (a) demonstration of a cooperation task by the experimenters for the children, (b) swinging treatment (2.5 min), and (c) a test period assessing children’s performance on the cooperation task. Children underwent these three phases for one cooperation task (e.g., Cooperative Button Pressing) followed by the same phases for another behavioral task (e.g., Give-and-Take with red tubes), followed by the same phases for the Give-and-Take task with food tubes. The order of the Cooperative Button Pressing and Give-and-Take with red tubes tasks were counterbalanced; the Give-and-Take with food tubes task was presented last (see below for rationale).
Each cooperation task was verbally explained and demonstrated prior to a swinging treatment in order to reduce the delay between the treatment and the test of the cooperation tasks. For the test of cooperation, the children simply dismounted from the swings and immediately participated in the behavioral cooperation assessment, because the task directions had already been explained to them. Dyad members of all groups were briefly introduced to each other by first name at the start of the experiment. There was no other rapport-building phase.
Cooperative give-and-take procedure
For the demonstration of this task, the experimenters showed the children how the tubes could be passed through the hole from beneath the tabletop surface and retrieved. As the children watched, Experimenter 1 (“Giver”) picked each of the four red tubes from her bucket and passed it under the tabletop surface and through the hole to Experimenter 2 (“Taker”) who retrieved the toy and put it in her bucket. The same demonstration was later done for the four food tubes. The children were not allowed to touch or handle the toys or try the game during this explanation period. Next, the children received the swinging treatment. After they dismounted from the swing, the experimenters reminded the children that the Giver needed to pass the toys through the hole one at a time to the Taker, and the children were asked to perform the task as quickly as they could. The roles of the Giver and Taker were assigned randomly across dyads. For the red plastic tubes, all dyads except one successfully transferred all four tubes, resulting in one missing trial of 432 possible trials (108 dyads x 4 red tube trials each). For the food tube trials, there were 4.63% missing trials of the 432. The rationale for testing the food tubes last was that this task was an addition to the Rabinowitch and Meltzoff11 study, and we sought to keep the procedures as similar as possible. In addition, we were not sure how the children would handle the food tubes. For example, we were concerned that the children could become so distracted by the food that they might ignore the specific task directions and try to open the tubes to get at the treats. Alternatively, the receiving child might stop the game once they received the first tube-with-food to eat the candy.
Cooperative button-press procedure
For the demonstration of this task, the two adult experimenters sat at the table where the apparatus (buttons and computer screen) was placed, and the children watched. Experimenter 1 explained to the children how to play the game. Then, Experimenters 1 and 2 each pressed their own buttons, showing that simultaneous button pushing led to the visual event. Following this, children were informed where they would sit (in the chairs in which the adults now sat), and which button each would use. Importantly, the children did not try the game themselves during the demonstration—they had no hands-on experience or a chance to practice before they were administered the swinging treatment. Immediately after the swinging treatment and prior to testing the cooperation behavior, the experimenters reminded the children that they needed to press their buttons together. The experimental task then began, and children performed the task for a block of four trials (using a different animated pop-up figure for each trial). For each trial, the children were allowed to push their buttons repeatedly until “successful completion” (defined as both children pushed the buttons simultaneously). Two dyads did not finish this task and were included in the analytic sample only for the tasks they completed.
Scoring and dependent measures
Cooperative give-and-take task
This measure of successful cooperation was scored from the video recordings. Time to success was defined as the latency between the time when each object became visible in the hole to when the Taker successfully grabbed it. Each dyad received a mean score for their time to success (in seconds) for the red tubes and also for the food tubes. The data were scored from the video records by coders who were blind to the children’s treatment group. Scoring agreement was calculated using a random sample of 20% of the trials. The intraclass correlation coefficients (two-way mixed model for absolute agreement) were high for interscorer agreement for both the red tubes and the food tubes (respectively, 0.95 and 0.99).
Cooperative button-press behavior
The dependent measure was the number of non-simultaneous button presses preceding the first simultaneous (“successful”) button press during each of the four trials (i.e., for each of the four boxes that appeared on the screen). This measure was objectively obtained using the computer software recording (Inquisit 4). The number of non-simultaneous button presses from both children for each trial was averaged across the four test trials (each trial ended with the first simultaneous button push).
Communicative signaling during the button-press task
Rabinowitch and Meltzoff11reported that children often used an interesting strategy to synchronize their button presses with each other. The strategy was never modeled to them and arose spontaneously within many of the dyads: Children would signal their intention to press the button by saliently and prominently lifting their hand (defined as > 5 cm) above the button as if showing it to their partner before moving it down to hit the button. In the present study, coders who were blind to the children’s treatment group, scored the hand-lift signaling behavior following the Rabinowitch and Meltzoff11 criteria. As in the previous study, dyads were dichotomously categorized as being either “high signaling” dyads if both children signaled on all four trials, or “low signaling” dyads otherwise. The scoring agreement was assessed using kappa for a random sample of 19% of the trials. The inter-scorer agreement was k = 0.99.
Looking behavior
Coders who were blind to the children’s cooperation behaviors scored children’s looking from the video records to determine the times (at the level of video frames) during each 2.5 min swinging treatment that children looked at the face of the other child. The duration of looking was the dependent measure submitted to analysis. Scoring agreement was calculated using a random sample of 20% of the trials. The intraclass correlation coefficient (two-way mixed model for absolute agreement) was 0.97 for the inter-scorer agreement. One video could not be scored because of a technical failure in the video processing.
Results
Preliminary analyses
We evaluated whether dyad sex composition and/or task order had an effect on cooperation scores. Analyses of variance (ANOVAs) showed that dyad sex was not a significant factor as either a main effect or interaction, all ps > 0.10. Task order was also not a significant factor as a main effect or interaction, all ps > 0.10. Therefore, the analyses reported below collapse across these factors. All analyses in this paper used two-tailed tests.
Cooperative give-and-take task
A 3 (treatment) × 2 (tube item type: red vs. food) mixed ANOVA with treatment as the between-subjects factor and tube item type as the within-subjects factor revealed a significant main effect of treatment, F(2, 104) = 5.56, p = 0.005, partial η2 = 0.10. As shown in Fig. 1, follow-up pairwise comparisons (Tukey HSD) showed that dyads in the asynchronous translucent group required significantly longer time to successfully transfer objects than did the synchronous translucent group (p = 0.030) and the synchronous transparent group (p = 0.007), whereas the two synchronous groups did not differ in their time to success (p = 0.849). The main effect of tube item type and its interaction (treatment × tube item type) were not significant, respectively: F(1, 104) = 0.004, p = 0.950, partial η2 = 0.00; F(2, 104) = 0.29, p = 0.749, partial η2 = 0.01.
Cooperative button press task
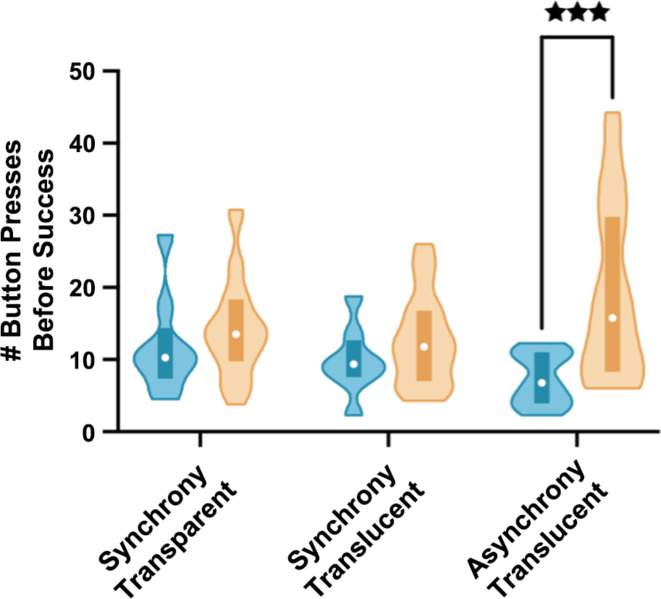
In this study, the classification of dyads into high versus low levels of communicative signaling (see “Scoring” for details) did not significantly vary as a function of treatment, χ2(2, N = 106) = 1.05, p = 0.591, with signaling levels similarly distributed across treatments, and this allowed us to use it as a factor in the ANOVA. A 3 (treatment) × 2 (signaling: high vs. low) ANOVA showed a significant main effect of signaling on the number of button presses before success, F(2, 100) = 11.02, p = 0.0013, partial η2 = 0.10. Across treatment groups, high signaling dyads needed significantly fewer button presses before success (M = 9.37, SD = 5.30, n = 29) than low signaling dyads (M = 15.29, SD = 9.03, n = 77). The main effect of treatment was not significant, F(2, 100) = 0.50, p = 0.609, ηp2 = 0.01; but the treatment × signaling interaction was significant, F(2, 100) = 3.42, p = 0.036, ηp2 = 0.06. As shown in Fig. 2, the follow-up pairwise comparisons within each treatment group revealed that within the synchronous treatment groups there was no significant difference as a function of signaling, ps > 0.24; but within the asynchronous translucent group, the low signaling dyads performed very poorly and required many more tries (M = 19.27, n = 24) before they succeeded in achieving a simultaneous button press than did the high signaling dyads (M = 7.25, n = 12), t(34) = 4.44, p = 0.0001, d = 1.17, which is interpreted in the “Discussion” section below.
Fig. 2.
Cooperative Button Press Task: Number of non-simultaneous button presses before success as a function of treatment and dyad signaling level. Violin plots showing the number of non-simultaneous button presses before success as a function of treatment group and dyad signaling level (blue color indicates a high level of dyad signaling and orange color indicates a low level of dyad signaling). Higher numbers of button presses indicate poorer performance. Plots span the data range (lower extent corresponds to minimal value and upper extent to maximal value), with the white circle indicating the median and the bottom and top edges of the shaded rectangle indicating the first and third quartiles, respectively. *** p = 0.0001.
Children’s looking
The key difference between the transparent and translucent barriers is the visibility of the partner—the translucent barrier limits the child’s ability to visually resolve the internal details of the peer-child’s face, their direction of eye gaze, and emotional expressions. As expected, a one-way ANOVA showed that looking time towards the partner’s face significantly varied as a function of groups, F(2,104) = 106.53, p < 0.0001, ηp2 = 0.67. Pairwise comparisons (Tukey HSD) revealed that children in the synchrony transparent group looked about ten times longer at the partner’s face (M = 33.62 s, SD = 17.00) than in either the synchrony translucent group (M = 3.84 s, SD = 5.13), p < 0.00001, or the asynchrony translucent group (M = 1.91 s, SD = 2.06), p < 0.00001. There were no other significant differences (ps > 0.70).
These results were expected inasmuch as the translucent conditions were specifically designed to exclude visibility of the details of the partner’s face, but the significant differences in looking data as a function of translucency/transparency are highly informative, as discussed next. (For completeness, we also note that across groups there were no significant correlations between children’s looking during swinging and the task scores: give-and-take task: r = -0.14, p = 0.152 and cooperative button press task: r = 0.05, p = 0.581)
Discussion
An experimental intervention that provided controlled experience of synchronous swinging enhanced cooperation in 4-year-old children compared to asynchronous swinging experience. The impact of interpersonal synchrony on cooperation was similar regardless of whether there was a detailed view of the synchronized partner (transparent barrier) or a blurry view that eliminated resolution of facial expressions and eye gaze of the partner (translucent barrier). This enhanced cooperativity was obtained even though we also documented a (predicted) difference in looking time to the partner’s face: As expected, in the transparent barrier group there was a high level of looking at the partner’s face (M = 33.62 s), whereas for the translucent barrier groups, in which the facial features could not be visually resolved, there was minimal looking at the partner’s face (M < 4 s), confirming that the translucent barrier worked as designed. Evidently, swinging in synchrony with another person has a positive effect on subsequent cooperative behavior towards that other person, and this does not require seeing your partner’s emotional facial expressions or mutual gaze during the synchrony experience.
This is an interesting combination of effects, because vision clearly provides a rich source of information in a wide range of social interaction tasks23–26. For example, in adult musical performances, detailed visual information provided by the conductor is helpful27; and shared visual information between vocalists while singing has a positive effect on the precision of vocal synchronization28 (but see29).
As appealing as it is that visible information about mutual gaze and/or positive facial expressions might be a necessary condition for the social effects of synchrony in child dyads, the results do not support this hypothesis under the present conditions. Rather, we demonstrated with the translucent barrier that perceiving global movements of the body in space, without being able to resolve the mutuality of facial expressions and eye contact, is sufficient for children to benefit from the interpersonal rhythmic synchrony. Moreover, the current results, like others11,18,30 demonstrate that self-initiated synchronous movements—the type typically found in dancing, singing, music-performance, and human couples walking—is a not a necessary prerequisite either. In the present experiment, the children did not freely initiate interpersonal synchronization through their own agency, but rather were put into synchrony by the adult pushing their swing. Not only were children’s movements synchronized outside of their own choosing, but children were randomly assigned to be moved in synchrony with a stranger (a child that they had never met before coming to the laboratory). It is also interesting to note that the effects of non-self-initiated synchronous movements is rarely tested in adult studies. This raises the possibility that self-initiation of movement may actually be less important for children and infants, who are used to being moved around by others, than it is for adults. Based on the current findings, it would be interesting to do research with adults to discover whether positive effects of non-self-initiated synchronous movement could also be found at older age groups.
Two further points about the present data patterns bear discussion. First, we note that in the Button-Press task, children spontaneously adopted hand signaling to facilitate task execution (raising and holding one’s hand above the button-panel as if to communicate about the simultaneous button press with one’s partner). In the ANOVA results, we observed a main effect of signaling, such that dyads who engaged in high signaling had better coordinated performance than low signalers, which makes sense because the high signalers provided visual information to each other about how to synchronize their efforts to strike the button at the same time. There was also an interaction between treatment group and signaling on the number of unsuccessful efforts prior to achieving success. Speculatively, we interpret this interaction to mean that without the experience of interpersonal synchrony, children who did not signal to one another were particularly poor in being able to time their actions to be coordinated with the actions with the peer partner. We acknowledge that further research will be needed to fully unpack the interaction.
A further result also bears discussion. We found in the Give-and-Take task that the synchrony experience led to better performance regardless of the type of object used in the task, red tubes versus transparent tubes filled with desirable food (M & M candy in the tubes). The task of transferring candy could have elicited higher motivation, or conversely could have produced a reluctance to pass on the candy to the partner child, interfering with performance. Prior studies have indicated that young children working together on a common resource can collaborate both with toy and food rewards by the age of 331, and studies with toddlers show a willingness to altruistically share desirable food at similar levels as they share non-food items under certain circumstances32. With these studies in mind, we wished to explore whether the type of object to be shared would make a difference for young children’s cooperation in the context of interpersonal behavioral synchrony. We found cooperative sharing regardless of whether the red tubes or the food tubes were used. That said, it is also worth noting that given the demographics of the participants in this study, the children are unlikely to have experienced food insecurity, and they may not have been so hungry as to want to seize the candy for themselves. Further studies systematically varying the perceived “value” of the objects to-be-shared with others in the cooperation task could be done (for example, see32).
Taking all of this together leads us to consider a more overarching theoretical question. We have found that seeing a partner’s facial expressions and self-initiation of the synchronous movement, are not necessary prerequisites for synchrony-induced cooperation effects. What then are the processes that underlie the effects of synchrony? It is possible that the experience of moving together in time extends beyond a sense of physical connectivity (movement-as-one), into a feeling of the dyad being connected psychologically and sharing in non-physical ways as well. If so, it may not be crucial whether or not one is able to visually detect a partner’s facial expressions and mutual gaze cues, or even whether partners have agentive control over their own movements. Rather, the experience of being together in time—literally “in sync”—may be enough to bind people together and foster greater connectivity and cooperation. This “in sync”—or in this case “in swing”—experience may increase peoples’ motivation to collaborate, their attitudes towards one another, and/or their capacity for collaborative success. An example of this latter point is that the experience of rhythmically swinging in time together may have helped children adopt a shared rhythm for smoothly and rapidly giving-and-taking toys from one another on that task.
We acknowledge that the pattern of findings in the present study moves us along the path of ruling out certain factors as necessary (e.g., mutual eye contact), but that additional experiments systematically varying other factors will be needed to better further isolate the social, cognitive, and neural processes that convert the experience of interpersonal synchrony into enhanced cooperative behaviors. It also remains to be determined whether seeing the partner’s face is similarly unnecessary for other synchrony-induced enhancements—such as for helping behavior or for feeling oneself to be closer to or more “like”33 the synchronized interacting partner.
In conclusion, a solid body of research in both children and adults has demonstrated that interpersonal synchrony enhances diverse facets of social interaction11,12,14,18,34–36. How this occurs and what features of the synchrony experience are required to induce subsequent positive behavior towards a partner raises tractable questions for future scientific research. The present study sheds light on this question by ruling out visual facial cues and mutual gaze as necessary, at least in the context of cooperation between pairs of young children. It will be important to establish which components of the synchrony experience—including the proprioceptive feeling of one’s own body movement, perception of the other’s movements, and physical proximity between self and other—contribute to enhancing the positive outcomes. The present study provides a paradigm and initial data that add to a burgeoning line of research designed to explore the bases, scope, development, and consequences of childhood interpersonal synchrony under experimental control.
Acknowledgements
This research was supported by the Templeton Foundation (44076), the Israel Science Foundation (100008920) to T.C.R, and the Bezos Family Foundation to A.N.M.
Author contributions
T.C.R. and A.N.M. conceptualized the study and wrote the original manuscript. T.C.R. performed the study. T.C.R. and R.B. analyzed the data. All of the authors participated in reviewing and editing the manuscript.
Data availability
Datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clayton, M. et al. Interpersonal entrainment in music performance: theory, method, and model. Music Percept.38, 136–194. 10.1525/mp.2020.38.2.136 (2020). [Google Scholar]
- 2.Feldman, R. Parent–infant synchrony and the construction of shared timing; Physiological precursors, developmental outcomes, and risk conditions. J. Child. Psychol. Psychiatry48, 329–354. 10.1111/j.1469-7610.2006.01701.x (2007). [DOI] [PubMed] [Google Scholar]
- 3.Konvalinka, I. et al. Frontal alpha oscillations distinguish leaders from followers: Multivariate decoding of mutually interacting brains. NeuroImage94, 79–88. 10.1016/j.neuroimage.2014.03.003 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Bosseler, A. N. et al. Infants’ brain responses to social interaction predict future language growth. Curr. Biol.34, 1731–1738. 10.1016/j.cub.2024.03.020 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durkheim, E. The Elementary Forms of the Religious Life (Allen & Unwin, 1915).
- 6.Vicaria, I. M. & Dickens, L. Meta-analyses of the intra- and interpersonal outcomes of interpersonal coordination. J. Nonverbal Behav.40, 335–361. 10.1007/s10919-016-0238-8 (2016). [Google Scholar]
- 7.Cross, L., Turgeon, M. & Atherton, G. How moving together binds us together: The social consequences of interpersonal entrainment and group processes. Open Psychol.1, 273–302. 10.1515/psych-2018-0018 (2019). [Google Scholar]
- 8.Mogan, R., Fischer, R. & Bulbulia, J. A. To be in synchrony or not? A meta-analysis of synchrony’s effects on behavior, perception, cognition and affect. J. Exp. Soc. Psychol.72, 13–20. 10.1016/j.jesp.2017.03.009 (2017). [Google Scholar]
- 9.Rennung, M. & Göritz, A. S. Prosocial consequences of interpersonal synchrony. Z. Für Psychol.224, 168–189. 10.1027/2151-2604/a000252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tunçgenç, B. & Cohen, E. Interpersonal movement synchrony facilitates pro-social behavior in children’s peer-play. Dev. Sci.21, e12505. 10.1111/desc.12505 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Rabinowitch, T. C. & Meltzoff, A. N. Synchronized movement experience enhances peer cooperation in preschool children. J. Exp. Child. Psychol.160, 21–32. 10.1016/j.jecp.2017.03.001 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Reddish, P., Fischer, R. & Bulbulia, J. Let’s dance together: Synchrony, shared intentionality and cooperation. PLOS ONE8, e71182. 10.1371/journal.pone.0071182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valdesolo, P., Ouyang, J. & DeSteno, D. The rhythm of joint action: Synchrony promotes cooperative ability. J. Exp. Soc. Psychol.46, 693–695. 10.1016/j.jesp.2010.03.004 (2010). [Google Scholar]
- 14.Wiltermuth, S. S. & Heath, C. Synchrony and cooperation. Psychol. Sci.20, 1–5. 10.1111/j.1467-9280.2008.02253.x (2009). [DOI] [PubMed] [Google Scholar]
- 15.Kirschner, S. & Tomasello, M. Joint music making promotes prosocial behavior in 4-year-old children. Evol. Hum. Behav.31, 354–364. 10.1016/j.evolhumbehav.2010.04.004 (2010). [Google Scholar]
- 16.Rabinowitch, T. C. & Knafo-Noam, A. Synchronous rhythmic interaction enhances children’s perceived similarity and closeness towards each other. PLOS ONE10, e0120878. 10.1371/journal.pone.0120878 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdesolo, P. & DeSteno, D. Synchrony and the social tuning of compassion. Emotion11, 262–266. 10.1037/a0021302 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Cirelli, L. K., Einarson, K. M. & Trainor, L. J. Interpersonal synchrony increases prosocial behavior in infants. Dev. Sci.17, 1003–1011. 10.1111/desc.12193 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Cirelli, L. K., Wan, S. J. & Trainor, L. J. Fourteen-month-old infants use interpersonal synchrony as a cue to direct helpfulness. Philos. Trans. R Soc. B Biol. Sci.369, 20130400. 10.1098/rstb.2013.0400 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Held, R. & Hein, A. Movement-produced stimulation in the development of visually guided behavior. J. Comp. Physiol. Psychol.56, 872–876. 10.1037/h0040546 (1963). [DOI] [PubMed] [Google Scholar]
- 21.Warneken, F., Gräfenhain, M. & Tomasello, M. Collaborative partner or social tool? New evidence for young children’s understanding of joint intentions in collaborative activities. Dev. Sci.15, 54–61. 10.1111/j.1467-7687.2011.01107.x (2012). [DOI] [PubMed] [Google Scholar]
- 22.Brownell, C. A., Ramani, G. B. & Zerwas, S. Becoming a social partner with peers: Cooperation and social understanding in one- and two-year-olds. Child. Dev.77, 803–821. 10.1111/j.1467-8624.2006.t01-1-.x-i1 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks, R. & Meltzoff, A. N. Connecting the dots from infancy to childhood: a longitudinal study connecting gaze following, language, and explicit theory of mind. J. Exp. Child. Psychol.130, 67–78. 10.1016/j.jecp.2014.09.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doherty-Sneddon, G. & Kent, G. Visual signals and the communication abilities of children. J. Child. Psychol. Psychiatry37, 949–959. 10.1111/j.1469-7610.1996.tb01492.x (1996). [DOI] [PubMed] [Google Scholar]
- 25.Klein, J. T., Shepherd, S. V. & Platt, M. L. Social attention and the brain. Curr. Biol.19, R958–R962. 10.1016/j.cub.2009.08.010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Repacholi, B. M., Meltzoff, A. N., Toub, T. S. & Ruba, A. L. Infants’ generalizations about other people’s emotions: Foundations for trait-like attributions. Dev. Psychol.52, 364–378. 10.1037/dev0000097 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Fredrickson, W. E. Band musicians’ performance and eye contact as influenced by loss of a visual and/or aural simulus. J. Res. Music Educ.42, 306–317. 10.2307/3345738 (1994). [Google Scholar]
- 28.D’Amario, S., Daffern, H. & Bailes, F. Synchronization in singing duo performances: The roles of visual contact and leadership instruction. Front. Psychol.9, 1208. 10.3389/fpsyg.2018.01208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawase, S. Gazing behavior and coordination during piano duo performance. Atten. Percept. Psychophys.76, 527–540. 10.3758/s13414-013-0568-0 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Rabinowitch, T. C. & Meltzoff, A. N. Joint rhythmic movement increases 4-year-old children’s prosocial sharing and fairness toward peers. Front. Psychol.8, 1050. 10.3389/fpsyg.2017.01050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warneken, F., Lohse, K., Melis, A. P. & Tomasello, M. Young children share the spoils after collaboration. Psychol. Sci.22, 267–273. 10.1177/0956797610395392 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Barragan, R. C., Brooks, R. & Meltzoff, A. N. Altruistic food sharing behavior by human infants after a hunger manipulation. Sci. Rep.10, 1785. 10.1038/s41598-020-58645-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meltzoff, A. N. Origins of social cognition: Bidirectional self-other mapping and the like-me hypothesis. In Navigating the Social World: What Infants, Children, and Other Species Can Teach Us (eds Banaji, M. & Gelman, S.) 139–144 (Oxford University Press, 2013). 10.1093/acprof:oso/9780199890712.003.0025.
- 34.Abraham, R., Grinspun, N. & Rabinowitch, T. C. Children’s perception of interpersonal coordination during joint painting. Sci. Rep.12, 18897. 10.1038/s41598-022-22516-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hove, M. J. & Risen, J. L. It’s all in the timing: Interpersonal synchrony increases affiliation. Soc. Cogn.27, 949–960. 10.1521/soco.2009.27.6.949 (2009). [Google Scholar]
- 36.Miles, L. K., Nind, L. K. & Macrae, C. N. The rhythm of rapport: Interpersonal synchrony and social perception. J. Exp. Soc. Psychol.45, 585–589. 10.1016/j.jesp.2009.02.002 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.