Abstract
The integration of trifluoromethyl groups and three-dimensional quaternary carbon moieties into organic molecules has emerged as a prominent strategy in medicinal chemistry to augment drug efficacy. Although trifluoromethyl (hetero)aromatic amines and derivatives are prevalent frameworks in pharmaceuticals, the development of trifluoromethyl-embedded, intricately structured alkyl amine scaffolds for medicinal research remains a significant challenge. Herein, we present a metallaphotoredox multicomponent amination strategy employing 3,3,3-trifluoropropene, nitroarenes, tertiary alkylamines, and carboxylic acids. This synthetic pathway offers notable advantages, including the accessibility and cost-effectiveness of starting materials, high levels of chemo- and regioselectivity, and modularity. Furthermore, this approach enables the synthesis of a broad spectrum of aniline compounds featuring both trifluoromethyl group and distal quaternary carbon motifs along the aliphatic chains. The accelerated access to such elaborate N-trifluoroalkyl anilines likely involves three sequential radical-mediated coupling events, providing insightful implications for the retrosynthesis of potential compounds in organic synthesis and drug discovery.
Subject terms: Synthetic chemistry methodology, Photocatalysis, Synthetic chemistry methodology
The trifluoromethyl group can confer advantageous properties to biologically relevant molecules, but methods for its incorporation into aliphatic carbons that are more embedded within the core of the structure are less available. Here, the authors present a multicomponent coupling to synthesize carbon skeletons with a branched trifluoromethyl group via iridium metallaphotoredox catalysis.
Introduction
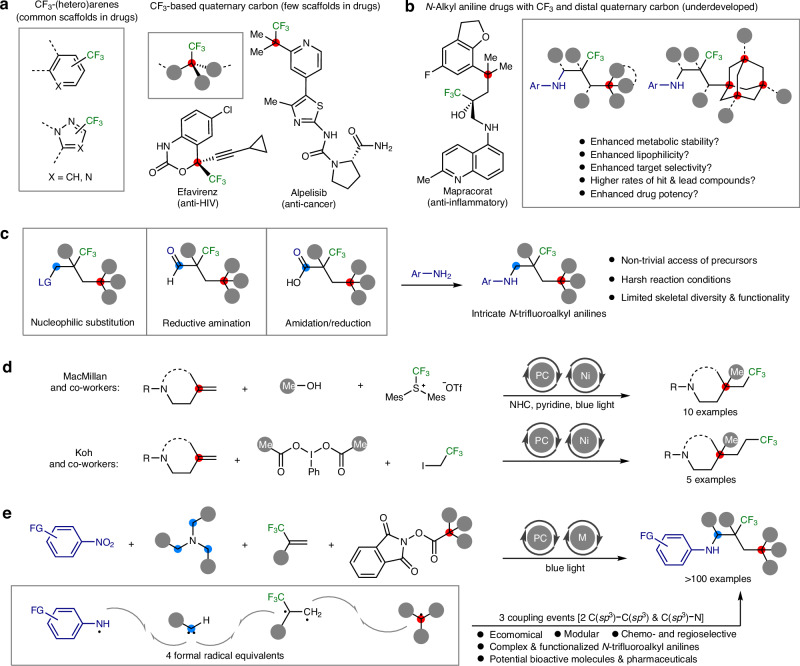
The strategic incorporation of fluorine atom and fluoroalkyl groups into drug molecules has emerged as a fundamental approach for fine-tuning physicochemical properties and optimizing the structure-activity relationship of pharmaceuticals1–3. Among various fluorination and fluoroalkylation strategies, the trifluoromethyl (CF3) group is particularly favored in medicinal chemistry for its ability to augment drug effectiveness through improved solubility, lipophilicity, metabolic stability, and protein-ligand interactions4,5. Despite the prevalence of trifluoromethyl arenes and heterocycles in pharmaceuticals6,7 (Fig. 1a, left), the exploration of trifluoromethyl-based aliphatic bioactive molecules8,9 has been somewhat limited, with the 2,2,2-trifluoroethyl moiety being the most commonly incorporated group in drug design6,7. The transition towards employing three-dimensional sp3-hybridized carbon structures, in place of planar sp2-hybridized carbons, as alternative bioisosteres, represents an innovative strategy for designing highly effective drug candidates10–12. This shift aims to leverage the advantageous pharmacological properties, including metabolic stability, target affinity and site specificity. Yet, the development of intricate trifluoroalkyl molecules, akin to approved drugs like efavirenz and alpelisib6,7 which feature quaternary carbons and α-CF3 substituents (Fig. 1a, right), remains a work in progress.
Fig. 1. Trifluoromethylated bioactive molecules and the construction of intricately structured complex N-trifluoroalkyl anilines.
a Trifluoromethyl (CF3) groups are widespread in pharmaceuticals, but three-dimensional CF3-based aliphatic drugs remain uncommon. b Intricate anilines with CF3 and remote quaternary carbons could be potentially more potent drug but remains underdeveloped. c Intricate N-fluoroalkyl anilines are non-trivial and challenging to access. d Photocatalytic multicomponent reactions have been developed to access complex CF3-substituted alkyl amines. e Metallaphotocatalysis would offer a modular approach to access elaborate N-trifluoroalkyl anilines using simple feedstocks. The grey circles represent hydrogen, alkyls, aryls or heteroatoms. PC, photocatalyst; M, transition metal catalyst; Me, methyl; Cbz, benzyloxycarbonyl; Ar, aryl; LG, leaving group; FG, functional groups.
Amines, particularly anilines, play a pivotal role as structural scaffolds in the creation of biologically active compounds and the synthesis of pharmaceuticals13–15. Introducing a trifluoroalkyl group to the nitrogen atom in aniline drugs, instead of the traditional acyl and sulfonyl groups, offers an alternative strategy to reduce oxidation and improve bioavailability16,17. Furthermore, the synthesis of intricate N-trifluoroalkyl aniline drugs, exemplified by mapracorat6,18 (Fig. 1b, left), incorporating both trifluoromethyl groups and distal quaternary carbon centers, illustrates a promising direction for designing potent pharmaceuticals. By expanding the molecular complexity and functionality of N-trifluoroalkyl chains, we can generate a wider array of pertinent aniline derivatives, facilitating extensive structure-activity relationship (SAR) studies and the discovery of novel drugs with enhanced physicochemical properties and drug efficacy19,20 (Fig. 1b, right). The current methodologies for synthesizing N-trifluoroalkyl anilines primarily involve the use of preformed complex trifluoroalkyl electrophiles in various synthetic routes such as substitution, reductive amination, and amidation/reduction sequences with anilines21–24 (Fig. 1c). However, the preparation of these substrates is often challenging and limited, which largely narrows the scope of attainable N-trifluoroalkyl anilines and derivatives.
Over the last decade, innovative approaches such as cross-coupling25, hydroamination26,27, C − H functionalization28, and imine addition29 have emerged as powerful strategies for the synthesis of a wide variety of amines and derivatives30. Within this realm, photocatalytic amination reactions, particularly the multicomponent version, stand out for their ability to efficiently combine simple and readily available chemical feedstocks into complex amines31–34. A pivotal advancement by MacMillan and co-workers was the introduction of a dual nickel/photoredox-catalyzed 1,2-dialkylation process that incorporates methanol and an electrophilic trifluoromethylating agent into amino-substituted alkenes35 (Fig. 1d, top). Additionally, Koh and co-workers developed a method that leverages (diacetoxyiodo)benzene and 1,1,1-trifluoro-2-iodoethane for metallaphotocatalytic 1,2-dialkylation of amino-based alkenes36 (Fig. 1d, bottom). These pioneering studies illustrate the effectiveness of photoinduced multicomponent reactions in enhancing molecular complexity and broadening the diversity of CF3-substituted alkyl amine compounds via the challenging construction of two contiguous C(sp3)−C(sp3) bonds35–38, offering streamlined disconnections in synthetic chemistry.
Here, we introduce a metallaphotoredox-catalyzed amination process that employs nitroarenes39, tertiary alkylamines40,41, 3,3,3-trifluoropropene42, and redox-active esters derived from aliphatic carboxylic acids43,44, inspired by these significant developments (Fig. 1e). These accessible and cost-effective reactants formally serve as precursors for aminyl radicals, carbenes, 1,2-dicarboradicals, and tertiary carbon radicals, respectively. Leveraging the synergistic effect of dual transition metal/photoredox catalysts45, the four substrates participate in three distinct coupling events, orchestrated in a modular and systematic fashion, to synthesize an array of elaborate N-trifluoroalkyl anilines. Particularly, the structurally diverse nature of nitroarenes and carboxylic acids greatly enriches the complexity and functionality of the building molecules. This advancement broadens the chemical landscape, facilitating the construction of intricately structured N-trifluoroalkyl anilines for organic synthesis and pharmaceutical development.
Results
Reaction optimization
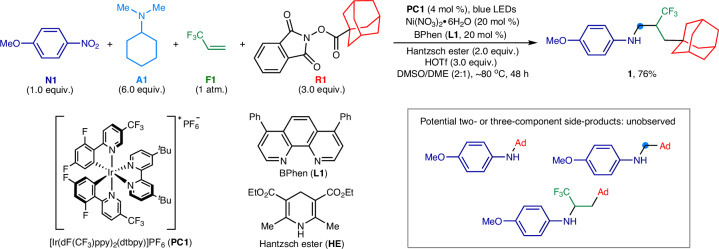
In our previous research, we successfully developed a nickel/photoredox-catalyzed three-component amination process that utilizes nitroarenes, tertiary alkylamines, and redox-active esters for synthesizing architecturally complex N-alkyl anilines46. Building upon this groundwork and employing similar reaction parameters, we aimed to test the feasibility of a more challenging four-component variation. This new approach incorporated an additional reactant, 3,3,3-trifluoropropene42 (F1), known for its utility in 1,2-functionalization reactions47–49. The investigation commenced with the use of 4-nitroanisole (N1, 1.0 equiv.), 3,3,3-trifluoropropene (F1, 1 atm.), a redox-active ester derived from 1-adamantanecarboxylic acid (R1, 3.0 equiv.), and a range of tertiary alkylamines (A1 − A6) (Supplementary Table 1). These components served as the arylamino source, trifluoromethylated skeleton, sp3-hybridized tertiary carbon precursor, and methylene linker, respectively. Employing a metallaphotoredox catalysis system consisting of [Ir(dF(CF3)ppy)2(dtbpy)]PF6 (PC1, 4 mol %) and nickel(II) nitrate/bathophenanthroline (BPhen) [Ni(NO3)2•6H2O/L1, 20 mol %], N,N-dimethylaminocyclohexane (A1, 6.0 equiv.) emerged as the optimal methylenating agent. The combination of dimethyl sulfoxide (DMSO) and 1,2-dimethoxyethane (DME) was identified as the ideal solvent system for the reaction. With the addition of Hantzsch ester (HE, 2.0 equiv.) and triflic acid (HOTf, 3.0 equiv.) serving as the reductant and proton source, respectively, a smooth reaction ensued at an ambient temperature of ~80 °C. This process yielded the intricate N-trifluoroalkyl aniline 1, feathering both a CF3 group and a remote adamantyl moiety attached to the N-aliphatic chain, in 76% yield (Fig. 2; Supplementary Table 1, entry 18). Notably, the transformation exhibited both chemo- and regioselectivity, without any detectable side-products from the two- or three-component reactions. Control experiments further affirmed that the photocatalyst, Ni salt, and Hantzsch ester are crucial for enhancing the product yields.
Fig. 2. The optimized conditions for the metallaphotocatalytic four-component amination.
Me, methyl; Et, ethyl; Ph, phenyl; Ad, 1-adamantyl.
Substrate scope
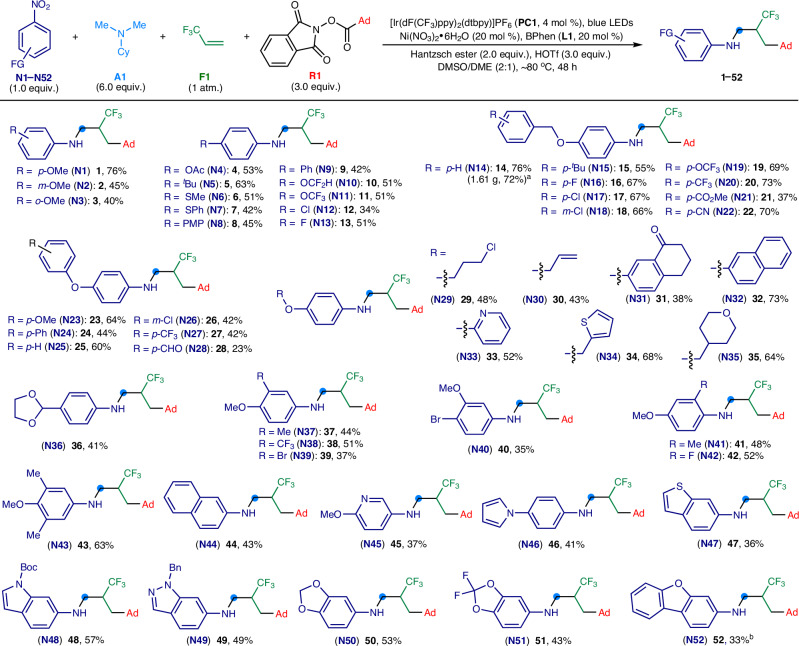
Equipped with the optimal conditions, we delved into the scope of nitroarenes (N1 − N52) in the four-component amination process (Fig. 3). We found that nitroarenes featuring various substitution patterns, including para- (N1), meta- (N2), and ortho-substitutions (N3), as well as di- (N37 − N42) and tri-substitutions (N43), were all compatible substrates. This protocol demonstrated remarkable tolerance for a wide array of functional groups, enabling the synthesis of a diverse array of N-trifluoroalkyl anilines (1 − 52). These compounds were adorned with a rich variety of functional moieties, including esters (4 and 21), tert-alkyls (5 and 15), aryls (8, 9 and 24), thioethers (6, 7), ethers (1 − 3, 14, 23, 25, 37, 41, and 43), fluoroalkoxies (10, 11, and 19), trifluoromethyls (20, 27, and 38), chloros (12, 17, 18, 26, and 29), fluoros (13, 16, and 42), bromos (39 and 40), nitriles (22), protected (36) and unprotected aldehydes (28), ketones (31), and alkenyls (30). The inclusion of naphthalene (32 and 44) into the nitroarenes was successful as well in the transformations. Next, our exploration extended to the synthesis of N-trifluoroalkyl anilines featuring heteroaryl substitutions as well as N-, S-, and O-fused heterocycles, employing corresponding nitroaromatic compounds that included pyridine (33 and 45), thiophene (34), tetrahydropyran (35), pyrrole (46), benzothiophene (47), indole (48), indazole (49), benzodioxole (50 and 51), and dibenzofuran (52). Furthermore, the reaction was amendable to gram-scale manipulation, yielding the product 14 with similar efficiency as observed in the 0.2 mmol-scale reaction. The intricate structure of N-trifluoroalkyl aniline compounds was further validated through the X-ray crystallography of compound 14 (see Supplementary Information). Highly electron-deficient nitroarenes, including 4-nitrobenzotrifluoride, 4-nitrobenzonitrile, 4-nitroacetophenone, and 4-nitrobenzophenone, were not tolerated and proved non-productive under the reaction conditions. Nevertheless, this broad compatibility with a variety of functionalities and heterocycles significantly extends the versatility of N-trifluoroalkyl aniline derivatives, opening new avenues for the discovery of novel biologically active molecules and the advancement of pharmaceutical research.
Fig. 3. Scope of nitroarenes.
General reaction conditions: nitroarene (N1–N52, 1.0 equiv., 0.15 mmol), tertiary alkylamine (A1, 6.0 equiv., 0.90 mmol), 3,3,3,-trifluoropropene (F1, 1 atm.), redox-active ester (R1, 3.0 equiv., 0.45 mmol), PC1 (4 mol %), Ni(NO3)2•6H2O (20 mol %), bathophenanthroline (BPhen, L1: 20 mol %), Hantzsch ester (2.0 equiv., 0.30 mmol), HOTf (3.0 equiv., 0.45 mmol), DMSO/DME (v/v = 2:1, 6 mL), ~80 oC, blue LEDs (30 W, 456–460 nm), 48 h. aGram-scale synthesis based on 5 mmol of N14, 96 h. bKessil LEDs (40 W, 456 nm) were used instead. Ad, 1-adamantyl; Me, methyl; Ac, acetyl; tBu, tert-butyl; Ph, phenyl; PMP, p-methoxyphenyl; Bn, benzyl; Boc, tert-butyloxycarbonyl.
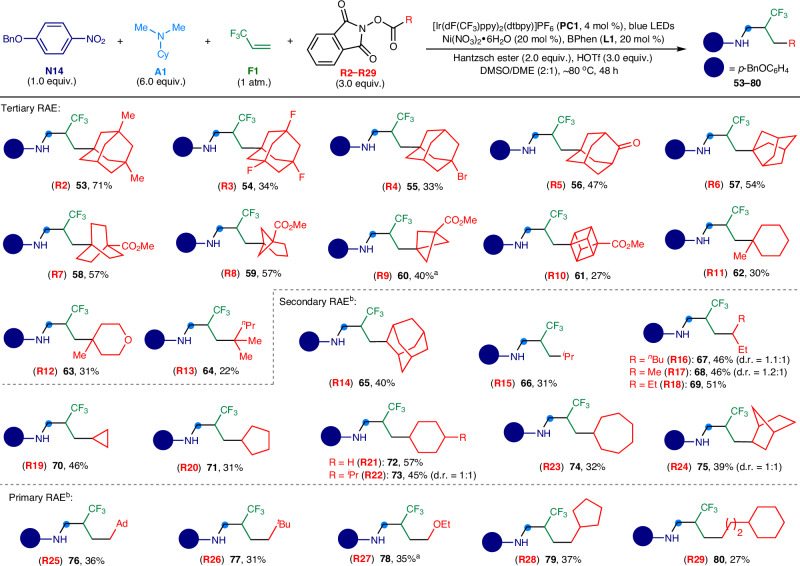
Aliphatic carboxylic acids represent cost-effective and structurally diverse building blocks in organic chemistry. Transforming these acids into redox-active esters through the straightforward addition of a photosensitive N-hydroxyphthalimide group has emerged as an efficient strategy for generating sp3-hybridized carbon radicals, facilitating various bond formation reactions43,44. Under our reaction protocol, a broad spectrum of tertiary alkyl carboxylic acid redox-active esters (R2 − R29) could be utilized as reactants (Fig. 4). The synthesis process enabled the creation of N-trifluoroalkyl anilines (53 − 64) with a range of quaternary carbon-centered skeletons, including the dimethyl- (R2), trifluoro- (R3), monobromo- (R4) and oxo-substituted (R5) variants of 1-adamantane, octahydro-2,5-methanopentalene (R6), bicyclo[2.2.2]octane (R7), bicyclo[3.1.1]heptane (R8), bicyclo[1.1.1]pentane (R9), cubane (R10), 1-methylcyclohexane (R11), 4-methyltetrahydropyran (R12), and 2-methylpentane (R13). These three-dimensional aliphatic moieties can function as bioisosteres for flat aromatic rings in drug discovery12. Furthermore, the protocol proved compatible with secondary (R14 − R24) and primary carboxylic acid redox-active esters (R25 − R29) as coupling partners. A diverse array of aliphatic groups, including 2-adamantyl (65), isopropyl (66), 3-heptyl (67), 2-butyl (68), 3-pentyl (69), cyclopropyl (70), cyclopentyl (71), cyclohexyl (72 and 73), cycloheptyl (74), bicycloheptyl (75), adamandylmethyl (76), 2,2-dimethylpropyl (77), ethoxymethyl (78), cyclopentylmethyl (79), and 2-cyclohexylethyl (80), could be integrated into the N-trifluoroalkyl anilines. The use of several tertiary and secondary RAEs and all primary redox-active esters tended to result in modest product yields, due to competition from two- and three-component reactions involving nitroarenes, tertiary alkylamines, and redox-active esters. Nonetheless, the four-component synthetic approach offers a direct route to a variety of N-trifluoroalkyl anilines with diverse skeletal complexity. These compounds hold promise as valuable scaffolds for the development of novel, more effective drug molecules.
Fig. 4. Scope of redox active esters.
General reaction conditions: nitroarene (N14, 1.0 equiv., 0.15 mmol), tertiary alkylamine (A1, 6.0 equiv., 0.90 mmol), 3,3,3,-trifluoropropene (F1, 1 atm.), redox-active ester (R2 − R29, 3.0 equiv., 0.45 mmol), PC1 (4 mol %), Ni(NO3)2•6H2O (20 mol %), bathophenanthroline (BPhen, L1: 20 mol %), Hantzsch ester (2.0 equiv., 0.30 mmol), HOTf (3.0 equiv., 0.45 mmol), DMSO/DCE (v/v = 2:1, 6 mL), ~80 oC, blue LEDs (30 W, 456–460 nm), 48 h. aKessil LEDs (40 W, 456 nm) were used instead. bNi(bipy)Cl2 (20 mol %) were used instead. Ad, 1-adamantyl; Me, methyl; nPr, n-propyl; iPr, isopropyl, nBu, n-butyl; Et, ethyl; tBu, tert-butyl; bipy = 2,2′-bipyridine.
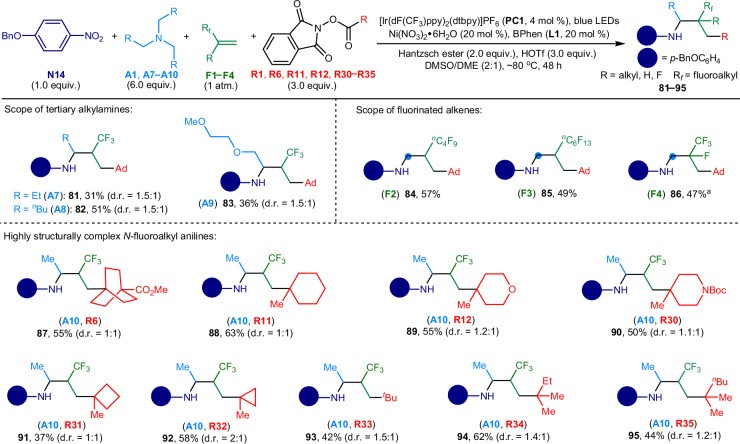
Our reaction protocol successfully accommodated higher-membered tertiary alkylamines and fluoro-substituted alkenes (Fig. 5). Specifically, tripropylamine (A7), tripentylamine (A8), and tris[2-(2-methoxyethoxy)ethyl]amine (A9) each contributed an alkyl chain in the amination reactions. This led to the production of α-ethyl (81), α-n-butyl (82), and α-(2-methoxyethoxy)methyl-substituted N-trifluoroalkyl anilines (83), respectively. Additionally, fluoroalkenes, such as nonafluorohex-1-ene (F2), tridecafluorooct-1-ene (F3), and 2,3,3,3-tetrafluoroprop-1-ene (F4) proved to be effective reaction partners, yielding β-perfluoro-substituted (84 and 85) and β-fluoro-β-trifluoromethyl substituted N-alkyl aniline derivatives (86). The strategic incorporation of methyl groups into drug-like molecules has been shown to significantly enhance their ADME (Absorption, Distribution, Metabolism, and Excretion) properties and overall drug efficacy50–52. Leveraging triethylamine (A10) and a variety of tertiary sp3-carbon-based redox active esters as substrates (R6, R11, R12, and R30 − R35), we successfully synthesized a broader scope of N-trifluoroalkyl anilines (87 − 95). These compounds, characterized by an aliphatic skeleton that includes an α-methyl group, a β-CF3 group, and either a cyclic (87 − 92) or acyclic distal quaternary carbon moiety (93 − 95), represent a leap forward in molecular complexity. These amine compounds (81 − 95) would benefit from the enhanced physicochemical properties conferred by multiple fluorination, enhanced saturation, multifaceted three-dimensionality and the ‘magic methyl effect’50–52, providing potential applications in the development of more effective drug-like molecules.
Fig. 5. Scope of tertiary alkylamines and fluoro-based alkenes.
General reaction conditions: nitroarene (N14, 1.0 equiv., 0.15 mmol), tertiary alkylamine (A1, A7 − A10, 6.0 equiv., 0.90 mmol.), fluoro-substituted propene (F1, F4: 1 atm.; F2, F3: 20 equiv.), redox-active ester (R1, R6, R11, R12, and R30 − R35, 3.0 equiv., 0.45 mmol), PC1 (4 mol %), Ni(NO3)2•6H2O (20 mol %), bathophenanthroline (BPhen, L1: 20 mol %), Hantzsch ester (2.0 equiv., 0.30 mmol), HOTf (3.0 equiv., 0.45 mmol), DMSO/DME (v/v = 2:1, 6 mL), ~80 oC, blue LEDs (30 W, 456–460 nm), 48 h. Unless otherwise noted, A1, F1 and R1 were used as reaction substrates. aThe reaction was conducted at 40 oC for 24 h. Ad, 1-adamantyl; Me, methyl; Boc, tert-butyloxycarbonyl, tBu, tert-butyl; Et, ethyl, nBu, n-butyl.
Utilizing readily available and cost-efficient starting materials such as nitroarenes, tertiary alkylamines, fluoro-substituted alkenes, and carboxylic acids, our modular synthetic strategy allows for swift access to a diverse array of elaborate N-trifluoroalkyl anilines. These compounds feature varied electronic, steric and spatial properties, enabling precise structural modifications for structure-activity relationship (SAR) studies for drug discovery. This approach underscores the versatility and efficacy of our protocol, enabling the exploration of new chemical space in organic synthesis and medicinal chemistry.
Synthetic utility
The development of structurally complex trifluoroalkyl components represents a significant challenge in medicinal chemistry, hindering the production of relevant amine compounds. A streamlined approach would involve using simpler building blocks to synthesize sophisticated trifluoroalkyl anilines, thereby facilitating the construction of novel biologically active molecules for study.
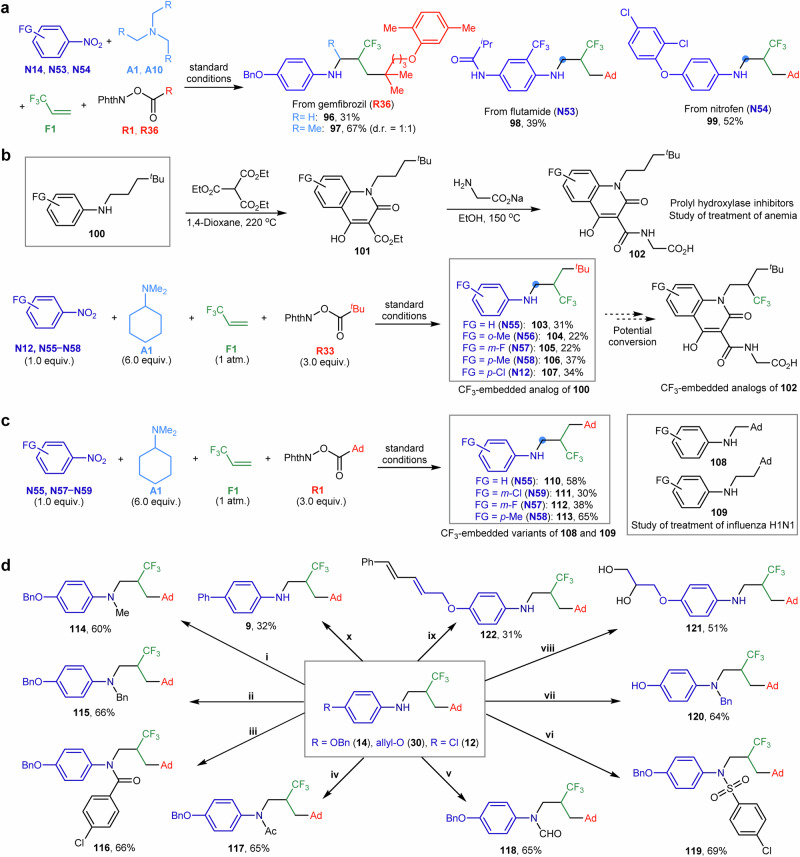
In our reaction protocol (Fig. 6a), gemfibrozil (R36), a lipid-regulating drug, served as a tertiary C(sp3)-radical precursor to synthesize the N-trifluoroalkyl aniline variants (96 and 97). Furthermore, nitroarene-based flutamide (N53), a nonsteroidal antiandrogen, and nitrofen (N54), an herbicide, were transformed to the trifluoroalkyl-decorated aniline derivatives (98 and 99).
Fig. 6. Synthetic utility of the metallaphotocatalytic four-component amination reaction.
a Synthesis of N-trifluoroalkyl anilines bearing drug- and herbicide moieties. b Synthesis of N-(4,4-dimethyl-2-(trifluoromethyl)pentyl) anilines as potential prolyl hydroxylase inhibitor intermediates. c Synthesis of N-(3-adamantyl-2-(trifluoromethyl)propyl) anilines as potential anti-influenza agents. d Derivatization of N-trifluoroalkyl aniline via N- and peripheral functionalization: (i) 14, MeI, NaH; (ii) 14, BnBr, NaH; (iii) 14, 4-chlorobenzoyl chloride, Et3N; (iv) 14, acetic anhydride; (v) 14, acetic anhydride, formic acid; (vi) 14, 4-chlorophenylsulfonyl chloride, Et3N; (vii) 14, BBr3, then K2CO3; (viii) 30, potassium osmate, N-methylmorpholine N-oxide; (ix) 30, (E)−1-bromo-2-phenylethene, Pd catalyst, Cs2CO3; (x) 12, phenylboronic acid, Pd catalyst, K3PO4. Ad, 1-adamantyl; Me, methyl; Et; ethyl, iPr, isopropyl; tBu, tert-butyl; Bn = benzyl; Ac = acetyl; NPhth: phthalimidyl; FG, functional group.
A series of N-(4,4-dimethylpentyl) anilines (100) has been prepared as structural components for heterocycles 101 (Fig. 6b), aiming at synthesizing prolyl hydroxylase inhibitors 102 for treating anemia53. Our reaction protocol allowed for modular assembly of nitroarenes (N12, and N55 − N58), N,N-dimethylcyclohexylamine (A1), 3,3,3-trifluoropropene (F1), and redox-active esters (R33) derived from 2,2-dimethylpropionic acid, yielding several N-(4,4-dimethyl-2-(trifluoromethyl)pentyl) anilines (103 − 107). These CF3-embedded analogues of 100 could potentially be modified into CF3-substituted inhibitors 102 for biological assessment.
For the study of influenza treatments (Fig. 6c), adamantyl-incorporated N-alkyl compounds such as 108 and 109 have been synthesized54. Using 1-adamantylcarboxylic acid redox-active ester (R1), we generated several variants, N-(3-adamantyl-2-(trifluoromethyl)propyl) anilines (110 − 113), under our reaction protocol. The introduction of an additional CF3 group could help develop more effective anti-influenza agents.
The N-trifluoroalkyl aniline products proved versatile for further derivatization (Fig. 6d), enhancing their functionality and structural diversity. Processes such as N-alkylation, amidation, and sulfonamidation produced N-methylated (114) and N-benzylated anilines (115), as well as amides (116 and 117), formamides (118), and sulfonamides (119). Benzyloxy-substituted N-trifluoroalkyl aniline (14) underwent a tandem C − O decoupling and C − N reconstruction, delivering the N-benzyl-N-phenol isomeric variant 120. Additionally, the oxidation of allyloxy-substituted N-trifluoroalkyl aniline (30) selectively produced the 1,2-diol derivative 121. The Heck reaction of 30 and the Suzuki coupling reaction of the 4-chloroaniline product (12) also afforded the 1,3-diene (122) and biphenyl (9) derivatives. These functionalized and intricate anilines and derivatives represent valuable synthetic scaffolds for the potential development of novel drug-like molecules and more potent pharmaceuticals.
Mechanistic study
The synthesis of N-trifluoroalkyl anilines through a four-component amination process entails a series of intricate reaction sequences. To elucidate the mechanisms and identify intermediates and co-products, a comprehensive suite of experiments was undertaken:
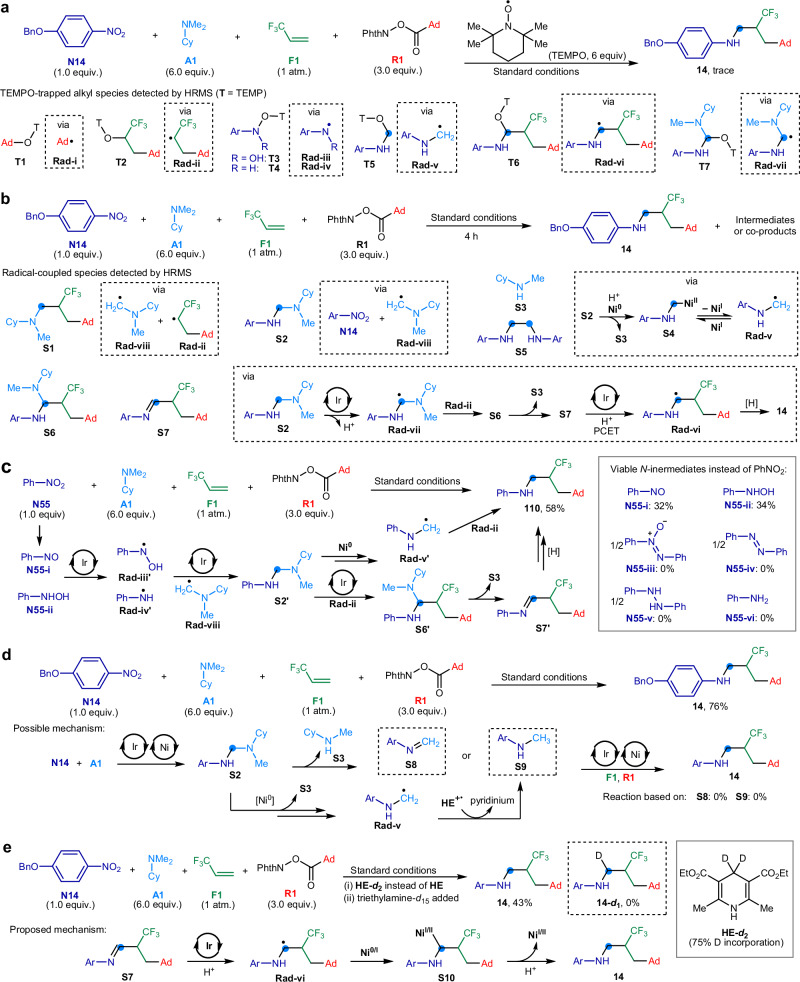
Radical species detection (Fig. 7a): When 2,2,6,6-tetramethylpiperidine−1-oxyl (TEMPO), acting as a radical scavenger, was introduced to the model reaction, only a minimal amount of N-trifluoroalkyl aniline 14 was produced. High-resolution mass spectrometry (HRMS) analysis identified several TEMPO-captured adducts (T1 − T7), corresponding to the adamantyl radical (Rad-i), the adamantyl-trifluoroalkyl radical (Rad-ii), the N-aryl-N-hydroxy-aminyl radical (Rad-iii), the N-aryl-aminyl radical (Rad-iv), the N-aryl-aminomethyl radical (Rad-v), the (α-amino)trifluoroalkyl radical (Rad-vi), and the α,α-di(amino)methyl radical (Rad-vii). Additionally, the radical clock experiment employing the RAE derived from hept-6-enoic acid yielded the ring-closing product, an N-trifluoroalkyl aniline featuring an incorporated cyclopentylmethyl group. This outcome suggested the formation of a hex-5-enyl radical species, which rapidly cyclizes to produce the cyclopentylmethyl radical intermediate (See Supplementary Figs. 2 and 3 for details). These findings indicated the presence of these radical intermediates, which are implicated in the reaction pathway leading to the formation of N-trifluoroalkyl aniline product 14.
Identification of reaction co-products (Fig. 7b): At the onset of the model reaction, the reaction mixture underwent HRMS analysis to identify emerging reaction intermediates and co-products (See Supplementary Fig. 4 for details). This analysis revealed a variety of species, including: (i) trifluoroalkyl amine S1, believed to originate from the coupling of the N-cyclohexyl-N-methylaminomethyl radical (Rad-viii) with Rad-ii; (ii) aminal S2, likely produced through the reaction between nitroarene N14 and Rad-viii46; (iii) N-methylcyclohexylamine (S3), which appears to result from the nucleophilic displacement of aminal S2 by low-valent nickel species55, coinciding with the formation of N-aryl-aminomethyl-Ni species (S4); (iv) N,N’-diaryl ethylenediamine (S5), suggested as the dimerization outcome of the N-aryl-aminomethyl radical (Rad-v)56,57, itself derived from the Ni−C homolysis58–60 of S4; (v) trifluoroalkyl aminal (S6), presumed to be synthesized through the photocatalytic oxidation of aminal S2 into α,α-diaminomethyl radical Rad-vii61, followed by its integration with Rad-ii, and (vi) trifluoroalkyl imine (S7), expected to develop from the removal of amine S3 from aminal S656. Our hypothesis posits that S7 further undergoes a proton-coupled electron transfer29 (PCET) facilitated by iridium photocatalysis, forming the (α-amino)trifluoroalkyl radical species (Rad-vi). This radical is then believed to undergo a formal hydrogen atom transfer (HAT), leading to the formation of the N-trifluoroalkyl aniline product 14.
Nitrogen intermediate exploration (Fig. 7c): Within the photocatalytic process, nitrobenzene (N55) could be reduced to a variety of nitrogen-containing intermediates, including nitrosobenzene (N55-i), N-phenyl hydroxylamine (N55-ii), azoxybenzene (N55-iii), azobenzene (N55-iv), N,N’-diphenyl hydrazine (N55-v), and aniline (N55-vi)62. Specifically, nitrobenzene was converted into N-trifluoroalkyl aniline (110) with a 58% yield under standard reaction conditions. Control experiments using N55-i and N55-ii yielded the target compound in 32% and 34% yields, respectively, while other nitrogen-containing intermediates (N55-iii − N55-vi) failed to produce the desired outcome. These results suggested that nitrosoarenes and N-aryl hydroxylamines could be the principal intermediates for synthesizing the target products. Experimental and analytical data led us to theorize that nitrobenzene undergoes a photocatalytic reduction, facilitated by HOTf as a proton source46, to form N55-i and N55-ii. These intermediates are then further reduced to generate nitrogen-centered radical species Rad-iii’ and Rad-iv’63, which subsequently interact with Rad-viii to produce the aminal intermediate S2′46. This intermediate undergoes deamination55, catalyzed by Ni, to form the N-phenyl-aminomethyl radical57 (Rad-v′), which combines with Rad-ii to yield the product. Alternatively, S2′ reacts under photocatalysis with Rad-ii to produce a trifluoroalkyl aminal (S6′). This aminal species then transitions through the formation of imine S7′56 and reduction, culminating in the formation of the product.
Analysis of intermediates from nitroarenes and tertiary alkylamines (Fig. 7d). The aminal species S2, arising from the photocatalytic formation of C–N bonds between nitroarene N14 and N,N-dimethylcyclohexylamine A1, is anticipated to undergo deamination46,56, yielding N-aryl imine S8. Concurrently, the N-aryl-aminoalkyl radical Rad-v, generated from S2, is expected to produce N-methyl aniline S9 through HAT with Hantzsch ester. However, the reaction with in-situ formed S8 and commercially available S9 did not lead to the synthesis of the target compound 14, indicating that these species are probably not the key reaction intermediates. This observation led us to conclude that S2 and its subsequent radical form Rad-v are most likely the reaction intermediates, facilitating the formation of the desired product through radical-driven processes.
(α-Amino)trifluoroalkyl radical reduction study (Fig. 7e): The α-amino-trifluoroalkyl radical species (Rad-vi) would engage in HAT with Hantzsch ester (HE) or its radical cation form (HE+•), identified as having the weakest C–H bonds {BDE (C4–H) of HE = 69 kcal mol−1; BDE (C4–H) of HE+• = 31 kcal mol−1}64, resulting in the formation of N-trifluoroalkyl aniline 14. However, when the model reaction employed C4-deuterium-enriched Hantzsch ester (HE-d2), it yielded the conventional product 14 without incorporating deuterium (Fig. 4e (i)). In addition, the C–H bond of tertiary alkylamines could serve as another HAT reagent for Rad-vi. However, when excess triethylamine-d15 was added to the model reaction, no deuterium incorporation into product 14 was observed (Fig. 4e (ii)). The results implied that Rad-vi likely undergoes an inner-sphere electron transfer with low-valent nickel to form a Ni-(α-amino)trifluoroalkyl complex (S10). This complex is then protonated, releasing the target product 14.
Stern–Volmer Quenching Analysis (Supplementary Figs. 11–16). To elucidate the photoexcitation processes initiated by the iridium photocatalyst (PC1, IrIII), we conducted Stern–Volmer quenching experiments. These experiments revealed that various quenchable species, including Hantzsch ester (HE), N,N-dimethylcyclohexylamine (A1), 4-benzyloxy-1-nitroarene (N14), redox-active ester (R1), and the in-situ formed nickel(II)/bathophenanthroline complex [Ni(NO3)2/L1], are capable of quenching the photoexcited state of IrIII*. Notably, the nitroarene (N14) emerged as the most potent quencher among them. This observation indicated that the photoexcited IrIII* species preferentially reduce nitroarenes, leading to the formation of an IrIV species and the corresponding reduced intermediates of nitroarenes. The half-wave potential for the reduction of N14 was determined to be –0.88 V vs SCE {E1/2red [p-BnOC6H4NO2/p-BnOC6H4NO2–•] = –0.88 V vs SCE in MeCN} (Supplementary Fig. 10), which falls within the redox window of the iridium photocatalyst31, further supporting the viability of this preferential redox process. This sequence of events triggers the subsequent photocatalytic reactions.
Fig. 7. Mechanistic study of the metallaphotocatalytic four-component amination.
a Identification of radical species using TEMPO as the radical trap. b Analysis of reaction co-products in the early reaction stage. c Examination of nitrogen-containing intermediates from nitroarenes in product formation. d Study of N-aryl imine and N-methyl aniline as reaction intermediates for product formation. e Investigation of the α-hydrogen source in N-trifluoroalkyl aniline product. Ar denotes the 4-benzyloxyphenyl group. Ad, 1-adamantyl; Me, methyl; Et = ethyl; Bn, benzyl; Cy: cyclohexyl; Ph, phenyl; T (TEMP), 2,2,6,6-tetramethylpiperidyl.
These experiments collectively advance our understanding of the sophisticated mechanisms underpinning the synthesis of N-trifluoroalkyl anilines, shedding light on the intricacies of catalysts, radical formation, and reaction intermediates in achieving selective formation of product.
Proposed mechanism
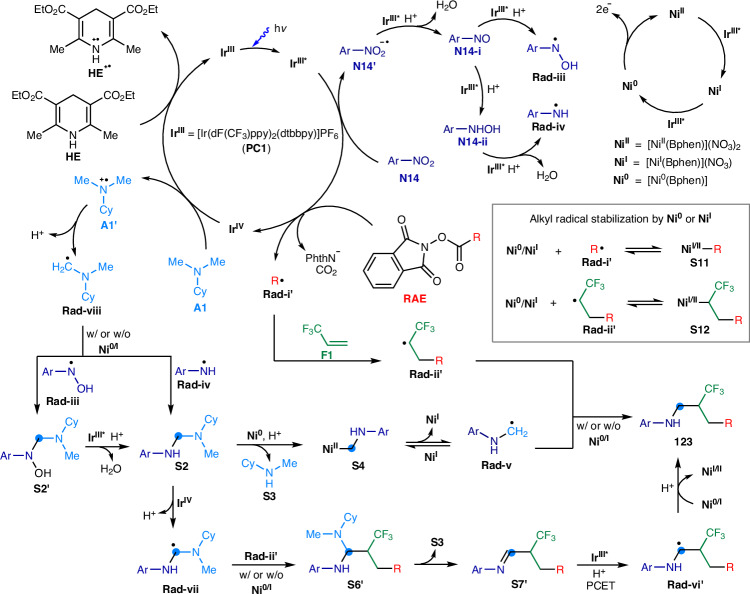
Based on the gathered experimental and instrumental evidence, we formulated a mechanism for the metallaphotocatalytic four-component amination process (Fig. 8). The iridium photocatalyst (PC1, IrIII) is activated under blue light to reach a high-energy, long-lived excited state (IrIII*){E1/2red [IrIV/IrIII*] = –0.89 V vs saturated calomel electrode (SCE)}31. This excited state can feasibly reduce nitroarene (N14) to radical anion species (N14′) {E1/2red [p-BnOC6H4NO2/ p-BnOC6H4NO2–•] = –0.88 vs SCE} (Supplementary Fig. 10)65, especially under conditions facilitated by HOTf through oxygen protonation46. Further photocatalytic reduction yields nitrosoarene (N14-i) and N-aryl hydroxylamine (N14-ii), followed by N-aryl-N-hydroxy-aminyl radical (Rad-iii) and N-aryl-aminyl radical Rad-iv), respectively. Concurrently, IrIII* reduces redox active esters (RAE) to generate alkyl radicals (Rad-i′), despite a redox potential of approximately –1.2 V {E1/2red [R11/R11–•] = –1.26 V vs SCE}66. The acidic reaction medium is believed to aid in overcoming the potential discrepancy, promoting the photoreduction of RAEs. The resulting oxidized iridium species (IrIV) {E1/2red [IrIV/IrIII] = +1.69 V vs SCE)}31 then oxidizes N,N-dimethylcyclohexylamine (A1) to a nitrogen-centered radical cation (A1′) {E1/2red [iPrMe2N+•/iPrMe2N] = +0.72 V vs SCE}67. This step is followed by a facile deprotonation to produce the N-cyclohexyl-N-methylaminomethyl radical (Rad-viii). Additionally, IrIV is regenerated to its IrIII state by interacting with Hantzsch ester (HE) {E1/2red [HE+•/HE] = +0.89 V vs SCE}68, thus completing the catalytic cycle and facilitating continuous reaction progression.
Fig. 8. Proposed mechanism of the metallaphotocatalytic four-component amination.
Ar denotes the 4-benzyloxyphenyl group. Me, methyl; Et, ethyl; Cy, cyclohexyl; R, alkyl; NPhth, phthalimidyl.
Furthermore, the [NiII(BPhen)](NO3)2 catalyst (NiII) is reduced by the excited iridium species IrIII*, resulting in the formation of low-valent nickel species, [NiI(BPhen)](NO3) (NiI) and [Ni0(BPhen)] (Ni0). Given the higher dissociation tendency of the nitrate ion and the increased electrophilicity of the [NiII(BPhen)](NO3)2 complex compared to its chloride counterpart {E1/2red [NiII(BPhen)Cl2/Ni0(BPhen)] ~ –1.2 V vs SCE}69, we hypothesized that the nitrate complex exhibits greater oxidizing properties70. This makes the reduction of NiII to NiI and Ni0 by IrIII* thermodynamically favorable.
Alkyl radicals (Rad-i′), generated from RAEs, react with 3,3,3-trifluoropropene (F1), leading to the formation of trifluoroalkyl radicals (Rad-ii′). Concurrently, Rad-viii engages with the resulting nitrogen-centered radicals (Rad-iii and Rad-iv) in a photoreduction process, yielding the N-aryl aminal species (S2). S2 likely interacts with low-valent Ni species (Ni0) via nucleophilic substitution or oxidative addition55, resulting in the formation of a nickel(II) N-aryl-aminomethyl species (S4)58–60. S4 then undergoes Ni–C homolysis58–60, releasing the N-aryl-aminomethyl radical57 (Rad-v). The electrophilic trifluoroalkyl radicals (Rad-ii′) and nucleophilic N-aryl-aminomethyl radical (Rad-v) efficiently couple to produce the N-trifluoroalkyl aniline products (123). The polarity match of these radical species underscores the chemoselective multiple-bond connections that lead to product formation.
In an alternative pathway, S2 is subject to photooxidation by IrIV {E1/2red [((Me2N)2CH2)+•/(Me2N)2CH2] = +0.87 V vs SCE}71, generating the α,α-di(amino)methyl radical (Rad-vii). The highly nucleophilic Rad-vii then combines with Rad-ii′ to form the trifluoroalkyl aminal species (S6′). S6′ readily dissociates into trifluoroalkyl imine (S7′)56, which, upon undergoing PCET29, produces the (α-amino)trifluoroalkyl radical species (Rad-vi′) {E1/2red [S7′/Rad-vi′] ~ –1 V vs SCE}29. Both Ni0 and NiI facilitate the inner-sphere reduction of Rad-vi′58–60, followed by protonation to yield the product.
Based on the HRMS analysis (Fig. 7a and b), the relative concentration of the trifluoroalkyl radical (Rad-ii) -based species T2 was significantly higher than that of the other detected radical-based species (T3 to T7 originated from Rad-iii to Rad-vii). This suggested that the concentrations of these other radical components are generally low. We proposed that Ni-mediated C–N and C–C bond formation reactions involving these radicals play a crucial role in the overall process, beyond the free radical interactions that contribute to product formation.
We proposed that the electron-rich, low-valent nickel species, NiI and NiII, capture the alkyl radical (Rad-i′) and trifluoroalkyl radical (Rad-ii′) to form Ni-alkyl complexes (S11 and S12) through equilibrium processes58–60. This interaction is key to minimizing the undesired alkyl-alkyl dimerization and two/three-component coupling reactions. Indeed, the yields of target products dropped significantly in the reactions of primary and secondary alkyl RAEs without the Ni catalyst (Supplementary Table 2), underscoring the crucial role of Ni in stabilizing the less nucleophilic and smaller-sized primary and secondary alkyl radicals46.
Moreover, the light/dark experiment demonstrated that the reaction occurs exclusively during the irradiation period (Supplementary Fig. 8), suggesting that it likely follows a non-chain photocatalytic mechanism rather than a radical chain process. Further 19F NMR spectroscopic analysis suggested that fluoride is likely formed in the reaction (Supplementary Fig. 9). We surmised that the over-reduction of the trifluoroalkyl radical (Rad-ii) via photocatalysis or Ni catalysis is inevitable, leading to the generation of a trifluoroalkyl anion that results in fluorine elimination72,73. However, the addition of excess RAEs and F1 would ensure sufficient loading of Rad-ii, thereby compensating for the defluorination side-reaction and maintaining the reaction productivity.
This comprehensive mechanism highlights the synchronized interplay between various radicals and catalysts, leading to the efficient and selective synthesis of N-trifluoroalkyl aniline compounds.
In summary, we have successfully developed a metallaphotoredox-catalyzed multicomponent amination reaction that employs nitroarenes, tertiary alkylamines, 3,3,3-trifluoropropene, and carboxylic acids. This approach enables the synthesis of a diverse array of intricate, three-dimensional N-trifluoroalkyl aniline compounds in a modular and cost-effective fashion. Our method facilitates the efficient generation of trifluoromethylated synthetic intermediates and compounds with pharmaceutical potential, aiming at the discovery of more potent therapeutic agents. The capability for various derivatizations enhances both the structural complexity and functional diversity of the produced N-trifluoroalkyl anilines. We anticipate that this versatile four-component reaction will open new avenues in the exploration of uncharted fluorinated, high C(sp3)-contented structural realms, leading to the identification of novel bioactive molecules and the advancement of drug development.
Methods
General procedure for photocatalytic four-component amination using nitroarenes, tertiary alkylamines, 3,3,3-trifluoropropene and redox-active esters
An oven-dried, transparent 20 mL Schlenk tube equipped with a stir bar was sequentially charged with nitroarene (N1–N59, 1.0 equiv., 0.15 mmol), redox active ester (R1–R36, 3.0 equiv., 0.45 mmol), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (PC1, 4 mol %, 0.0060 mmol), Ni(NO3)2•6H2O (20 mol %, 0.030 mmol), bathophenanthroline (BPhen, L1, 20 mol %, 0.030 mmol), and Hantzsch ester (diethyl 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate, HE, 2.0 equiv., 0.30 mmol). Dried dimethyl sulfoxide (DMSO, 4.0 mL) and dried 1,2-dimethoxyethane (DME, 2.0 mL) were then transferred into the tube via syringe. Subsequently, tertiary alkylamine (A1–A5, 6.0 equiv., 0.90 mmol) and triflic acid (HOTf, 3.0 equiv., 0.45 mmol) were transferred into the tube via syringe. The resulting mixture was degassed via blowing for 2 min with a needle equipped with a 3,3,3-trifluoropropene-filled-balloon (F1, ~1 L), after which time the tube was quickly capped with a Teflon screw cap such that it was filled with F1 in atmospheric pressure. The reaction mixture was vigorously stirred and irradiated using 30 W blue LEDs (λ = 456–460 nm) for 48 h under the ambient temperature of approximately ~80 oC (without the use of fans for cooling). At this point, the reaction mixture was diluted with ethyl acetate ( ~ 100 mL) and washed with water ( ~ 50 mL × 4). The organic fraction was further dried with anhydrous Na2SO4 and concentrated in vacuo with the aid of rotary evaporator. The residue was purified by preparative thin-layer chromatography using a mixture of petroleum ether and ethyl acetate as an eluent to afford the N-trifluoroalkyl aniline product.
Supplementary information
Acknowledgements
We acknowledge the National Natural Science Foundation of China [Nos. 21971186 (C.W.C.), 22271216 (C.W.C.), and 21961142015 (J.-A.M.)], the National Key Research and Development Program of China [No. 2019YFA0905100 (J.-A.M. and C.W.C.)], and the Graduate Outstanding Innovation Award Program for Humanities and Sciences 2023 Year Project from the Graduate School of Tianjin University [No. B1-2023-002 (C.W.C.)] for financial support. We also thank Zhe Feng for assistance with spectroscopic analysis and Professor Libing Zhang’s group (Tianjin University) for their help with the cyclic voltammetry study.
Author contributions
T.Z., Z.-W.Z., J.-A.M., and C.W.C. discovered the reactions. T.Z. optimized the reactions and investigated the reaction scope, synthetic utility, and mechanisms. C.W.C. wrote the manuscript with input and suggestions from T.Z., Z.-W.Z., J.N., F.Y.K., and J.-A.M. C.W.C. and J.-A.M. conceived the project, directed the research, and designed the experiments.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The experimental and analytical procedures and full spectral data are available in the supplementary materials. Crystallographic data for the structure reported in this Article has been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2350147 (14). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Additional data is available upon request from the corresponding authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jun-An Ma, Email: majun_an68@tju.edu.cn.
Chi Wai Cheung, Email: cw.cheung@cuhk.edu.hk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-53828-8.
References
- 1.Böhm, H.-J. et al. Fluorine in Medicinal Chemistry. ChemBioChem5, 637–643 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev.37, 320–330 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem.58, 8315–8359 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Zhu, W. et al. J. Fluor. Chem.167, 37–54 (2014). [Google Scholar]
- 5.Meanwell, N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem.61, 5822–5880 (2018). [DOI] [PubMed] [Google Scholar]
- 6.DrugBank Online. https://go.drugbank.com (accessed March 22, 2024).
- 7.Williams, R. E. & Njardarson, J. T. Top 200 Small Molecule Drugs by Retail Sales in 2022 Poster. https://sites.arizona.edu/njardarson-lab/top200-posters/ (accessed March 22, 2024).
- 8.Inoue, M., Sumii, Y. & Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega5, 10633–10640 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeffries, B. et al. Systematic Investigation of Lipophilicity Modulation by Aliphatic Fluorination Motifs. J. Med. Chem.63, 1002–1031 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Lovering, F., Bikker, J. & Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem.52, 6752–6756 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Talele, T. T. Opportunities for Tapping into Three-Dimensional Chemical Space through a Quaternary Carbon. J. Med. Chem.63, 13291–13315 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Meanwell, N. A. Applications of Bioisosteres in the Design of Biologically Active Compounds. J. Agric. Food Chem.71, 18087–18122 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Ricci, A. & Bernardi, L. Eds. Methodologies in amine synthesis: challenges and applications (Wiley, 2021).
- 14.Roughley, S. D. & Jordan, A. M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem.54, 3451–3479 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem.10, 383–394 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Brusoe, A. T. & Hartwig, J. F. Palladium-Catalyzed Arylation of Fluoroalkylamines. J. Am. Chem. Soc.137, 8460–8468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire, R. T., Yadav, A. A. & Stradiotto, M. Nickel-Catalyzed N-Arylation of Fluoroalkylamines. Angew. Chem. Int. Ed.60, 4080–4084 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Vollmer, T. R., Stockhausen, A. & Zhang, J.-Z. Anti-inflammatory Effects of Mapracorat, a Novel Selective Glucocorticoid Receptor Agonist, Is Partially Mediated by MAP Kinase Phosphatase-1 (MKP-1). J. Biol. Chem.287, 35212–35221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruijter, E., Scheffelaar, R. & Orru, R. V. A. Multicomponent Reaction Design in the Quest for Molecular Complexity and Diversity. Angew. Chem. Int. Ed.50, 6234–6246 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Buskes, M. J., Coffin, A., Troast, D. M., Stein, R. & Blanco, M.-J. Accelerating Drug Discovery: Synthesis of Complex Chemotypes via Multicomponent Reactions. ACS Med. Chem. Lett.14, 376–385 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biggadike, K. et al. Nonsteroidal Glucocorticoid Agonists: Tetrahydronaphthalenes with Alternative Steroidal A-Ring Mimetics Possessing Dissociated (Transrepression/Transactivation) Efficacy Selectivity. J. Med. Chem.50, 6519–6534 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Berger, M. et al. Discovery of new selective glucocorticoid receptor agonist leads. Bioorg. Med. Chem. Lett.27, 437–442 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Jaroch, S. et al. Preparation of quinoline and related compounds for use as anti-inflammatory agents. WO2003082827 (2003).
- 24.Edwards, C., Fenton, G., MacDonald, S. J. F. & Weingarten, G. G. Preparation of tetrahydronaphthalene derivatives as glucocorticoid receptor modulators. WO2006015870 (2006).
- 25.Ruiz-Castillo, P. & Buchwald, S. L. Applications of Palladium-Catalyzed C−N Cross-Coupling Reactions. Chem. Rev.116, 12564–12649 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganley, J. M., Murray, P. R. D. & Knowles, R. R. Photocatalytic Generation of Aminium Radical Cations for C−N Bond Formation. ACS Catal.10, 11712–11738 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, R. Y. & Buchwald, S. L. CuH-Catalyzed Olefin Functionalization: From Hydroamination to Carbonyl Addition. Acc. Chem. Res.53, 1229–1243 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park, Y., Kim, Y. & Chang, S. Transition Metal-Catalyzed C−H Amination: Scope, Mechanism, and Applications. Chem. Rev.117, 9247–9301 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Leitch, J. A., Rossolini, T., Rogova, T., Maitland, J. A. P. & Dixon, D. J. α-Amino Radicals via Photocatalytic Single-Electron Reduction of Imine Derivatives. ACS Catal.10, 2009–2025 (2020). [Google Scholar]
- 30.Trowbridge, A., Walton, S. M. & Gaunt, M. J. New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev.120, 2613–2692 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Kwon, K., Simons, R. T., Nandakumar, M. & Roizen, J. L. Strategies to Generate Nitrogen-centered Radicals That May Rely on Photoredox Catalysis: Development in Reaction Methodology and Applications in Organic Synthesis. Chem. Rev.122, 2353–2428 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pratley, C., Fenner, S. & Murphy, J. A. Nitrogen-Centered Radicals in Functionalization of sp2 Systems: Generation, Reactivity, and Applications in Synthesis. Chem. Rev.122, 8181–8260 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Rivas, M., Palchykov, V., Jia, X. & Gevorgyan, V. Recent advances in visible light induced C(sp3)–N bond formation. Nat. Rev. Chem.6, 544–561 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellotti, P., Huang, H.-M., Faber, T. & Glorius, F. Photocatalytic Late-Stage C−H Functionalization. Chem. Rev.123, 4237–4352 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Wang, J. Z., Lyon, W. L. & MacMillan, D. W. C. Alkene dialkylation by triple radical sorting. Nature628, 104–109 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cong, F. et al. A Bimolecular Homolytic Substitution-Enabled Platform for Multicomponent Cross-Coupling of Unactivated Alkenes. J. Am. Chem. Soc.146, 10274–10280 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Tan, T.-D. et al. Congested C(sp3)-rich architectures enabled by iron-catalysed conjunctive alkylation. Nat. Catal.7, 321–329 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao, C., Zhang, T., Liu, H. & Huang, H. Double alkyl–alkyl bond construction across alkenes enabled by nickel electron-shuttle catalysis. Nat. Catal.6, 847–857 (2023). [Google Scholar]
- 39.Qin, J.-H., Wang, Y., Ouyang, J.-Y., Liu, M. & Ouyang, X.-H. Recent progress in the synthesis of N-substituted arylamines by reductive cross-coupling of nitroarenes. Org. Chem. Front.11, 2638–2664 (2024). [Google Scholar]
- 40.Shen, Y., Funez-Ardoiz, I., Schoenebeck, F. & Rovis, T. Site-Selective α-C−H Functionalization of Trialkylamines via Reversible Hydrogen Atom Transfer Catalysis. J. Am. Chem. Soc.143, 18952–18959 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen, Y. & Rovis, T. Late-Stage N-Me Selective Arylation of Trialkylamines Enabled by Ni/Photoredox Dual Catalysis. J. Am. Chem. Soc.143, 16364–16369 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, Y.-Z. et al. Enantioselective nickel-catalyzed dicarbofunctionalization of 3,3,3-trifluoropropene. Nat. Commun.13, 5539 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karmakar, S., Silamkoti, A., Meanwell, N. A., Mathur, A. & Gupta, A. K. Utilization of C(sp3)-Carboxylic Acids and Their Redox-Active Esters in Decarboxylative Carbon-Carbon Bond Formation. Adv. Synth. Catal.363, 3693–3736 (2021). [Google Scholar]
- 44.Parida, S. K. et al. Single Electron Transfer-Induced Redox Processes Involving N-(Acyloxy)phthalimides. ACS Catal. 11, 1640−1683 (2021).
- 45.Chan, A. Y. et al. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev.122, 1485–1542 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Z.-W., Feng, Z., Ma, J.-A. & Cheung, C. W. Metallaphotocatalytic synthesis of anilines through tandem C–N transposition and C–H alkylation of alkylamines. Nat. Synth.2, 1171–1183 (2023). [Google Scholar]
- 47.Zhang, Y., Zhang, Y., Guo, Y., Liu, S. & Shen, X. Reductive quenching-initiated catalyst-controlled divergent alkylation of α-CF3-olefins. Chem. Catal.2, 1380–1393 (2022). [Google Scholar]
- 48.Zhang, Y. et al. Photocatalyzed cascade reactions of cyclopropanols and α-trifluoromethyl-substituted olefins for the synthesis of fused gem-difluorooxetanes. Angew. Chem. Int. Ed.61, e202212201 (2022). [DOI] [PubMed] [Google Scholar]
- 49.Li, Z., Zhang, Y., Zhang, Y., He, X. & Shen, X. Diastereoselective synthesis of monofluorocyclohexenes through photocatalyzed cascade cyclization of gem-difluoroalkenes and α,β-unsaturated carbonyl compounds. Angew. Chem. Int. Ed.62, e202303218 (2023). [DOI] [PubMed] [Google Scholar]
- 50.Barreiro, E. J., Kümmerle, A. E. & Fraga, C. A. M. The Methylation Effect in Medicinal Chemistry. Chem. Rev.111, 5215–5246 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Leung, C. S., Leung, S. S. F., Tirado-Rives, J. & Jorgensen, W. L. Methyl Effects on Protein−Ligand Binding. J. Med. Chem.55, 4489–4500 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schönherr, H. & Cernak, T. Profound Methyl Effects in Drug Discovery and a Call for New C–H Methylation Reactions. Angew. Chem. Int. Ed.52, 12256–12267 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Chai, D. et al. Preparation of N-[(4-hydroxy-2-oxo-1,2-dihydro-3-quinolinyl)carbonyl]glycine derivatives as prolyl hydroxylase inhibitors. WO2007038571 (2007).
- 54.Leiva, R. et al. Aniline-Based Inhibitors of Influenza H1N1 Virus Acting on Hemagglutinin-Mediated Fusion. J. Med. Chem.61, 98–118 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Xie, Y., Hu, J., Wang, Y., Xia, C. & Huang, H. Palladium-Catalyzed Vinylation of Aminals with Simple Alkenes: A New Strategy To Construct Allylamines. J. Am. Chem. Soc.134, 20613–20616 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Meng, Y. et al. Photocatalytic synthesis of 2,3-diamines from anilines and DIPEA via C–N bond cleavage and C–C bond formation. Green. Chem.26, 300–305 (2024). [Google Scholar]
- 57.Zheng, T., Feng, Z., Ma, J.-A. & Cheung, C. W. Dual Nickel/Photoredox-Catalyzed Synthesis of N-formyl N,N’-Diaryl Ethylenediamines via Multiple C-N/C-C Coupling of Nitroarenes with Trimethylamine. Adv. Synth. Catal.365, 2377–2384 (2023). [Google Scholar]
- 58.Diccianni, J., Lin, Q. & Diao, T. Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization. Acc. Chem. Res.53, 906–919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, C.-S., Zhang, B.-B., Zhong, L., Chen, X.-Y. & Wang, Z.-X. DFT insight into asymmetric alkyl–alkyl bond formation via nickel-catalysed enantioconvergent reductive coupling of racemic electrophiles with olefins. Chem. Sci.13, 3728–3379 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dawson, G. A., Spielvogel, E. H. & Diao, T. Nickel-Catalyzed Radical Mechanisms: Informing Cross-Coupling for Synthesizing Non-Canonical Biomolecules. Acc. Chem. Res.56, 3640–3653 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kostromitin, V. S., Sorokin, A. O., Levin, V. V. & Dilman, A. D. Aminals as powerful XAT-reagents: activation of fluorinated alkyl chlorides. Chem. Sci.14, 3229–3234 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blaser, H.-U. A Golden Boost to an Old Reaction. Science313, 312–313 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Maldotti, A. et al. Photochemical and photocatalytic reduction of nitrobenzene in the presence of cyclohexene. J. Photochem. Photobiol. A: Chem.133, 129–133 (2000). [Google Scholar]
- 64.Wu, J., Grant, P. S., Li, X., Noble, A. & Aggarwal, V. K. Catalyst-Free Deaminative Functionalizations of Primary Amines by Photoinduced Single-Electron Transfer. Angew. Chem. Int. Ed.58, 5697–5701 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wardman, P. Some Reactions and Properties of Nitro Radical-Anions Important in Biology and Medicine. Environ. Health Perspect.64, 309–320 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lackner, G. L., Quasdorf, K. W., Pratsch, G. & Overman, L. E. Fragment Coupling and the Construction of Quaternary Carbons Using Tertiary Radicals Generated From tert-Alkyl N‑Phthalimidoyl Oxalates By Visible-Light Photocatalysis. J. Org. Chem.80, 6012–6024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masui, M., Sayo, H. & Tsuda, Y. Anodic Oxidation of Amines. Part 1. Cyclic Voltammetry of Aliphatic Amines at a Stationary Glassy-carbon Electrode. J. Chem. Soc. B, 973–976 (1968).
- 68.Yang, Y.-F., Lin, J.-H. & Xiao, J.-C. Starting from Styrene: A Unified Protocol for Hydrotrifluoromethylation of Diversified Alkenes. Org. Lett.23, 9277–9282 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Zuo, Z. et al. Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides. Science345, 437–440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin, Q., Dawson, G. & Diao, T. Experimental Electrochemical Potentials of Nickel Complexes. Synlett32, 1606–1620 (2021). [Google Scholar]
- 71.Nelsen, S. F. & Hintz, P. J. Electrochemical Oxidation of Tertiary Amines. The Effect of Structure upon Reversibility. J. Am. Chem. Soc.94, 7114–7117 (1972). [Google Scholar]
- 72.Ding, D., Lan, Y., Lin, Z. & Wang, C. Synthesis of gem-difluoroalkenes by merging Ni-catalyzed C−F and C−C bond activation in cross-electrophile coupling. Org. Lett.21, 2723–2730 (2019). [DOI] [PubMed] [Google Scholar]
- 73.Cheng, R., Xu, C. & Zhang, X. Nickel-catalyzed regioselective coupling of 3,3,3-trifluoropropene with arylzinc reagents. Chin. J. Org. Chem.40, 3307–3313 (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The experimental and analytical procedures and full spectral data are available in the supplementary materials. Crystallographic data for the structure reported in this Article has been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2350147 (14). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Additional data is available upon request from the corresponding authors.