Abstract
The black soldier fly, Hermetia illucens (L. 1758), is an omnivorous saprophagous insect with a high potential for valorizing organic by-products rich in carbohydrates. Among carbohydrates, H. illucens relies on soluble sugars for the growth and storage lipid synthesis. This study aimed to assess the impact of common soluble sugars on the development, survival, and fatty acid composition of H. illucens. Monosaccharides and disaccharides were individually incorporated into a chicken feed diet. Cellulose was used as a control. Larvae fed glucose, fructose, sucrose, and maltose grew faster than control larvae. In contrast, lactose exhibited anti-nutritional effects on larvae, slowing down growth and reducing final individual weight. However, all soluble sugars produced larvae fatter than the control diet. Notably, the tested sugars shaped the fatty acid profile. Maltose and sucrose increased saturated fatty acid content compared to cellulose. Conversely, lactose increased the bioaccumulation of dietary unsaturated fatty acids. This study is the first to demonstrate the effect of soluble sugars on the fatty acid profile of H. illucens larvae. Our findings highlight that the tested carbohydrates have significant effects on black soldier fly fatty acid composition and can thus determine their final applications.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75730-5.
Keywords: Monosaccharides, Disaccharides, Insect lipid, Fatty acid, Insect performance
Subject terms: Animal physiology, Entomology
Introduction
Worldwide, global demand for energy and animal proteins continues to rise1. In the context of global warming, finding more environmentally friendly alternatives to fossil energy and conventional food production methods while improving production yields is essential. Insects are promising candidates to address these challenges due to their chemical composition and lower ecological footprint compared to conventional animal production2. Among insects, the black soldier fly (BSF), Hermetia illucens (L. 1758), a saprophagous species capable of feeding on a wide range of organic substrates, is an excellent candidate to face these challenges3. The valorization of these substrates through BSF breeding thus produces a new source of raw material that meets the demand of a wide range of sectors.
BSF larvae (BSFL) can be fed agricultural and agri-food by-products, such as brewers’ grains, surplus vegetables, fruit press cakes, and stale bread, which are particularly well-suited for developing BSFL growth due to their high carbohydrate (CH) content4–6. Mass production of BSFL yields two products: frass, a mix of substrate residues and dejections, which can be used as fertilizers for plant production7, and larvae, which are mainly composed of protein, lipids and chitin. Proteins and lipids are primarily intended for animal production, biofuel, and cosmetics industries8,9. Regarding chitin, this biopolymer finds applications in the agri-food, biotechnological, and healthcare sectors10.
The BSF is an autogenous holometabolous insect, meaning that its metamorphosis and reproduction, particularly energy-intensive stages of insect life cycle, can be entirely supported by nutrient reserves constituted during larval growth11. More specifically, the synthesis of proteins and lipids leads to the development of the fat body, a crucial storage organ that releases energy during nonfeeding stages of the BSF: prepupa (i.e. last larval stage during which the BSF larva, which has turned black, stops feeding and seeks a suitable environment for metamorphosis), pupa (i.e. immobile stage during which the insect undergoes metamorphosis) and adult12,13. CH serve as the primary source of energy in the BSF diet14. Among these nutrient, fibrous CH, such as hemicellulose, cellulose, and lignin, can’t be digested by BSFL unlike disaccharides and polysaccharides such as starch15,16. The digestion of CH is an essential preliminary step in the assimilation of carbohydrates, at the end of which they are hydrolyzed in the gut into monosaccharides16. Then, monosaccharides can be assimilated (i.e.passed through the gut peritrophic membrane) and metabolized to provide energy17. As introduced above, any excess energy is stored as lipids by the larva in the fat body12,18. Reserve lipids, composed of triglycerides - neutral lipids formed by one molecule of glycerol and three fatty acids- are synthesized by larvae from dietary monosaccharides. These CHs provide the acetyl-CoA substrate necessary for fatty acid (FA) biosynthesis via the fatty acid synthase and thioesterase pathways19. The FA profile of H. illucens lipids is naturally dominated by saturated fatty acids (SFA), with a high proportion of lauric acid (C12:0)19,20. Consequently, high lipid content and FA composition have quickly become a limiting factor for using entire larvae in animal feed, particularly aquaculture, where polyunsaturated fatty acids (PUFA) are required21.
Since discovering the BSF’s potential in reducing organic waste, research into the valorization of various by-products revealed that the BSFL composition is partly modulated by its diet. Currently, the modulation of the FA profile of H. illucens has continued to grow. Substrate enrichment with PUFAs via algae, fish waste, or oilseed cakes such as linseed has demonstrated the BSFL’s ability to bioaccumulate PUFAs, resulting in a higher quality FA profile for animal nutrition19,22,23. Conversely, with co-products not enriched in PUFAs, there is not always a correlation between dietary FAs and larval FA profile, suggesting the impact of other nutrients24,25. Indeed, digestible CH influence on the FA profile remains poorly understood and understudied24–27.
To our best knowledge, the nutritional effects of common monosaccharides and disaccharides remain primarily unexplored in H. illucens rearing despite their abundance in its diet. This research aims to decipher their influence on the rearing and lipid composition of BSFL. Larval growth, survival, and yield on different diets will be assessed. Then, lipid content and the FA profile will be characterized on each diet to highlight CH’s impact on the nutritional quality of BSFL.
We hypothesized that the nature of tested CH would influence (1) larval growth, (2) total lipid levels, and (3) modulate the FAs profile. Monosaccharides can be assimilated directly, unlike disaccharides, which must be hydrolyzed. Consequently, monosaccharides would be more readily available as a direct energy source or as precursors for lipogenesis via the FA synthase and thioesterase pathways, accelerating growth and promoting the accumulation of reserve lipids, particularly lauric acid, in H. illucens larvae.
Results
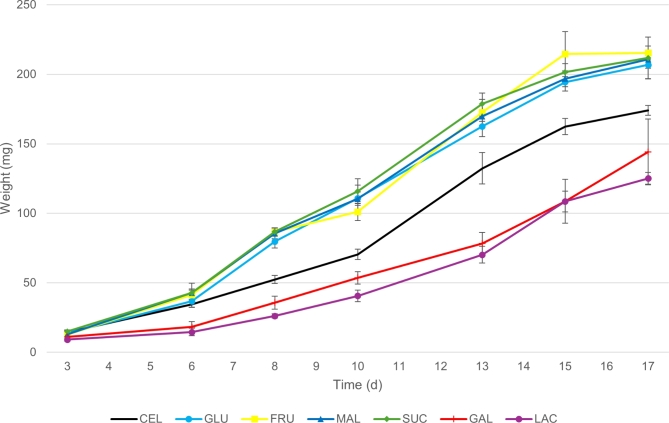
Tested CHs influenced the mean weight of the larvae during their growth (Fig. 1). FRU, GLU, SUC, and MAL increase larval weight gain in the same way as the control diet (CEL). In contrast, LAC and GAL appeared to decrease larval development. Notably, LAC significantly negatively impacted larval growth compared to SUC throughout the growth period, with respectively 9.16 ± 1.10 mg and 15.00 ± 1.01 mg at day 3 (F6,21=12.77, p < 0.001; Figs. 1) and 125.11 ± 4.26 mg and 211.79 ± 14.93 mg at day 17 (F6,21=38.57, p < 0.001; Fig. 1).
Fig. 1.
Larval growth of Hermetia illucens larvae fed different monosaccharides (fructose (FRU), galactose (GAL), glucose (GLU)), disaccharides (lactose (LAC), maltose (MAL), sucrose (SUC)) and cellulose (CEL) as control. Each point on the curves represents the mean individual weight (mg) calculated from weighing 20 larvae randomly selected from a population of 100 larvae (n = 4). Errors bars indicate SD.
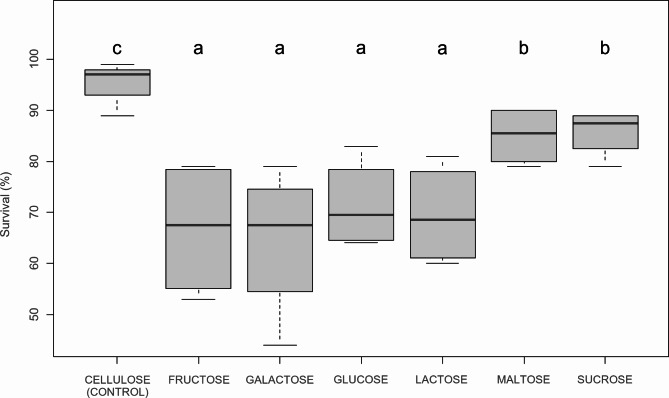
The CEL diet ensured an excellent larval survival rate of 95.5 ± 3.8%. Also, the survival of H. illucens fed diet containing soluble CH has been decreased (GLM: χ2 = 107.13, df = 21, p < 0.001), and among the studied CHs, MAL, and SUC (disaccharides) caused less mortality than GLU, FRU, GAL (monosaccharides) and LAC (EMM: p < 0.001, Fig. 2).
Fig. 2.
Boxplots of the survival of Hermetia illucens larvae fed different monosaccharides (fructose, galactose, glucose), disaccharides (lactose, maltose, sucrose) and cellulose as control. Treatments sharing the same letter are not significantly different from each other (EMM, p > 0,05).
All the tested diets enabled the larvae to reach the prepupal stage. However, tested CH tended to lengthen the larval development time (F6,21=9.60, p < 0.001; Table 1). Particularly, larvae fed GAL and LAC took longer to reach the prepupal stage compared to those reared on the CEL (CEL-GAL: p < 0.001; CEL-LAC: p < 0.001; Table 1).
Table 1.
Performance of Hermetia illucens larvae fed different monosaccharides (fructose, galactose, glucose), disaccharides (lactose, maltose, sucrose) and cellulose as control.
| Carbohydrates | Mean time to first prepupae ± SD (day) | Mean yield rate ± SD (g of larvae/day) | Mean lipid content ± SD (%MS) |
|---|---|---|---|
| CELLULOSE | 17.75 ± 0.96a | 0.95 ± 0.04c | 23.19 ± 0.70a |
| FRUCTOSE | 19.75 ± 2.06ab | 0.70 ± 0.09b | 31.92 ± 1.48b |
| GALACTOSE | 22.25 ± 0.50b | 0.51 ± 0.09a | 32.61 ± 2.02b |
| GLUCOSE | 19.50 ± 1.00ab | 0.74 ± 0.06b | 31.76 ± 0.80b |
| LACTOSE | 22.25 ± 0.50b | 0.48 ± 0.06a | 32.50 ± 0.40b |
| MALTOSE | 20.00 ± 0.00ab | 0.86 ± 0.04bc | 33.45 ± 1.02b |
| SUCROSE | 19.00 ± 1.15ab | 0.93 ± 0.09c | 32.37 ± 0.27b |
| Statistical analyses | F6,21 = 9.60, p < 0.001 | F6,21 = 22.39, p < 0.001 | F6,21 = 40.45, p < 0.001 |
Means with different letters significantly differ among columns.
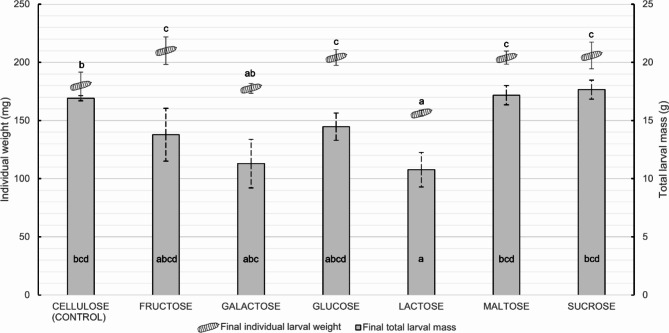
Tested CH also had various effects on the larvae’s body weight, which reached 180,19 ± 11,35 mg for larvae fed the CEL diet (F6,21=16.86, p < 0.001; Fig. 3). FRU, GLU, MAL, and SUC enabled larvae to achieve an average final weight of over 200 mg, which was significantly higher than CEL (p < 0.05). In contrast, larvae fed GAL and LAC had lower weights, averaging 177.64 ± 4.23 mg and 156.30 ± 2.59 mg respectively (p < 0.05). This impact is even more significant for LAC, with a lower final weight than the control diet (CEL-LAC: Diff = 23.89 mg; p = 0.03; Fig. 3).
Fig. 3.
Final individual larval mean weight indicated by larvae spot (mg) and total larvae mass indicated by bar plots (g) of Hermetia illucens fed different monosaccharides (fructose, galactose, glucose), disaccharides (lactose, maltose, sucrose) and cellulose as control. Bar plot letters indicate the significantly different groups for total larval mass (p < 0.001). Letters linked to larvae spots indicate a significantly different group for the individual larvae weight (p < 0.001). Error bars indicate SD.
The greatest individual weights were not correlated with the greatest final total mass of the larval population. Indeed, diets containing FRU, GLU, MAL, and SUC did not increase the total mass of larvae produced per tank compared to CEL (Fig. 3). However, LAC significantly decreased total mass (CEL-LAC: Diff = 9.14 g; p < 0.001; Fig. 3).
Table 1 presents the production yields (g of larvae per day). Interestingly, CEL, MAL, and SUC gave similar best yields (Table 1). In contrast, FRU, GAL, GLU, and LAC decreased the production yield compared to CEL (Table 1). GAL and LAC gave the worst results, halving the yield and reaching respectively only 0.51 ± 0.09 g of larvae/day and 0.48 ± 0.06 g of larvae/day (Table 1).
Monosaccharides and disaccharides increased the lipid content of BSF larvae (Table 1). The CEL diet produced larvae with a lipid content of 23.19 ± 0.70% of DM content. In contrast, larvae fed soluble sugars had a mean lipid content exceeding 30% (Table 1). However, tested CH increased the fat content equivalently.
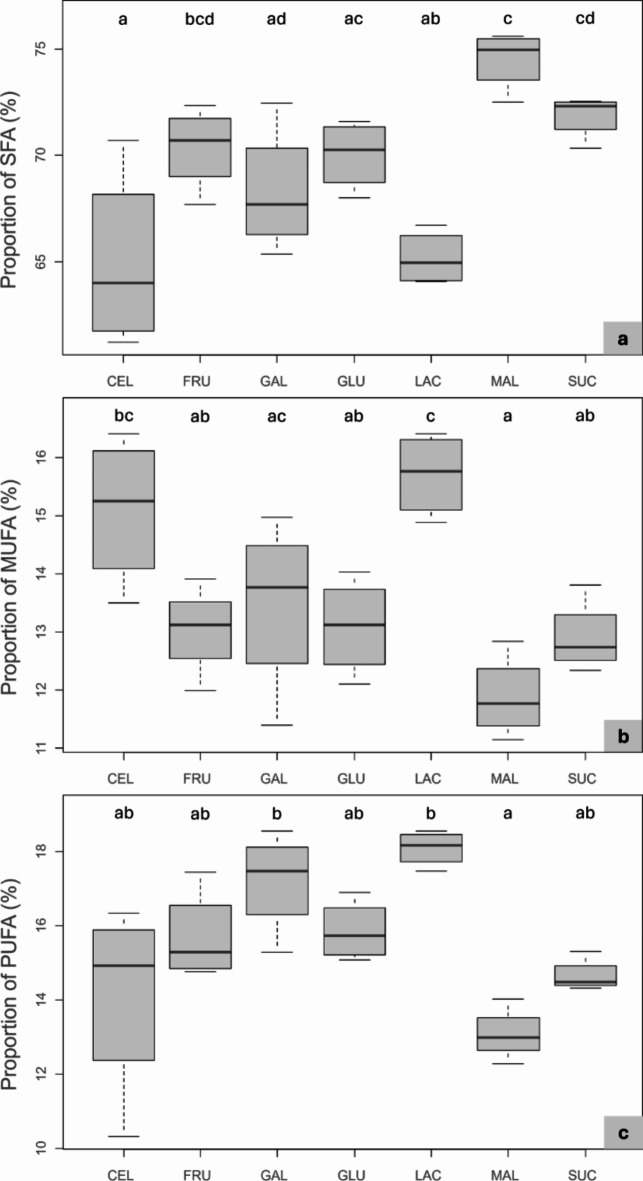
As hypothesized, tested CH variably shaped the FA profiles of the larvae (Fig. 4). The SFA amount remained higher for all diets, reaching more than 60%. MAL and SUC unbalance the FAs profile in increasing SFA content. In the case of MAL, on the one hand, this imbalance leads mainly to the decrease of mono-unsaturated fatty acids (MUFA) (F6,21= 7.47; p < 0.001; Fig. 4.). For SUC, on the other hand, the decrease is more evenly shared between MUFAs and PUFAs. LAC and MAL have opposite effects on FAs profile (SFA: F6,21= 8.74; p < 0.001; MUFA: F6,21= 7.47; p < 0.001; PUFA: χ2 = 19.60; Df = 6; p < 0.001; Fig. 4). The low proportion of SFA in LAC fed larvae appears to increase MUFA content. Specifically, MUFA levels were higher in LAC-fed larvae compared to other soluble sugars, except for GAL (F6,21=7.47; p < 0.001; Fig. 4).
Fig. 4.
Boxplots of fatty acids composition of Hermetia illucens larvae fed different monosaccharides (fructose (FRU), galactose (GAL), glucose (GLU)), disaccharides (lactose (LAC), maltose (MAL), sucrose (SUC)) and cellulose (CEL) as control. Results are presented as % of total FAMEs. Treatments denoted with different letters significantly differ (p < 0.001). (a) Proportion of saturated fatty acids; (b) monounsaturated fatty acids; (c) polyunsaturated fatty acids.
Concerning identified FAs, lauric acid (C12:0) predominates in all observed profiles (over 40%). Other SFAs present, in descending order, include palmitic acid (C16:0) (less than 10%), stearic acid (C18:0) (less than 2.5%), and decanoic acid (C10:0) (less than 1.5%). MUFAs are primarily represented by oleic acid (C18:1n9) (less than 9.5%), while PUFA are mainly comprised of linoleic acid (C18:2n6) (less than 13.0%) (see Supplementary Table S1). Additionally, a small percentage of compounds could not be identified, particularly in the profile of CEL larvae, where the unidentified compound number 9 (UND9) accounted for an average of 2.46 ± 0.52% (see Supplementary Table S1). GC×GC-FID analysis suggested it could be a fatty acid with 20 carbon atoms and five or six double bonds (see Supplementary Fig. S5).
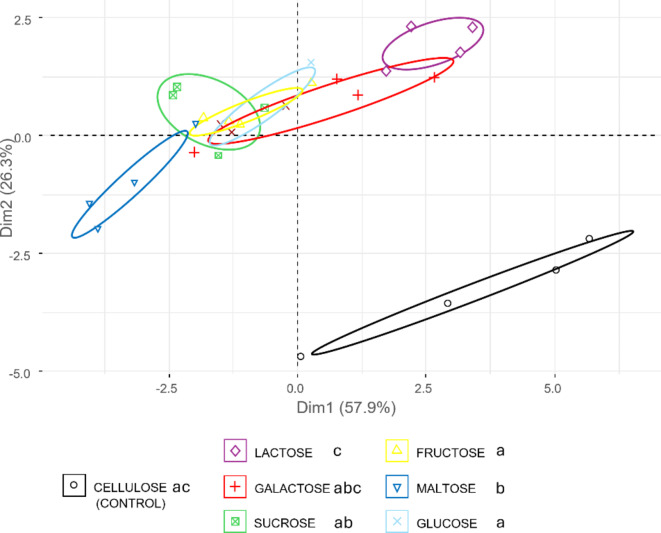
PERMANOVA analysis identified three distinct groups based on FAs profiles (F6,21=7.79, p < 0.001; Fig. 5). This is illustrated by the principal component analysis (PCA) of the FA profiles and is explained by two components (Fig. 5). The primary component accounts for 57.9% of the variance. It includes, in order of importance, lauric acid (C12:0), oleic acid (C18:1n9), palmitic acid (C16:0), stearic acid (C18:0), and linolenic acid (C18:3n3) (see Supplementary Fig. S4). The second component accounts for 26.3% of the variance and includes, in order of importance, decanoic acid (C10:0) and linoleic acid (C18:2n6 cis) (see Supplementary Fig. S4). Profiles from diets containing monosaccharides (FRU, GAL, and GLU) exhibit similar characteristics. In contrast, disaccharides produce distinct profiles, with MAL and SUC on one side and LAC on the other. Specifically, MAL is the only sugar that alters the FAs profile compared to CEL. Moreover, the MAL profile varies notably from the FRU and GLU profiles. Particularly, the MAL profile shows the highest proportion of C12:0 (54.59 ± 2.17%), setting it apart from the CEL (43.10 ± 5.01%), LAC (43.35 ± 1.31%), FRU (48.90 ± 1.97%), and GLU (48.38 ± 2.17%) profiles (see Supplementary Tab. S1). The MAL profile also exhibits the lowest content of C18:1n9 (9.52 ± 0.50%), further distinguishing it from the LAC (12.86 ± 0.52%) and CEL (12.40 ± 1.31%) profiles. A similar trend is observed for C16:0. On the second component, the LAC profile shows the highest content of C18:2n6 (17.22 ± 0.46%), contrasting with MAL, which has the lowest (12.58 ± 0.67%). C18:2n6 also distinguishes LAC from the control (CEL), which exhibits a lower level (13.41 ± 2.48%) (see Supplementary Tab. S1).
Fig. 5.
PCA score plot for fatty acid profiles of Hermetia illucens larvae fed different monosaccharides (fructose, galactose, glucose), disaccharides (lactose, maltose, sucrose) and cellulose as control.
Discussion
To investigate the nutritional impact of soluble sugars on H. illucens larvae, cellulose (CEL in chicken feed has been substituted by glucose (GLU), fructose (FRU), galactose (GAL), maltose (MAL), sucrose (SUC), and lactose (LAC). Here in, monosaccharides and disaccharides showed variable effects on BSF larvae development, survival and composition. For example, GLU, FRU, and their disaccharide forms (MAL and SUC) positively supported larval growth, allowing them to achieve higher final weights than CEL. Unlike CEL, which is indigestible, GLU, FRU, and SUC can bypass the intestinal barrier and serve as an important source of nutrients in formulated diets16,28. MAL lacks a specific animal transporter and is presumed to undergo hydrolysis into two glucose molecules before assimilation15. These molecules serve as immediate energy sources or are stored as lipids in the insect18. First, regarding the latter, some intramodal variation observed may be due to slight sex ratio differences. Indeed, in H. illucens, reproduction can be entirely autogenous, with adult females naturally possessing adequate egg-laying reserves and being heavier than males29. Still, lipid accumulation in BSFL correlates with dietary soluble CH intake, as previously observed with GLU and xylose26,30. For example, Li et al.30 observed that lipid content of BSF larvae was increased by 7.78% compared to the control when 8% of GLU was added to the larval diet. Our study’s findings align with these observations, showing that larvae fed soluble sugars exhibited higher fat content than those fed the CEL diet with, in comparison, an 8.57% increase for the addition of GLU. Surprisingly, similar results were observed in larvae fed GAL and LAC despite adverse effects on larval growth, final weight, and survival. LAC-fed larvae were notably smaller than those on the CEL diet, yet their fat content matched larvae fed other soluble sugars. These outcomes underscore lactose’s anti-nutritional impact on BSFL. Firstly, CH were present in large quantities in the feed. It is possible that a saturation of the absorption and hydrolysis systems was reached, respectively, for monosaccharides and disaccharides, creating a bottleneck in the assimilation process. Regarding hydrolysis, it is carried out by alpha- and beta-glucosidases31. These enzymes have preferred substrates depending on their size and the chemical bond between the constituent monosaccharides (alpha or beta bond)15. The hydrolysis of LAC into GLU and GAL is carried out by beta-galactosidase, an enzyme whose activity in the gut of BSF has already been demonstrated32. However, it might have been expressed in insufficient quantities compared to the amount of LAC ingested by the larva. In contrast, alpha-glucosidase maltase and sucrase, known to be abundantly expressed in insects15, would have been able to degrade a greater quantity of MAL and sucrose SUC, limiting this saturation effect. Secondly, the anti-nutritional effect could be possibly due to a reduced stimulation of the insect’s gut amylase activity and slower feeding behavior than other treatments. Indeed, soluble sugars have been identified as stimulants of enzymatic activities crucial for insect digestion, such as amylase, and as triggers of feeding responses33–35. The degree of stimulation varies with the sugar’s molecular structure. Indeed, disaccharides, which require hydrolysis before absorption, tend to stimulate amylase more than their constituent monosaccharides34. Conversely, LAC exhibits a more subdued effect, and its inability to support insect growth has been noted across various species33,35. For instance, in the pest Heliothis zea (Boddie 1850), no LAC hydrolytic activity was detected in caterpillar midgut enzymatic extracts36.
Regarding the FAs profile, our results indicated considerable modulation by the tested CH. Notably, lauric acid (C12:0) predominated in all profiles despite constituting less than 1% of the total FAs in the diet (see Supplementary Tab. S1). This aligns with previous findings that lauric acid is synthesized in H. illucens from dietary CH through pathways involving acetyl-CoA carboxylase and FA synthase19,27,37. Our findings confirm CEL is mainly indigestible and functions as a “bulk agent” in BSF control diets, as it’s considered in several studies on BSFL38–40. Substituting CEL with monosaccharides and disaccharides, except LAC, enhances the proportion of C12:0, indicating increased assimilation of CHs by the larvae. Interestingly, the MAL and SUC disaccharides promoted lauric acid synthesis more effectively than their constituent monosaccharides, suggesting that the availability of GLU and FRU is increased despite their polymerization degree and the presumed absence of disaccharides transporter in the larval gut of H. illucens15 given that D. melanogaster is only animal species where a sucrose transporter has been identified. However, while GLU and FRU are theoretically more assimilable for BSF, they are also more assimilable for substrate and gut microorganisms, potentially leading to their faster degradation and reduced availability to larvae compared to disaccharides.
At first glance, the comparable lipid content of LAC and MAL fed larvae suggests a similar bioavailability of these sugars. However, surprisingly, the FA profile of LAC appeared to be enriched in UFAs compared to MAL, notably with a lower C12:0. One hypothesis to explain this difference is that LAC may stimulate the bioaccumulation of dietary FAs at the expense of FA synthase from acetyl-CoA. Supporting this hypothesis, LAC larvae had the lowest proportion of capric acid (C10:0) (0.77 ± 0.13%), which was lower than the CEL diet (1.27 ± 0.16%), suggesting reduced activity of FA synthase and thioesterase enzymes19. Secondly, dietary FAs are recognized as the primary drivers influencing the UFA composition of H. illucens27. In our experiments, linoleic acid (C18:2n6) constituted 54.81% of the dietary fatty acids, with LAC larvae showing a proportion of 17.22 ± 0.46%, compared to 12.58 ± 0.67% in MAL. A similar trend was observed for oleic acid (cis + trans C18:1n9) (23.22% in the diet). The proportions of alpha-linolenic acid (C18:3n3) also support the bioaccumulation hypothesis. This FA is known to be accumulated by BSFL when enriched in the substrate, such as by adding linseed cake, reaching up to 6–9% of total larval FAs19. In enriched diet, C18:3n3 can reach as high as 35% of total dietary FAs. However, C18:3n3 accounted for only 2.51% of the FAs profile in our study. Although naturally occurring in low proportions in our larvae, it was higher in LAC larvae (0.87 ± 0.02%) compared to MAL (0.49 ± 0.04%) (p < 0.001; See Supplementary Tab.S1). The CEL diet showed an intermediate proportion of 0.72 ± 0.18%. Finally, the proportion of palmitic acid (C16:0) in BSF larvae reflects contributions from synthetic pathways and dietary FAs19. Hoc et al.19 observed a decrease in C16:0 synthesis when the diet was enriched with linseed meal, attributing this to reduced availability of the substrate acetyl-CoA due to decreased CH proportions. Surprisingly, MAL larvae exhibited the lowest proportion of C16:0 (10.46 ± 0.77%), whereas LAC showed a higher proportion at 12.85 ± 0.27% (p < 0.05; See Supplementary Tab.S1), despite both diets having identical CHs content and MAL demonstrating greater bioavailability. These findings underscore the intricate influence of nutrients on the digestion and metabolism of BSFL. At present, studies on this subject seem to have been more thorough in Lepidoptera than in Diptera. In caterpillars, LAC has been identified as a weak stimulator of feeding behavior compared to other soluble sugars like SUC and FRU34,35. Specifically, in Spodoptera littoralis (Boisduval 1833), MAL consumption stimulates amylolytic activities in the gut more than LAC34. Similar effects in BSFL could explain the heightened stimulation of C12:0 synthesis pathways in MAL larvae due to increased assimilable CH in the gut, sustained feeding, and the action of intestinal amylases. A less stimulated feeding rhythm in the presence of LAC could also account for the slower growth observed in LAC larvae. Additionally, Liu Yanxia et al.27 noted that lipids have a longer shelf life in the H. illucens substrate than CH. Consequently, LAC larvae may have relied more on dietary lipids to complete their development, potentially increasing their final lipid content and modulating their FAs profile.
To our knowledge, very few studies have tested the influence of including monosaccharides and disaccharides in the diet of BSF on its FA profile. First, Li et al.30 evaluated the impact of GLU and xylose and observed lipid levels similar to ours at inclusion rates of 8%. The FA profile was not detailed and predominantly composed of SFA, but no difference was reported between the two sugars or when provided simultaneously30. Furthermore, Cohn et al.41 did not demonstrate an effect on the related FA profile, including 20% GLU, SUC, FRU, and GAL in chicken feed. The profiles were obtained based on technical and not biological replicates, which may have constrained statistical analysis, as explained by the authors. Moreover, the absence of isoglucidic controls (with CEL) limits the interpretation of the results. Recently, two studies by Nugroho R.A. et al. showed abnormal FA profiles42,43. In the first study, Nugroho R.A. et al.43 tested the influence of adding FRU to fermented palm kernel meal. The FA profiles of the larvae obtained showed abnormally high PUFA levels, with over 90% in a diet containing 10% FRU (similar to our study). Although this diet contained fish pellets rich in PUFA, the reported values for the FA profile of larvae from the control diet composed of 100% fermented PKM do not match any previously reported profile, notably with an exceptional level of 17.77 ± 1.67% of C18:3n3 and the presence of 26.08 ± 0.20% of conjugated linoleic acid (C18:2n6t), a rare isomer of linoleic acid. The second study showed similar results, including FRU, GLU, MAL, and SUC in fermented palm kernel meal42. These studies, like ours, highlighted major difficulties in comparing dietary trial results on BSF larvae, such as control selection, interactions with other nutrient sources, and FA analysis methods.
During the experiment, we observed that the color and odors of the substrates evolved differently depending on the diets used. This suggests that microorganisms may have played a role in the observed results at the substrate and the larval digestive system. Indeed, monosaccharides and disaccharides are easily assimilated by colonizing microorganisms. Microorganisms’ rapid consumption of soluble sugars could have led to the massive release of microbial metabolic waste products such as ethanol, lactic acid, short-chain fatty acids like acetic, propionic, butyric acids, and CO244. Some of these compounds may explain the lethal toxic effect on larvae, an effect also observed by Cohn et al.41 under similar development conditions. Ethanol, for instance, is harmful to insects45. Significant CO2 emission can cause its accumulation at the bottom of bins, rendering the atmosphere anoxic if air circulation does not allow for its removal. As for short-chain fatty acids, their effects on insects, particularly H. illucens, are still understudied, although lactic, propionic, and butyric acids have shown lethal effects on Callosobruchus maculatus (Fabricius 1775)46. In Drosophila melanogaster Meigen 1830, these short-chain fatty acids are olfactory markers guiding females to oviposition sites, suggesting a beneficial role in larval development47. However, acetic acid has been categorized as harmful, significantly slowing larval development47. Conversely, in D. melanogaster, a protective role of microbial-derived lactic acid against invasive intestinal microbes has recently been identified48. Additionally, within the digestive system, microorganisms also play roles in insect CH digestion49. The physiological effects of short-chain fatty acids from gut microbiota, such as feeding rate and gene expression, have been described in vertebrates50. They could also have nutritional effects on H. illucens larvae and partly contribute to the modulation of the FA profile. A study on the nutritional effect of these microbial fermentation products would clarify their impact on H. illucens nutrition and guide future research on beneficial or detrimental microorganisms for its development and the valorization of CH-rich substrates. In this regard, the involvement of microorganisms in the digestion of mass-reared insects is increasingly being studied. The insect is beginning to be considered a bioreactor, offering pH and oxygenation conditions favorable to the development of microorganisms specialized in the degradation of nutrients that are difficult for the insect to digest or in detoxification51. Recently, Xiang et al.52 demonstrated, for example, that inoculating organic waste with a bacterial mix allowed BSF to recruit bacteria specialized in lignocellulose degradation, improving its breakdown in the substrate compared to substrate without larvae.
Finally, regarding organic waste valorization by H. illucens, CEL and SUC diets yield the highest larval mass production per day. This means that despite lower individual final weights, the total mass of larvae produced from the substrate composed of non-digestible CH is comparable to that from isoglucidic diets containing monosaccharides and disaccharides. In our study, it is important to note that the other nutrients were present in sufficient quantities to support the growth of the larval population, and that the inclusion of CEL must have some limit. However, the final composition of the larvae differs, highlighting the importance of choosing the right strategy for insect valorization. Whole fed-CEL larvae would be more suitable for animal feed due to their lower fat content and lower lauric acid levels, unlike larvae from the SUC or MAL diet, which would need to be de-fatted through pressing to valorize the oil, particularly in the biofuel sector. LAC is found in the dairy industry by-products such as whey from cheese production. Its use (at 3.5% lactose) recently improved larval final weight53. However, the control diet in this study contained half the lipid content. Therefore, the antinutritional effect of LAC may have been compensated by the bioaccumulation of dietary lipids by the larvae.
The nature of monosaccharides and disaccharides significantly impacts the growth of BSFL and modulates its FA profile, as previous research suggested. Specifically, LAC appears to play an antinutritional role in larval development by limiting the utilization of CH in favor of absorbing dietary lipids, thereby promoting the bioaccumulation of UFA. In this context, conducting bioassays with diets combining PUFA with LAC would be interesting. Furthermore, the role of microorganisms, especially the microbial metabolites derived from sugar fermentation processes such as SCFA’s, remains a research topic worthy of exploration.
Methods
Insect rearing
Insects come from the Laboratory of Functional and Evolutionary Entomology BSF colony in Gembloux Agro-Bio Tech (Belgium), established in 2017 (see Hoc et al.19 for more details on the rearing method). For experimental trials, 2.0 g of daily BSF eggs are randomly harvested in the reproduction cages and incubated on 2.0 kg of 70% moist chicken feed (Aveve, Leuven, Belgium). Five days after hatching, the larvae are sieved from the substrate and counted by hand for experimental purposes. The initial weight of each batch was measured. The mean individual weight was 7.125 ± 0.41 mg and means for each treatment are presented in Supplementary Tab. S2.
Feeding assays
The diet formulation has been adapted from Barragan-Fonseca et al.38. Briefly, a compromise was found between similar chicken feed mass per larva, close dry matter (DM) content, significative CH incorporation (10% based on fresh diet), and texture since mono- and di-saccharides do not have texturizing properties. Tested CH (i.e., soluble sugars) were individually incorporated as an autoclaved aqueous solution (15.9%) in grinded chicken feed composed of 16.0% protein, 5.0% total lipid, 11.9% ash and 4.8% cellulose according to the manufacturer’s information (Chicken Pellet, AVEVE, Leuven, Belgium). In each 750-mL tank (17.20 × 11.50 × 6.00 cm, AVA, Temse, Belgium), 101.9 g of autoclaved CH solution was mixed with 37.8 g of chicken feed. For each diet, the dry matter content was 37.0% and was isoprotein (11.7%), isolipid (3.7%), and isoglucid (26.9% of added CH). The tested CH were glucose (GLU), fructose (FRU), galactose (GAL), maltose (MAL), sucrose (SUC), and lactose (LAC). A control diet was made with cellulose (CEL), considered as a non-digestible for H. illucens larvae38. One hundred 5-day-old larvae were placed in trays fitted with a lid pierced in the center with a one-centimeter hole and covered with plastic mosquito netting. Four replicates per diet were performed.
Larval growth, survival, and yields
Weight measurements of the larvae began three days after the beginning of the experiment. For each measurement, twenty larvae were extracted from the substrate using sterile tepid tap water and tweezers, dried, and weighed (STX223, Ohaus Scout, Parsippany, USA). After weighing, the larvae were returned to the center of the substrate. Measurements were taken three times a week at regular intervals until the appearance of the first prepupae. At this point, all larvae were collected, counted, and weighed as described previously. Larvae in stage 6 (i.e. white larvae corresponding to the larval stage preceding the prepupal stage) and prepupae (i.e. last larval stage during which the BSF larva, which has turned black, stops feeding and seeks a suitable environment for metamorphosis) were separated and stored at − 18 °C for composition analysis. Yields were calculated as the ratio between the total mass of insect (stage 6 larvae and prepupae) produced per tray (g) and development time (d). All means are presented in the text as follows: mean ± SD.
Lipid content and fatty acid profile
All the subsequent steps involving solvents (hexane (Hex), chloroform (CHCl3), methanol (MeOH)) were carried out under a fume hood, and nitrile gloves, an apron, and protective goggles were required.
White larvae were dried for 72 h in a FreeZone6 freeze-dryer (Labconco Corp., Kansa City, MO, USA) and ground (IKA A10, Staufen, Germany). Total lipids were extracted from ±1 g powder by Folch’s method54. The remaining water content of each freeze-dried sample was determined in duplicate using a moisture analyzer (MA 150, Sartorius, Göttigen, Germany) to correct the amount of total lipids.
Total lipids were trans-esterified under acidic conditions to obtain fatty acid methyl esters. Briefly, ~ 10 mg of lipid/100 µL of CHCl3 solution (100 µL) was evaporated with nitrogen in an 8 mL Pyrex© tube (SciLabware - DWK Life Sciences, London, UK). The tube was heated in a water bath at 70 °C for 90 min in the presence of Hex (0.5 mL) (PESTINORM ®SUPRATRACE n-Hexane > 95% for organic trace analysis, VWR Chemicals, Radnor, PA, USA) and Hex/MeOH/BF3 (20/25/55) solution (0.5 mL). After cooling, 10% H2SO4 aqueous solution (0.2 mL) and saturated NaCl solution (0.5 mL) were added. The tube was stirred, and the mixture was covered with pure Hex (8.0 mL). A portion of the upper phase was transferred to a vial for gas chromatography coupled with a flame ionization detector (GC-FID) analysis. Samples were analyzed using a Trace GC Ultra instrument (Thermo Scientific, Waltham, Massachusetts, USA) equipped with a split/splitless injector (240 °C) in split mode (split flow: 10 mL/min), a Stabilwax®-DA column (30 m, 0.25 mmID, 0.25 μm, Restek Corp., Bellefonte, PA, USA) and an FID (250 °C). The temperature program was set as follows: 50 °C for 1 min, increase to 150 °C at 30 °C/min, increase to 240 °C at 4 °C/min and 240 °C for 5 min. Hex was used as a blank and a reference standard containing 37 fatty acid methyl esters for identification (Supelco 37 component FAMEmix, Sigma-Aldrich, Overijse, Belgium). The identification of unsaturated fatty acids (UFA) was confirmed by comprehensive two-dimensional GC (GC×GC-FID) to accurately determine the presence of isomers by slightly adapting the method from Ferrara et al.55. Instrumental details can be found in Supplementary Tab. S3 and results as Supplementary Fig. S5.
Statistical analysis
Data were implemented in an Excel table (Microsoft Corporation, Redmond, Washington, USA). Statistical analyses were conducted using R Studio (version 2023.12.1 + 402, Boston, USA)56. Data on larval weights, development time, and production yields were assessed using a linear model (LM) (“lm” command, R-package “stats”56), as they follow a Gaussian distribution. Survival, analyzed with a binomial model, was evaluated using a General Linear Model (GLM) (“glm” command, R-package “lme4”57), . Normality and homoscedasticity were confirmed using Shapiro tests (“shapiro.test” command, R-package “stats”56), and data dispersion analyses (“betadisper” command, R-package “vegan”58), . Significant p-values (p < 0.05) from the LM or GLM tests were followed by a pairwise analyses using EMM test (“emmeans” command, R-package “emmeans”59), to pinpoint substantial differences among groups.
The comparison of the complete FA profiles was conducted with a permutational multivariate analysis of variance (i.e., permMANOVA; “adonis2” command, R-package “vegan”58), using a Euclidean distance matrix and 999 permutations. This facilitated the identification of FAs influenced by the nature of dietary carbohydrates. Significant differences in FA profiles were further analyzed using pairwise comparison. The data were then visualized using principal component analysis (PCA) (“PCA” command, R-package “FactoMineR”60), . The FAs driving these differences were determined by interpreting the correlation circle. These candidates were confirmed using one-way analysis of variance (ANOVA) (“aov” command, R-package “stats”56), followed by a post-hoc Tukey test (“TukeyHSD” command, R-package “stats”56), . Before analysis, normality was assessed using the Shapiro-Wilk test, and homoscedasticity was checked using the Bartlett test (“bartlett.test” command, R-package “stats”56), . If one of both assumptions were not satisfied, comparisons were made using nonparametric analyses (“kruskal.test” command, R-package “stats”56), followed by post-hoc Dunn’s test was applied (“dunn.test” command, R-package “dunn.test”56).
AI writing tool
The final draft was reviewed using Grammarly Editor as an English corrector (Grammarly Inc., San Francisco, California, USA)61.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Pr. M-L Fauconnier (Laboratory of Chemistry of Natural Molecules, Gembloux Agro-Bio Tech, University of Liège, Belgium) for the support of laboratory equipment and Pr. Y. Brostaux (Applied Statistics, Computer Science and Modeling Laboratory, TERRA Teaching and Research Center, Gembloux Agro-Bio Tech, University of Liège, Belgium) for guidance in the statistical processing of data.
Author contributions
Conceptualization, J.C. and R.C.M.; Methodology, J.C. and R.C.M.; Investigation, J.C., C.M. and R.C.M; Data collection: J.C., N.D. and H.L.; Chemical analysis: J.C., D.F. and G.P.; Resources, G.P., C.B. and F.F.; Writing Original Draft, J.C. and R.C.M.; Writing Review & Editing, G.P., C.M., H.L. and F.F.; Supervision, R.C.M and F.F.; Funding Acquisition, F.F. and R.C.M. All the authors critically reviewed the manuscript for its intellectual content and gave their approval for the final version to be submitted.
Funding
Mr. Hugo Luttenschlager and Mr. Nicolas Deville are financially supported by the Walloon Region (Service Public de Wallonie; DGO6) from Belgium, as part of the ASTIPOR project (D65-1438) obtained under Walloon Recovery Plan (https://www.wallonie.be/en/plans-wallons/plan-de-relance-de-la-wallonie). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joachim Carpentier, Email: joachim.carpentier@uliege.be.
Rudy Caparros Megido, Email: r.caparros@uliege.be.
References
- 1.Kim, S. W. et al. Meeting global feed protein demand: Challenge, opportunity, and strategy. Annu. Rev. Anim. Biosci.7, 221–243 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Van Huis, A. et al. EDIBLE INSECTS Future Prospects Fo Food and Feed Security. vol. 171 (2013).
- 3.Caparros Megido, R. et al. A worldwide overview of the status and prospects of edible insect production. Entomol. Gen.44, (2024).
- 4.Rehman, K. ur et al. Black soldier fly, Hermetia illucens as a potential innovative and environmentally friendly tool for organic waste management: A mini-review. Waste Manag. Res.41, 81–97 (2023). [DOI] [PMC free article] [PubMed]
- 5.Scala, A. et al. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Scie. Rep.10, 19448 (2020). [DOI] [PMC free article] [PubMed]
- 6.Shu, M. K. et al. Antibacterial properties of oil extracts of black soldier fly larvae reared on bread waste. Anim. Prod. Sci.64, (2024).
- 7.Schmitt, E. & de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem.25, 100335 (2020). [Google Scholar]
- 8.Franco, A. et al. Lipids from Hermetia illucens, an Innovative and Sustainable Source. Sustainability13, (2021).
- 9.Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed6, 27–44 (2020). [Google Scholar]
- 10.Kaczor, M., Bulak, P., Proc-Pietrycha, K., Kirichenko-Babko, M. & Bieganowski, A. The variety of applications of Hermetia illucensin industrial and agricultural areas—review. Biology12, (2023). [DOI] [PMC free article] [PubMed]
- 11.Hoc, B., Noël, G., Carpentier, J., Francis, F. & Caparros Megido, R. Optimization of black soldier fly (Hermetia illucens) artificial reproduction. PLoS ONE14, (2019). [DOI] [PMC free article] [PubMed]
- 12.Arrese, E. L. & Soulages, J. L. Insect Fat Body: Energy, Metabolism, and Regulation. vol. 55 (2010). [DOI] [PMC free article] [PubMed]
- 13.Seyedalmoosavi, M. M., Mielenz, M., Veldkamp, T., Daş, G. & Metges, C. C. Growth efficiency, intestinal biology, and nutrient utilization and requirements of black soldier fly (Hermetia illucens) larvae compared to monogastric livestock species: a review. J. Anim. Sci. Biotechnol.13, 31 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinotti, L. & Ottoboni, M. Substrate as insect feed for bio-mass production. J. Insects Food Feed7, 585–596 (2021). [Google Scholar]
- 15.Holtof, M., Lenaerts, C., Cullen, D. & Vanden Broeck, J. Extracellular nutrient digestion and absorption in the insect gut. Cell Tissue Res.377, 397–414 (2019). [DOI] [PubMed]
- 16.Miguel-Aliaga, I., Jasper, H. & Lemaitre, B. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics210, 357–396 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rockstein, M. Biochemistry of Insects. (New York, 1978).
- 18.Bellezza Oddon, S., Biasato, I., Resconi, A. & Gasco, L. Determination of lipid requirements in black soldier fly through semi-purified diets. Sci. Rep.12, 10922 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoc, B. et al. About lipid metabolism in Hermetia illucens (L. 1758): on the origin of fatty acids in prepupae. Sci. Rep.10, (2020). [DOI] [PMC free article] [PubMed]
- 20.Spranghers, T. et al. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric.97, 2594–2600 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Gougbedji, A., Detilleux, J., Lalèyè, P. A., Francis, F. & Caparros Megido, R. Can insect meal replace fishmeal? A meta-analysis of the effects of black soldier fly on fish growth performances and nutritional values. Animals12, (2022). [DOI] [PMC free article] [PubMed]
- 22.Liland, N. S. et al. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLOS ONE12, e0183188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St-Hilaire, S. et al. Fish offal recycling by the black soldier fly produces a foodstuff high in omega-3 fatty acids. J. World Aquac. Soc.38, 309–313 (2007). [Google Scholar]
- 24.Franco, A. et al. Antimicrobial activity of lipids extracted from Hermetia illucens reared on different substrates. Appl. Microbiol. Biotechnol.108, 167 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meneguz, M. et al. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric.98, 5776–5784 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Danieli, P. P., Lussiana, C., Gasco, L., Amici, A. & Ronchi, B. The effects of diet formulation on the yield, proximate composition, and fatty acid profile of the black soldier fly (Hermetia illucens L.) prepupae intended for animal feed. Animals9, (2019). [DOI] [PMC free article] [PubMed]
- 27.Liu, Y. et al. Chronological and carbohydrate-dependent transformation of fatty acids in the larvae of black soldier fly following food waste treatment. Molecules28, (2023). [DOI] [PMC free article] [PubMed]
- 28.Meyer, H., Vitavska, O. & Wieczorek, H. Identification of an animal sucrose transporter. J. Cell Sci.124, 1984–1991 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Georgescu, B., Struti, D., Papuc, T., Cighi, V. & Boaru, A. Effect of the energy content of diets on the development and quality of the fat reserves of larvae and reproduction of adults of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). EJE118, 297–306 (2021). [Google Scholar]
- 30.Li, W. et al. Simultaneous utilization of glucose and xylose for lipid accumulation in black soldier fly. Biotechnol. Biofuels8, 117 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terra, W. R. & Ferreira, C. Insect digestive enzymes: properties, compartmentalization and function. Comp. Biochem. Physiol. B Biochem. Mol. Biol.109, 1–62 (1994). [Google Scholar]
- 32.Kim, W. et al. Biochemical characterization of digestive enzymes in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). J. Asia-Pac Entomol.14, 11–14 (2011). [Google Scholar]
- 33.Galun, R. & Fraenkel, G. Physiological effects of carbohydrates in the nutrition of a mosquito, Aedes aegypti and two flies, Sarcophaga bullata and Musca domestica. J. Cell Comp. Physiol.50, 1–23 (1957). [DOI] [PubMed] [Google Scholar]
- 34.Ishaaya, I. & Meisner, J. Physiological effect of sugars on various digestive enzymes of Spodoptera littoralis larvae. J. Comp. Physiol.86, 117–124 (1973). [Google Scholar]
- 35.Ito, T. Effect of sugars on feeding of larvae of the silkworm, Bombyx mori. J. Insect Physiol.5, 95–107 (1960). [Google Scholar]
- 36.Burton, R. L., Starks, K. J. & Sauer, J. R. Carbohydrate Digestion by the larval midgut of Heliothis zea. Ann. Entomol. Soc. Am.70, 477–480 (1977). [Google Scholar]
- 37.Giannetto, A. et al. Hermetia illucens (Diptera: Stratiomydae) larvae and prepupae: Biomass production, fatty acid profile and expression of key genes involved in lipid metabolism. J. Biotechnol.307, 44–54 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Barragan-Fonseca, K. B., Gort, G., Dicke, M. & van Loon, J. J. A. Effects of dietary protein and carbohydrate on life-history traits and body protein and fat contents of the black soldier fly Hermetia illucens. Physiol. Entomol.44, 148–159 (2019). [Google Scholar]
- 39.Barragan-Fonseca, K. B., Dicke, M. & van Loon, J. J. A. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl.166, 761–770 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barragan-Fonseca, K. B., Gort, G., Dicke, M. & Van Loon, J. J. A. Nutritional plasticity of the black soldier fly (Hermetia illucens) in response to artificial diets varying in protein and carbohydrate concentrations. J .Insects Food Feed7, 51–61 (2021). [Google Scholar]
- 41.Cohn, Z., Latty, T. & Abbas, A. Understanding dietary carbohydrates in black soldier fly larvae treatment of organic waste in the circular economy. Waste Manag.137, 9–19 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Nugroho, R. A. et al. Fermented palm kernel waste with different sugars as substrate for black soldier fly larvae. Global J. Environ. Sci. Manag.10, 503–516 (2024). [Google Scholar]
- 43.Nugroho, R. A. et al. Nutritive value, material reduction, biomass conversion rate, and survival of black solider fly larvae reared on palm kernel meal supplemented with fish pellets and fructose. Int. J. Trop. Insect Sci.43, 1243–1254 (2023). [Google Scholar]
- 44.Caplice, E. & Fitzgerald, G. F. Food fermentations: role of microorganisms in food production and preservation. Int. J. Food Microbiol.50, 131–149 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Moore, M. S. et al. Ethanol Intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell93, 997–1007 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Krzyżowski, M., Francikowski, J., Baran, B. & Babczyńska, A. The short-chain fatty acids as potential protective agents against Callosobruchus maculatus infestation. J. Stored Products Res.86, 101570 (2020). [Google Scholar]
- 47.Kim, G., Huang, J. H., McMullen, J. G., Newell, P. D. & Douglas, A. E. Physiological responses of insects to microbial fermentation products: Insights from the interactions between Drosophila and acetic acid. J. Insect Physiol.106, 13–19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barron, A. J. et al. Microbiome-derived acidity protects against microbial invasion in Drosophila. Cell Reports43, 114087 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Douglas, A. E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol.60, 17–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasubuchi, M., Hasegawa, S., Hiramatsu, T., Ichimura, A. & Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients7, 2839–2849 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carpentier, J. et al. Microorganism contribution to mass-reared edible insects: Opportunities and challenges. Insects15, (2024). [DOI] [PMC free article] [PubMed]
- 52.Xiang, F., Zhang, Q., Xu, X. & Zhang, Z. Black soldier fly larvae recruit functional microbiota into the intestines and residues to promote lignocellulosic degradation in domestic biodegradable waste. Environ. Pollut.340, 122676 (2024). [DOI] [PubMed] [Google Scholar]
- 53.Caltzontzin-Rabell, V., Escobar-Ortiz, A., Gutiérrez-Antonio, C., Feregrino-Pérez, A. A. & García-Trejo, J. F. Revaluation process of cheese whey through the cultivation of black soldier fly larvae (Hermetia illucens). Eng. Rep.6, e12853 (2024).
- 54.Folch, J., Lees, M. & Stanley, G. H. S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem.226, 497–509 (1957). [PubMed] [Google Scholar]
- 55.Ferrara, D. et al. Composition and nutritional values of fatty acids in marine organisms by one-step microwave-assisted extraction/derivatization and comprehensive two-dimensional gas chromatography -flame ionization detector. J. Chromatogr. B1236, 124074 (2024). [DOI] [PubMed] [Google Scholar]
- 56.R Core Team. R: A Language and Environment for Statistical Computing. Foundation for Statistical Computing (2024).
- 57.Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw.67(1), 1–48 (2015). [Google Scholar]
- 58.Oksanen J, et al. _vegan: Community Ecology Package_. R package version 2.6–4. (2022).
- 59.Lenth, R. _emmeans: Estimated Marginal Means, aka Least-Squares Means_. R package version 1.10.1. (2024).
- 60.Le, S., Josse, J. & Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw.25(1), 1–18 (2008). [Google Scholar]
- 61.Lider, D., Shevchenko, A. & Lytvyn, M. Grammarly Editor (v1.2.104.1487). (Grammarly, Inc, San Francisco).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.