Highlights
-
•
We identify critical liana traits that contribute to establishing a liana plant functional type in vegetation models for tropical rainforests.
-
•
We review key processes to properly represent lianas in ecosystem models.
-
•
We discuss a variety of possible liana implementation strategies with their associated strengths, limitations, computational costs and data requirements, based on our experience with implementing lianas in a cohort (ED2) and individual (FORMIND) based model.
Keywords: Lianas, Tropical forest, Vegetation model, Plant functional type
Abstract
Lianas (woody climbers) are crucial components of tropical forests and they have been increasingly recognized to have profound effects on tropical forest carbon dynamics. Despite their importance, lianas' representation in vegetation models remains limited, partly due to the complexity of liana-tree dynamics and the diversity in liana life history strategies. This paper provides a comprehensive review of advances and challenges for mechanistically representing lianas in forest ecosystem models and a proposed path towards effectively representing lianas in these models.
Defining a liana plant functional type is a significant challenge because of the high morphological and physiological diversity amongst liana species, and because of their structural association with trees. Here, we identify critical liana traits that likely should contribute to establishing a liana plant functional type, along with key processes to properly represent lianas in ecosystem models. Subsequently, we discuss a variety of possible liana implementation strategies with their associated strengths, limitations, computational costs and data requirements. A fundamental redesign of the tree-centric demographic vegetation models seems appropriate to accommodate the unique growth and competition strategies of lianas. We illustrate the potential of such models with a single-site case study where we disentangle putative mechanisms of liana increasing abundance. Furthermore, we underscore the critical need for comprehensive liana demographic and functional data (including long-term, physiological, and pantropical observations) for the qualitative implementation and evaluation in the proposed modeling efforts. Currently, there is a scarcity of liana data and the data that do exist have a neotropical bias. We finally introduce a new liana functional trait database that can centralize existing liana trait data, incentivize improved data gathering and thus facilitate model development and scientific analyses.
1. Introduction
Lianas (woody vines) are a common feature of tropical forests, where they contribute up to 35 percent of the woody plant species and up to 40 percent of the woody stems (Schnitzer and Bongers, 2011). These climbing plants use the structure of other stems to escape from the deep shade on the forest floor and reach the highly illuminated canopy. As such, canopy lianas can produce a large amount of leaf biomass in the upper canopy without the need to invest in self-supporting stems (Putz 1983; Wyka et al., 2013).
The relatively low liana contribution to forest woody biomass compared to trees (van der Heijden et al. 2013) makes lianas seem unimportant for forest dynamics and functioning. However, not only are lianas highly biodiverse (Gianoli 2015), they also affect many processes such as gap dynamics, secondary succession, and carbon, water and nutrient cycling (Schnitzer and Carson, 2010; Tymen et al., 2016; van der Heijden et al., 2015; Chen et al. 2015; Tang et al., 2012). Moreover, lianas negatively impact growth and mortality of host trees (Estrada-Villegas et al., 2022) as well as their structure (Moorthy et al., 2022) while disproportionately contributing to canopy gross and net primary productivity (Phillips et al. 2005; van der Heijden et al. 2013). Liana infestation is associated with an increased forest carbon turnover and a reduced sink strength and long-term carbon storage of the ecosystem (van der Heijden et al. 2015). Ongoing liana proliferation (increases in both biomass and abundance), confirmed in multiple studies across the Neotropics (e.g. Phillips et al. 2002, Schnitzer and Bongers 2011) and in some (but not all) forests in the Paleotropics (Wright et al. 2015; Bongers et al. 2020; Pandian and Parthsarathy 2016; Abiem et al. 2023), further increases their effect on forest dynamics and functioning.
The volume of observational and experimental research on lianas has been increasing exponentially in the past decades (Fig. 1a). Published literature review studies so far focused on empirical work on liana abundance, proliferation and carbon cycling (e.g., Schnitzer and Bongers 2002; Schnitzer and Bongers 2011, van der Heijden et al. 2013), on liana removal experiments (Estrada-Villegas and Schnitzer 2018), and on liana trait observations (Wyka et al. 2013). These review studies identified the need for more (long-term) data and experiments, but only a few of them identified the need to use vegetation models to advance liana ecological research (van der Heijden et al. 2013; Muller-Landau and Pacala 2020; Marshall et al. 2020).
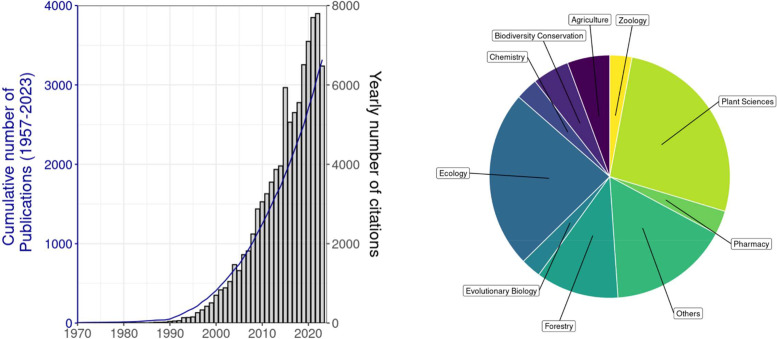
Fig. 1.
Literature overview of the research interest on lianas. Referencing data was obtained by entering the search terms (“liana” or “woody vine”) into the ISI Web of science engine. Cumulative number of publications and number of citations per year since 1957 (left) and frequency distribution of topics in those studies (right).
Critically, vegetation models can serve as platforms to integrate data and knowledge on multiple processes at different spatial and temporal resolutions. Hence models can be used to identify knowledge gaps, guide future empirical investigation and prioritize data collection. Implementation of lianas in vegetation models would, however, allow investigating a much wider range of open research questions (Table 1). First, vegetation models would allow us to exhaustively quantify the effect of lianas on comprehensive forest functioning, which is challenging in empirical studies. More precisely, vegetation model simulations including lianas would enable ecologists to determine: (i) the contribution of lianas to the different pools and fluxes of carbon, water, energy and nutrient cycles of tropical forests, (ii) the relative contribution of different putative mechanisms to liana abundance increases, (iii) the relative importance of resource competition (light, nutrients, water) to the performance of lianas and trees, (iv) the contribution of liana-induced tree mortality to total tree mortality (McDowell et al. 2018), (v) processes that may allow lianas and trees to coexist in tropical forest communities, and (vi) the phenomenon of arrested succession (i.e., stop or slowing down of ecological forest succession post-disturbance due to liana proliferation, see Schnitzer et al. 2000, Tymen et al. 2016). Moreover, empirical ecological studies in tropical forests often suffer from low replicability (Schnitzer and Carson 2016) and are limited in space and time (Estes et al. 2018), while vegetation models are essentially tools to overcome such limitations. In addition to characterizing the current effect of lianas, models also allow for identifying their effects in future and historical scenarios of climate and land-use change. As such, the power of a modeling framework to predict the effects of lianas also creates opportunities for practical applications. For example, forest managers could use such models to test the effect of (partial) liana removal as a management practice (Sfair et al. 2015), or policy-makers could account for lianas as an interacting factor in forest restoration projects (Marshall et al. 2017). Finally, quantifying the future changes of carbon dynamics due to lianas could result in improved estimates of carbon storage and exchange in tropical ecosystems and improved Earth System Models simulations of land-atmosphere feedbacks over a wide range of climatic and land-use scenarios.
Table 1.
Examples of liana-related ecological questions that could be addressed using appropriate vegetation model developments and simulations.
| Liana-related ecological questions | Role(s) vegetation models have to play |
|---|---|
| Demography | |
| What is/are the driver(s) of increasing liana abundance, and how do these drivers interact? | Understanding/quantifying |
| What is the future liana abundance locally? Regionally? Pantropically? | Predicting/upscaling |
| What are the effects of liana loading on hosting trees? | Quantifying |
| What drives the occurrence and duration of liana-induced arrested succession? | Understanding |
| How will lianas affect the forest structure and composition under future climate and land-use scenarios? | Predicting/quantifying |
| What determines the biogeographical distribution of lianas? | Upscaling |
| Forest biogeochemical cycles and carbon allocation | |
| What is the current and future liana contribution to the forest carbon/water/nutrient cycles? | Quantifying/predicting |
| How does differing biomass partitioning to plant organs between lianas and trees contribute to carbon residence time? | Understanding |
| What role do lianas play in the acceleration of the climate-change/forest sink strength decline feedback? | Understanding/quantifying |
| Competition for resources | |
| Do lianas and trees form separate niches with respect to water/nutrient uptake? | Integrating |
| Are lianas more drought-tolerant than trees? How? | Integrating/understanding |
| Do lianas aggravate the drought stress in tropical forests? | Understanding/quantifying |
| What are the amounts of water and nutrients ‘taken’ from trees by competing lianas? | Quantifying |
| Traits and diversity | |
| Do lianas with different functional traits exhibit different strategies and hence belong to different functional groups (PFTs)? | Integrating |
| How do key liana traits contribute to whole-plant carbon gain in lianas vs self-supporting plants? | Integrating/understanding |
| Which mechanisms allow the long-term coexistence of the different growth forms? | Understanding |
| Forest management | |
| Can lianas hamper/assist in rainforest restoration? | Understanding/predicting |
| How does liana removal affect forest demography and productivity? | Quantifying/predicting |
Incorporating lianas in vegetation models is therefore an obvious next step in the development of comprehensive tropical forest and global vegetation models. Adapting these models to incorporate lianas, however, is a challenging task. Throughout this paper, we give our vision on the most important challenges and possible ways to integrate one or multiple liana functional types into vegetation models, focusing on modeling concepts, process representation, parameterization and data requirements, illustrated with a simulation case-study.
2. Modeling concepts
2.1. Vegetation models
The continuous spectrum of vegetation models covers three big classes of models based on the way they aggregate the plants within a forest. Area-based models such as ORCHIDEE (Krinner et al. 2005) or CLM (Lawrence et al. 2019) aggregate all plants within a landscape grid cell into one (or multilayer) “big leaf” and represent the vegetation globally by a handful of PFTs (which are essentially biome types in these global area-based models). Cohort-based models such as ED2 (Longo et al. 2019), FATES (Koven et al. 2020) or LPJ-GUESS (Ahlström et al. 2012) represent forests by multiple interacting tree cohorts, aggregating trees belonging to the same functional and size class. These cohort-based models are in fact approximations of the third vegetation model class: stochastic, individual-based models, such as TROLL (Maréchaux and Chave, 2017) or FORMIND (Fischer et al. 2016) that simulate individual plants explicitly. Cohort and individual-based models are grouped under the name of “vegetation demography models” (VDMs; Fisher et al. 2018). Many VDMs aggregate species and functional diversity using the concept of plant functional types (PFT), even though some retain a species-level representation (Maréchaux and Chave, 2017) or rather uses a trait continuum approach (e.g. Sakschewski et al., 2015). The PFTs aggregate all individuals belonging to functionally similar species and their parameterization is typically prescribed, based on observed, calibrated or assumed values of traits.
2.2. Liana modeling approaches
Conceptually we see two main options to include lianas in vegetation models. A first approach is a host-parasite model, in which lianas are implemented implicitly as a (negative or positive) effect on trees. Here, there is no need to explicitly simulate the growth of lianas themselves. Instead, lianas are empirically implemented as the degree of infestation of the forest stand or individual trees, and process parameterization changes according to the infestation level. In area-based models, liana infestation could be represented as a fraction of the big-leaf canopy that is infested. In VDMs, a probability for each cohort or individual to get infested at any time could be assumed and tracked over time to represent the effect of lianas on ecosystem processes (Visser et al. 2018b; Muller-Landau and Pacala 2020; De Deurwaerder et al. 2024). Such a host-parasite model can then be calibrated with empirical data, such as the relationship between liana infestation levels and tree growth and mortality rates. We consider host-parasite models to be useful for studying the effects of liana abundance on the carbon stock dynamics in tropical forests. However, this implementation approach neglects aspects of resource competition and mutualism (Muller-Landau and Pacala, 2020). Moreover, to simulate the effect of lianas on the carbon cycle with a host-parasite model, empirically-based equations will be required to describe the effect of lianas on growth, productivity, and mortality of trees or forest stands. The core of such a model is, by essence, data-driven, and could mainly be used as a diagnostic tool (as has been recently done by De Deurwaerder et al. 2024) for data analysis rather than for the predictive and quantifying purposes listed in Table 1.
A second approach is to simulate the growth and development of lianas explicitly, as independent PFT in an operational vegetation model. In this case, one or more liana PFTs interact and compete for resources with the other PFTs (e.g. trees and grasses). In this conceptual framework, the effect of lianas on the biogeochemical cycle of the forest ecosystem emerges from the competition between lianas and the other PFTs for environmental resources. The development of such a model requires new processes and parameterizations to describe the specific functioning of lianas (di Porcia e Brugnera et al. 2019; Willson et al. 2022), for example in terms of their interaction with trees or their carbon allocations (Fig. 2). Many challenges exist to integrate and parameterize liana PFTs, but existing data and the current knowledge of liana ecology should already enable us to develop accurate and representative process-based equations of the effects of lianas on forests. Yet, model developers will need to make decisions on how to integrate the processes, which vegetation model type(s) to develop and how to integrate lianas in the structure of the chosen model.
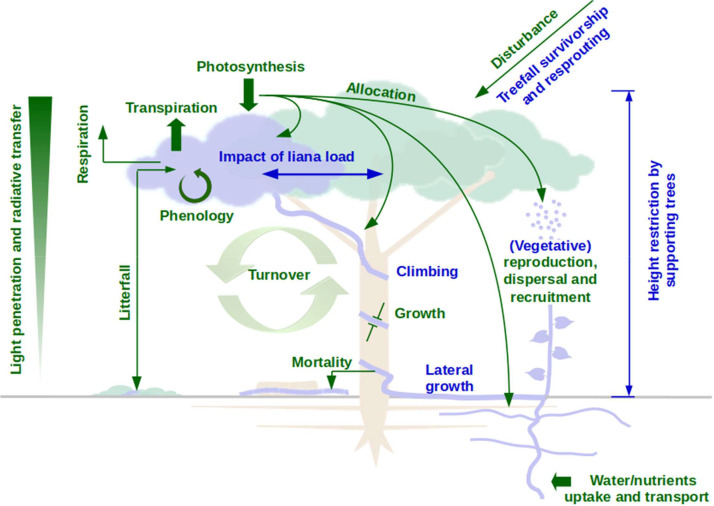
Fig. 2.
Overview of some of the most important liana-specific (blue) and host tree equivalent (green) processes to implement to mechanistically account for liana infestation in process-based vegetation models.
2.3. Model selection
The type of ecological questions that can be addressed with a specific model (e.g. Table 1), depends on the scope and structure of the model. Area-based models might be useful for liana-related upscaling studies, but are less suited to answer most of the ecological questions highlighted in Table 1, because in those models PFTs do not physically co-exist or compete.
The main advantage of VDMs compared to area-based models is their capacity to predict the distributions and compositions of plant communities directly from the user-prescribed parameterization of demographic and physiological traits and the appropriate environmental drivers (Fisher et al., 2015, 2018). Given the importance of temporal dynamics on liana community properties (Ingwell et al. 2010; Tymen et al. 2016; Mumbanza et al. 2022), demography should be explicitly accounted for in the models, which is only possible with VDMs. These models typically also account for trait variability across the PFTs that compose the vegetation community (e.g., Moorcroft et al. 2001; Maréchaux and Chave 2017), which is essential to simulate the observed effect of lianas on forest functional composition. The simulation of successional dynamics in VDMs is achieved via trait filtering, whereby plant traits affect resource acquisition, growth and survival. Those VDMs that also include mechanistic representations of ecophysiology and biogeochemistry may be the most suited to assess the combined effect of lianas on both vegetation functional composition and biogeochemistry. Therefore we advocate to implement lianas as a distinct PFT (as opposed to lianas as a parasite property of a host tree) into VDMs (as opposed to big-leaf models).
We expect that starting with a single liana PFT is a first step, leading to a useful initial model formulation. To date, we are aware of only a single liana PFT, which has been implemented in the ED2 model (di Porcia e Brugnera et al. 2019, Meunier et al. 2022) and the FORMIND model (di Porcia e Brugnera et al. 2020). In time, adding multiple liana PFTs could better reflect the many liana species covering a broad functional space (Schnitzer et al. 2012, 2021; Meunier et al. 2020; Coppieters et al. 2022), similar to what is already done for trees in many demographic models.
An important remark to make here, is that the operational VDMs in which lianas have been implemented so far, have different levels of detail (some processes are omitted) and resolution. The FORMIND model for example has a high level of detail in simulating the spatial structure of forests. It is individual-based and has spatially explicit patches (Fischer et al. 2016, di Porcia e Brugnera et al. 2020). The temporal resolution of FORMIND is on the other hand much coarser, with a typical annual simulation time step that does not allow to simulate seasonal patterns or responses to specific climatic events. In contrast, the ED2 model is resolving the spatial heterogeneity in a more aggregated way (with spatially implicit patches and cohorts), but has a sub-daily timestep for fast processes like photosynthesis, allowing to simulate physiological responses to climatic events in a much more realistic way (Meunier et al. 2021a). The parameter needs of specific models depend highly on those differences in model structure.
2.4. Data requirements
Data are at the center of all modeling activities, as they are necessary at each model development stage, from model formulation to evaluation (Dietze et al. 2013). With liana research expansion over the last few decades (Fig. 1), an increasing amount of liana data have been made available for a multitude of processes, including experimental results, trait measurements, and field observations. As liana research becomes more widespread, standard protocols to observe and census lianas have also been increasingly formalized, leading to more comparable and homogeneous datasets that can be used for model development (e.g. Gerwing et al. 2006).
Depending on the direction taken for modeling lianas and on the chosen model, different quantities and types of data are necessary to build, constrain, and validate the liana PFT. These data requirements will depend on the research question, which dictates factors such as the spatio-temporal scale of the simulations. Snapshot models that look at relatively short time intervals (months to years) may need fewer observations. In a site study that focused on resource competition between growth forms (Meunier et al. 2021a), a process-based liana model was run for a few years. By focusing at time scales where some processes can safely be ignored (in that study e.g. drivers of liana abundance), model outputs can inform on other research questions, such as the relative importance of water and light competition (Meunier et al. 2021a). VDMs are often the most demanding in terms of data because they require mechanistic representation for a wide number of processes. The degree of sophistication of VDMs is variable and different models may be structured around more or less coarse-grained representations of natural phenomena. Yet, these models require trait data for parameter estimation, calibration data to constrain unobserved (or unobservable) parameters, and independent data for model evaluation. The source and type of those data will necessarily depend on the selected model as well as the data availability and the research question. For example, the liana modelling work done with the FORMIND model by di Porcia e Brugnera et al. (2020) made use of trait and allometry data to constrain PFT parameters, while inventory data were used to compute some of the growth parameters and for model evaluation. The work on lianas with the ED2 model (which has more detailed description of some processes like plant hydraulics) used trait and allometry data in a similar way, but used a larger number of trait variables, including spectral (Meunier et al. 2022) and hydraulic traits (Meunier et al. 2021a). Inventory data were used both for prescribing initial conditions and for model evaluation in these studies (Meunier et al. 2021b; van der Heijden et al. 2024). The ED2 model version with lianas was also evaluated by various independent datasets, such as fluxtower data (e.g. Meunier et al. 2021a) and repeated inventories from a liana removal experiment (Meunier et al. 2021b). So far, both models have been used for site-level studies. But when these models will be used for spatial upscaling, we expect the use of data from pantropical plot networks and various remote sensing products for model initialization and evaluation.
3. Liana-specific processes to account for in models
Several processes specific to the liana growth form will require new process formulations within the model, specifically when implementing a liana PFT into a VDM. Modeling concepts for these processes are discussed, and potential issues are identified below. Other plant-generic processes do not require new representations, but will solely require liana-specific PFT parameterizations. These plant-generic processes and parameterizations are discussed separately in the next section.
3.1. Aboveground liana-tree competition
The aboveground liana-tree interaction is a critical aspect to account for when modeling lianas, as an inherent characteristic of the liana life-form as a structural parasite, and three main components of this interaction should be accounted for: (1) a critical competition for light, (2) the displacement of tree leaves, and (3) the induced mechanical stress on the hosting tree.
Conceptually, it is important to consider to what extent specific liana-tree interactions should be represented explicitly in a VDM, or can be simulated as a model outcome without explicit model formulation. In a first approach, lianas could be considered as freestanding living plants with a specific set of traits and competing with trees for resources, but without representation of interaction with host trees. Here, lianas would compete with neighboring plants just like tree PFTs compete with one another. Yet, to capture the essence of the lianescent growth form (lianas lacking self-supporting tissues), liana's maximal vertical height should be limited by the height of their host tree. Such a height limitation for lianas has been implemented in the ED2 model (di Porcia e Brugnera et al. 2019, Meunier et al. 2021a). The height and crown size of trees in VDMs is typically determined by a PFT-specific allometry and it was recently shown that the height allometry of trees is affected by their liana load (Moorthy et al. 2022). A prescribed liana-impacted tree allometry has so far no been accounted for by the ED2 model, the impact on the tree crown is rather an emergent result of competition for light and replacement of tree leaves by liana leaves. For that aspect the FORMIND liana PFT is more complex and realistic. Here, liana individuals track specific host trees and have a specific prescribed impact on the tree crown allometry (di Porcia e Brugnera et al. 2020). In a first model approximation, it may be tempting to permanently fix a liana to its host in a VDM. If instead lianas are allowed to change host or colonize multiple tree crowns in the model, an individual liana can be more dominant and persist for a longer part of the succession, as it likely happens in nature (Schnitzer et al. 2021). The individual based model FORMIND allows multiple lianas to colonize a single host, but so far does not account for the possibility for a single liana to colonize multiple trees (di Porcia e Brugnera et al. 2020). For the cohort-based model ED2 accounting for such explicit liana-host interactions is more challenging. An ED2 version with liana-cohort ‘tracking’ has been tested, in which liana cohorts are attached to specific tree cohorts (di Porcia e Brugnera et al. 2019). However, a more implicit approach where specific liana-host interactions are omitted, but liana height is limited by tree height and resource competition is constraining the growth of lianas and trees has proven to be effective in simulating demography and biogeochemistry of tropical forests (Meunier et al. 2021a).
The vertical distribution of leaf biomass of lianas and trees is a crucial component to simulate both large-scale and local stand dynamics. Liana leaves are considered to displace tree leaves on a one-to-one ratio in the canopy (Kira and Ogawa 1971; Rodríguez-Ronderos et al. 2016), both in the upper and mid canopy layers (Rodríguez-Ronderos et al. 2016; di Porcia e Brugnera et al. 2019). Vertical light profiles throughout the canopy can significantly be altered due to liana covering, related to differences in liana leaf architectural and spectral traits, with restriction of light penetration even found very close to the canopy top (Fauset et al. 2017). Clumps of liana stems have also been shown to significantly lower the light interception for understory vegetation (Sanchez-Azofeifa et al. 2009; Paul and Yavitt 2011).
It is essential to account for the way liana and tree crowns fill the vertical and horizontal canopy space. In VDMs multiple approaches are used to organize the tree crowns spatially, e.g. assuming flat-topped crowns or the perfect plasticity approximation (Fisher et al. 2018). Lianas, however, may violate these assumptions by growing into tree canopies and replacing tree leaves. To address this issue, VDMs could benefit from implementing explicit liana-to-host attachment. Because lianas grow into or on top of their host canopy, the competitive effect on their host is stronger than the effect of competing neighboring trees (Tobin et al. 2012). This means that an ideal vegetation model should differentiate between liana-tree and tree-tree competition for light. Data on individual tree crown infestation is essential to build such models, and is mostly available through ground-based crown occupancy index (COI) assessments (van der Heijden et al. 2010), although detection using remote sensing is underway (Guzmán et al. 2018; Visser et al. 2021; Waite et al. 2019). Infestation of tree crowns by lianas is dynamic, and new or increased colonization or liberation is observed over time (Ingwell et al. 2010). An additional level of complication in the liana-tree interaction term could be added to a model by distinguishing host tree characteristics, which could affect their vulnerability to be infested by or liberated from lianas and their tolerance to survive liana infestation (e.g. Visser et al. 2018a).
When implementing the liana-tree interaction in models, the fate of the liana when their host dies should also be considered and will likely require new processes. In this case, the liana often survives, either remaining (partially) in the canopy on connected hosts, or will start the process of re-ascension via new hosts (Putz 1984a). Accordingly, interconnected host trees via the liana might have an increased mortality probability by being pulled in a tree fall. Depending on the model representation of mortality, these new processes will need to be evaluated.
The mechanical stress and torque imposed by lianas on trees through both the use of the tree as structural support and by connecting trees together (Putz, 1984a) could also be implemented in vegetation models. The most straightforward approach would be to increase branch turnover (Visser et al. 2018b) and the mortality risk of infested or inter-connected trees (Schnitzer and Bongers 2002; Schnitzer and Carson 2001).
3.2. Succession and gap dynamics
The recruitment, growth and survival of lianas during various stages of succession and gap formation are very different compared to trees. Recruitment of lianas in the light-abundant environment of early succession or in treefall gaps is stronger compared to trees, because lianas have other avenues (e.g. lateral growth) than only dispersing their seeds to recruit a gap. Vegetative reproduction is a common form of reproduction for lianas, by which lianas produce multiple stems from vegetative offshoots of mature stems (Schnitzer et al. 2021). Such clonal reproduction is an important strategy after natural disturbance in treefall gaps (Schnitzer and Carson 2001, 2010; Letcher 2015), and gap-phase regeneration is imperative for the recruitment of many liana species (Schnitzer et al. 2000; Schnitzer and Carson 2001, 2010; Letcher 2015; Ledo and Schnitzer 2014). A mature liana pulled down with treefall will often survive (around 90 % of the time according to Putz 1984a) and can resprout vigorously in the beneficial light environment of the treefall gap (Putz 1984a; Fisher and Ewers 1991). Although no genetically new individuals are produced, for modeling purposes the clonal stems could be considered as new recruits.
The specific liana growth patterns give them a particular dynamic role in forest succession. As climbers, lianas are characterized by slender stems, rapid horizontal growth, and particular searching and attachment mechanisms, which provide them with the ability to explore the forest understory efficiently, especially in highly disturbed environments such as treefall gaps (Putz, 1984a; Nabe-Nielsen and Hal 2002; Ledo and Schnitzer 2014; Mori et al. 2018).
The rate of vertical ascension through the canopy of lianas also needs specific attention. While for trees canopy height is generally derived from (increasing) diameter (e.g. Feldpausch et al. 2012), for lianas no straightforward allometric relation exists to derive its height location within the canopy. Quantification on the rate of liana canopy ascension is very rare for tropical forests (Medina-Vega et al. 2021b). Derivations could however be made by assessing the size of lianas present in the forest canopy. Kurzel et al. (2006) predicted on average a 50 % probability of a liana with a stem diameter > 2 cm to reach the canopy, increasing to 80 % for lianas > 2.5 cm, although it depended on forest type. Explicitly modeling rates of vertical ascension and canopy position will be important for any research question related to forest dynamics and liana-tree competition, as lianas will reach optimal light conditions in the top of the canopy and shading of the host tree becomes an important factor.
3.3. Liana ontogeny
Lianas are characterized by ontogenetic changes throughout their life cycle, including in their allometric relationships (Smith-Martin et al. 2020). Allometries are particularly important for modeling because most models use these functions for the structural description of PFTs and hence for the allocation of carbon to pools with various turnover times. Since lianas depend on the structural support of their host, they may possess even stronger ontogenetic variability than trees. Some models (Seidl et al. 2012) account for this changing allometries throughout the plant life history, for example by distinguishing between saplings and adult trees. In the case of lianas, a partitioning could be made by creating three different growth stages as has so far only been done in the FORMIND model (di Porcia e Brugnera et al. 2020). In the first stage, lianas are assumed to be self-supporting and are represented like other self-supporting plants. The second stage starts when lianas attach to their host and persists as long as they have not reached the canopy. The FORMIND model assumes that lianas focus exclusively on vertical elongation in this second stage. In the final third stage, lianas are in the canopy and deploy their leaves to maximize light acquisition. In such a model the height of self-supporting lianas can follow a tree-like allometry and when reaching the canopy, the liana height may be constrained to the height of the host. However, during the climbing phase height cannot be derived from the host and thus needs either specific measurements or an indirect derivation.
Scattered observations suggest that some properties, for example structural shape or leaf area, may differ radically across these stages (Yuan et al. 2016). A deeper understanding of liana ontogeny may come from empirical measurements that parse these multiple phases of growth. We consider the simulation of the timing of transition between these stages as a challenging task. Although some research has focused on these mechanisms (Ménard et al. 2009; Gallenmuller et al. 2004; Putz 1984b), a quantitative understanding of these phases and of the transition mechanisms is yet to be achieved.
4. Plant-generic processes requiring new parameterization for lianas
Various plant-generic processes of a liana PFT require specific parameterization, as is done for all growth forms in PFT-based models. Parameter values of process-based models are based on actual trait measurements, estimations or optimizations. Here, we present multiple processes relevant to the parameterization of a liana PFT, and identify if sufficient data is currently available for parameterization.
4.1. Belowground competition
Lianas have a substantial effect on belowground processes, and are in direct competition with trees for water and nutrients. Often, lianas are considered to have a competitive advantage on trees related to deeper roots that are better at resource uptake than trees (Schnitzer 2005; Putz 2023), although alternative rooting structures through fast and wide lateral root expansion (Putz 2023) and a multifocal root growth strategy (i.e. at multiple depths) (Tang et al. 2012; De Deurwaerder et al. 2018, 2020), and hydraulic redistribution (de Azevedo Amorim et al. 2018) have been postulated. Other root traits including rapid growth, faster turnover, a higher specific root length, high nutrient uptake and assimilation, and poor structural integrity can increase their competitive edge (Schnitzer 2005; Collins et al. 2016). However, there is a real lack of available data on liana rooting depth and fine root traits. Such data are critical for modelling purposes.
Belowground competition between lianas and trees have so far only been studied in detail with the ED2 model (Meunier et al. 2021a), which has an explicit representation of roots and their hydraulic traits. The FORMIND model is only considering above-ground competition (di Porcia e Brugnera et al. 2020).
4.2. Carbon allocation
The reduced need of lianas for structural tissue, as a structural parasite, should be reflected in the carbon allocation parameterization of the model. Lianas may allocate proportionally more carbon to their acquisitive tissues (leaf and fine roots) than to supportive woody biomass as compared to freestanding plants, combined with a lower relative allocation to coarse roots (Wyka et al. 2013). Accordingly, lower mean wood density, lower stem mass ratios, higher ratios of leaf mass and leaf area to stem cross-section, and higher leaf-to-sapwood area are reported (Zhu and Cao 2009; Gallenmüller, Rowe, and Speck 2004; Putz 1983; Gerwing and Farias 2000; Isnard and Silk 2009), in addition to higher non-structural carbon concentrations compared to trees (Signori-Müller et al. 2023).
It should be noted that liana allometric relationships (e.g. relating leaf biomass to stem diameter; Putz 1983; Gerwing and Farias 2000; Gehring et al. 2004) are highly uncertain because they are based on relatively low replication from only a few sites, do not use wood density, and omit large lianas. On top of that, lianas likely have a high structural plasticity (with stem length growth often independent of diameter growth), adding variability and uncertainty to allometric relations. We see a lot of potential in terrestrial LIDAR data to replace the weak aboveground allometric relationships that have been the only feasible way to estimate liana biomass (Moorthy et al. 2020). However, belowground carbon allocation remains largely unknown.
Current modeling efforts for lianas have however shown that carbon allocation is a key process, and reliable allometric relations are essential for realistic carbon cycle simulations. In the ED2 model, the high C allocation of lianas to reproduction (including towards clonal stems) is essential to close the carbon balance for the liana PFT (di Porcia e Brugnera et al. 2019, Meunier et al. 2021a).
4.3. Wood hydraulic traits
An important functional difference between lianas and trees lies in their hydraulic architecture (Isnard and Silk 2009). As structural parasites, the wood anatomical structure of lianas, often comprising of long, wide and long-lived vessels (Ewers et al. 1989, 1990; Caballé 1993; Chen et al. 2017; Putz 1983), allows for a higher sapwood- (and often leaf-) specific conductivity and a high water transport capacity (Jiménez-Castillo and Lusk 2013). On the other hand, lianas are potentially more vulnerable to embolism evidenced by their less negative xylem pressure at 50 % conductivity loss (P50) (Chen et al. 2015; De Guzman et al. 2016; Johnson et al. 2013; Zhu and Cao 2009), although adaptations could be present (Isnard and Silk 2009; De Guzman et al. 2016; Angyalossy et al. 2012; Bastos et al. 2016; Maréchaux et al. 2017).
Altogether, it is likely that the specific hydraulic traits of lianas play a key role in their performance, abundance and effect on ecosystem scale processes. A considerable amount of data on wood hydraulic traits of lianas is already available and has been used to parameterize the ED2 model (Meunier et al. 2021a) (which in contrast to FORMIND considers detailed plant hydraulics), so we consider the collection of additional wood trait data less of a priority, compared to some of the other parameter values needed. However, when aiming to represent in a model the variation of hydraulic strategies via multiple liana PFTs, additional data would be needed.
4.4. Leaf traits
Lianas are considered to belong to the ‘fast turnover/quick return’ end of the leaf economic spectrum (Wyka et al. 2013) even if liana species present a wide variety of acquisitive strategies (Schnitzer et al. 2012; Mello et al. 2020; Medina-Vega et al. 2021a). This is often reflected through a high maximum photosynthetic capacity (Cai et al. 2009; Zhu and Cao 2010), high leaf N and P concentrations (Asner and Martin 2011; 2012; Santiago and Wright 2007), high mass-based foliar nutrients concentrations (including K, Ca, Mg, Zn, Mn, B and Fe) (Asner and Martin 2012), high respiration rates per leaf area (Slot et al. 2013), lower Q10 values (Slot et al. 2013; Cavaleri et al., 2008), and a larger specific leaf area (Han et al. 2010; Santiago and Wright 2007; Asner and Martin 2012), specifically as compared to leaf trait values of trees.
This contrast with trees (Mello et al. 2020) must be represented in vegetation models, and in general enough leaf trait data are available to do so. However, it is important to mention here that leaf lifespan plays an important role in the leaf economic spectrum. It is unclear if liana leaf lifespans are shorter (Wright et al. 2005), similar (Wright et al. 2004), or even larger than those of trees (Tang et al. 2012). Finally, it is unknown if lianas are adapting their leaf traits to changing climate similarly or differently compared to trees. Such adaptation patterns are key determinants for simulating demographic processes, and we therefore advocate doing more experimental work to understand the leaf trait adaptation strategies of lianas versus trees. Contrasting leaf traits between lianas and trees including a different leaf life span have been implemented in the ED2 model (di Porcia e Brugnera et al. 2019, Meunier et al. 2021a), while in the FORMIND model only a limited number of leaf traits are accounted for (e.g. max photosynthetic rate) (di Porcia e Brugnera et al. 2020), given the fact that physiological processes are less at the center of this model.
4.5. Phenology
While a high variation in leaf phenological patterns is found for lianas (Putz and Windsor 1987; Hegarty 1990), in general a higher fraction of tropical liana species might retain and produce new leaves during the dry season as compared to trees (Putz and Windsor 1987; Opler et al. 1991; Kalácska et al. 2005; Medina-Vega et al. 2022). Asynchronous timing between lianas and trees has also been reported regarding reproductive phenology, with lianas often producing flowers and fruits during the dry season (Putz and Windsor 1987). The difference in phenological timing between the growth forms could be related to evolutionary processes leading to growth-form specific niche partitioning in time, their respective canopy position being affected by a different microclimate or the lianas’ competitive advantage to access belowground resources during the dry season (Schnitzer 2005; Putz 2023). Given these deviating phenological patterns between lianas and trees, it is important to consider whether and how phenology is represented in vegetation models. For modeling purposes, it could be sufficient to keep similar constraints for leaf fall and leaf production between lianas and trees (liana modelling efforts so far in ED2 and FORMIND considered lianas to be evergreen only). The difference in timing could be an emerging outcome of the simulations due to differences in resource availability and needs. However, good data on liana (leaf) phenology is lacking and we consider the collection of more and better data on tropical liana and tree phenology as a priority, as we expect the asynchronous phenology between growth forms a key element to simulate their competition.
4.6. Demographic processes
4.6.1. Reproduction and successful establishment
Lianas reproduce both by seed reproduction and clonal reproduction, of which the proportion is highly variable and dependent on local conditions (Putz 1984a; Schnitzer et al. 2021). In our opinion, the added complication of distinguishing clonal and seed reproduction into a VDM as two distinct carbon pools is not worth the effort when investigating whole-forest dynamics. A liana PFT that attains efficient establishment and recruitment would be sufficient to capture their effect at this level. For detailed understory or gap dynamic studies, the added investment would however potentially improve insights gained from a model.
4.6.2. Growth
The growth rates attained by lianas in a vegetation model like ED2 will be a model outcome related to the parameterization of various other processes (including photosynthesis, allocation etc.) (di Porcia e Brugnera et al. 2019). While in the individual based model FORMIND the growth rates of lianas and trees are calculated by a prescribed size dependent growth function (di Porcia e Brugnera et al. 2020). Attaining calibration or evaluation data for modeled growth rates can be difficult, mainly related to the complication of assessing stem elongation (in addition to diameter / height as done for trees). Some information on aboveground biomass growth rates could be derived using allometric equations (e.g. Schnitzer et al. 2006, van der Heijden et al. 2019, Moorthy et al. 2020), although these are poorly constrained.
4.6.3. Mortality
Mortality of lianas distinguishes between ramet mortality, i.e. mortality of individual stems that have originated from the vegetative iteration of a parent stem, and genet mortality, i.e. mortality of the distinct genetic individual. For most modeling purposes it would be too complicated to distinguish between both and thus sufficient to cluster ramet and genet mortality in one mortality rate. Reports on mortality rates of larger liana individuals can generally be found in long-term population demography studies based on repeated inventories in large permanent forest monitoring plots.
The implementation of liana mortality in a VDM depends on the type of model. In the individual based model FORMIND, mortality is implemented stochastically (each individual has a certain chance to die each time step). The mortality of lianas is here assumed to depend on the growth stage of the liana but also on the fate of the host tree (di Porcia e Brugnera et al. 2020). In the cohort-based model ED2, mortality is implemented as a fraction of the individuals within a cohort that die off at a certain time step. Here, specific mortality parameters can be implemented for lianas (Meunier et al. 2021a). Both models assume a higher survival rate of lianas (compared to trees) in case of a tree fall disturbance.
There is a relatively large amount of liana inventories available across the tropics. However, the data is scattered and some regions have only very limited data coverage (e.g. Central Africa, Bongers et al. 2020). Moreover, often liana censuses are done in small plots, there is only a limited number of censuses available and in many cases only large lianas (>10 cm DBH) are included in the census. Therefore, we advocate to include small lianas in census protocols of the pantropical inventory networks. Long-term monitoring of liana composition is imperative for validating decadal-long model simulation. Therefore, data on population demographics along successional chrono-sequences and in mature forests are critically required.
5. A database for liana PFT development
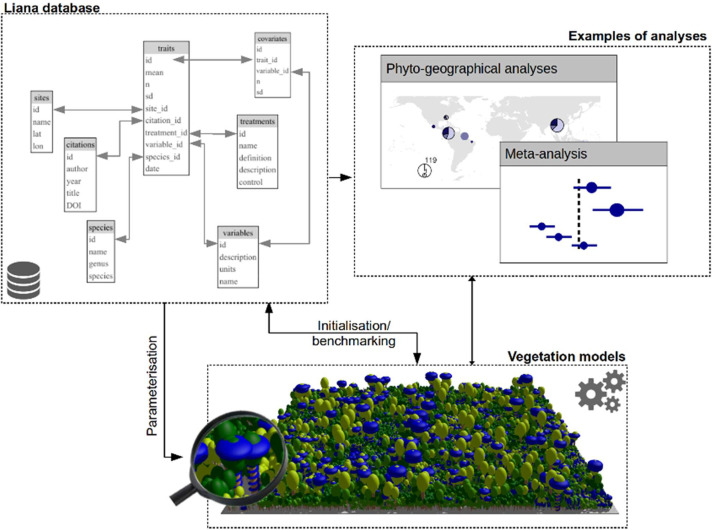
We initiated a new liana-specific, open-access database available for the development of liana PFTs (see code availability section). In our database some existing liana functional trait data are centralized, quality-checked, and made available for developers and other scientists. In the future, such a liana database will facilitate the work of model developers, as well as help avoid duplicated work of data assembling and increase data leverage paving the road towards new phytogeographical studies and meta-analyses (Fig. 3). As more data are added to the database, important data gaps will also be highlighted.
Fig. 3.
Illustration of structure for the liana database and how it can interact with models to produce new scientific outputs and analyses.
Because different models will use different data types, we made the first version of the database flexible enough to host monitoring and experimental plot data, and provide life-stage-specific information. Our database was first established for building the liana PFT in ED2 (Meunier et al. 2021a), and further supplemented for subsequent analyses (Meunier et al. 2021a; 2021b and 2022). It illustrates how data meta-analysis can help refine parameter values: in the initial model formulation, liana parameters were simply copied from the pioneer tropical tree PFT but those values substantially diverge from the trait observations pulled from the literature. The liana trait database currently includes +1100 records for 18 different traits, 161 unique species across 17 sites in the Tropics (Table 2). Yet it remains limited compared to the existing literature. To reach its goal, we aim and invite the scientific community to enrich it, e.g. with other liana meta-analyses (e.g. Willson et al. 2022).
Table 2.
Overview of the liana database that was used to constrain and parameterize the liana PFT in ED2 and how it modified the initial value of the parameter initially taken from the early successional PFT, the actual reference for each of the data points can be found in the database.
| Parameter[Units] | Description | Number of data points (Number of species - number of references) | Mean value[min - max] | Reference ED2 value (Early successional) |
|---|---|---|---|---|
| Hydraulics | ||||
| ks [kg.m-1.s-1.MPa-1] |
Maximum hydraulic conductivity of the stem per unit sapwood area | 72 (60 −13) |
18.2 [0.5 - 210] |
3.1 |
| kl [kg.m-1.s-1.MPa-1] |
Maximum hydraulic conductivity of the stem per unit leaf area | 51 (44–8) |
0.0013 [0.000085 - 0.0039] |
0.0008 |
| Al:As [m2.cm-2] |
Leaf to sapwood ratio | 51 (43–8) |
1.26 [0.047 - 9.75] |
0.39 |
| ax [%] |
Slope of the vulnerability | 51 (43–9) |
55.6 [13.6 - 140.8] |
20.3 |
| P50 [MPa] |
Water potential at which 50 % of stem conductivity is lost | 69 (47–12) |
−1.2 [−2.7 - −0.2] |
−2.3 |
| Wood capacitance [kg.m-3.MPa-1] |
Wood hydraulic capacitance | 6 (6–1) |
352 [134 - 590] |
62 |
| Leaf capacitance [kg.kg-1.MPa-1] |
Leaf hydraulic capacitance | 7 (7–1) |
0.21 [0.11 - 0.28] |
0.18 |
| leaf TLP [MPa] |
Leaf turgor loss point | 62 (45–7) |
−1.6 [−3.2 - −0.8] |
−2.1 |
| Leaf traits | ||||
| LNC [mg.g-1] |
Leaf nitrogen content | 34 (11–1) |
28.5 [13.5–49.8] |
– |
| LPC [mg.g-1] |
Leaf phosphorus content | 34 (11–1) |
1.8 [20.7–3.1] |
– |
| SLA [m2.kg-1] |
Leaf area per leaf mass | 239 (120–18) |
16.0 [2.5 - 39.5] |
16 |
| Vcmax [µmol.m-2.s-1] |
Maximum photosynthetic capacity at a reference temperature (25 °C) | 23 (9–1) |
43.9 [22.5 - 73.3] |
45 |
| Jmax [µmol.m-2.s-1] |
Maximum rate of electron transport at a reference temperature (25 °C) | 25 (10–1) |
69.7 [40.1 - 107.6] |
54 |
| Q10 [-] |
Rate of change of a as a consequence of increasing the temperature by 100 C | 14 (14–1) |
2.5 [1.9 - 2.9] |
2.4 |
| Aboveground carbon allocation | ||||
| WD [g.cm-3] |
Wood density | 152 (75–18) |
0.47 [0.2 - 0.83] |
0.53 |
| Demography | ||||
| mort [yr-1] |
Density-independent mortality | 18 (1) | 0.05 [0.02 - 0.09] |
0.07 |
We envision the liana database not necessarily to be an independent effort of the other big data initiatives (e.g. TRY), but to be effective, it must take into account liana specificities and meta-data required for model development (e.g. species does not always determine the growth form, which can change across life stages) and be further co-developed by the community. The effort to expand the database could also be facilitated by the compilation of the liana references and metadata in the Liana Ecology Project database, which is freely available at https://www.lianaecologyproject.com/database. Finally, we are convinced that recent and future advances in remote sensing to study lianas at larger scales (Visser et al. 2021; van der Heijden et al. 2022) will provide critical datasets to test and validate upscaled model simulations.
6. Case study: liana proliferation mechanisms
To illustrate the power of vegetation models to study ecological questions, we present here a case study showing the potential of VDMs to study the putative mechanisms of liana proliferation. The only operational VDM that currently has a liana PFT is the ED2 model (di Porcia e Brugnera et al. 2019, Meunier et al. 2021a), although some preliminary efforts have been done for other models too (e.g. the FORMIND model, di Porcia e Brugnera et al. 2020). So far, the ED2 liana PFT has been used for site-level studies on liana-tree competition (Meunier et al. 2021a), liana removal (Meunier et al. 2021b) and energy balance effects (Meunier et al. 2022).
We used the ED2-liana model, as published by Meunier et al. (2021a), to run simulations on Barro Colorado Island (BCI), Panama. We simulated liana-tree interactions for five years starting from prescribed vegetation initial conditions (the liana census of 2007 (Schnitzer et al. 2012) and the tree inventory of 2010 (Condit et al. 2019). The model was driven by the same climatic drivers as in previous publications and we refer the reader to Meunier et al. (2021a) and di Porcia e Brugnera et al. (2019) for more details. Compared to the baseline run presented in Meunier et al. (2021a), we considered three different scenarios: elevated CO2 (450 ppm instead of 400 ppm), increased treefall disturbance by 20 %, and drier climate (achieved by recycling the two driest years of our 15-year time-series). We ran the simulations for each of those scenarios alone and their combinations (eight simulations in total). We evaluated the effect of the different drivers by computing the relative change of the simulated liana abundance (with a DBH cutoff of 1 cm) at the end of the simulation with the baseline run.
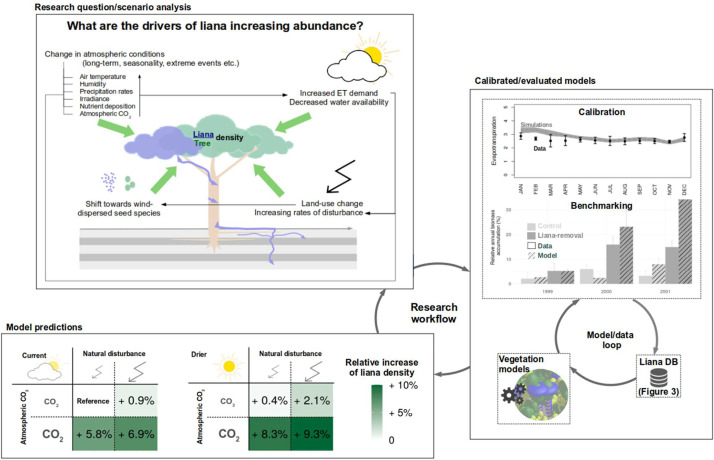
The results of this case study (Fig. 4) illustrate the potential workflow of a modeling study. A research question is developed and translated into model development. In our case, we investigated three putative mechanisms for liana proliferation, namely elevated CO2, enhanced drought, and increased disturbance, for a single site (BCI). A suited model is then improved and (re-)developed through a continuous integration of data that serves to calibrate and validate the model. In our case study, we used a model that was already calibrated against flux tower measurements and validated it against independent biomass accumulation data from a liana removal experiment (Meunier et al. 2021b). After model development, the model can be used to make predictions or learn something new. Our results show that all three factors have a positive impact on the simulated liana density, with elevated CO2 leading to the strongest increase in liana density (5.8 %), when applied as a sole mechanism. However, the combined scenarios illustrated important interaction effects with a liana density increase of up to 9.3 % when all mechanisms were combined. We must emphasize that this is a simplified single-site study to illustrate the potential of simulation studies. When aiming to fundamentally answer the research question on the drivers of liana proliferation, a more thorough simulation study with well-developed scenarios at multiple sites is needed. However, the research workflow of such a study would be similar to the one presented here. A key bottleneck emerging from the case study is the need for calibration and evaluation data, as discussed in the previous sections as well as the need to perform high-quality sensitivity analyses and to quantify model (structural) uncertainties.
Fig. 4.
The scientific method applied to liana-related ecological questions. Case study for the BCI site. Top left: research questions/scenarios analysis, right: model calibration/evaluation, bottom left: model prediction results.
7. Conclusion
Great challenges for accurately simulating tropical forest dynamics in vegetation models remain. In this synthesis study, we argue that the development of liana PFTs is key, but currently at an early stage of development. Lianas have indeed multiple effects on ecosystem processes, including effects on species diversity, carbon dynamics, and the carbon sink capacity of the forests. Vegetation models ignoring lianas are likely biased for forests where liana abundance is high.
Now is an ideal time to start building vegetation models that include lianas. Starting to actively develop liana PFTs and the unique mechanisms that drive liana-tree competition for light, water and nutrients would also guide future experimental and observational efforts, and highlight critical data and knowledge gaps to represent lianas, including how to close their carbon balance and accommodate their functional diversity.
Our opinion is that liana ecological research, while on the rise, will greatly benefit from working with vegetation models. Significant advances in liana-related research will benefit from active model development and upscaling exercises. Liana proliferation is one of the most obvious examples: with calibrated models, we can assess the relative importance of different drivers and project the effect of their changes in the future. However, such model projections can only be trusted if a wide variety of models (ensemble) and common liana databases are further developed.
CRediT authorship contribution statement
Hans Verbeeck: Writing – review & editing, Writing – original draft, Investigation, Funding acquisition, Conceptualization. Hannes P.T. De Deurwaerder: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Elizabeth Kearsley: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Sruthi M.Krishna Moorthy: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Francis Mumbanza Mundondo: Writing – review & editing, Writing – original draft, Investigation. Kasper Coppieters: Writing – review & editing, Writing – original draft, Investigation. Stefan A. Schnitzer: Writing – review & editing, Writing – original draft, Investigation. Marcos Longo: Writing – review & editing, Investigation. Marc Peaucelle: Writing – review & editing, Investigation. Marijn Bauters: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Félicien Meunier: Writing – review & editing, Writing – original draft, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the European Research Council Starting Grant 637643 (TREECLIMBERS) and the Research Foundation Flanders (FWO), senior research project G002321N. ML was supported by the Next Generation Ecosystem Experiments-Tropics, funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research. H.D.D. was supported by the Carbon Mitigation Initiative at Princeton University. M.P. was supported by the H2020 Marie Skłodowska-Curie Actions (LEAF-2-TBM grant no. 891369) and would like to acknowledge the financial support from the European Research Council Starting grant ERC-2023-STG 101117001 LEAFPACE. F.M. was funded by the FWO under junior and senior postdoc fellowships (grant no 1214720 N/1214723 N) and is thankful to this organization for its financial support.
Data availability
The liana database is available on github (https://github.com/femeunier/LianaDB), as MySQL and R storage files. The database can also be accessed via https://www.lianaecologyproject.com Script files to manipulate the database (e.g. to build, insert new records, pull data, and plot) are provided as well.
References
- Abiem I., Kenfack D., Chapman H.M. Assessing the impact of abiotic and biotic factors on seedling survival in an African montane forest. Front. For. Glob. Change. 2023;6 doi: 10.3389/ffgc.2023.1108257. [DOI] [Google Scholar]
- Angyalossy, V., Angeles, G., Pace, M.R., Lima, A.C., Dias-Leme, C.L., Lohmann, L.G., & Madero-Vega, C. (2012). An overview of the anatomy, development and evolution of the vascular system of lianas. https://doi.org/10.1080/17550874.2011.615574, 5(2), 167–182. 10.1080/17550874.2011.615574. [DOI]
- Ahlström A., Schurgers G., Arneth A., Smith B. Robustness and uncertainty in terrestrial ecosystem carbon response to CMIP5 climate change projections. Environmental Research Letters. 2012;7(4):44008. doi: 10.1088/1748-9326/7/4/044008. [DOI] [Google Scholar]
- Asner G.P., Martin R.E. Canopy phylogenetic, chemical and spectral assembly in a lowland Amazonian forest. New Phytologist. 2011;189(4):999–1012. doi: 10.1111/J.1469-8137.2010.03549.X. [DOI] [PubMed] [Google Scholar]
- Asner G.P., Martin R.E. Contrasting leaf chemical traits in tropical lianas and trees: implications for future forest composition. Ecol. Lett. 2012;15(9):1001–1007. doi: 10.1111/j.1461-0248.2012.01821.x. [DOI] [PubMed] [Google Scholar]
- Bastos C.L., Tamaio N., Angyalossy V. Unravelling roots of lianas: a case study in Sapindaceae. Ann. Bot. 2016;118(4):733–746. doi: 10.1093/AOB/MCW091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers F., Ewango C.E.N., van der Sande M.T., Poorter L. Liana species decline in Congo basin contrasts with global patterns. Ecology. 2020;101(5):e03004. doi: 10.1002/ECY.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballé G. Liana structure, function and selection: a comparative study of xylem cylinders of tropical rainforest species in Africa and America. Botanical Journal of the Linnean Society. 1993;113(1):41–60. doi: 10.1111/J.1095-8339.1993.TB00328.X. [DOI] [Google Scholar]
- Cai Z.Q., Schnitzer S., Bongers F. Seasonal differences in leaf-level physiology give lianas a competitive advantage over trees in a tropical seasonal forest. Oecologia. 2009;161(1):25–33. doi: 10.1007/s00442-009-1355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaleri M.A., Oberbauer S.F., Ryan M.G. Foliar and ecosystem respiration in an old-growth tropical rain forest. Plant Cell Environ. 2008;31(4):473–483. doi: 10.1111/j.1365-3040.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- Chen Y.J., Cao K.F., Schnitzer S.A., Fan Z.X., Zhang J.L., Bongers F. Water-use advantage for lianas over trees in tropical seasonal forests. New Phytologist. 2015;205(1):128–136. doi: 10.1111/nph.13036. [DOI] [PubMed] [Google Scholar]
- Chen Y.J., Schnitzer S.A., Zhang Y.J., Fan Z.X., Goldstein G., Tomlinson K.W., Lin H., Zhang J.L., Cao K.F. Physiological regulation and efficient xylem water transport regulate diurnal water and carbon balances of tropical lianas. Funct. Ecol. 2017;31(2):306–317. doi: 10.1111/1365-2435.12724. [DOI] [Google Scholar]
- Collins C.G., Wright S.J., Wurzburger N. Root and leaf traits reflect distinct resource acquisition strategies in tropical lianas and trees. Oecologia. 2016;180(4):1037–1047. doi: 10.1007/S00442-015-3410-7/FIGURES/3. [DOI] [PubMed] [Google Scholar]
- Condit, Richard et al. (2019), Complete data from the Barro Colorado 50-ha plot: 423617 trees, 35 years, Dryad, Dataset, 10.15146/5xcp-0d46. [DOI]
- Coppieters K., Verbeeck H., Dequeker S., Powers J.S., Vargas G., Smith-Martin C.M., Steppe K., Meunier F. Two Co-occurring Liana Species Strongly Differ in Their Hydraulic Traits in a Water-Limited Neotropical Forest. Front. For. Glob. Change. 2022;5 doi: 10.3389/ffgc.2022.836711. G. [DOI] [Google Scholar]
- de Azevedo Amorim T., Nunes-Freitas A.F., Rosado B.H.P. Revisiting the hypothesis for increasing liana abundance in seasonal forests: a theoretical review. Plant Soil. 2018;430(1–2):1–6. doi: 10.1007/S11104-018-3730-6/FIGURES/1. [DOI] [Google Scholar]
- De Deurwaerder H., Hervé-Fernández P., Stahl C., Burban B., Petronelli P., Hoffman B., Bonal D., Boeckx P., Verbeeck H. Liana and tree below-ground water competition—Evidence for water resource partitioning during the dry season. Tree Physiol. 2018;38(7):1071–1083. doi: 10.1093/treephys/tpy002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Deurwaerder H.P.T., Visser M.D., Detto M., Boeckx P., Meunier F., Kuehnhammer K., Magh R.K., Marshall J.D., Wang L., Zhao L., Verbeeck H. Causes and consequences of pronounced variation in the isotope composition of plant xylem water. Biogeosciences. 2020;17(19):4853–4870. doi: 10.5194/bg-17-4853-2020. [DOI] [Google Scholar]
- de Deurwaerder H.P.T., Detto M., Visser M.D., Schnitzer S., Pacala S.W. Linking physiology, epidemiology, and demography: Understanding how lianas outcompete trees in a changing world. Proceedings of the National Academy of Sciences. 2024;121(34) doi: 10.1073/pnas.2319487121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guzman M.E., Santiago L.S., Schnitzer S.A., Alvarez-Cansino L. Trade-offs between water transport capacity and drought resistance in neotropical canopy liana and tree species. Tree Physiol. 2016 doi: 10.1093/treephys/tpw086. [DOI] [PubMed] [Google Scholar]
- Dietze M.C., Lebauer D.S., Kooper R.O.B. On improving the communication between models and data. Plant Cell Environ. 2013;36(9):1575–1585. doi: 10.1111/pce.12043. [DOI] [PubMed] [Google Scholar]
- di Porcia e Brugnera M., Meunier F., Longo M., Krishna Moorthy S.M., de Deurwaerder H., Schnitzer S.A., Bonal D., Faybishenko B., Verbeeck H. Modeling the impact of liana infestation on the demography and carbon cycle of tropical forests. Glob. Chang. Biol. 2019;25(11):3767–3780. doi: 10.1111/gcb.14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Porcia e Brugnera M., Fischer R., Taubert F., Huth A., Verbeeck H. Lianas in silico, ecological insights from a model of structural parasitism. Ecol. Modell. 2020;431 doi: 10.1016/j.ecolmodel.2020.109159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes L., Elsen P.R., Treuer T., Ahmed L., Caylor K., Chang J., Choi J.J., Ellis E.C. The spatial and temporal domains of modern ecology. Nature Ecology and Evolution. 2018;2(5):819–826. doi: 10.1038/s41559-018-0524-4. [DOI] [PubMed] [Google Scholar]
- Estrada-Villegas S., Schnitzer S.A. A comprehensive synthesis of liana removal experiments in tropical forests. Biotropica. 2018;50(5):729–739. doi: 10.1111/BTP.12571. [DOI] [Google Scholar]
- Estrada-Villegas S., Pedraza Narvaez S.S., Sanchez A., Schnitzer S.A. Lianas Significantly Reduce Tree Performance and Biomass Accumulation Across Tropical Forests: A Global Meta-Analysis. Front. For. Glob. Change. 2022;4 doi: 10.3389/FFGC.2021.812066/BIBTEX. [DOI] [Google Scholar]
- Ewers F.W., Fisher J.B., Chiu S.T., Garden T., Miami F. Water Transport in the Liana Bauhinia fassoglensis (Fabaceae) PlantPhysiology. 1989;91(4):1625–1631. doi: 10.1104/PP.91.4.1625. Fairchild. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers F.W., Fisher J.B., Chiu S.T. A survey of vessel dimensions in stems of tropical lianas and other growth forms. Oecologia. 1990;84(4):544–552. doi: 10.1007/BF00328172/METRICS. [DOI] [PubMed] [Google Scholar]
- Fauset S., Gloor M.U., Aidar M.P.M., Freitas H.C., Fyllas N.M., Marabesi M.A., Rochelle A.L.C., Shenkin A., Vieira S.A., Joly C.A. Tropical forest light regimes in a human-modified landscape. Ecosphere. 2017;8(11):e02002. doi: 10.1002/ecs2.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldpausch T.R., Lloyd J., Lewis S.L., Brienen R.J.W., Gloor M., Monteagudo Mendoza A., Lopez-Gonzalez G., Banin L., Abu Salim K., Affum-Baffoe K., Alexiades M., Almeida S., Amaral I., Andrade A., Aragão L.E.O.C., Araujo Murakami A., Arets E.J.M., Arroyo L., Aymard C.…Phillips O.L. Tree height integrated into pantropical forest biomass estimates. Biogeosciences. 2012;9(8):3381–3403. doi: 10.5194/BG-9-3381-2012. [DOI] [Google Scholar]
- Fischer R., Bohn F., Dantas de Paula M., Dislich C., Groeneveld J., Gutiérrez A.G., Kazmierczak M., Knapp N., Lehmann S., Paulick S., Pütz S., Rödig E., Taubert F., Köhler P., Huth A. Lessons learned from applying a forest gap model to understand ecosystem and carbon dynamics of complex tropical forests. Ecol. Modell. 2016;326:124–133. doi: 10.1016/J.ECOLMODEL.2015.11.018. [DOI] [Google Scholar]
- Fisher J.B., Ewers F.W. In: The Biology of Vines. Putz F.E., Mooney H.A., editors. Cambridge University Press; Cambridge: 1991. Structural responses to stem injury in vines; pp. 99–126. [Google Scholar]
- Fisher R.A., Muszala S., Verteinstein M., Lawrence P., Xu C., McDowell N.G., Knox R.G., Koven C., Holm J., Rogers B.M., Spessa A., Lawrence D., Bonan G. Taking off the training wheels: The properties of a dynamic vegetation model without climate envelopes, CLM4.5(ED) Geosci. Model. Dev. 2015;8(11):3593–3619. doi: 10.5194/GMD-8-3593-2015. [DOI] [Google Scholar]
- Fisher R.A., Koven C.D., Anderegg W.R.L., Christoffersen B.O., Dietze M.C., Farrior C.E., Holm J.A., Hurtt G.C., Knox R.G., Lawrence P.J., Lichstein J.W., Longo M., Matheny A.M., Medvigy D., Muller-Landau H.C., Powell T.L., Serbin S.P., Sato H., Shuman J.K.…Moorcroft P.R. Vegetation demographics in Earth System Models: A review of progress and priorities. Glob. Chang. Biol. 2018;24(1):35–54. doi: 10.1111/gcb.13910. [DOI] [PubMed] [Google Scholar]
- Gallenmüller F., Rowe N., Speck T. Development and growth form of the neotropical liana Croton nuntians: The effect of light and mode of attachment on the biomechanics of the stem. J. Plant Growth Regul. 2004;23(2):83–97. doi: 10.1007/S00344-004-0045-Z/FIGURES/12. [DOI] [Google Scholar]
- Gehring C., Park S., Denich M. Liana allometric biomass equations for Amazonian primary and secondary forest. For. Ecol. Manage. 2004;195(1–2):69–83. doi: 10.1016/J.FORECO.2004.02.054. [DOI] [Google Scholar]
- Gerwing, J.J., & Farias, D.L. (2000). Integrating liana abundance and forest stature into an estimate of total aboveground biomass for an eastern Amazonian forest. Journal of Tropical Ecology, 16(03), 327–335. null.
- Gerwing J.J., Schnitzer S.A., Burnham R.J., Bongers F., Chave J., DeWalt S.J., Ewango C.E.N., Foster R., Kenfack D., Martínez-Ramos M., Parren M., Parthasarathy N., Pérez-Salicrup D.R., Putz F.E., Thomas D.W. A Standard Protocol for Liana Censuses1. Biotropica. 2006;38(2):256–261. doi: 10.1111/j.1744-7429.2006.00134.x. [DOI] [Google Scholar]
- Gianoli E. The behavioural ecology of climbing plants. AoB Plants. 2015;7:plv013. doi: 10.1093/aobpla/plv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán Q., Rivard B., Sánchez-Azofeifa G.A. Discrimination of liana and tree leaves from a Neotropical Dry Forest using visible-near infrared and longwave infrared reflectance spectra. Remote Sens. Environ. 2018;219:135–144. doi: 10.1016/j.rse.2018.10.014. J. A. [DOI] [Google Scholar]
- Han L., Xie L.J., Dai K.J., Yang Q., Cai Z.Q. Contrasting leaf characteristics of trees and lianas in secondary and mature forests in southwestern China. Photosynthetica. 2010;48(4):559–566. doi: 10.1007/S11099-010-0073-9/METRICS. [DOI] [Google Scholar]
- Hegarty E.E. Leaf Life-Span and Leafing Phenology of Lianes and Associated Trees During a Rainforest Succession. Journal of Ecology. 1990;78(2):300–312. doi: 10.2307/2261113. [DOI] [Google Scholar]
- Ingwell L.L., Joseph Wright S., Becklund K.K., Hubbell S.P., Schnitzer S.A. The impact of lianas on 10 years of tree growth and mortality on Barro Colorado Island, Panama. Journal of Ecology. 2010;98(4):879–887. doi: 10.1111/j.1365-2745.2010.01676.x. [DOI] [Google Scholar]
- Isnard S., Silk W.K. Moving with climbing plants from Charles Darwin's time into the 21st century. Am. J. Bot. 2009;96(7):1205–1221. doi: 10.3732/ajb.0900045. [DOI] [PubMed] [Google Scholar]
- Johnson D.M., Domec J.C., Woodruff D.R., McCulloh K.A., Meinzer F.C. Contrasting hydraulic strategies in two tropical lianas and their host trees. Am. J. Bot. 2013;100(2):374–383. doi: 10.3732/AJB.1200590. [DOI] [PubMed] [Google Scholar]
- Jiménez-Castillo M., Lusk C.H. Vascular performance of woody plants in a temperate rain forest: lianas suffer higher levels of freeze–thaw embolism than associated trees. Funct. Ecol. 2013;27(2):403–412. doi: 10.1111/1365-2435.12045. [DOI] [Google Scholar]
- Kalácska M., Calvo-Alvarado J.C., Sánchez-Azofeifa G.A. Calibration and assessment of seasonal changes in leaf area index of a tropical dry forest in different stages of succession. Tree Physiol. 2005;25:733–744. doi: 10.1093/treephys/25.6.733. [DOI] [PubMed] [Google Scholar]
- Kira T., Ogawa H. In: Productivity of Forest Ecosystems. Duvigneaud P., editor. UNESCO; Paris, France: 1971. Productivity of Forest Ecosystems Assessment of primary production in tropical and equatorial forests; pp. 309–321. [Google Scholar]
- Koven C.D., Knox R.G., Fisher R.A., Chambers J.Q., Christoffersen B.O., Davies S.J., Detto M., Dietze M.C., Faybishenko B., Holm J., Huang M., Kovenock M., Kueppers L.M., Lemieux G., Massoud E., McDowell N.G., Muller-Landau H.C., Needham J.F., Norby R.J.…Xu C. Benchmarking and parameter sensitivity of physiological and vegetation dynamics using the Functionally Assembled Terrestrial Ecosystem Simulator (FATES) at Barro Colorado Island, Panama. Biogeosciences. 2020;17(11):3017–3044. doi: 10.5194/bg-17-3017-2020. [DOI] [Google Scholar]
- Krinner G., Viovy N., de Noblet-Ducoudré N., Ogée J., Polcher J., Friedlingstein P., Ciais P., Sitch S., Prentice I.C. A dynamic global vegetation model for studies of the coupled atmosphere-biosphere system. Global. Biogeochem. Cycles. 2005;19:GB1015. [Google Scholar]
- Kurzel B.P., Schnitzer S.A., Carson W.P. Predicting liana crown location from stem diameter in three Panamanian lowland forests. Biotropica. 2006;38(2):262–266. doi: 10.1111/j.1744-7429.2006.00135.x. [DOI] [Google Scholar]
- Lawrence D.M., Fisher R.A., Koven C.D., Oleson K.W., Swenson S.C., Bonan G., Collier N., Ghimire B., van Kampenhout L., Kennedy D., Kluzek E., Lawrence P.J., Li F., Li H., Lombardozzi D., Riley W.J., Sacks W.J., Shi M., Vertenstein M.…Zeng X. The Community Land Model Version 5: Description of New Features, Benchmarking, and Impact of Forcing Uncertainty. J. Adv. Model. Earth. Syst. 2019;11(12):4245–4287. doi: 10.1029/2018MS001583. [DOI] [Google Scholar]
- Ledo A., Schnitzer S.A. Disturbance and clonal reproduction determine liana distribution and maintain liana diversity in a tropical forest. Ecology. 2014;95(8):2169–2178. doi: 10.1890/13-1775.1. [DOI] [PubMed] [Google Scholar]
- Letcher S.G. In: The Ecology of Lianas. Schnitzer S.A., Bongers F., Burnham R., Putz F.E., editors. Wiley-Blackwell Publishers; Oxford: 2015. Patterns of liana succession in tropical forests. [Google Scholar]
- Longo M., Knox R.G., Medvigy D.M., Levine N.M., Dietze M.C., Kim Y., Swann A.L.S., Zhang K., Rollinson C.R., Bras R.L., Wofsy S.C., Moorcroft P.R. The biophysics, ecology, and biogeochemistry of functionally diverse, vertically and horizontally heterogeneous ecosystems: the Ecosystem Demography model, version 2.2 – Part 1: Model description. Geosci. Model. Dev. 2019;12(10):4309–4346. doi: 10.5194/gmd-12-4309-2019. [DOI] [Google Scholar]
- Maréchaux I., Chave J. An individual-based forest model to jointly simulate carbon and tree diversity in Amazonia: description and applications. Ecol. Monogr. 2017;87(4):632–664. doi: 10.1002/ecm.1271. [DOI] [Google Scholar]
- Maréchaux I., Bartlett M.K., Iribar A., Sack L., Chave J. Stronger seasonal adjustment in leaf turgor loss point in lianas than trees in an Amazonian forest. Biol. Lett. 2017;(1):13. doi: 10.1098/RSBL.2016.0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A.R., Coates M.A., Archer J., Kivambe E., Mnendendo H., Mtoka S., Mwakisoma R., de Figueiredo R.J.R.L., Njilima F.M. Liana cutting for restoring tropical forests: a rare palaeotropical trial. Afr. J. Ecol. 2017;55(3):282–297. doi: 10.1111/aje.12349. [DOI] [Google Scholar]
- Marshall A.R., Platts P.J., Chazdon R.L., Seki H., Campbell M.J., Phillips O.L., Gereau R.E., Marchant R., Liang J., Herbohn J., Malhi Y., Pfeifer M. Conceptualising the Global Forest Response to Liana Proliferation. Front. For. Glob. Change. 2020;3(35) doi: 10.3389/ffgc.2020.00035. [DOI] [Google Scholar]
- McDowell N., Allen C.D., Anderson-Teixeira K., Brando P., Brienen R., Chambers J., Christoffersen B., Davies S., Doughty C., Duque A., Espirito-Santo F., Fisher R., Fontes C.G., Galbraith D., Goodsman D., Grossiord C., Hartmann H., Holm J., Johnson D.J.…Xu X. Drivers and mechanisms of tree mortality in moist tropical forests. New Phytologist. 2018;219(3):851–869. doi: 10.1111/NPH.15027. [DOI] [PubMed] [Google Scholar]
- Medina-Vega J.A., Bongers F., Poorter L., Schnitzer S.A., Sterck F.J. Lianas have more acquisitive traits than trees in a dry but not in a wet forest. Journal of Ecology. 2021;109(6):2367–2384. doi: 10.1111/1365-2745.13644. [DOI] [Google Scholar]
- Medina-Vega J.A., Bongers F., Schnitzer S.A., Sterck F.J. Lianas explore the forest canopy more effectively than trees under drier conditions. Funct. Ecol. 2021;35(2):318–329. doi: 10.1111/1365-2435.13717. [DOI] [Google Scholar]
- Medina-Vega J.A., Wright S.J., Bongers F., Schnitzer S.A., Sterck F.J. Vegetative phenologies of lianas and trees in two Neotropical forests with contrasting rainfall regimes. New Phytologist. 2022;235(2):457–471. doi: 10.1111/nph.18150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello F.N.A., Estrada-Villegas S., Defilippis D.M., Schnitzer S.A. Can functional traits explain plant coexistence? A case study with tropical lianas and trees. Diversity. (Basel) 2020;12(10):1–15. doi: 10.3390/d12100397. [DOI] [Google Scholar]
- Ménard L., McKey D., Rowe N. Developmental plasticity and biomechanics of treelets and lianas in Manihot aff. quinquepartita (Euphorbiaceae): a branch-angle climber of French Guiana. Ann. Bot. 2009;103(8):1249–1259. doi: 10.1093/AOB/MCP078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier F., Krishna Moorthy S.M., de Deurwaerder H.P.T., Kreus R., den Bulcke J., Lehnebach R., Verbeeck H. Within-Site Variability of Liana Wood Anatomical Traits: A Case Study in Laussat, French Guiana. Forests. 2020;11(5) doi: 10.3390/f11050523µ. [DOI] [Google Scholar]
- Meunier F., Verbeeck H., Cowdery B., Schnitzer S.A., Smith-Martin C.M., Powers J.S., Xu X., Slot M., de Deurwaerder H.P.T., Detto M., Bonal D., Longo M., Santiago L.S., Dietze M. Unraveling the relative role of light and water competition between lianas and trees in tropical forests: A vegetation model analysis. Journal of Ecology. 2021;109(1):519–540. doi: 10.1111/1365-2745.13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier F., van der Heijden G.M.F., Schnitzer S.A., de Deurwaerder H.P.T., Verbeeck H. Lianas Significantly Reduce Aboveground and Belowground Carbon Storage: A Virtual Removal Experiment. Front. For. Glob. Change. 2021;4:75. doi: 10.3389/ffgc.2021.663291. [DOI] [Google Scholar]
- Meunier F., Visser M.D., Shiklomanov A., Dietze M.C., Guzmán Q., Sanchez-Azofeifa G.A., de Deurwaerder H.P.T., Krishna Moorthy S.M., Schnitzer S.A., Marvin D.C., Longo M., Liu C., Broadbent E.N., Almeyda Zambrano A.M., Muller-Landau H.C., Detto M., Verbeeck H. Liana optical traits increase tropical forest albedo and reduce ecosystem productivity. Glob. Chang. Biol. 2022;28(1):227–244. doi: 10.1111/GCB.15928. J. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorcroft P.R., Hurtt G.C., Pacala S.W. A METHOD FOR SCALING VEGETATION DYNAMICS: THE ECOSYSTEM DEMOGRAPHY MODEL (ED) Ecol. Monogr. 2001;71(4):557–586. doi: 10.1890/0012-9615(2001)071[0557:amfsvd]2.0.co;2. [DOI] [Google Scholar]
- Moorthy S.M.K., Raumonen P., Bulcke den, van J., Calders K., Verbeeck H. Terrestrial laser scanning for non-destructive estimates of liana stem biomass. For. Ecol. Manage. 2020;456 doi: 10.1016/j.foreco.2019.117751. [DOI] [Google Scholar]
- Moorthy, S.M.K., Meunier, F., Calders, K., Aguilar, A., Pausenberger, N., Schnitzer, S.A., Visser, M.D., Muller-Landau, H., & Verbeeck, H. (2022). Strong impacts of lianas on tree allometry lead to overestimation of tropical forest carbon stocks and sink. 10.21203/rs.3.rs-2094059/v1. [DOI]
- Mori H., Ueno S., Matsumoto A., Kamijo T., Tsumura Y., Masaki T. Large contribution of clonal reproduction to the distribution of deciduous liana species (Wisteria floribunda) in an old-growth cool temperate forest: evidence from genetic analysis. Ann. Bot. 2018;121(2):359–365. doi: 10.1093/AOB/MCX153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Landau Helene, Pacala Stephen W. In: Unsolved Problems in Ecology. Dobson Andrew, Tilman David, Holt Robert D., editors. Princeton University Press; Princeton, New Jersey: 2020. What determines the abundance of lianas and vines? pp. 239–264. editors. [Google Scholar]
- Mumbanza F.M., Bauters M., Makelele I., Meunier F., Kearsley E., Boeckx P., Verheyen K., Ewango C., Mbula I.M., Lubini C.A., Verbeeck H. Lianas rapidly colonize early stages of tropical forests, presumably through leaf trait diversification. Journal of Vegetation Science. 2022;(6):33. doi: 10.1111/jvs.13162. [DOI] [Google Scholar]
- Nabe-Nielsen J., Hall P. Environmentally induced clonal reproduction and life history traits of the liana Machaerium cuspidatum in an Amazonian rain forest, Ecuador. Plant Ecol. 2002;162(2):215–226. doi: 10.1023/A:1020561923488/METRICS. [DOI] [Google Scholar]
- Opler P.A., Baker H.G., Frankie G.W. In: The Biology of Vines. Putz FE, Mooney HA, editors. Cambridge University Press; Cambridge: 1991. Seasonality of climbers: a review and example from Costa Rican dry forest; pp. 377–391. [Google Scholar]
- Pandian E., Parthasarathy N. Decadal (2003–2013) changes in liana diversity, abundance and aboveground biomass in four inland tropical dry evergreen forest sites of peninsular India. J. For. Res. (Harbin) 2016;27(1):133–146. doi: 10.1007/s11676-015-0146-5. [DOI] [Google Scholar]
- Paul G.S., Yavitt J.B. Tropical Vine Growth and the Effects on Forest Succession: A Review of the Ecology and Management of Tropical Climbing Plants. Botanical Review. 2011;77(1):11–30. doi: 10.1007/S12229-010-9059-3/TABLES/2. [DOI] [Google Scholar]
- Phillips O.L., Martinez R.v, Arroyo L., Baker T.R., Killeen T., Lewis S.L., Malhi Y., Mendoza A.M., Neill D., Vargas P.N., Alexiades M., Ceron C., di Fiore A., Erwin T., Jardim A., Palacios W., Saldias M., Vinceti B. Increasing dominance of large lianas in Amazonian forests. Nature. 2002;418(6899):770–774. doi: 10.1038/nature00926. [DOI] [PubMed] [Google Scholar]
- Phillips O.L., Martinez R.v, Mendoza A.M., Baker T.R., Vargas P.N. Large lianas as hyperdynamic elements of the tropical forest canopy. Ecology. 2005;86(5):1250–1258. doi: 10.1890/04-1446. [DOI] [Google Scholar]
- Putz F.E. Liana Biomass and Leaf Area of a “Tierra Firme” Forest in the Rio Negro Basin, Venezuela. Biotropica. 1983;15(3):185–189. doi: 10.2307/2387827. [DOI] [Google Scholar]
- Putz F.E. The Natural History of Lianas on Barro Colorado Island, Panama. Ecology. 1984;65(6):1713–1724. doi: 10.2307/1937767. [DOI] [Google Scholar]
- Putz F.E. How trees avoid and shed lianas. Biotropica. 1984;16:19–23. [Google Scholar]
- Putz F.E., Windsor D.M. Liana Phenology on Barro Colorado Island, Panama. Biotropica. 1987;19(4):334–341. doi: 10.2307/2388631. [DOI] [Google Scholar]
- Putz F.E. Climbing plants beat trees to soil nutrient patches. Current Biology. 2023;33(12):R675–R676. doi: 10.1016/j.cub.2023.04.035. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ronderos M.E., Bohrer G., Sanchez-Azofeifa A., Powers J.S., Schnitzer S.A. Contribution of lianas to plant area index and canopy structure in a Panamanian forest. Ecology. 2016;97(12):3271–3277. doi: 10.1002/ecy.1597. [DOI] [PubMed] [Google Scholar]
- Sánchez-Azofeifa G.A., Kalácska M., do Espírito-Santo M.M., Fernandes G.W., Schnitzer S. Tropical dry forest succession and the contribution of lianas to wood area index (WAI) For. Ecol. Manage. 2009;258(6):941–948. doi: 10.1016/j.foreco.2008.10.007. [DOI] [Google Scholar]
- Santiago L.S., Wright S.J. Leaf functional traits of tropical forest plants in relation to growth form. Funct. Ecol. 2007;21(1):19–27. doi: 10.1111/j.1365-2435.2006.01218.x. [DOI] [Google Scholar]
- Schnitzer S.A., Dalling J.W., Carson W.P. The impact of lianas on tree regeneration in tropical forest canopy gaps: Evidence for an alternative pathway of gap-phase regeneration. Journal of Ecology. 2000;88(4):655–666. doi: 10.1046/j.1365-2745.2000.00489.x. [DOI] [Google Scholar]
- Schnitzer S.A., Carson W.P. TREEFALL GAPS AND THE MAINTENANCE OF SPECIES DIVERSITY IN A TROPICAL FOREST. Ecology. 2001;82(4):913–919. doi: 10.1890/0012-9658(2001)082[0913:TGATMO]2.0.CO;2. [DOI] [Google Scholar]
- Schnitzer S.A., Carson W.P. Would Ecology Fail the Repeatability Test? Bioscience. 2016;66(2):98–99. doi: 10.1093/biosci/biv176. [DOI] [Google Scholar]
- Schnitzer S.A., Bongers F. The ecology of lianas and their role in forests. Trends. Ecol. Evol. 2002;17(5):223–230. [Google Scholar]
- Schnitzer S.A. A mechanistic explanation for global patterns of liana abundance and distribution. American Naturalist. 2005;166(2):262–276. doi: 10.1086/431250. [DOI] [PubMed] [Google Scholar]
- Schnitzer S.A., DeWalt S.J., Chave J. Censusing and measuring lianas: A quantitative comparison of the common methods. Biotropica. 2006;38(5):581–591. doi: 10.1111/j.1744-7429.2006.00187.x. [DOI] [Google Scholar]
- Schnitzer S.A., Carson W.P. Lianas suppress tree regeneration and diversity in treefall gaps. Ecol. Lett. 2010;13(7):849–857. doi: 10.1111/j.1461-0248.2010.01480.x. [DOI] [PubMed] [Google Scholar]
- Schnitzer S.A., Bongers F. Increasing liana abundance and biomass in tropical forests: emerging patterns and putative mechanisms. Ecol. Lett. 2011;14:397–406. doi: 10.1111/j.1461-0248.2011.01590.x. [DOI] [PubMed] [Google Scholar]
- Schnitzer S.A., Mangan S.A., Dalling J.W., Baldeck C.A., Hubbell S.P., Ledo A., Muller-Landau H., Tobin M.F., Aguilar S., Brassfield D., Hernandez A., Lao S., Perez R., Valdes O., Yorke S.R. Liana Abundance, Diversity, and Distribution on Barro Colorado Island, Panama. PLoS. One. 2012;(12):7. doi: 10.1371/journal.pone.0052114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer S.A., DeFilippis D.M., Visser M., Estrada-Villegas S., Rivera-Camaña R., Bernal B., Peréz S., Valdéz A., Valdéz S., Aguilar A., Dalling J.W., Broadbent E.N., Almeyda Zambrano A.M., Hubbell S.P., Garcia-Leon M. Local canopy disturbance as an explanation for long-term increases in liana abundance. Ecol. Lett. 2021;24(12):2635–2647. doi: 10.1111/ELE.13881. [DOI] [PubMed] [Google Scholar]
- Seidl R., Rammer W., Scheller R.M., Spies T.A. An individual-based process model to simulate landscape-scale forest ecosystem dynamics. Ecol. Modell. 2012;231:87–100. doi: 10.1016/J.ECOLMODEL.2012.02.015. [DOI] [Google Scholar]
- Sfair J.C., et al. Theoretical approaches to liana management: a search for a less harmful method. Int. J. Biodivers. Sci. Ecosyst. Serv. Manage. 2015;11.2 [Google Scholar]
- Signori-Müller C., Galbraith D., Tavares J.v, Reis S.M., Diniz F.C., Gilpin M., Marimon B.S., van der Heijden G.M.F., Borges C., Cintra B.B.L., Mião S., Morandi P.S., Nina A., Salas Yupayccana C.A., Marca Zevallos M.J., Cosio E.G., Junior B.H.M., Mendoza A.M., Phillips O.…Oliveira R.S. Tropical forest lianas have greater non-structural carbohydrate concentrations in the stem xylem than trees. Tree Physiol. 2023 doi: 10.1093/treephys/tpad096. [DOI] [PubMed] [Google Scholar]
- Slot M., Wright S.J., Kitajima K. Foliar respiration and its temperature sensitivity in trees and lianas: in situ measurements in the upper canopy of a tropical forest. Tree Physiol. 2013;33(5):505–515. doi: 10.1093/treephys/tpt026. [DOI] [PubMed] [Google Scholar]
- Smith-Martin C.M., Xu X., Medvigy D., Schnitzer S.A., Powers J.S. Allometric scaling laws linking biomass and rooting depth vary across ontogeny and functional groups in tropical dry forest lianas and trees. New Phytologist. 2020;226(3):714–726. doi: 10.1111/NPH.16275. [DOI] [PubMed] [Google Scholar]
- Tang Y., Kitching R.L., Cao M. Lianas as structural parasites: A re-evaluation. Chinese Science Bulletin. 2012;57(4):307–312. doi: 10.1007/s11434-011-4690-x. [DOI] [Google Scholar]
- Tobin M.F., Wright A.J., Mangan S.A., Schnitzer S.A. Lianas have a greater competitive effect than trees of similar biomass on tropical canopy trees. Ecosphere. 2012;3(2):1–11. doi: 10.1890/es11-00322.1. [DOI] [Google Scholar]
- Tymen B., Réjou-Méchain M., Dalling J.W., Fauset S., Feldpausch T.R., Norden N., Phillips O.L., Turner B.L., Viers J., Chave J. Evidence for arrested succession in a liana-infested Amazonian forest. Journal of Ecology. 2016;104(1):149–159. doi: 10.1111/1365-2745.12504. [DOI] [Google Scholar]
- van der Heijden G.M.F., Feldpausch T.R., Herrero A., de la F., van der Velden N.K., Phillips O.L. Calibrating the liana crown occupancy index in Amazonian forests. For. Ecol. Manage. 2010;260(4):549–555. doi: 10.1016/j.foreco.2010.05.011. [DOI] [Google Scholar]
- van der Heijden G.M., Schnitzer S.A., Powers J.S., Phillips O.L. Liana Impacts on Carbon Cycling, Storage and Sequestration in Tropical Forests. Biotropica. 2013;45(6):682–692. doi: 10.1111/btp.12060. [DOI] [Google Scholar]
- van der Heijden G.M.F., Powers J.S., Schnitzer S.A. Lianas reduce carbon accumulation and storage in tropical forests. Proceedings of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1504869112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden G.M.F., Powers J.S., Schnitzer S.A. Effect of lianas on forest-level tree carbon accumulation does not differ between seasons: Results from a liana removal experiment in Panama. Journal of Ecology. 2019;107(4):1890–1900. doi: 10.1111/1365-2745.13155. [DOI] [Google Scholar]
- van der Heijden G.M.F., Proctor A.D.C., Calders K., Chandler C.J., Field R., Foody G.M., Krishna Moorthy S.M., Schnitzer S.A., Waite C.E., Boyd D.S. Making (remote) sense of lianas. Journal of Ecology. 2022;110(3):498–513. doi: 10.1111/1365-2745.13844. [DOI] [Google Scholar]
- van der Heijden, G., Meunier, F., Verbeeck, H., & Schnitzer, S. (2024). Lianas detrimentally affect carbon storage potential and recovery times of tropical secondary forests. 10.21203/rs.3.rs-4696533/v1. [DOI]
- Visser M.D., Schnitzer S.A., Muller-Landau H.C., Jongejans E., de Kroon H., Comita L.S., Hubbell S.P., Wright S.J. Tree species vary widely in their tolerance for liana infestation: A case study of differential host response to generalist parasites. Journal of Ecology. 2018;106(2):781–794. doi: 10.1111/1365-2745.12815. [DOI] [Google Scholar]
- Visser M.D., Muller-Landau H.C., Schnitzer S.A., de Kroon H., Jongejans E., Wright S.J. A host–parasite model explains variation in liana infestation among co-occurring tree species. Journal of Ecology. 2018;106(6):2435–2445. doi: 10.1111/1365-2745.12997. [DOI] [Google Scholar]
- Visser M.D., Detto M., Meunier F., Wu J., Foster J., Marvin D.C., Bongalov B., Nunes M.H., Coomes D., Verbeeck H., Q J.A.G., Sanchez-Azofeifa A., Chandler C.J., Heijden G.M.F.van der, Boyd D.S., Foody G.M., Cutler M.E.J., Broadbent E.N., Serbin S.S.…Pacala S. Why can we detect lianas from space? bioRxiv. 2021 doi: 10.1101/2021.09.30.462145. 2021.09.30.462145. [DOI] [Google Scholar]
- Waite C.E., van der Heijden G.M.F., Field R., Boyd D.S. A view from above: Unmanned aerial vehicles (UAVs) provide a new tool for assessing liana infestation in tropical forest canopies. Journal of Applied Ecology. 2019;56(4):902–912. doi: 10.1111/1365-2664.13318. [DOI] [Google Scholar]
- Willson A.M., Trugman A.T., Powers J.S., Smith-Martin C.M., Medvigy D. Climate and hydraulic traits interact to set thresholds for liana viability. Nat. Commun. 2022;13(1):1–11. doi: 10.1038/s41467-022-30993-2. 2022 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright I.J., Reich P.B., Westoby M., Ackerly D.D., Baruch Z., Bongers F., Cavender-Bares J., Chapin T., Cornellssen J.H.C., Diemer M., Flexas J., Garnier E., Groom P.K., Gulias J., Hikosaka K., Lamont B.B., Lee T., Lee W., Lusk C.…Villar R. The worldwide leaf economics spectrum. Nature. 2004;428(6985):821–827. doi: 10.1038/nature02403. 2004 428:6985. [DOI] [PubMed] [Google Scholar]
- Wright I.J., Reich P.B., Cornelissen J.H.C., Falster D.S., Garnier E., Hikosaka K., Lamont B.B., Lee W., Oleksyn J., Osada N., Poorter H., Villar R., Warton D.I., Westoby M. Assessing the generality of global leaf trait relationships. New Phytologist. 2005;166(2):485–496. doi: 10.1111/J.1469-8137.2005.01349.X. [DOI] [PubMed] [Google Scholar]
- Wright S.J., Sun I.F., Pickering M., Fletcher C.D., Chen Y.Y. Long-term changes in liana loads and tree dynamics in a Malaysian forest. Ecology. 2015;96(10):2748–2757. doi: 10.1890/14-1985.1. [DOI] [PubMed] [Google Scholar]
- Wyka T.P., Oleksyn J., Karolewski P., Schnitzer S.A. Phenotypic correlates of the lianescent growth form: a review. Ann. Bot. 2013;112(9):1667–1681. doi: 10.1093/aob/mct236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C.ming, Wu T., Geng Y.fen, Chai Y., Hao J.bo. Phenotypic plasticity of lianas in response to altered light environment. Ecol. Res. 2016;31(3):375–384. doi: 10.1007/s11284-016-1343-1. [DOI] [Google Scholar]
- Zhu S.D., Cao K.F. Hydraulic properties and photosynthetic rates in co-occurring lianas and trees in a seasonal tropical rainforest in Southwestern China. Plant Ecol. 2009;204(2):295–304. doi: 10.1007/S11258-009-9592-5/TABLES/3. [DOI] [Google Scholar]
- Zhu S.D., Cao K.F. Contrasting cost–benefit strategy between lianas and trees in a tropical seasonal rain forest in southwestern China. Oecologia. 2010;163(3):591–599. doi: 10.1007/s00442-010-1579-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The liana database is available on github (https://github.com/femeunier/LianaDB), as MySQL and R storage files. The database can also be accessed via https://www.lianaecologyproject.com Script files to manipulate the database (e.g. to build, insert new records, pull data, and plot) are provided as well.