Abstract
Architect-HIV Ag/Ab combo chemiluminescence assay is globally recognized for its sensitivity but has a notable false-positive rate. In this study, we aim to evaluate the performance of a new cost-effective screening alternative, the chemiluminescence Ag/Ab combo assay (CL-900i-HIV) from Mindray, China. We selected 195 archived samples categorized according to the INNO-LIA™ HIV I/II, the gold standard confirmatory assay. These samples included true positive (n = 38; positive by Architect-HIV & INNO-LIA-HIV), true negative (n = 101; negative by Architect-HIV & INNO-LIA-HIV), false positive (n = 20; positive by Architect-HIV & negative by INNO-LIA-HIV), and indeterminate results (n = 26). We tested all samples using the Mindray CL-900i-HIV and all positive Architect-HIV samples (n = 80) were confirmed by PCR. Compared to INNO-LIA™ HIVI/II line immunoassay confirmatory assay, Mindray CL-900i-HIV demonstrated a sensitivity of 100% (95% CI 90.7–100), specificity of 100% (95% CI 97.0–100), overall percent agreement (OPA) of 100% (95% CI 97.7–100.0), and perfect agreement with the INNO-LIA confirmatory assay (κ = 1.00). Additionally, Mindray’s CL-900i-HIV exhibited a significantly lower false-positive rate (8.75%) compared to Architect-HIV’s (55%). Mindray CL900i demonstrated high sensitivity and very low false-positive rate, thus, has the potential to serve as an excellent, cost-effective surrogate for HIV screening, overcoming the limitations of existing automated assays.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78271-z.
Keywords: AIDS, HIV, CLIA, Infectious disease, Diagnosis
Subject terms: HIV infections, Infectious-disease diagnostics
Introduction
Acquired Immunodeficiency Syndrome (AIDS) caused by the human immunodeficiency virus (HIV) remains a significant global public health concern. According to the World Health Organization (WHO), an estimated 39.9 million people were living with HIV by the end of 2023, including 1.4 million children under the age of 141. Over the years, HIV infection has claimed the lives of approximately 42.3 million people. Despite significant advances in treatment and prevention, the virus continues to have a profound impact, with 630,000 people dying from HIV-related causes in 2023 alone1. HIV is a retrovirus that mainly targets CD4 + T helper lymphocytes and leads to their gradual loss, causing severe immunodeficiency in the affected patients. As the CD4 + T count drops below critical levels, the body becomes increasingly unable to fight infections and disease and vulnerable to opportunistic infections and cancers2–6. The main routes of HIV transmission includes unprotected sexual intercourse, sharing contaminated needles, mother-to-child transmission, and blood transfusions7–9. Thus, early and accurate HIV diagnosis is crucial in order to start antiretroviral therapy promptly and prevent the spread of the virus10. There are several diagnostic options available, such as rapid diagnostic tests (RDTs), enzyme-linked immunosorbent assays (ELISAs), chemiluminescence immunoassays (CLIA), and nucleic acid tests (NATs)11,12.
In Qatar, communicable diseases account for approximately 8% of all deaths, and continue to present a challenge to public health services13. The Medical Commission (MC) is an essential pillar operating under the Qatar Ministry of Public Health (MOPH), which is responsible for screening newcomers to Qatar to keep the country free from serious infectious diseases, including AIDS. As a precautionary measure against the import and spread of serious infectious diseases, the MOPH requires individuals to undergo a medical examination upon their arrival in Qatar. Currently, the MC employs ARCHITECT®HIV Ag/Ab combo assay (Abbott Diagnostics, Abbott Park, Illinois, USA) as the primary screening method for HIV detection. Although the assay is highly sensitive, it presents significant challenges due to its tendency to produce false-positive results. The high false positive results lead to the need for repeated and confirmatory testing, which increases costs and burdens the healthcare system14,15. The confirmatory testing includes line immunoassay (LIA); and PCR testing. Nevertheless, the LIA assay does have several limitations, with one of the main drawbacks being the occurrence of indeterminate results16. Indeterminate results require additional testing and follow-up, leading to increased costs, reporting delays, and potential social implications. Additionally, PCR testing has its own limitations in HIV diagnosis. For instance, if the individual is on treatment, the PCR result might show as “Not Detected” or “Below Detection Limit,” while the INNO-LIA result is positive. Furthermore, the applicant might be one of the rare cases of “Elite Controllers” who have the unique ability to naturally suppress the replication of the HIV virus. As a result, their PCR test may show a result of “Not Detected” or “Below Detection Limit,” but they would still test positive on the INNO-LIA test.
To address the challenges posed by the current screening and confirmatory assay employed in the MC, it is essential to explore alternative approaches that balance sensitivity, specificity, and cost-effectiveness. In our study, we aim to evaluate the performance of a new cost-effective Mindray CL-900i-HIV Ag/Ab combo assay from Mindray-China as an alternative screening method to the well-established ARCHITECT®HIV Ag/Ab combo assay17–19. In addition, we compared the performance of the Mindray CL-900i-HIV Ag/Ab combo assay against the gold standard INNO-LIA™ HIV I/II confirmatory assay20. We also included a surrogate reference method, PCR, in our evaluation. Our findings aim to is to provide valuable insights for healthcare professionals in Qatar to improve their diagnostic methods for HIV infection and enhance the effectiveness of their screening protocols. This initiative aims to result in a more effective distribution of healthcare resources, improved patient management, and enhanced control of HIV transmission within the population.
Methods
Ethical approval
This study was approved by IRB at Qatar University (QU-IRB 017/2024-E).
Study population and study design
The MC is part of the Ministry of Public Health (MOPH) and plays a vital role in implementing the ministry’s health strategies. For this study, the MC provided archived samples initially collected during routine HIV screening as part of the visa application process for individuals entering the country. Thus, no patients or applicants were recruited, and there was no direct or indirect interaction with any human subject. To ensure participant confidentiality, unique codes were generated and assigned to each sample, categorized by testing outcomes, to avoid the use of actual sample barcode numbers that could potentially disclose identities. For example, samples were coded as N001 for those negative by both ARCHITECT® HIV Ag/Ab combo assay and INNO-LIA™ HIVI/II confirmatory assay, PN401 for samples positive by ARCHITECT® HIV Ag/Ab combo assay but negative by INNO-LIA™ HIVI/II confirmatory assay, PI401 for samples positive by ARCHITECT® HIV Ag/Ab combo assay but indeterminant by INNO-LIA™ HIVI/II confirmatory assay, and PP401 for samples positive by both assays.
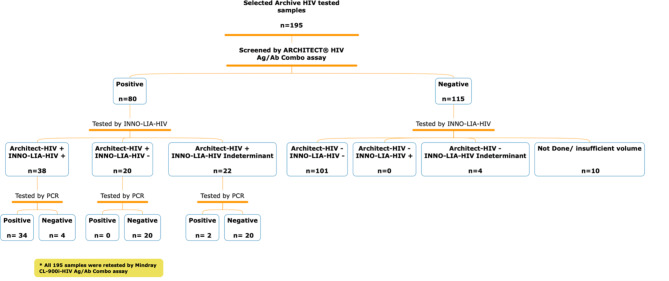
We collected retrospective data from a large population of individuals who underwent HIV screening at the MC between 2022 and 2023 using the Architect-HIV assay. From this, we specifically selected 195 samples that showed agreement and discrepancies between the ARCHITECT® HIV Ag/Ab combo assay and the gold standard, INNO-LIA™ HIVI/II confirmatory assay, for inclusion in the current study. The selected samples comprised 38 cases that tested positive by both methods, 20 cases positive by ARCHITECT® HIV Ag/Ab combo assay but negative by INNO-LIA™ HIVI/II confirmatory assay, 22 cases positive by ARCHITECT® HIV Ag/Ab combo assay with indeterminate results by INNO-LIA™ HIVI/II confirmatory assay, 4 samples negative by ARCHITECT® HIV Ag/Ab combo assay with indeterminate results by INNO-LIA™ HIVI/II confirmatory assay and 101 cases negative by both assays (Fig. 1). All samples were re-tested using the Mindray CL-900i-HIV Ag/Ab combo assay, and all positive ARCHITECT® HIV Ag/Ab combo samples were additionally confirmed by PCR as a surrogate reference method. This approach allows for a comprehensive evaluation of the Mindray CL-900i-HIV accuracy, specifically for samples with potential false-positive results, providing robust evidence for its performance and suitability as an alternative screening method.
Fig. 1.
Flowchart depicting the sample selection process for inclusion in the study. 10 negative samples were not tested by INNO-LIA-HIV because of insufficient sample volume.
Mindray CL-900i-HIV Ag/Ab combo assay
The Mindray CL-900i-HIV Ag/Ab combo assay (Shenzhen Mindray Bio-Medical Electronics Co. Ltd., Shenzhen, China) is a 2-site sandwich CLIA. The assay utilizes monoclonal antibodies and HIV-1 or HIV-2 antigen-coated on microparticles, enabling the simultaneous testing for both antigens and antibodies associated with HIV infection. Briefly, 100 µL of sample was combined with assay diluent and paramagnetic microparticles coated with HIV-1/HIV-2 antigen and HIV p24 mouse monoclonal antibody. After magnetic capture and washing of unbound substances, alkaline phosphatase (ALP)-labeled antigens and antibodies form a sandwich complex with the microparticles. Then, the substrate is added, resulting in a chemiluminescence reaction that will be measured as relative light units (RLUs) by the photomultiplier. The amount of positive reaction is directly proportional to the RLUs measured. Specimens are classified as reactive or non-reactive by comparing the chemiluminescent signals to cut-off values determined through calibration.
ARCHITECTHIV Ag/Ab combo assay
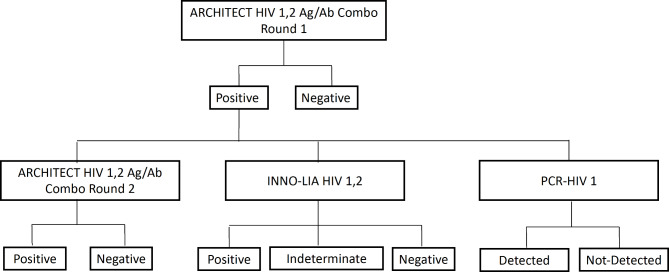
The fully automated ARCHITECT®HIV Ag/Ab combo assay (Abbott Diagnostics, Abbott Park, Illinois, USA) is a qualitative antigen/antibody immunoassay. ARCHITECT® HIV Ag/Ab combo assay can simultaneously detect both the p24 antigen and antibody of HIV-1/HIV-221. Unlike the Mindray assay, it uses acridinium as the fluorescent label. Samples are considered reactive if signal-to-cut-off (S/CO) values are ≥ 1.00, indicating presence of HIV-1 p24 antigen or HIV-1/HIV-2 antibodies; values < 1.00 are deemed non-reactive22. According to MC laboratory protocols, any reactive sample undergoes duplicate retesting. If both replicates are non-reactive, the result is considered non-reactive. If one or both replicates are reactive, the sample is confirmed as reactive. All initially reactive samples from the ARCHITECT® assay, were re-tested using the ARCHITECT® analyzer, the INNO-LIA™ HIV I/II confirmatory assay, and PCR, using freshly drawn blood samples as depicted in Fig. 2.
Fig. 2.
A chart summary of HIV diagnosis workflow in the MC. ARCHITECT® HIV Ag/Ab Combo analyzer B, INNO-LIA™ HIVI/II confirmatory assay, and PCR tests were done on a freshly drawn sample. Note: in this study, we tested all negative samples by the INNO-LIA™ HIVI/II confirmatory assay.
INNO-LIA™ HIV I/II confirmatory assay
All positive samples from the ARCHITECT®HIV Ag/Ab combo assay were previously tested using the gold standard INNO-LIA™ HIVI/II confirmatory assay (Innogenetics, Ghent, Belgium; now: Fujirebio Europe N.V.). Additionally we also tested the negative samples, except 10 samples because of insufficient sample volume. The INNO-LIA™ assay is a Line Immunoassay that utilizes a nylon strip coated with recombinant proteins and synthetic peptides specific to HIV-1 (sgp120, gp41, p31, p24, and p17) and HIV-2 (gp36 and sgp105)23. The assay was performed using AUTO-LIA™ 48 automated machine according to the manufacturer’s protocol24. Briefly, a 20 µL aliquot of each sample was diluted 1:50 in sample diluent and incubated at room temperature (20 °C) for three hours with the test strip. This step was followed by three wash cycles using a washing buffer. A goat anti-human IgG conjugated to alkaline phosphatase was then applied, succeeded by additional wash cycles. The reaction was developed using a chromogen solution and halted using a stopping solution. The strips were analyzed and interpreted using LiRAS for Infectious Diseases software, tailored for LIA results interpretation.
COBAS AmpliPrep/COBAS TaqMan HIV-1 test, version 2.0
The COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test (Roche Molecular Systems, Branchburg, USA) version 2.0 (v2.0) is a nucleic acid amplification test used for quantitating HIV-1 RNA in human plasma. It specifically targets the gag and LTR regions of the HIV-1 genome to optimize detection and account for genetic variability. Following the manufacturer’s protocol25, 850 µL of plasma is processed to isolate the HIV-1 RNA, which is then reverse-transcribed to create complementary DNA (cDNA). Subsequent PCR amplification of this cDNA integrates the use of a dual-labeled oligonucleotide probe specific to the HIV-1 targets. Amplification and detection are performed using the automated COBAS® TaqMan® 48 analyzer. This assay can quantify HIV-1 RNA within a range of 20 to 10,000,000 copies/ml.
Statistical analysis
We conducted a descriptive statistical analysis to summarize categorical variables. To assess the agreement between the Mindray CL-900i-HIV Ag/Ab combo assay and reference methods, we performed a concordance analysis that includes measures of sensitivity, specificity, overall percent agreement (OPA), positive predictive value (PPV), negative predictive value (NPV), accuracy, and the Cohen’s Kappa coefficient. The Cohen’s Kappa values are interpreted as follows: ≤ 0 indicating no agreement, 0.01–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement26,27. All statistical analyses were executed using GraphPad Prism software (Version 9, San Diego, CA, USA) at https://www.graphpad.com.
Results
Mindray CL-900i-HIV Ag/Ab combo assay demonstrates no false positives, high sensitivity, and specificity compared to the gold standard INNO-LIA™ HIVI/II confirmatory assay
The comparative analysis was performed between Mindray CL-900i-HIV Ag/Ab combo assay compared to INNO-LIA™ HIVI/II confirmatory assay as the gold standard reference method. 10 samples were excluded due to insufficient volume. Among these samples, 20.5% (38/185) were confirmed as true positive, while 65.4% (121/185) were confirmed as true negative when compared to the INNO-LIA™ HIVI/II confirmatory assay (Table 1). Most importantly, no false-negative or false-positive samples were observed with Mindray CL-900i-HIV Ag/Ab combo assay compared to INNO-LIA™ HIVI/II confirmatory assay.
Table 1.
Comparison of Mindray CL-900i-HIV Ag/Ab combo results with INNO-LIA™ HIVI/II confirmatory assay (n = 185).
| INNO-LIA-HIV + | INNO-LIA-HIV− | INNO-LIA-HIV IND | Total | |
|---|---|---|---|---|
| CL-900i-HIV+ | 38 (20.5%) | 0 (0%) | 5 (2.7%) | 43 (23.2%) |
| CL-900i-HIV− | 0 (0%) | 121 (65.4%) | 21 (11.4%) | 142 (76.8%) |
| Total | 38 (20.5%) | 121 (65.4%) | 26 (14.1%) | 185 (100%) |
10 samples were not tested by INNO-LIA™ HIVI/II confirmatory assay due to insufficient volume.
A total of 159 samples were included in the analysis excluding 26 samples with indeterminate results and 10 samples due to insufficient volume. Notably, the Mindray CL-900i-HIV assay demonstrated excellent sensitivity of 100% (95% CI 90.7–100) and specificity of 100% (95% CI 97.0–100). The OPA was 100% (97.7–100.0), further establishing the reliability of the Mindray CL-900i-HIV Ag/Ab combo assay. The PPV was 100% (90.7–100), indicating the assay’s ability to accurately identify true positive cases. The NPV was 100% (95% CI 97.0–100.0), underscoring the assay’s effectiveness in correctly identifying true negative cases. Cohen’s kappa statistic (κ = 1.00) demonstrated excellent agreement between the Mindray CL-900i-HIV Ag/Ab combo assay and the INNO-LIA™ HIVI/II confirmatory assay (Table 2).
Table 2.
Concordance assessment between Mindray CL-900i-HIV Ag/Ab combo in comparison to INNO-LIA™ HIVI/II (n = 159).
| Performance parameters | |
|---|---|
| Sensitivity (%) | 100% (90.7–100) |
| Specificity (%) | 100% (97.0–100) |
| PPV (%) | 100% (90.7–100) |
| NPV (%) | 100% (97.0–100) |
| OPA (%) | 100% (97.7–100) |
| PPA (%) | 100% (90.7–100) |
| NPA (%) | 100% (97.0–100) |
| Efficiency/accuracy (%) | 100% (97.7–100) |
| Cohen’s Kappa coefficient | 1.00 (1.00–1.00) |
Mindray CL-900i-HIV Ag/Ab combo assay showed a low level of false positives, high sensitivity, and specificity comparable to the surrogate reference method, PCR
To validate the results of ARCHITECT® HIV Ag/Ab combo positive samples and assess the false positive rate, we conducted retesting using PCR as a surrogate reference method. It is important to note that only Architect-HIV positive samples underwent PCR testing, which limits our ability to directly calculate the sensitivity and specificity of the ARCHITECT® HIV Ag/Ab Combo assay.
Upon PCR confirmation, the ARCHITECT® HIV Ag/Ab Combo assay identified 36 out of 80 samples (45%) as true positive, correctly detecting the presence of HIV RNA. However, it also demonstrated a high false positive rate, with 44 out of 80 samples (55%) showing false positive results (Table 3).
Table 3.
Comparison of ARCHITECT® HIV Ag/Ab combo assay with PCR (n = 80).
| PCR-HIV+ | PCR-HIV− | Total | PPV | PPA | |
|---|---|---|---|---|---|
| Architect-HIV+ | 36 | 44a | 80 | 45.0% (33.8–56.5%) | 100% (90.3–100%) |
| Architect-HIV− | NA | NA | NA |
aINNO-LIA-HIV was done on these 44 samples, 4 were positive by INNO-LIA-HIV, 20 were indeterminate and 20 were negative by INNO-LIA-HIV.
NA Not applicable .
Further, we assessed the performance of Mindray CL-900i-HIV Ag/Ab combo assay in comparison to the surrogate reference method, PCR. Using Mindray CL-900i-HIV Ag/Ab combo assay, 8.75% (7/80) samples were false positive out of 46.3% (37 /80) true negative results, while it correctly identified 45.0% (36/80) true positive cases. (Table 4).
Table 4.
Comparison of Mindray CL-900i-HIV results with PCR (n = 80).
| PCR-HIV+ | PCR-HIV− | Total | |
|---|---|---|---|
| CL-900i-HIV+ | 36 (45.0%) | 7 (8.75%)a | 43 (53.8%) |
| CL-900i-HIV− | 0 (0.00%) | 37 (46.3%) | 37 (46.2%) |
| Totals | 36 (45.0%) | 44 (55.0%) | 80 (100%) |
aINNO-LIA was done on these 7 samples, 4 were positive by INNO-LIA and, 3 were indeterminant.
In addition, the Mindray CL-900i-HIV Ag/Ab combo assay exhibited excellent performance, with remarkable sensitivity of 100% (95% CI 90.0–100) and specificity of 95.0% (95% CI 90.4–97.8). The assay demonstrated perfect agreement with the PCR (κ = 0.83), highlighting its reliability and accuracy in HIV diagnosis (Table 5).
Table 5.
Performance evaluation of Mindray CL-900i-HIV Ag/Ab combo in comparison to the surrogate reference test, PCR.
| Performance parameters | |
|---|---|
| Sensitivity (%) | 100% (90.0–100) |
| Specificity (%) | 95.0% (90.4–97.8) |
| PPV (%) | 81.4% (66.6–91.6) |
| NPV (%) | 100% (97.6–100) |
| OPA (%) | 95.9% (92.1–98.2) |
| PPA (%) | 100% (90.0–100) |
| NPA (%) | 95.0% (90.4–97.8) |
| Efficiency/accuracy (%) | 95.9% (92.1–98.2) |
| Cohen’s Kappa coefficient | 0.83 (0.70–0.95) |
Discussion
Accurate and reliable diagnostic assays play a crucial role in detecting HIV infection, allowing the timely initiation of antiretroviral therapy and effective patient management. In this study, we aim to evaluate the performance of the Mindray CL-900i-HIV Ag/Ab combo assay, a new cost-effective chemiluminescence assay from Mindray-China. To achieve this aim, we selected 195 archived samples, classified based on the gold standard INNO-LIA™ HIVI/II line immunoassay confirmatory assay. All samples were tested using the Mindray CL-900i-HIV, and all positive Architect-HIV samples were additionally confirmed by PCR as a surrogate reference method. By comparing the diagnostic capabilities of the Mindray CL-900i-HIV Ag/Ab combo assay to the currently used ARCHITECT® HIV Ag/Ab Combo assay, our findings provide valuable insights into its performance and suitability for routine screening purposes.
Mindray CL-900i-HIV Ag/Ab combo assay demonstrated excellent performance when compared to the gold standard, INNO-LIA™ HIVI/II confirmatory assay. With a sensitivity of 100% and specificity of 100%, the Mindray CL-900i-HIV Ag/Ab combo assay exhibited a high accuracy in detecting true positive and true negative cases of HIV infection. The high sensitivity of the Mindray CL-900i-HIV Ag/Ab combo assay ensures the identification of all infected individuals, while the specificity minimizes the occurrence of false-positive results, thus reducing unnecessary confirmatory testing and associated costs. It is worth noting that Mindray CL-900i-HIV Ag/Ab combo assay demonstrated perfect agreement with INNO-LIA™ HIVI/II confirmatory assay (κ = 1.00) and the PCR (κ = 0.83), as indicated by the Cohen’s kappa statistic, highlighting the effectiveness of the Mindray CL-900i-HIV Ag/Ab combo assay in accurately detecting HIV infection.
In our previous study, we assessed the performance of ARCHITECT® HIV Ag/Ab Combo assay15. We demonstrated that the ARCHITECT® HIV Ag/Ab Combo assay performs with very high reproducibility (99.8%) with the two-run repeats done on two different analyzers tested by two different technicians on two different samples taken on two different occasions from the same individuals. However, The PPV between ARCHITECT® HIV Ag/Ab Combo and the INNO-LIA™ HIVI/II confirmatory assay was 31.8%. In addition, the PPV between ARCHITECT® HIV Ag/Ab Combo and HIV-PCR assay was 26.8%. In this study, the PPV was calculated to be 45.0% (95% CI 33.8–56.5). Our findings suggest a higher likelihood of false-positive results with the ARCHITECT® HIV Ag/Ab Combo assay, which may lead to unnecessary confirmatory testing and potential psychological distress for patients. The moderate agreement observed between the ARCHITECT® HIV Ag/Ab Combo assay and PCR is also consistent with previous studies that have highlighted the challenges associated with false-positive results and the need for caution when interpreting ARCHITECT® HIV Ag/Ab Combo assay outcomes14,15,28.
Among the 44 false positive results (Table S1) initially detected by the ARCHITECT® HIV Ag/Ab Combo assay, the Mindray CL-900i-HIV Ag/Ab combo assay successfully resolved 37 samples, showing negative results consistent with the PCR results. On the other hand, out of the 7 remaining false positive results detected by Mindray CL-900i-HIV Ag/Ab combo, four were also positive when tested using the INNO-LIA™ HIVI/II confirmatory assay. However, these four samples were confirmed negative by PCR, raising the possibility of a potential issue with the PCR results as these four samples were positive in all three assays. False-negative results by PCR could occur due to several reasons, including antiviral treatment, viral load fluctuations, low RNA levels, sampling errors, or technical issues during the PCR process, or that the applicant might be one of the rare cases of the " Elite Controllers” who can naturally suppress HIV virus replication and as a result the PCR will show up as “Not Detected” or “Below Detection Limit” but at the same time INNO-LIA result is Positive29–31. Additionally, it is worth mentioning that among the 44 false positive results from the ARCHITECT® HIV Ag/Ab Combo assay, 20 of them were initially deemed indeterminate by the INNO-LIA™ HIVI/II confirmatory assay. However, 17 of these 20 samples were ultimately resolved as negative by the Mindray CL-900i-HIV Ag/Ab combo, further emphasizing the reliability of the Mindray CL-900i-HIV Ag/Ab combo assay in confirming true negative cases (Table S1).
The evaluation of the INNO-LIA™ HIVI/II confirmatory assay revealed excellent performance in comparison to the reference method, PCR. These results align with previous studies that have reported the superior performance of the INNO-LIA™ HIVI/II confirmatory assay16,32. Several previous studies have also reported a notable proportion of indeterminate results when using the INNO-LIA™ HIVI/II confirmatory assay15,33,34. The presence of indeterminate results can pose challenges in clinical settings as it introduces uncertainty into the diagnostic process. Indeterminate results require further evaluation, such as repeat testing or additional laboratory investigations, to establish a conclusive diagnosis, which poses a challenge in terms of cost-effectiveness.
One potential limitation of our study is the relatively small sample size, and the subjectivity of sample selection according to ARCHITECT® HIV. This may impact the generalizability of the findings. A larger sample size over one year testing, as we have previously performed15 would provide more statistical power and enhance the reliability of the results. Additionally, our study evaluated the performance of the assay using the INNO-LIA™ HIVI/II confirmatory assay and PCR as reference methods, but there may be inherent limitations or variability in the accuracy of these reference methods themselves. Finally, we did not assess other potential factors that could influence assay performance, such as sample storage conditions or the presence of interfering substances. These limitations should be taken into consideration when interpreting the results of our study.
Conclusion
Our study highlights the performance evaluation of the Mindray CL-900i-HIV Ag/Ab combo assay for the detection of HIV in comparison to the current screening method, ARCHITECT® HIV Ag/Ab Combo assay. The results demonstrate that the Mindray CL-900i-HIV Ag/Ab combo assay exhibits excellent sensitivity and specificity, outperforming the ARCHITECT® HIV Ag/Ab Combo assay. Furthermore, the Mindray CL-900i-HIV Ag/Ab combo assay shows strong agreement with the INNO-LIA™ HIVI/II confirmatory assay, indicating its reliability in confirming HIV infection. These findings highlight the robustness and accuracy of the Mindray CL-900i-HIV Ag/Ab combo assay in detecting HIV antibodies and support its adoption as a reliable diagnostic tool.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
G.K.N and A.I., conceived the study and led the laboratory testing. N.Y., J.A.A., H.M.K., S.Y., M.N.A., M.E., I.W.K., M.A.I., M.M.A., I.A.S., P.B.N, conducted the lab testing of the specimens. G.K.N., N.Y., H.M.Y., H.A., A.I validated and interpreted the laboratory results. G.K.N., N.Y., and A.I, developed the study design, managed the databases, performed the data analyses, and wrote the first draft of the article. G.K.N and A.I., led the statistical analyses and contributed to the first draft of the article. G.K.N., H.M.Y., M.N.A., M.E., I.W.K., M.A.I., M.M.A., I.A.S., P.B.N., S.Y., N.L., H.A., L.J.A.-R., A.I critically reviewed the manuscript. All authors have read and approved the final manuscript.
Funding
Gheyath Nasrallah would like to acknowledge receiving funds from NPRP13S-0128-200185 and UREP30-041-3-014 grants from Qatar National Research Fund (QNRF), a member of Qatar Foundation, as well as QUCG-CHS-23/24–170 from Qatar University. Houssein Ayoub, Laith Abu-Raddad and Gheyath Nasrallah would like to acknowledge receiving funds from Qatar University under grant QUCG-CAS-23/24–114. Nadin Younes would like to acknowledge receiving funds from GSRA8-L-1-0501-21022 from QNRF. It is important to note that the funders played no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. All statements made in this report are solely the responsibility of the authors.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Competing interests
All kits of the Mindray CL-900i-HIV Ag/Ab combo assay used in this study were received from Mindray as in-kind support to Dr. Gheyath. However, it is important to note that Mindray had no influence or involvement in the study design, data collection, analysis, interpretation, or the decision to publish the results.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. HIV data and statistics 2024 https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics.
- 2.Walker, B. & McMichael, A. The T-cell response to HIV. Cold Spring Harb Perspect. Med.2(11). (2012). [DOI] [PMC free article] [PubMed]
- 3.Balasubramaniam, M., Pandhare, J. & Dash, C. Immune control of HIV. J. Life Sci. (Westlake Village). 1 (1), 4–37 (2019). [PMC free article] [PubMed] [Google Scholar]
- 4.Lv, T., Cao, W. & Li, T. HIV-related Immune activation and inflammation: current understanding and strategies. J. Immunol. Res.2021, 7316456 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boasso, A., Shearer, G. M. & Chougnet, C. Immune dysregulation in human immunodeficiency virus infection: know it, fix it, prevent it? J. Intern. Med.265 (1), 78–96 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganczak, M., Barss, P. & Nosocomial HIV infection: epidemiology and prevention—a global perspective. AIDS Rev.10 (1), 47–61 (2008). [PubMed] [Google Scholar]
- 7.Volmink, J. & Marais, B. HIV: mother-to-child transmission. BMJ Clin. Evid.2008 (2008). [PMC free article] [PubMed]
- 8.Shaw, G. M. & Hunter, E. HIV transmission. Cold Spring Harb Perspect. Med.2(11). (2012). [DOI] [PMC free article] [PubMed]
- 9.Stoltey, J. E. & Cohen, S. E. Syphilis transmission: a review of the current evidence. Sex. Health. 12 (2), 103–109 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May, M. T. Better to know: the importance of early HIV diagnosis. Lancet Public. Health. 2 (1), e6–e7 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Fearon, M. The laboratory diagnosis of HIV infections. Can. J. Infect. Dis. Med. Microbiol.16 (1), 26–30 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parekh, B. S. et al. Diagnosis of human immunodeficiency virus infection. Clin. Microbiol. Rev.32(1). (2019). [DOI] [PMC free article] [PubMed]
- 13.MOPH. Communicable diseases https://www.moph.gov.qa/english/strategies/Supporting-Strategies-and-Frameworks/QatarPublicHealthStrategy/Pages/Communicable-diseases.aspx.
- 14.Alonso, R., Pérez-García, F., Gijón, P., Collazos, A. & Bouza, E. Evaluation of the Architect HIV Ag/Ab combo assay in a low-prevalence setting: the role of samples with a low S/CO ratio. J. Clin. Virol.103, 43–47 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Nasrallah, G. K. et al. Screening and diagnostic testing protocols for HIV and Syphilis infections in health care setting in Qatar: evaluation and recommendations. PLoS One. 18 (2), e0278079 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong, C. C. et al. Performance of the HIV blot 2.2, INNO-LIA HIV I/II score, and Geenius HIV 1/2 confirmatory assay for use in HIV confirmation. PLoS One. 13 (6), e0199502 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan, C. A., Yamaguchi, J., Vallari, A., Swanson, P. & Hackett, J. R. Jr ARCHITECT® HIV Ag/Ab combo assay: correlation of HIV-1 p24 antigen sensitivity and RNA viral load using genetically diverse virus isolates. J. Clin. Virol.57 (2), 169–172 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Chavez, P., Wesolowski, L., Patel, P., Delaney, K. & Owen, S. M. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab combo assay. J. Clin. Virol.52, S51–S5 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Adhikari, E. et al. Diagnostic accuracy of 4 th generation ARCHITECT HIV Ag/Ab combo assay and utility of signal-to-cutoff ratio to predict false positive HIV tests in pregnancy. Am. J. Obstet. Gynecol.219 (2018). [DOI] [PubMed]
- 20.Guiraud, V. et al. Are confirmatory assays reliable for HIV-1/HIV-2 infection differentiation? A multicenter study. J. Clin. Microbiol.61 (8), e0061923 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diagnostics, A. ARCHITECT HIV Ag/Ab Combo Reagent Insert.
- 22.Ag/Ab, A. H. I. V. Combo (2009). https://www.ilexmedical.com/files/PDF/HIVAgAbCombo.pdf.
- 23.WHO. WHO Prequalification of. In Vitro Diagnostics Programme PUBLIC REPORT: NNO-LIA HIV I/II Score (2015).
- 24.INNOGENETICS & INNO-LIATM* HIV I/II Score https://search.cosmobio.co.jp/cosmo_search_p/search_gate2/docs/IGT_/80540.20070926.pdf.
- 25.Roche. COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test.
- 26.Li, M. & Yu, T. Methodological issues on evaluating agreement between two detection methods by Cohen’s kappa analysis. Parasit. Vectors. 15 (1), 270 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conger, A. J. Kappa and rater accuracy: paradigms and parameters. Educ. Psychol. Meas.77 (6), 1019–1047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, L. et al. The characteristics of screening and confirmatory test results for HIV in Xi’an, China. PLoS One. 12 (7), e0180071 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oladokun, R. et al. False-negative HIV-1 polymerase chain reaction in a 15-month-old boy with HIV-1 subtype C infection. S Afr. Med. J.105 (10), 877 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Alharthy, A., Faqihi, F., Noor, A., Memish, Z. A. & Karakitsos, D. Co-infection of human immunodeficiency virus, herpes simplex virus-2 and SARS-CoV-2 in a patient with false-negative real-time polymerase chain reaction results. Singap. Med. J.63 (6), 345–347 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen, L. et al. Contradictory results of serological confirmatory test and real-time PCR assays in diagnosis a patient of HIV-1 infection. Int. J. Infect. Dis.74, 38–40 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Schüpbach, J. et al. High specificity of line-immunoassay based algorithms for recent HIV-1 infection independent of viral subtype and stage of disease. BMC Infect. Dis.11, 254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serhir, B. et al. Evaluation of the Bio-rad Geenius HIV 1/2 assay as part of a confirmatory HIV Testing Strategy for Quebec, Canada: comparison with western blot and Inno-Lia Assays. J. Clin. Microbiol.57(6). (2019). [DOI] [PMC free article] [PubMed]
- 34.Al-Kindi, H. & Al-Jardani, A. HIV serology false positivity among expatriates from Africa: a screening dilemma. J. Med. Microbiol.69 (6), 812–816 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].