Abstract
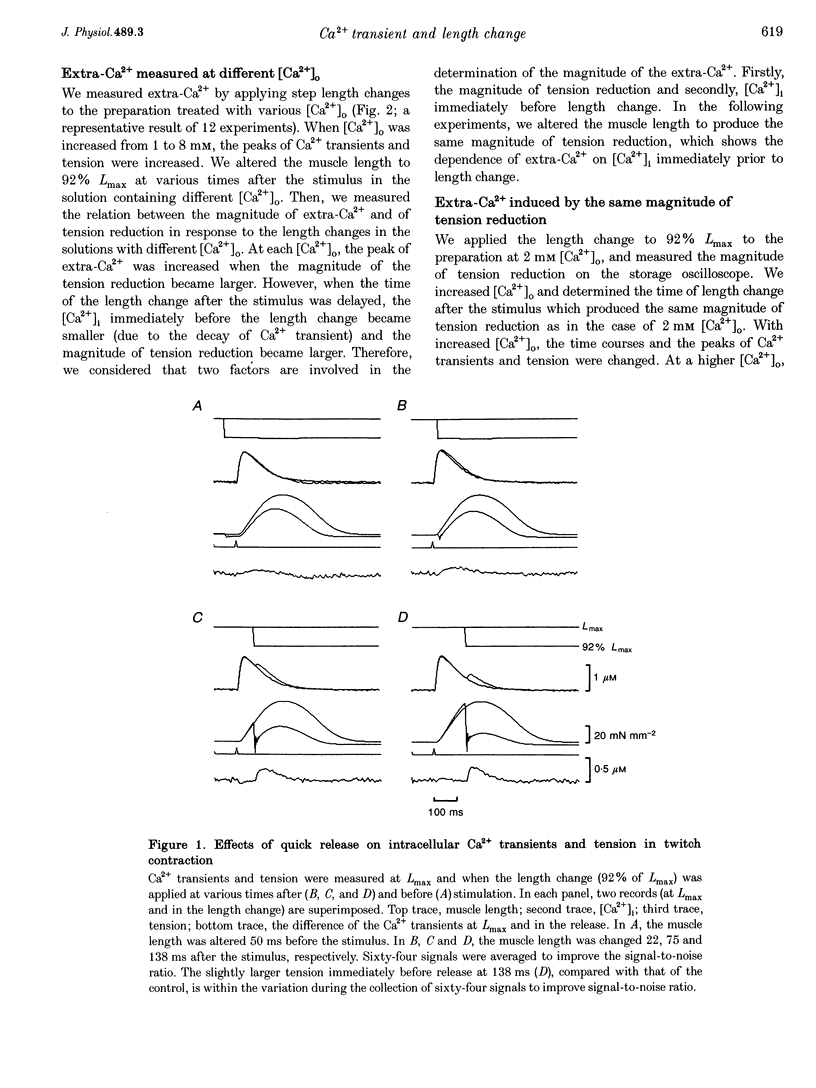
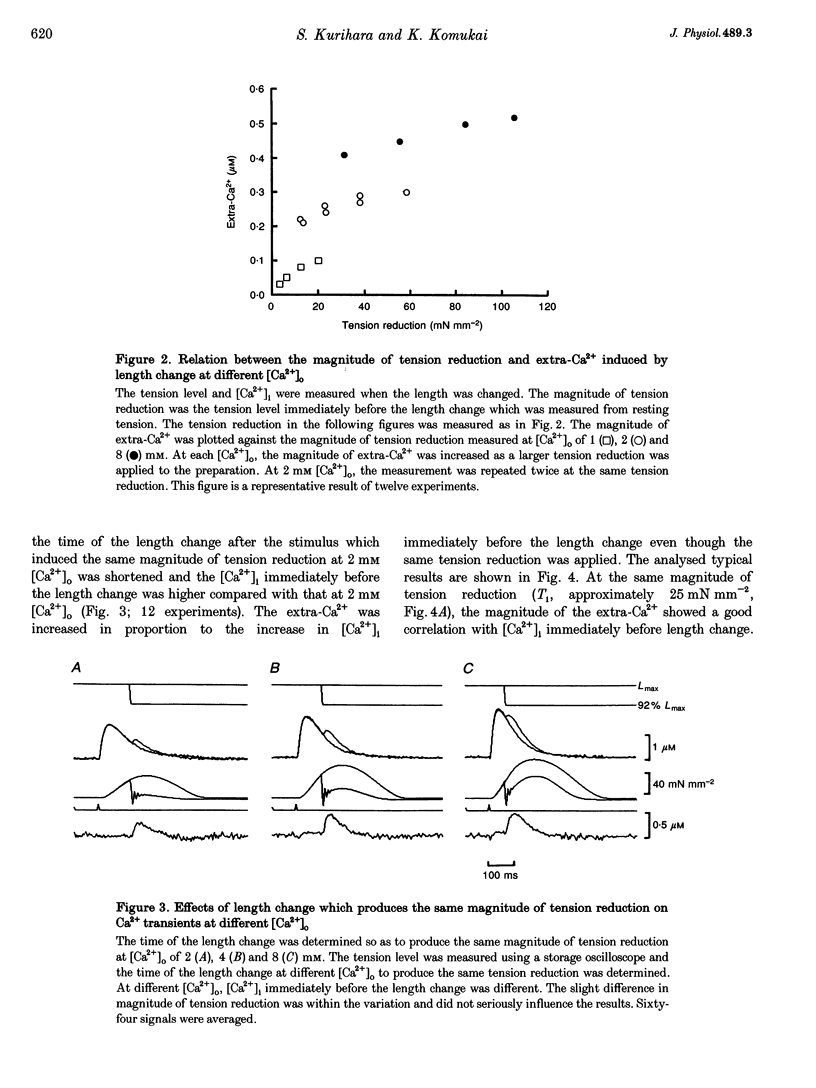
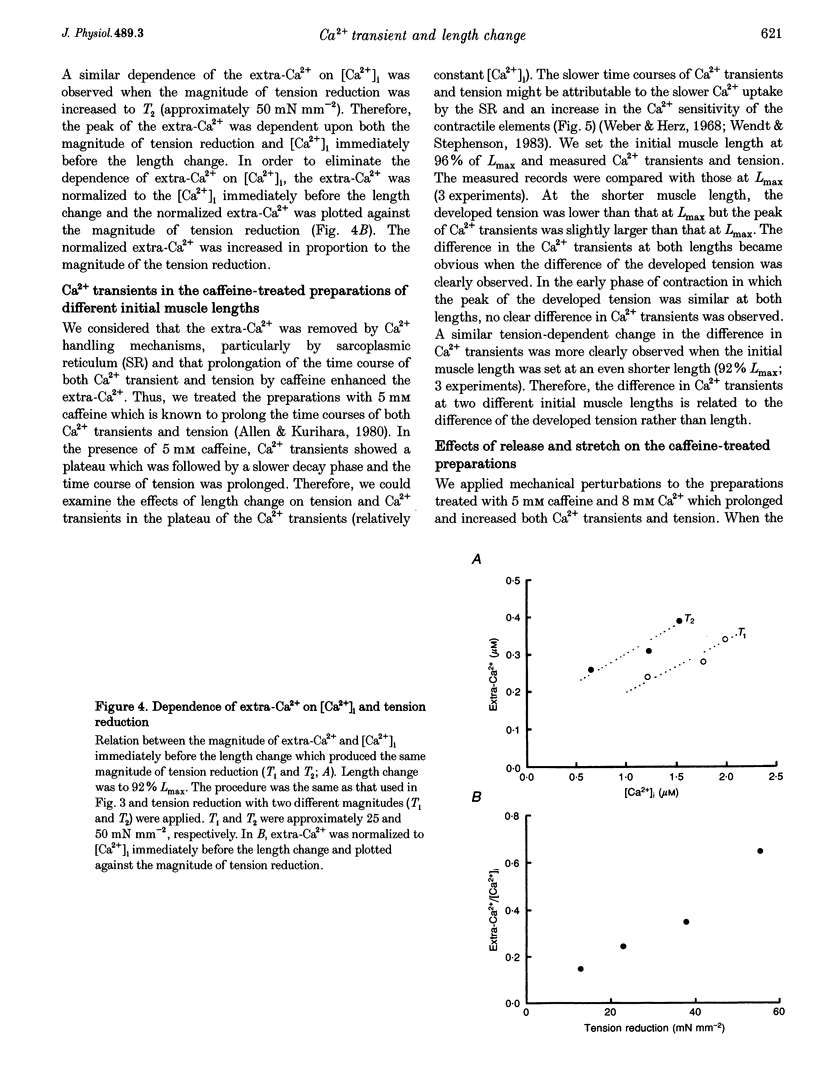
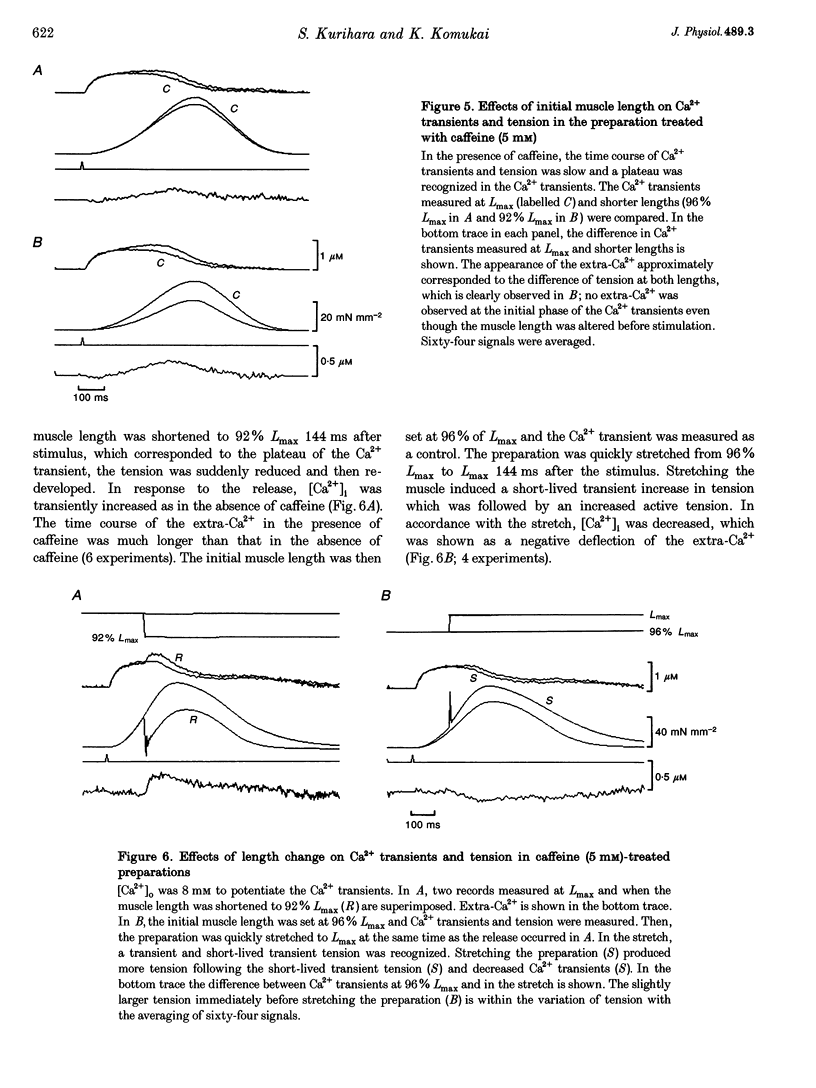
1. We measured the change in intracellular Ca2+ transients, using aequorin, in response to muscle length change during twitch contraction in ferret ventricular muscles. 2. Intracellular Ca2+ concentration ([Ca2+]i) was transiently increased when the muscle length was quickly shortened to 92% of maximum length (Lmax) at various times after stimulation (this increase in [Ca2+] is termed extra-Ca2+). The magnitude of extra-Ca2+, measured at different extracellular Ca2+ concentrations ([Ca2+]o), showed a dependence upon the magnitude of tension reduction and upon [Ca2+]i immediately before the length change. 3. In the presence of caffeine (5 mM), the difference between the Ca2+ transient at Lmax and at shorter lengths showed a time course similar to the difference between the developed tension at both lengths. A quick release in the caffeine-treated preparation produced the extra-Ca2+ with a slower time course compared with that observed in the absence of caffeine. Stretching the muscle from 96% Lmax to Lmax produced more active tension and decreased [Ca2+]i. 4. These results indicate that the affinity of troponin-C, a major Ca2+ binding protein, which controls contraction, is influenced by developed tension i.e. cross-bridge attachment and detachment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Kentish J. C. Calcium concentration in the myoplasm of skinned ferret ventricular muscle following changes in muscle length. J Physiol. 1988 Dec;407:489–503. doi: 10.1113/jphysiol.1988.sp017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Kentish J. C. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. doi: 10.1093/eurheartj/1.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982 Jun;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backx P. H., Ter Keurs H. E. Fluorescent properties of rat cardiac trabeculae microinjected with fura-2 salt. Am J Physiol. 1993 Apr;264(4 Pt 2):H1098–H1110. doi: 10.1152/ajpheart.1993.264.4.H1098. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Ridgway E. B. Stretch of active muscle during the declining phase of the calcium transient produces biphasic changes in calcium binding to the activating sites. J Gen Physiol. 1990 Nov;96(5):1013–1035. doi: 10.1085/jgp.96.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Sonnenblick E., Babu A. The role of troponin C in the length dependence of Ca(2+)-sensitive force of mammalian skeletal and cardiac muscles. J Physiol. 1991 Sep;441:305–324. doi: 10.1113/jphysiol.1991.sp018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güth K., Potter J. D. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987 Oct 5;262(28):13627–13635. [PubMed] [Google Scholar]
- Hofmann P. A., Fuchs F. Effect of length and cross-bridge attachment on Ca2+ binding to cardiac troponin C. Am J Physiol. 1987 Jul;253(1 Pt 1):C90–C96. doi: 10.1152/ajpcell.1987.253.1.C90. [DOI] [PubMed] [Google Scholar]
- Hofmann P. A., Fuchs F. Evidence for a force-dependent component of calcium binding to cardiac troponin C. Am J Physiol. 1987 Oct;253(4 Pt 1):C541–C546. doi: 10.1152/ajpcell.1987.253.4.C541. [DOI] [PubMed] [Google Scholar]
- Holroyde M. J., Robertson S. P., Johnson J. D., Solaro R. J., Potter J. D. The calcium and magnesium binding sites on cardiac troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1980 Dec 25;255(24):11688–11693. [PubMed] [Google Scholar]
- Housmans P. R., Lee N. K., Blinks J. R. Active shortening retards the decline of the intracellular calcium transient in mammalian heart muscle. Science. 1983 Jul 8;221(4606):159–161. doi: 10.1126/science.6857274. [DOI] [PubMed] [Google Scholar]
- Kurihara S., Saeki Y., Hongo K., Tanaka E., Sudo N. Effects of length change on intracellular Ca2+ transients in ferret ventricular muscle treated with 2,3-butanedione monoxime (BDM). Jpn J Physiol. 1990;40(6):915–920. doi: 10.2170/jjphysiol.40.915. [DOI] [PubMed] [Google Scholar]
- Lab M. J., Allen D. G., Orchard C. H. The effects of shortening on myoplasmic calcium concentration and on the action potential in mammalian ventricular muscle. Circ Res. 1984 Dec;55(6):825–829. doi: 10.1161/01.res.55.6.825. [DOI] [PubMed] [Google Scholar]
- Liao R., Gwathmey J. K. Effects of MCI-154 and caffeine on Ca(++)-regulated interactions between troponin subunits from bovine heart. J Pharmacol Exp Ther. 1994 Aug;270(2):831–839. [PubMed] [Google Scholar]
- Palmer S., Kentish J. C. The role of troponin C in modulating the Ca2+ sensitivity of mammalian skinned cardiac and skeletal muscle fibres. J Physiol. 1994 Oct 1;480(Pt 1):45–60. doi: 10.1113/jphysiol.1994.sp020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B. S., Solaro R. J. Calcium-binding properties of troponin C in detergent-skinned heart muscle fibers. J Biol Chem. 1987 Jun 5;262(16):7839–7849. [PubMed] [Google Scholar]
- Ridgway E. B., Gordon A. M. Muscle calcium transient. Effect of post-stimulus length changes in single fibers. J Gen Physiol. 1984 Jan;83(1):75–103. doi: 10.1085/jgp.83.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki Y., Kurihara S., Hongo K., Tanaka E. Alterations in intracellular calcium and tension of activated ferret papillary muscle in response to step length changes. J Physiol. 1993 Apr;463:291–306. doi: 10.1113/jphysiol.1993.sp019595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt I. R., Stephenson D. G. Effects of caffeine on Ca-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflugers Arch. 1983 Aug;398(3):210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]



