Abstract
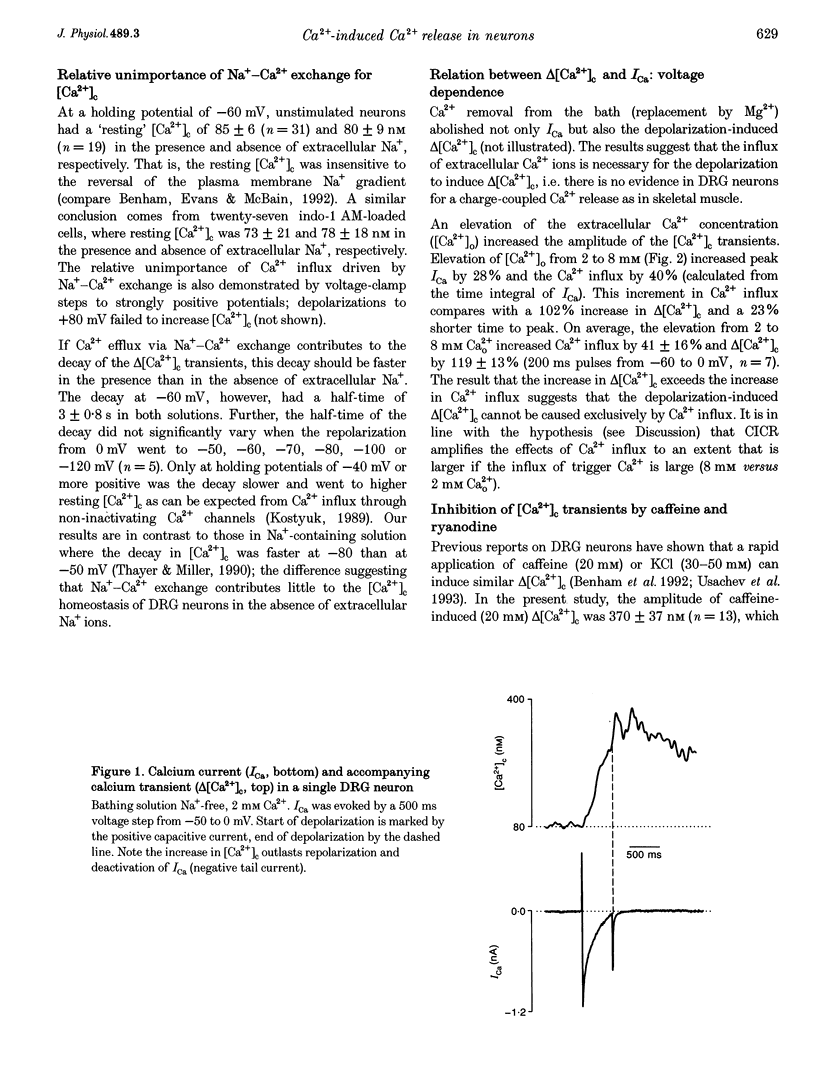
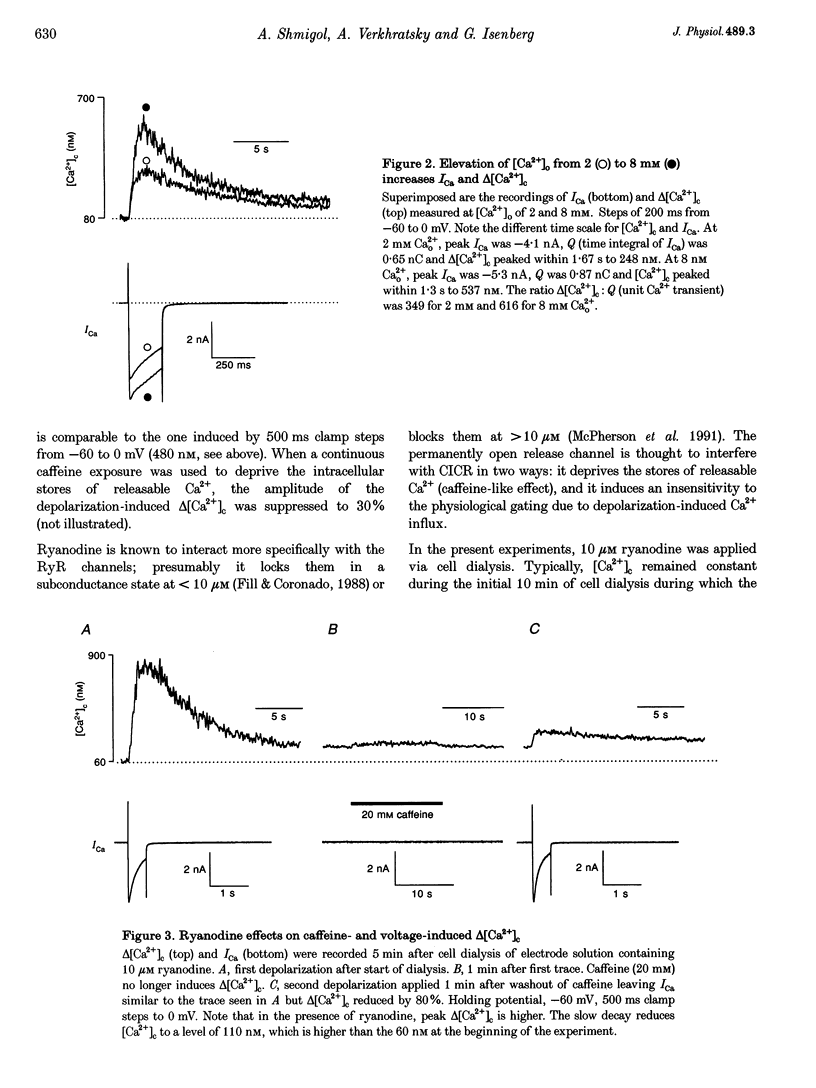
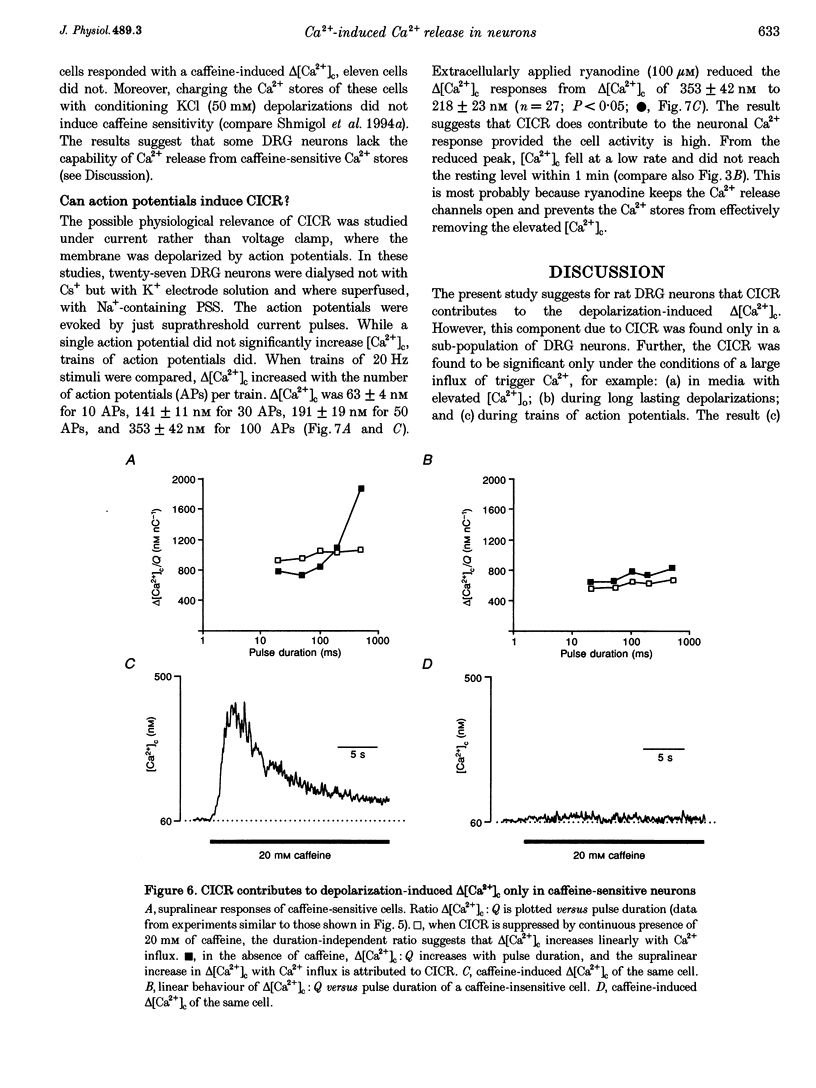
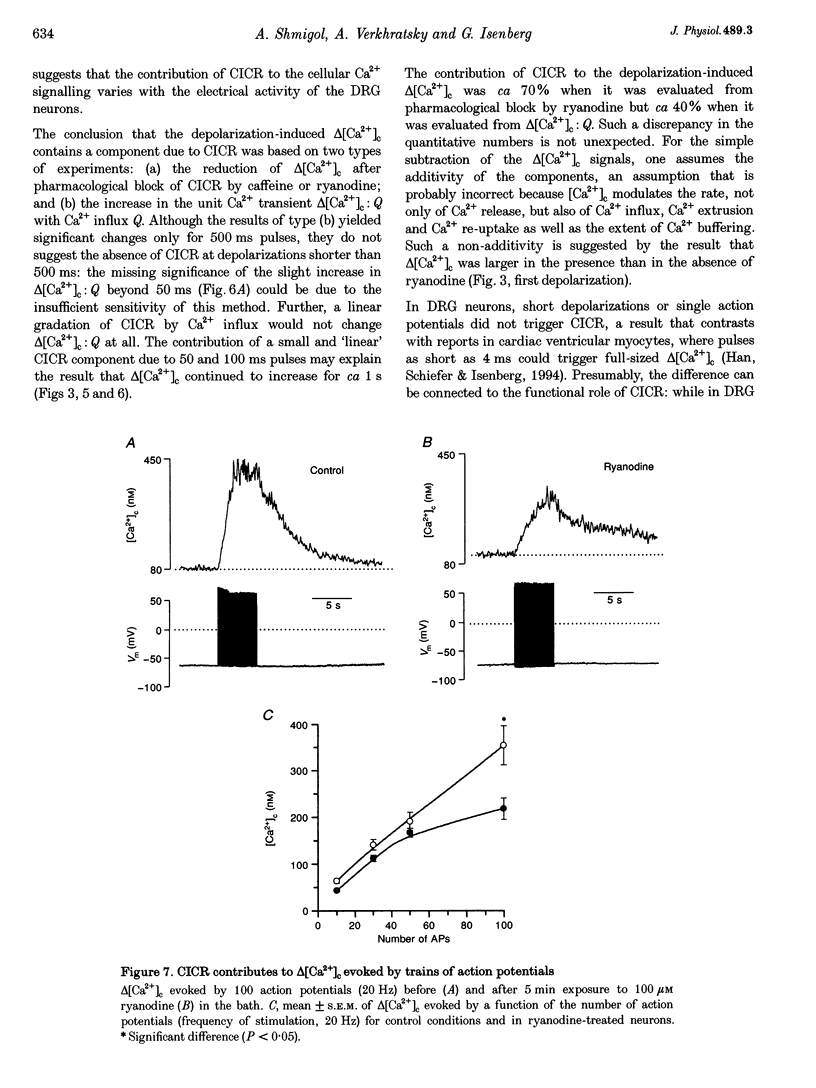
1. In isolated dorsal root ganglion cells (DRG neurons), changes in the concentration of global cytosolic Ca2+ (delta [Ca2+]c) were measured by the fluorescence of K5-indo-1. Depolarizations from -60 to 0 mV (500 ms) and Ca2+ influx through Ca2+ channels (ICa) increased [Ca2+]c by 480 +/- 113 nM, the peak occurring 542 +/- 76 ms (mean +/- S.E.M.) after repolarization. 2. Ryanodine (10 microM) reduced depolarization-induced delta [Ca2+]c by up to 80% and blocked delta [Ca2+]c induced by 20 mM caffeine. 3. Peak delta [Ca2+]c and peak ICa followed a similar bell-shaped voltage dependence. Removal of extracellular Ca2+ abolished depolarization-induced delta [Ca2+]c; its elevation from 2 to 8 mM increased peak ICa by 30% and delta [Ca2+]c by 108%. 4. Ca2+ influx at 0 mV was graded by pulse durations between 20 and 500 ms. Up to 200 ms, delta [Ca2+]c increased linearly with Ca2+ influx. Depolarizations longer than 200 ms induced a supralinear increase in delta [Ca2+]c that was abolished by caffeine (20 mM). 5. The supralinear increase in delta [Ca2+]c and the caffeine-induced delta [Ca2+]c were measured only in thirteen of nineteen DRG neurons; in the other six of nineteen cells both properties were absent. The results suggest that Ca(2+)-induced Ca2+ release (CICR) is expressed differently in different populations of DRG neurons. 6. A single action potential did not significantly increase [Ca2+]c. Trains of stimuli (20 Hz) induced delta [Ca2+]c that linearly increased with the number of action potentials. Delta [Ca2+]c due to 100 action potentials had a significant ryanodine-sensitive component. 7. It is discussed that CICR can contribute to the depolarization-induced [Ca2+]c, provided the Ca2+ influx lasts for a certain minimum period of time.
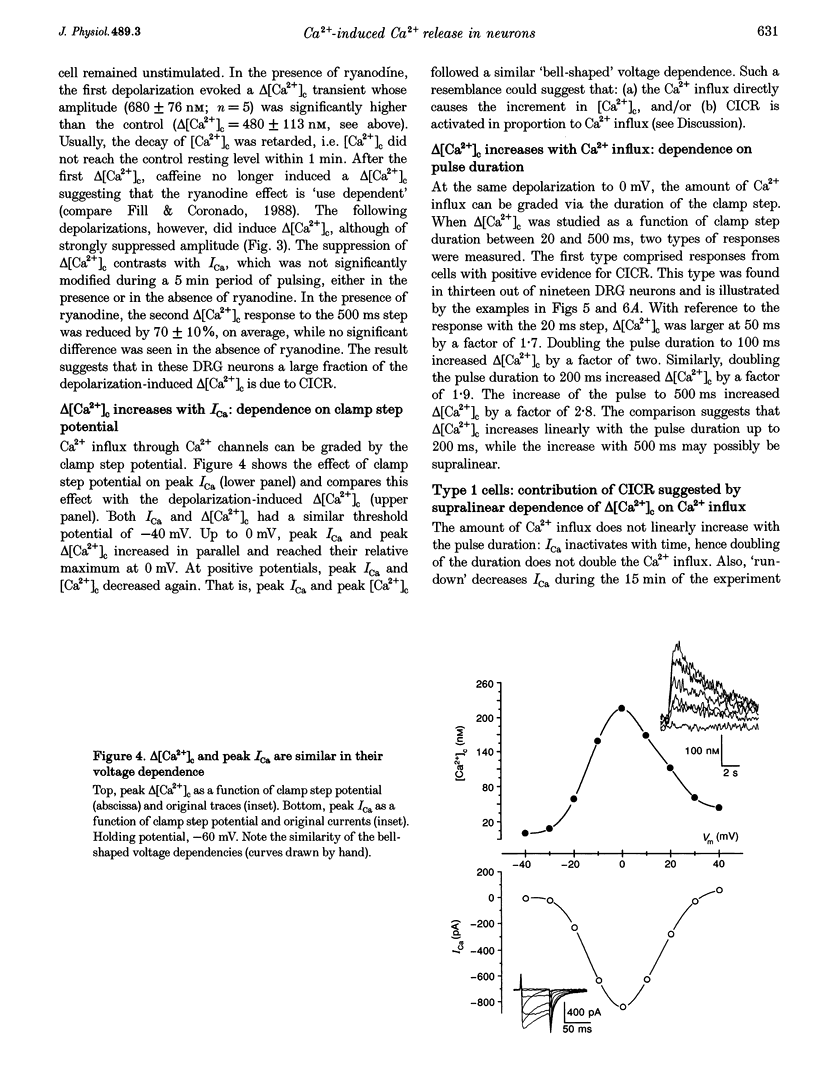
Full text
PDF

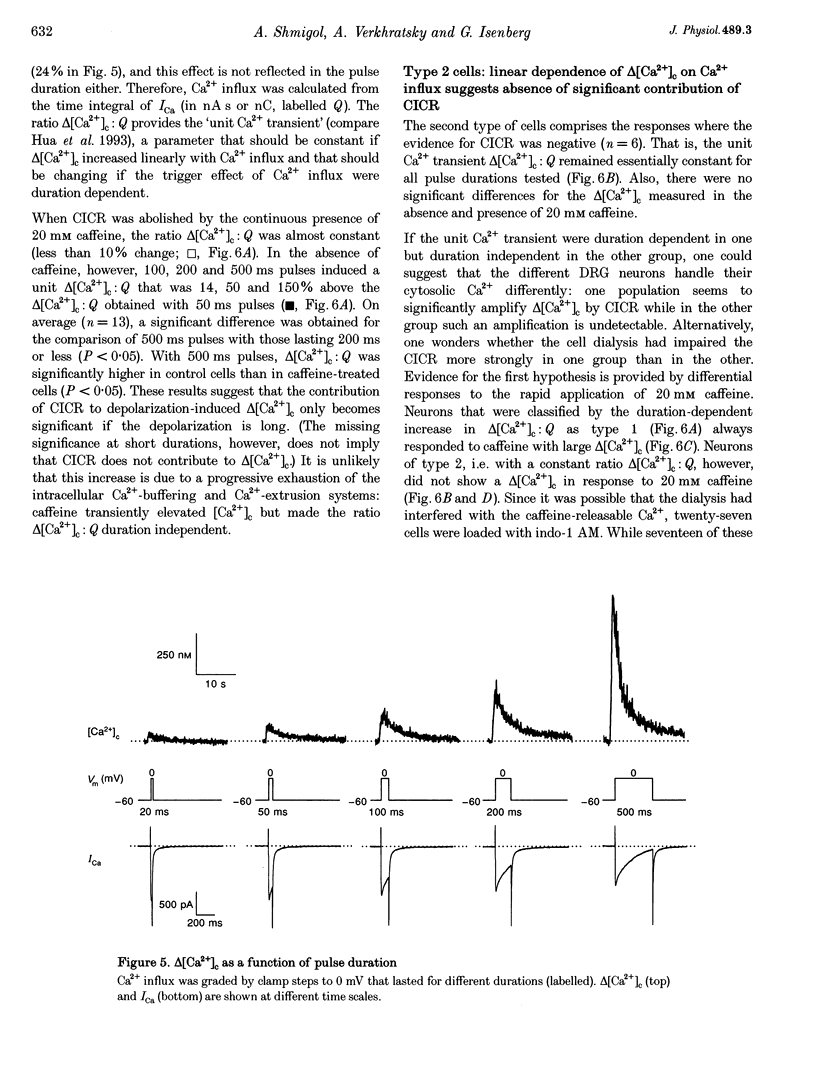


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bleakman D., Roback J. D., Wainer B. H., Miller R. J., Harrison N. L. Calcium homeostasis in rat septal neurons in tissue culture. Brain Res. 1993 Jan 15;600(2):257–267. doi: 10.1016/0006-8993(93)91381-2. [DOI] [PubMed] [Google Scholar]
- Brorson J. R., Bleakman D., Gibbons S. J., Miller R. J. The properties of intracellular calcium stores in cultured rat cerebellar neurons. J Neurosci. 1991 Dec;11(12):4024–4043. doi: 10.1523/JNEUROSCI.11-12-04024.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M., Coronado R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci. 1988 Oct;11(10):453–457. doi: 10.1016/0166-2236(88)90198-1. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992 May;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich V Y. a., Isenberg G. Depolarization-mediated intracellular calcium transients in isolated smooth muscle cells of guinea-pig urinary bladder. J Physiol. 1991 Apr;435:187–205. doi: 10.1113/jphysiol.1991.sp018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Han S., Schiefer A., Isenberg G. Ca2+ load of guinea-pig ventricular myocytes determines efficacy of brief Ca2+ currents as trigger for Ca2+ release. J Physiol. 1994 Nov 1;480(Pt 3):411–421. doi: 10.1113/jphysiol.1994.sp020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S. Y., Nohmi M., Kuba K. Characteristics of Ca2+ release induced by Ca2+ influx in cultured bullfrog sympathetic neurones. J Physiol. 1993 May;464:245–272. doi: 10.1113/jphysiol.1993.sp019633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Han S. Gradation of Ca(2+)-induced Ca2+ release by voltage-clamp pulse duration in potentiated guinea-pig ventricular myocytes. J Physiol. 1994 Nov 1;480(Pt 3):423–438. doi: 10.1113/jphysiol.1994.sp020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko A., Baring M. D., Airey J. A., Sutko J. L., Kenyon J. L. A caffeine- and ryanodine-sensitive Ca2+ store in avian sensory neurons. J Neurophysiol. 1993 Aug;70(2):710–722. doi: 10.1152/jn.1993.70.2.710. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Diversity of calcium ion channels in cellular membranes. Neuroscience. 1989;28(2):253–261. doi: 10.1016/0306-4522(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Kostyuk P., Verkhratsky A. Calcium stores in neurons and glia. Neuroscience. 1994 Nov;63(2):381–404. doi: 10.1016/0306-4522(94)90537-1. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. W., Tsien R. Y. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988 Jul;1(5):355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Llano I., DiPolo R., Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994 Mar;12(3):663–673. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Hillman D. E., Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992 Sep;15(9):351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- Marrion N. V., Adams P. R. Release of intracellular calcium and modulation of membrane currents by caffeine in bull-frog sympathetic neurones. J Physiol. 1992 Jan;445:515–535. doi: 10.1113/jphysiol.1992.sp018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson P. S., Kim Y. K., Valdivia H., Knudson C. M., Takekura H., Franzini-Armstrong C., Coronado R., Campbell K. P. The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron. 1991 Jul;7(1):17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- Miller R. J. The control of neuronal Ca2+ homeostasis. Prog Neurobiol. 1991;37(3):255–285. doi: 10.1016/0301-0082(91)90028-y. [DOI] [PubMed] [Google Scholar]
- Neering I. R., McBurney R. N. Role for microsomal Ca storage in mammalian neurones? Nature. 1984 May 10;309(5964):158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- Nohmi M., Hua S. Y., Kuba K. Basal Ca2+ and the oscillation of Ca2+ in caffeine-treated bullfrog sympathetic neurones. J Physiol. 1992 May;450:513–528. doi: 10.1113/jphysiol.1992.sp019140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol A., Kirischuk S., Kostyuk P., Verkhratsky A. Different properties of caffeine-sensitive Ca2+ stores in peripheral and central mammalian neurones. Pflugers Arch. 1994 Jan;426(1-2):174–176. doi: 10.1007/BF00374686. [DOI] [PubMed] [Google Scholar]
- Shmigol A., Kostyuk P., Verkhratsky A. Role of caffeine-sensitive Ca2+ stores in Ca2+ signal termination in adult mouse DRG neurones. Neuroreport. 1994 Oct 27;5(16):2073–2076. doi: 10.1097/00001756-199410270-00021. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Hirning L. D., Miller R. J. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988 Nov;34(5):664–673. [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usachev Y., Shmigol A., Pronchuk N., Kostyuk P., Verkhratsky A. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience. 1993 Dec;57(3):845–859. doi: 10.1016/0306-4522(93)90029-f. [DOI] [PubMed] [Google Scholar]
- Wier W. G. Cytoplasmic [Ca2+] in mammalian ventricle: dynamic control by cellular processes. Annu Rev Physiol. 1990;52:467–485. doi: 10.1146/annurev.ph.52.030190.002343. [DOI] [PubMed] [Google Scholar]
- Yamada W. M., Zucker R. S. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophys J. 1992 Mar;61(3):671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


