Abstract
Study Objectives
To evaluate sex-specific associations between known or suspected obstructive sleep apnea (OSA) and dementia risk over 10 years among older women and men.
Methods
This study included 18 815 women and men age 50+ years (dementia-free at baseline) who participated in the Health and Retirement Study (HRS), a nationally representative cohort of US adults. Presence of OSA was defined by self-reported diagnosis or key HRS items that correspond to elements of a validated OSA screening tool (STOP-Bang). Incident dementia cases were identified using a validated, HRS-based algorithm derived from objective cognitive assessments. Survey-weighted regression models based on pseudo-values were utilized to estimate sex- and age-specific differences in cumulative incidence of dementia by OSA status.
Results
Data from 18 815 adults were analyzed, of which 9% of women and 8% of men (weighted proportions) met criteria for incident dementia. Known/suspected OSA was more prevalent in men than in women (weighted proportions 68% vs. 31%). Unadjusted sex-stratified analyses showed that known/suspected OSA was associated with higher cumulative incidence of dementia across ages 60–84 years for women and men. By age 80, relative to adults without known/suspected OSA, the cumulative incidence of dementia was 4.7% higher (CI 2.8%, 6.7%) for women with known/suspected OSA, and 2.5% (CI 0.5%, 4.5%) for men with known/suspected OSA, respectively. Adjusted associations between age-specific OSA and cumulative incidence of dementia attenuated for both women and men but remained statistically significant.
Conclusions
OSA contributes to dementia risk in older adults, particularly women. This study illuminates the impact of a potentially modifiable yet frequently overlooked risk factor for dementia onset.
Keywords: OSA, neurodegenerative disorders, neurological disorders, cognitive function, women’s health, dementia
Statement of Significance.
This study utilized population-based, objective, longitudinal data from the Health and Retirement Study to estimate sex-specific associations between known or suspected obstructive sleep apnea (OSA) and dementia incidence. Based on biennial surveys and cognitive performance assessments, we determined the 10-year population-based risk of dementia among a stratified cohort of women and men with known or suspected OSA. As 80% of adults with OSA are undiagnosed, this study implemented methodology to identify most of the population at risk for OSA who do not carry an OSA diagnosis (a frequently overlooked cohort), to better characterize the longitudinal relationship between OSA and dementia.
Introduction
Nearly seven million Americans are currently living with dementia [1]—a term that describes a group of progressive, incurable neurological syndromes associated with irreversible decline in cognitive function and behavioral changes. Alzheimer’s dementia (AD) accounts for approximately 70% of dementia cases and is the sixth leading cause of death in the USA [2]. A critical need exists to identify modifiable risk factors that can be effectively addressed prior to the onset of irreversible cognitive decline. Further, as older women are more likely to experience dementia than men [3–9], additional research is needed to understand how common comorbidities and environmental exposures that disproportionately impact women could influence sex disparities in dementia incidence.
Among potentially modifiable risk factors for dementia, obstructive sleep apnea (OSA)—a chronic sleep disorder characterized by repeated episodes of upper airway obstruction, sleep fragmentation, and hypoxia during sleep—is associated with cognitive decline [10, 11]. The prevalence of OSA increases with age, making this condition particularly germane when considering causal pathways to dementia onset in older adults. While prior studies have associated OSA and dementia incidence [12, 13], prospective, sex-stratified, population-based data that captures potential undiagnosed OSA cases, includes longer follow up periods and incorporates more granular cognitive measures is still needed to understand the scope and nature of the relationship, and to more firmly establish causal pathways between OSA and cognitive decline across women and men who are most at risk. To characterize pathways between OSA and dementia risk, this study evaluated sex-specific differences in cumulative dementia incidence among midlife and older women and men with known or suspected OSA over a 10-year observation interval using a population-based cohort.
Methods
Data source and analytic sample
All study data were extracted from core files of The Health and Retirement Study (HRS). The HRS, supported by the National Institute on Aging and the Social Security Administration, is a longitudinal cohort study that captures multidisciplinary aspects of health and wellbeing in a nationally representative cohort of Americans aged 50+ years. The survey has been conducted biennially since 1992, with recruitment of individuals aged 51–56 years old every 6 years. As of 2020, the HRS has collected responses from over 43 000 individuals in 26 000 households. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline [14]. Local institutional review board approval (IRBMED) was obtained prior to extant HRS data access. As all data were obtained from the HRS core (publicly available) dataset, the investigators in this study were not involved in the consenting process of HRS participants.
The present analysis utilized 2010–2020 HRS datasets (waves), which include follow up assessments every 2 years. Of 28 058 participants interviewed in 2010–2020, the initial sample contained 115 116 observations. We first excluded 3 699 observations from respondents below age 50 years at the time of interview, 5828 observations from proxy interviews, and 792 observations from respondents residing in the nursing home at the time of interview. We further restricted the sample to individuals with an ascertainable OSA status who were dementia-free at baseline and had complete sociodemographic information, resulting in a final analytic sample of 89 155 observations from 18 815 respondents (Table 1). The longitudinal attrition rate was low. On average, there were 5.2 observations per respondent among people who entered HRS prior to 2016, and 2.5 observations among those who entered the study in 2016.
Table 1.
Sample size crosswalk 2010–2020
| 2010 | 2012 | 2014 | 2016 | 2018 | 2020 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | |
| HRS sample | 12 815 | 9219 | 12 014 | 8540 | 11 048 | 7699 | 12 247 | 8665 | 10 115 | 7031 | 9334 | 6389 |
| Exclusion criteria | ||||||||||||
| Age < 50 | 761 | 239 | 523 | 172 | 341 | 117 | 594 | 177 | 353 | 106 | 236 | 80 |
| Proxy interviews | 653 | 705 | 563 | 564 | 525 | 515 | 469 | 461 | 319 | 339 | 340 | 375 |
| Nursing home participants | 97 | 32 | 116 | 62 | 118 | 60 | 64 | 36 | 46 | 35 | 80 | 46 |
| No ascertainable OSA status | 2 937 | 2511 | 2208 | 1885 | 1329 | 1062 | 147 | 91 | 60 | 21 | 54 | 27 |
| Incomplete demographic information* | 44 | 36 | 72 | 53 | 87 | 69 | 547 | 454 | 519 | 433 | 539 | 455 |
| Analytic sample | 8323 | 5696 | 8532 | 5804 | 8648 | 5876 | 10 425 | 7445 | 8818 | 6097 | 8085 | 5406 |
Abbreviations: HRS, Health and Retirement Study; OSA, obstructive sleep apnea.
*Sociodemographic information included race/ethnicity, educational attainment and cohabitation status.
Assessment of known or suspected OSA
Known or suspected OSA was the primary exposure of interest, identified by presence of self-reported diagnosis or, in the absence of diagnosis, a positive screen on an adapted version of the STOP-Bang Questionnaire that was constructed from medical history and demographic data (age, sex, BMI, and hypertension history collected at participant’s entry into HRS) and new sleep items (snoring, witnessed apneas) that were introduced in the 2016–2020 HRS waves. As up to 80% of adults with OSA remain undiagnosed, the addition of a validated symptom screening approach to the self-report measure was employed. Evidence suggests that assessment of the STOP-Bang questionnaire items can capture the majority of undiagnosed cases, allowing for a more comprehensive assessment of the scope of OSA among older adults [15].
The validated STOP-Bang questionnaire consists of eight items that form the acronym “STOP-BANG” (snoring, tiredness, observed apneas, high blood pressure, BMI, age, neck circumference, and gender) [16]. All items are yes/no questions or measures. Scoring is based on the number of positive items. In general, a score of zero to two reflects low risk for OSA, three to four as moderate risk, and five or above as high risk. In addition, a modified scoring rubric, with at least two positive STOP items and male sex or BMI >35 kg/m2, has been demonstrated to improve the prediction performance of the original scale [17].
Variables assessed in seven of the eight STOP-Bang items were available in the HRS dataset; only neck circumference was missing (Table 2). As the STOP-Bang utilizes binary responses, we dichotomized corresponding HRS items, with cutoffs described previously [18]. Probable OSA cases were defined by (1) total STOP-Bang score of at least four; or (2) at least two positive STOP items plus either male sex or BMI >35 kg/m2. OSA status was defined at the respondent level. Specifically, participants who met criteria (1) or (2) were considered to have “known or suspected” OSA. These assumptions were applied to the entire assessment interval, as OSA is a chronic disorder that typically develops in midlife prior to HRS enrollment [19].
Table 2.
Probable OSA assessment corresponding to STOP-Bang questionnaire items
| Questions from the HRS physical health file | Cutoffs utilized for identifying positive items | Corresponding STOP-Bang items |
|---|---|---|
| In the past 12 months, how often did you snore while you were sleeping?† | Occasionally (3–4 nights/week), frequently (5 or more nights/week) | Do you snore loudly? |
| How often do you really feel rested when you wake up in the morning? | Sometimes, most of the time | Do you often feel tired, fatigued, or sleepy during the daytime? |
| In the past 12 months, how often did you snort, gasp or stop breathing while you were sleeping?*,† | Occasionally (3–4 nights/week), frequently (5 or more nights/week) | Has anyone observed you stop breathing during sleep? |
| Has a doctor ever told you that you have high blood pressure or hypertension? In order to lower your blood pressure, are you now taking any medication? |
Yes, to either one of two questions | Do you have (or are you being treated for) high blood pressure? |
| About how tall are you? About how much do you weigh? |
BMI > 35 kg/m2 | BMI |
| Year born | Age ≥ 50 | Age |
| N/A | N/A | Neck circumference |
| Sex | Men | Sex |
Abbreviations: OSA, obstructive sleep apnea; HRS, Health and Retirement Study; BMI, body mass index; N/A, not available.
*The question on gasping behaviour was only administered to respondents who endorsed snoring; non-snorers did not receive a point for gasping.
†Questions that were only asked during the 2016 wave or later.
Assessment of dementia
Dementia status at each wave of surveys for all enrolled HRS respondents was identified through either self-reported diagnosis of dementia or Alzheimer’s disease, or based on performance on an objective cognitive functioning assessment that was conducted during each wave. Every 2 years, the HRS core interview evaluates total cognitive functioning of self-respondents through a set of measures. Using Weir-Langa classifications [20], scores from immediate and delayed word recall, Serial Sevens Task and backward count were mapped to a 27-point scale. Scores below seven are considered a reliable proxy for dementia. Details on these four measures are described in a prior publication [18]. We used the HRS imputed values on cognitive measurements to account for missing data [21].
Covariates
We selected covariates that could confound the association between OSA and dementia. The HRS provides age, sex, and race/ethnicity, educational attainment, and cohabitation status for all respondents. Self-reported race/ethnicity was categorized into four groups in the HRS dataset: non-Hispanic White, nonHispanic Black, Hispanic, and other. Educational attainment was characterized as below and above college (i.e., a 2-year or 4-year college degree or higher). Cohabitation status was defined by whether the respondents ever had a spouse/partner from 2010 to 2020. Although excessive body weight and hypertension are associated with both OSA and dementia, they are both already captured in the STOP-Bang questionnaire used for exposure definition and therefore not included as confounders in regression models.
Statistical analysis
Baseline sociodemographic and health characteristics were summarized at the respondent’s first assessment during 2010–2020. Respondent-level sampling weights were incorporated to ensure results were representative of US community-dwelling adults over age 50 in 2016. Sampling weights in the analytic sample were adjusted with the raking procedure (also known as iterative proportional fitting) [22], according to marginal distributions of sex and race/ethnicity in the full HRS sample.
Cumulative incidence (CI) was used to quantify dementia risk by exposures of interest, while accounting for the competing risk of mortality. For each respondent, we utilized information from all waves to calculate cumulative dementia incidence for a specific age group. For example, the cumulative dementia incidence at age A is the proportion of the population having dementia by age A, regardless of whether they are still alive at age A. For extreme advanced age, the cumulative dementia risk becomes the lifetime dementia risk. As HRS assessments are spaced 2 years apart (and data can be missing during any given wave), we used methods for interval censored data. Furthermore, the HRS does not consistently capture mortality, so we used sex-specific mortality from the Social Security Administration life tables [23] instead of inferring mortality directly from the HRS. The sex-specific hazard for dementia at age can be obtained as , where is the Turnbull estimate of the dementia-free survival function and the are the sorted centers of the Turnbull intervals. The sex-specific cumulative incidence at age A was obtained by summing over all , where denotes sex-specific mortality.
To assess associations between exposures and dementia CI, we used a pseudo-value approach [24]. Jack-knifed CI estimates at a sequence of ages between 60 to 84 were calculated for each respondent, and linear regression models were fit using the dementia CI estimates as the outcome and known or suspected OSA as the exposure, while adjusting for confounders. Both linear and quadratic terms of age were included in all models to capture potential nonlinear relationships between age and dementia CI. Cluster-robust standard errors were used to account for the repeated CI estimates for each subject. Models were fit to assess: (1) sex differences in the relationship between known or suspected OSA and dementia CI, (2) sex- and age-specific differences in dementia CI between respondents with and without known or suspected OSA, and (3) attenuation of any relationship between known or suspected OSA and dementia CI in sex-stratified analysis when controlling for confounders. Cluster-robust chi-squared tests were used to assess each of these hypotheses. Contrasts of dementia CI were presented as point estimates and 95% confidence intervals using cluster-robust standard errors. The known or suspected OSA population attributable risk for dementia by sex was also estimated using unadjusted regression models. All statistical analyses were performed in R (version 4.2.3, with packages ipfr, icenReg, sandwich, and survey). Unless otherwise stated, data are reported as unweighted frequencies and survey-weighted proportions.
Results
The analytic sample encompassed 89 155 observations from 18 815 respondents, including 11 023 women (54%) and 7792 men (46%), Supplementary Table. The mean (SD) age was 61 (9) years for women and 60 (9) years for men at the time of their first cognitive assessment, with more than 80% younger than age 70 years. Most respondents were White (74% of women and men), reported cohabitation (65% of women, 77% of men) and did not obtain a college degree (62% of women, 58% of men). A total of 2 233 incident dementia cases [1 322 women (9%) and 911 men (8%)] were identified. Forty-eight percent of respondents met criteria for “known or suspected” OSA. The prevalence of known or suspected OSA was more than twice as high in men as in women (68% vs. 31%). Seventeen percent of the entire sample endorsed a clinical OSA diagnosis. Among those who screened positive for suspected OSA with the adapted STOP-Bang, 29% of men and 30% of women also endorsed a clinical OSA diagnosis. Among those who screened negative for suspected OSA, a clinical OSA diagnosis was endorsed in 10% of men and 7% of women. Regardless of sex, respondents with suspected/known OSA tended to have lower educational level compared to those at low risk or without OSA. Additionally, cohabitation was more common in men with OSA than those without OSA (Table 3).
Table 3.
Sample characteristics overall and stratified by sex in the Health and Retirement Study [1]
| Overall N = 18 815 | Low risk OSA*N = 10 018 | Known or suspected OSA*N = 8797 | ||||
|---|---|---|---|---|---|---|
| Characteristic | Women† | Men† | Women† | Men† | Women† | Men† |
| Sample size (%) | 11 023 (54) | 7792 (46) | 7482 (69)‡ | 2536 (32)‡ | 3541 (31)‡ | 5256 (68)‡ |
| Age at the first cognitive assessment for analysis | ||||||
| 50–54 | 3481 (33) | 2307 (32) | 2200 (30) | 733 (33) | 1,281 (38) | 1574 (32) |
| 55–59 | 2273 (22) | 1,880 (26) | 1433 (22) | 560 (23) | 840 (24) | 1320 (28) |
| 60–64 | 1431 (16) | 961 (15) | 950 (16) | 281 (15) | 481 (16) | 680 (15) |
| 65–69 | 1138 (10) | 760 (11) | 772 (10) | 224 (10) | 366 (10) | 536 (11) |
| 70–74 | 1255 (8) | 920 (7) | 940 (9) | 311 (8) | 315 (6) | 609 (7) |
| 75–79 | 821 (5) | 588 (5) | 651 (6) | 233 (6) | 170 (4) | 355 (4) |
| 80–84 | 421 (3) | 260 (2) | 355 (4) | 125 (3) | 66 (2) | 135 (2) |
| 85+ | 203 (2) | 116 (1) | 181 (3) | 69 (2) | 22 (1) | 47 (1) |
| Race/Ethnicity§ | ||||||
| White | 6354 (74) | 4621 (74) | 4494 (76) | 1553 (76) | 1860 (70) | 3068 (74) |
| Hispanic | 1753 (10) | 1264 (10) | 1,179 (10) | 411 (10) | 574 (11) | 853 (10) |
| Black | 2450 (11) | 1510 (10) | 1484 (10) | 437 (8) | 966 (14) | 1073 (10) |
| Other | 466 (5) | 397 (6) | 325 (5) | 135 (7) | 141 (5) | 262 (6) |
| Education | ||||||
| Below college | 7554 (62) | 5083 (58) | 5066 (61) | 1577 (54) | 2488 (65) | 3506 (60) |
| College/Graduate degree | 3469 (38) | 2709 (42) | 2416 (39) | 959 (46) | 1053 (35) | 1750 (40) |
| Cohabitation | ||||||
| Yes | 6576 (65) | 6067 (77) | 4500 (65) | 1881 (73) | 2076 (64) | 4186 (80) |
| No | 4447 (35) | 1725 (23) | 2982 (35) | 655 (27) | 1465 (36) | 1070 (20) |
*OSA = obstructive sleep apnea.
†Unweighted sample size n and weighted proportions (%).
‡Weighted frequencies reflected prevalence of low risk OSA and known or suspected OSA by sex.
§Race and ethnicity information were self-reported by study participants.
In unadjusted analyses of the overall sample, sex was found to moderate the relationship between known or suspected OSA and cumulative incidence (CI) of dementia. Further sex-stratified unadjusted analyses revealed that, relative to absence of known/suspected OSA, the presence of known/suspected OSA was associated with increased dementia CI across ages 60-84 years for both women and men (Figure 1, left panels). In addition, differences in dementia CI by OSA status were found to be age- and sex-dependent (Figure 1, right panels). Specifically, as age increased, the increase in dementia CI difference among those with known or suspected OSA grew larger for women but stabilized for men. Moreover, at every age level, OSA was associated with a higher increase of dementia CI for women compared with men. For example, at age 80 years, women with versus without known or suspected OSA had a 4.7% higher cumulative dementia incidence (95% CI: 2.8%, 6.7%) whereas men with versus without known or suspected OSA had only a 2.5% higher cumulative dementia incidence (95% CI: 0.5%, 4.5%) (Table 4). Using age 80 years as an example, the population attributable risk percent (PAR%) of dementia associated with OSA, i.e., the fraction of dementia cases due to OSA, was estimated as 10.3% for women and 13.2% for men. The lower PAR% of dementia in women relative to men can be explained by the lower prevalence of OSA in women versus men (31% vs. 68%).
Figure 1.
Line charts of unadjusted age-specific cumulative incidence of dementia and difference in cumulative incidence of dementia by known or suspected OSA status in women and men aged 60–84 years. Left panels represent age-specific cumulative incidence (CI) of dementia by OSA status among women (top) and men (bottom). Right panels reflect age-specific differences in cumulative dementia incidence for women (top) and men (bottom) with known/suspected OSA versus no/low risk OSA. Figure 1 demonstrates that, regardless of sex, known or suspected OSA is associated with higher cumulative dementia incidence across ages. Moreover, as age advances, the increased impact of OSA on cumulative dementia incidence increases for women but decreases for men.
Table 4.
Difference in age- and sex-specific cumulative dementia incidence for known or suspected OSA versus no OSA among women and men from the Health and Retirement Study
| Women | Men | |||
|---|---|---|---|---|
| Age | Unadjusted cumulative dementia incidence difference (95% CI)* | Adjusted cumulative dementia incidence difference (95% CI)*,† | Unadjusted cumulative dementia incidence difference (95% CI)* | Adjusted cumulative dementia incidence difference (95% CI)*,† |
| 60 | 2.4 (1.3, 3.5) | 1.4 (0.3, 2.5) | 1.0 (0.0, 2.0) | 0.7 (−0.3, 1.7) |
| 62 | 2.6 (1.6, 3.6) | 1.5 (0.5, 2.6) | 1.2 (0.3, 2.2) | 0.9 (−0.1, 1.9) |
| 64 | 2.7 (1.7, 3.8) | 1.7 (0.7, 2.8) | 1.4 (0.4, 2.5) | 1.1 (0.1, 2.1) |
| 66 | 2.9 (1.8, 4.1) | 1.9 (0.8, 3.0) | 1.6 (0.5, 2.7) | 1.3 (0.2, 2.4) |
| 68 | 3.2 (1.9, 4.4) | 2.1 (0.9, 3.4) | 1.8 (0.6, 3.0) | 1.4 (0.2, 2.7) |
| 70 | 3.4 (2.0, 4.7) | 2.3 (1.0, 3.7) | 1.9 (0.6, 3.3) | 1.6 (0.3, 2.9) |
| 72 | 3.6 (2.2, 5.0) | 2.6 (1.2, 4.0) | 2.1 (0.6, 3.5) | 1.7 (0.3, 3.1) |
| 74 | 3.9 (2.4, 5.4) | 2.8 (1.4, 4.3) | 2.2 (0.7, 3.7) | 1.9 (0.4, 3.4) |
| 76 | 4.2 (2.5, 5.8) | 3.1 (1.5, 4.7) | 2.3 (0.7, 4.0) | 2.0 (0.4, 3.6) |
| 78 | 4.4 (2.7, 6.2) | 3.4 (1.7, 5.1) | 2.4 (0.6, 4.2) | 2.1 (0.3, 3.8) |
| 80 | 4.7 (2.8, 6.7) | 3.7 (1.8, 5.6) | 2.5 (0.5, 4.5) | 2.1 (0.1, 4.1) |
| 82 | 5.1 (2.8, 7.3) | 4.0 (1.8, 6.2) | N/A | N/A |
| 84 | 5.4 (2.8, 8.0) | 4.4 (1.8, 6.9) | N/A | N/A |
Abbreviations: OSA, obstructive sleep apnea; N/A, cumulative incidence differences are not available for men older than 80 years.
*Difference in cumulative incidence (%).
†Adjusted for race/ethnicity, educational attainment and cohabitation status.
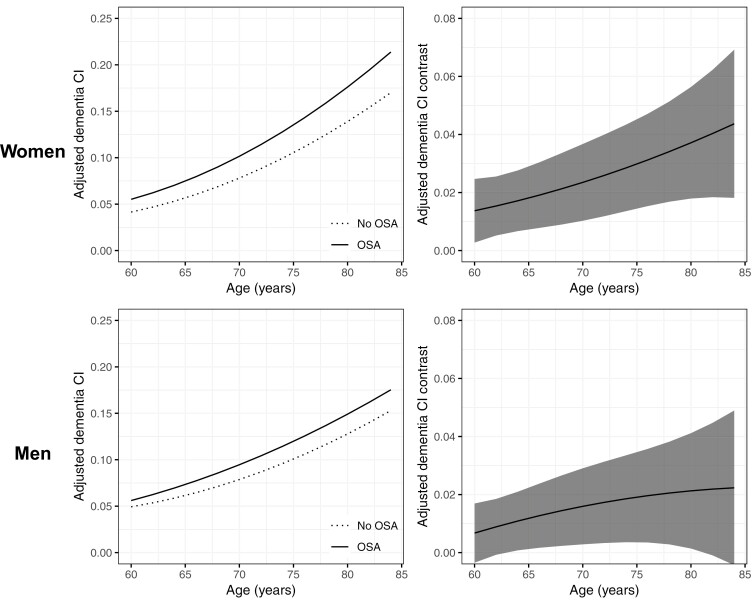
After adjusting for race/ethnicity, educational attainment, and cohabitation status, the impact of known or suspected OSA on age-specific dementia CI attenuated for both women and men but remained statistically significant (Figure 2). As shown in Table 4, at age 80 years and relative to their counterparts without OSA, women with known or suspected OSA had 3.7% greater cumulative dementia risk (95% CI: 1.8%, 5.6%) while men with known OSA had 2.1% greater cumulative dementia risk (95% CI: 0.1%, 4.1%).
Figure 2.
Line charts of differences in age-specific cumulative dementia incidence of known or suspected OSA status in women and men aged 60–84 years, adjusted for sociodemographic factors. Left panels represent age-specific cumulative incidence (CI) of dementia by OSA status among women (top) and men (bottom). Right panels reflect age-specific differences in cumulative dementia incidence for women (top) and men (bottom) with known/suspected OSA versus no/low risk OSA. Figure 2 demonstrates that associations between known or suspected OSA and age-specific cumulative dementia incidence for both women and men remained significant following adjustment for sociodemographic factors.
Discussion
These population-based data implicate a common midlife sleep disorder as a significant risk factor for dementia onset, with disproportionate impact on women. Our findings offer new evidence of a sleep-based pathway that underlies sex-specific differences in dementia risk. Further, as OSA is a treatable condition, this study illuminates the impact of a potentially modifiable yet frequently overlooked risk factor for dementia.
Temporal relationships between OSA and dementia onset demonstrated in this study substantiate growing support for a causal role of OSA in the development of dementia. Indeed, prior work has revealed compelling relationships between OSA and objective measures of cognitive performance within and outside of AD, and between OSA and measures of AD-associated biomarker signatures in the cerebrospinal fluid and central nervous system [25–28]. Associations between OSA and dementia diagnoses have been also suggested. In the Study of Osteoporotic Fractures [12], community-dwelling older women (mostly white) with evidence of moderate sleep apnea were 85% more likely to develop mild cognitive impairment (MCI) or dementia. Further, recent analyses of HRS data from our group revealed cross-sectional associations between OSA and dementia among older adults [18]. Recent evidence suggests that sleep deprivation or insufficient sleep (salient features of OSA) are independently associated with increased risk for AD progression and all-cause cognitive decline, as well as accumulation of pathological AD-associated biomarkers (Aβ42 and tau protein) in cognitively intact individuals [29–32]. Chronic inflammation associated with OSA and sleep deprivation may also contribute to impairment in microglial function and to decreased clearance of Aβ [33–37], as well as atherosclerotic disease that underlies vascular dementia. Proinflammatory cytokines that are commonly elevated in the plasma of patients with OSA are linked to reductions in brain volume in AD and vascular dementia.
Despite this foundational work, use of regional samples with low racial and ethnic diversity, cross-sectional designs, or limitations in sleep data have stymied conclusions regarding sex-specific temporal relationships between OSA and dementia onset, on a population level. To date, prospective studies and big dataset analyses focused on OSA and dementia have not allowed for simultaneous evaluation of OSA (including undiagnosed cases) and objective cognitive data over an extended period to examine accrual of dementia cases. Despite the high prevalence of OSA in the general population, the large majority of adults with OSA remain undiagnosed [38]. Consequently, datasets, particularly insurance claims that are restricted to OSA diagnostic or treatment codes, are subject to misclassification bias since the majority of undiagnosed cases will be missed with this approach [15]. Datasets such as the HRS that include more granular data regarding sleep offer a more reliable means to identify undiagnosed cases and minimize potential misclassification bias. Further, implementation of cutting-edge statistical approaches to HRS data now allows for causal inferences that could augment understanding of the differential impact of OSA on dementia incidence and inform subsequent, more definitive prospective studies of the OSA and dementia.
Our findings also add a new facet to recent reports on a growing list of comorbidities and health behaviors that may influence dementia onset. In a recent analysis, Lee et al. calculated contemporary estimates of the fraction of dementia cases in the USA that are associated with 12 potentially modifiable dementia risk factors [39]. In this study, the estimated proportion of the population with dementia that was attributable to these collective modifiable factors was 41%. Notably, OSA was not included in this study; however, several of the 12 risk factors identified including hypertension, obesity, and depression are closely related to OSA [40–46]. A separate systematic review and meta-analysis suggested that approximately 15% of AD cases may be attributable to sleep problems; however, no studies to date have conducted dedicated analyses to assess the proportion of dementia cases that may be attributable to OSA, a common sleep disorder, or to what extent this relationship could be mitigated with OSA treatment. That said, evidence summarized by a recent systematic review suggests a possible therapeutic benefit of positive airway pressure (PAP) therapy on MCI and AD incidence, slower cognitive decline, or progression to AD [47]. Further, in a recent analysis of a nationally-representative dataset from n = 53 321 Medicare beneficiaries with OSA, PAP use was associated with lower incident diagnoses of MCI, AD, and dementia not-otherwise-specified in older adults at three years, highlighting a potentially protective role for PAP therapy on short-term dementia risk [48]. This possibility merits further exploration over longer intervals and statistical methods shaped by this current work.
Reasons for observed sex-specific differences in cumulative dementia incidence by OSA status are still speculative. The nearly twofold greater prevalence of dementia among women relative to men has historically been attributed mainly to differences in the longevity of women; however, our findings suggest there may be more complex explanations. Although OSA is estimated to be more prevalent in men, women are more likely to suffer from several downstream consequences of OSA that could impact dementia risk [49]. Comparisons of women and men with untreated OSA suggest that women are more likely to experience worse quality of life and sleep quality, fatigue, anxiety, and depression [50, 51]. Relative to men, women with moderate OSA may have a greater risk of cardiovascular disease [52] and are more likely to present with insomnia [53, 54], which could impose detrimental impact on cognitive function [55, 56]. Moreover, sex-specific differences at the intersection between OSA and ApoEε4 allelic status [57], an understudied yet important risk factor for dementia [58], could uncover mechanistic pathways that contribute to the vulnerability of women in brain aging.
The disproportional burden of dementia among women also warrants attention to interactions between sleep and reproductive determinants of cognitive health later in life. OSA risk in women increases post-menopause [59, 60]. Later age at menopause, which could be associated with fewer years of OSA exposure, is associated with lower risk of dementia [61].
This study has several strengths. As a large, population-based study, this cohort is representative of women and men aged 50+ who live in the USA, which allows for generalizability of the findings. Prospectively designed, this study collects longitudinal, rich data that provides unique opportunity to examine decade-long temporal associations between OSA and dementia while accounting for important risk factors for both OSA and dementia. In addition, with a low attrition rate (13%), we were able to continually follow up cognitive trajectories of most participants and retain a good internal validity. Finally, as a flexible method to analyze censored survival data in the presence of competing risks, the pseudo-value regression approach provides more interpretable estimates of covariate effects on cumulative incidence compared with cause-specific hazard models and Fine-Gray sub-distribution hazard models.
Some limitations should be noted. While the STOP-Bang is not a diagnostic tool for OSA, it is routinely and widely used for OSA screening with good validity. The HRS did not include an item for neck circumference; however, prior work that has examined alternative scoring methods for the STOP-Bang has shown neck circumference to have less predictive value than other STOP-Bang items [17]. Rather than at baseline, collection of two sleep-based items utilized in the adapted STOP-Bang (snoring, witnessed apneas) began in 2016. However, other items factored into OSA classification (age, sex, BMI, and hypertension) were collected at time of HRS enrollment. Further, as OSA is a chronic, irreversible condition that mostly occurs in midlife, reverse causality due to potential misclassification of OSA cases prior to 2016 is unlikely, and sensitivity analyses that evaluated dementia incidence from the subsample who enrolled from 2016 to 2020 confirmed similar associations between OSA and cumulative dementia incidence, retaining significance for women specifically. Moreover, recent longitudinal data from >27 000 adults suggests that OSA risk plateaus at age 55–59 years among men and 65–69 years among women [62], prior to dementia onset that increases at age 70 years and peaks after age 80 years [63]. Although the HRS cognitive scoring criteria provide a reliable means to estimate cognitive function in HRS respondents, detailed neuropsychological testing data or claims data were not used to confirm dementia diagnoses. Although the HRS dataset included a survey question regarding presence and type of OSA treatment, it did not allow for the assessment of OSA treatment adherence, including PAP therapy which could influence cognitive function [47]. Further, this could only be assessed in those 15% who endorsed an OSA diagnosis, and absent responses could not be confirmed as negative, precluding assessment of the impact of OSA treatment on dementia risk. As the STOP-Bang is in part weighted by male gender, our findings regarding gender-specific differences could partially stem from the higher number of OSA risk factors that women must have outside of gender (including those independently associated with dementia) to screen positive for OSA. Additional research that examines the weights of STOP-Bang components alone and in various combinations could provide more nuanced predictions of dementia risk [48]. Weighting of the STOP-Bang toward male gender could also explain the higher prevalence of known or suspected OSA among men. As specificity of the full STOP-Bang may be lower among older adults, modified OSA screening tools for this age demographic could enhance future studies [64]. Finally, as in most large, longitudinal studies, missing data are expected. To account for missingness in cognitive data (22%), the HRS harnessed powerful imputation techniques.
In conclusion, we demonstrated a sex-specific association between known or suspected OSA and a decade-long cumulative incidence of dementia. Further research is warranted to examine the role of OSA therapy and potential mediators that lie in the pathways between OSA and dementia, including genetic mutations and comorbidities. Identification of potential mediators could support sex-specific interventions to reduce the incidence of dementia in older women and men with OSA.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Huchen Liu for his assistance in data acquisition for this study.
Contributor Information
Tiffany J Braley, Division of Sleep Medicine, Department of Neurology, University of Michigan, Ann Arbor, MI, USA; Division of Neuroimmunology, Department of Neurology, University of Michigan, Ann Arbor, MI, USA.
Xiru Lyu, Consulting for Statistics, Computing and Analytics Research, University of Michigan, Ann Arbor, MI, USA.
Galit Levi Dunietz, Division of Sleep Medicine, Department of Neurology, University of Michigan, Ann Arbor, MI, USA.
Paul C Schulz, Population Studies Center, Center for Social Research, University of Michigan, Ann Arbor, MI, USA.
Riley Bove, Division of Neuroimmunology, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
Ronald D Chervin, Division of Sleep Medicine, Department of Neurology, University of Michigan, Ann Arbor, MI, USA.
Henry L Paulson, Division of Cognitive Disorders, Department of Neurology, University of Michigan, Ann Arbor, MI, Michigan Alzheimer’s Disease Center, Ann Arbor, MI, USA.
Kerby Shedden, Consulting for Statistics, Computing and Analytics Research, University of Michigan, Ann Arbor, MI, USA; Department of Statistics, University of Michigan, Ann Arbor, MI, USA.
Funding
This work was funded by National Institute on Aging R01AG074342 (Braley and Dunietz PIs). Dr. Paulson’s contributions were supported by the Michigan Alzheimer’s Disease Research Center grant (P30AG072931 Paulson PI).
Disclosure Statement
Financial Disclosures: This study was supported by funding from the NIH/NIA (R01AG074342, PIs Braley and Dunietz). TJB reports current or recent research support from the National Institutes of Health, the International Progressive MS Alliance, and the National MS Society, and an award from the Patient-Centered Outcomes Research Institute. She has received support from Michigan Humane for her research. She has received travel and meeting support from the American Academy of Neurology and the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). GLD reports funding support from the National Institutes of Health. RDC reports grant support from the National Institutes of Health, and royalties from UpToDate. He consults for Eli Lilly & Company through a contract between Eli Lilly and Michigan Medicine. HLP reports support from the National Institutes of Health. RB receives research support from: Biogen, Roche Genentech, Novartis, Eli Lilly. She has received personal consulting fees from: Alexion, Amgen, Sanofi Genzyme, TG Therapeutics. XL, PCS, and KS report no relevant financial disclosures. Nonfinancial Disclosures: TJB reports a patent held by the University of Michigan related to treatment of sleep apnea. She receives material support, but no financial compensation, from Jazz Pharma for an NIH-funded investigator-initiated trial. She holds unpaid service and leadership positions for the National MS Society and the ACTRIMS Program Committee. RDC is named in patents and copyrighted material, owned by the University of Michigan, focused on diagnosis and treatment of sleep disorders including obstructive sleep apnea. XL, GLD, PCS, RB, HLP, and KS report no relevant non-financial disclosures.
Author Contributions
Tiffany Braley (Conceptualization [lead], Funding acquisition [lead], Investigation [lead], Methodology [lead], Project administration [lead], Supervision [lead], Writing—original draft [lead], Writing—review & editing [lead]), Xiru Lyu (Formal analysis [lead], Methodology [supporting], Writing—original draft [equal]), Galit Dunietz (Conceptualization [lead], Formal analysis [equal], Funding acquisition [lead], Investigation [equal], Methodology [equal], Supervision [lead], Writing—original draft [equal], Writing—review & editing [equal]), Paul Schultz (Formal analysis [supporting], Writing—review & editing [equal]), Riley Bove (Writing—review & editing [supporting]), Ronald Chervin (Writing—review & editing [equal]), Henry Paulson (Writing—review & editing [equal]), and Kerby Shedden (Formal analysis [lead], Methodology [lead], Supervision [equal], Writing—review & editing [equal])
Data Availability
Core survey data for the Health and Retirement Study is publicly available at https://hrsdata.isr.umich.edu/data-products/public-survey-data?_gl=1*1hu7f0c*_ga*MTk5MDc2MDM5LjE2OTUzMjMxNTk.*_ga_FF28MW3MW2*MTY5NjM2NzYwMC4xLjEuMTY5NjM2NzkxMC4wLjAuMA. &_ga=2.266139215.1336083028.1696342728-199076039.1695323159.
References
- 1. Hudomiet P, Hurd MD, Rohwedder S.. Trends in inequalities in the prevalence of dementia in the United States. Proc Natl Acad Sci U S A. 2022;119(46):e2212205119. doi: https://doi.org/ 10.1073/pnas.2212205119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA.. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020-2060). Alzheimers Dement. 2021;17(12):1966–1975. doi: https://doi.org/ 10.1002/alz.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dilworth-Anderson P, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S; Social, Behavioral and Diversity Research Workgroup of the Alzheimer’s Association. Diagnosis and assessment of Alzheimer’s disease in diverse populations. Alzheimers Dement. 2008;4(4):305–309. doi: https://doi.org/ 10.1016/j.jalz.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 4. Manly JJ, Mayeux R.. Ethnic Differences in Dementia and Alzheimer’s Disease. In: Anderson NB, Bulatao RA, Cohen B, eds. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington (DC): National Academies Press; 2004: 95–141. [PubMed] [Google Scholar]
- 5. Demirovic J, Prineas R, Loewenstein D, et al. Prevalence of dementia in three ethnic groups: the South Florida program on aging and health. Ann Epidemiol. 2003;13(6):472–478. doi: https://doi.org/ 10.1016/s1047-2797(02)00437-4 [DOI] [PubMed] [Google Scholar]
- 6. Harwood DG, Ownby RL.. Ethnicity and dementia. Curr Psychiatry Rep. 2000;2(1):40–45. doi: https://doi.org/ 10.1007/s11920-000-0040-4 [DOI] [PubMed] [Google Scholar]
- 7. Perkins P, Annegers JF, Doody RS, Cooke N, Aday L, Vernon SW.. Incidence and prevalence of dementia in a multiethnic cohort of municipal retirees. Neurology. 1997;49(1):44–50. doi: https://doi.org/ 10.1212/wnl.49.1.44 [DOI] [PubMed] [Google Scholar]
- 8. Steenland K, Goldstein FC, Levey A, Wharton W.. A meta-analysis of Alzheimer’s Disease incidence and prevalence comparing African-Americans and Caucasians. J Alzheimers Dis. 2016;50(1):71–76. doi: https://doi.org/ 10.3233/JAD-150778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Power MC, Bennett EE, Turner RW, et al. Trends in relative incidence and prevalence of dementia across non-hispanic black and white individuals in the United States, 2000-2016. JAMA Neurol. 2021;78(3):275–284. doi: https://doi.org/ 10.1001/jamaneurol.2020.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O.. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. doi: https://doi.org/ 10.1093/sleep/14.6.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bubu OM, Andrade AG, Umasabor-Bubu OQ, et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: A systematic review integrating three decades of multidisciplinary research. Sleep Med Rev. 2020;50:101250. doi: https://doi.org/ 10.1016/j.smrv.2019.101250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi: https://doi.org/ 10.1001/jama.2011.1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guay-Gagnon M, Vat S, Forget MF, et al. Sleep apnea and the risk of dementia: A systematic review and meta-analysis. J Sleep Res. 2022;31(5):e13589. doi: https://doi.org/ 10.1111/jsr.13589 [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England). 2007;370(9596):1453–1457. doi: https://doi.org/ 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 15. Braley TJ, Dunietz GL, Chervin RD, Lisabeth LD, Skolarus LE, Burke JF.. Recognition and Diagnosis of obstructive sleep apnea in older Americans. J Am Geriatr Soc. 2018;66(7):1296–1302. doi: https://doi.org/ 10.1111/jgs.15372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: https://doi.org/ 10.1097/ALN.0b013e31816d83e4 [DOI] [PubMed] [Google Scholar]
- 17. Chung F, Yang Y, Brown R, Liao P.. Alternative scoring models of STOP-bang questionnaire improve specificity to detect undiagnosed obstructive sleep apnea. J Clin Sleep Med. 2014;10(9):951–958. doi: https://doi.org/ 10.5664/jcsm.4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shieu MM, Dunietz GL, Paulson HL, Chervin RD, Braley TJ.. The association between obstructive sleep apnea risk and cognitive disorders: a population-based study. J Clin Sleep Med. 2022;18(4):1177–1185. doi: https://doi.org/ 10.5664/jcsm.9832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jennum P, Riha RL.. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33(4):907–914. doi: https://doi.org/ 10.1183/09031936.00180108 [DOI] [PubMed] [Google Scholar]
- 20. Crimmins EM, Kim JK, Langa KM, Weir DR.. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1Suppl 1):i162–i171. doi: https://doi.org/ 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCammon RJ, G. FG, Hassan H, Faul JD, Rodgers WL, Weir DR.. Health and Retirement Study Imputation of Cognitive Functioning Measures: 1992–2020 Data Description. 2023. [Google Scholar]
- 22. Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9(8):1–19. doi: https://doi.org/ 10.18637/jss.v009.i08 [DOI] [Google Scholar]
- 23. SSA.gov. Period Life Table, 2019, as used in the 2022 Trustees Report. Accessed Feb 15, 2023. https://www.ssa.gov/oact/STATS/table4c6_2019_TR2022.html [Google Scholar]
- 24. Andersen PK, Perme MP.. Pseudo-observations in survival analysis. Stat Methods Med Res. 2010;19(1):71–99. doi: https://doi.org/ 10.1177/0962280209105020 [DOI] [PubMed] [Google Scholar]
- 25. Elias A, Cummins T, Tyrrell R, et al. Risk of Alzheimer’s Disease in Obstructive Sleep Apnea Syndrome: Amyloid-beta and Tau Imaging. J Alzheimers Dis. 2018;66(2):733–741. doi: https://doi.org/ 10.3233/JAD-180640 [DOI] [PubMed] [Google Scholar]
- 26. Liguori C, Mercuri NB, Izzi F, et al. Obstructive Sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep. 2017;40(5). doi: https://doi.org/ 10.1093/sleep/zsx011 [DOI] [PubMed] [Google Scholar]
- 27. Sharma RA, Varga AW, Bubu OM, et al. Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly. A Longitudinal Study. Am J Respir Crit Care Med. 2018;197(7):933–943. doi: https://doi.org/ 10.1164/rccm.201704-0704OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diaz-Roman M, Pulopulos MM, Baquero M, et al. Obstructive sleep apnea and Alzheimer’s disease-related cerebrospinal fluid biomarkers in mild cognitive impairment. Sleep. 2021;44(1). doi: https://doi.org/ 10.1093/sleep/zsaa133 [DOI] [PubMed] [Google Scholar]
- 29. Kam K, Parekh A, Sharma RA, et al. Sleep oscillation-specific associations with Alzheimer’s disease CSF biomarkers: novel roles for sleep spindles and tau. Mol Neurodegener. 2019;14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu W, Tan C-C, Zou J-J, Cao X-P, Tan L.. Sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91(3):236–244. doi: https://doi.org/ 10.1136/jnnp-2019-321896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sabia S, Fayosse A, Dumurgier J, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289. doi: https://doi.org/ 10.1038/s41467-021-22354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Insel PS, Mohlenhoff BS, Neylan TC, Krystal AD, Mackin RS.. Association of sleep and β-amyloid pathology among older cognitively unimpaired adults. JAMA Network Open. 2021;4(7):e2117573–e2117573. doi: https://doi.org/ 10.1001/jamanetworkopen.2021.17573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee CD, Landreth GE.. The role of microglia in amyloid clearance from the AD brain. J Neural Transm. 2010;117:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bergeron C, Kimoff J, Hamid Q.. Obstructive sleep apnea syndrome and inflammation. J Allergy Clin Immunol. 2005;116(6):1393–1396. doi: https://doi.org/ 10.1016/j.jaci.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 35. Heneka MT, Golenbock DT, Latz E.. Innate immunity in Alzheimer’s disease. Nat Immunol. 2015;16(3):229–236. doi: https://doi.org/ 10.1038/ni.3102 [DOI] [PubMed] [Google Scholar]
- 36. Dearborn JL, Zhang Y, Qiao Y, et al. Intracranial atherosclerosis and dementia: the Atherosclerosis Risk in Communities (ARIC) study. Neurology. 2017;88(16):1556–1563. doi: https://doi.org/ 10.1212/WNL.0000000000003837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jefferson A, Massaro J, Wolf P, et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68(13):1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S.. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: https://doi.org/ 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 39. Lee M, Whitsel E, Avery C, et al. Variation in population attributable fraction of dementia associated with potentially modifiable risk factors by race and ethnicity in the US. JAMA Netw Open. 2022;5(7):e2219672. doi: https://doi.org/ 10.1001/jamanetworkopen.2022.19672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guillot M, Sforza E, Achour-Crawford E, et al. Association between severe obstructive sleep apnea and incident arterial hypertension in the older people population. Sleep Med. 2013;14(9):838–842. doi: https://doi.org/ 10.1016/j.sleep.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 41. Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37(9):2317–2321. doi: https://doi.org/ 10.1161/01.STR.0000236560.15735.0f [DOI] [PubMed] [Google Scholar]
- 42. Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B.. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology. 1999;92(2):79–84. doi: https://doi.org/ 10.1159/000006952 [DOI] [PubMed] [Google Scholar]
- 43. Harris M, Glozier N, Ratnavadivel R, Grunstein RR.. Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13(6):437–444. doi: https://doi.org/ 10.1016/j.smrv.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 44. Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP.. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25(9):735–741. doi: https://doi.org/ 10.1016/j.ehj.2004.02.021 [DOI] [PubMed] [Google Scholar]
- 45. Tasali E, Ip MS.. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5(2):207–217. doi: https://doi.org/ 10.1513/pats.200708-139MG [DOI] [PubMed] [Google Scholar]
- 46. Silva GE, Goodwin JL, Vana KD, Quan SF.. Obstructive sleep apnea and quality of life: comparison of the SAQLI, FOSQ, and SF-36 questionnaires. Southwest J Pulm Crit Care. 2016;13(3):137–149. doi: https://doi.org/ 10.13175/swjpcc082-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shieu MM, Zaheed A, Shannon C, et al. Positive airway pressure and cognitive disorders in adults with obstructive sleep apnea: A systematic review of the literature. Neurology. 2022;99(4):e334–e346. doi: https://doi.org/ 10.1212/WNL.0000000000200383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dunietz GL, Chervin RD, Burke JF, Conceicao AS, Braley TJ.. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep. 2021;44(9). doi: https://doi.org/ 10.1093/sleep/zsab076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koo P, McCool FD, Hale L, Stone K, Eaton CB.. Association of obstructive sleep apnea risk factors with nocturnal enuresis in postmenopausal women. Menopause. 2016;23(2):175–182. doi: https://doi.org/ 10.1097/GME.0000000000000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sampaio R, Pereira MG, Winck JC.. Psychological morbidity, illness representations, and quality of life in female and male patients with obstructive sleep apnea syndrome. Psychol Health Med. 2012;17(2):136–149. doi: https://doi.org/ 10.1080/13548506.2011.579986 [DOI] [PubMed] [Google Scholar]
- 51. Greenberg-Dotan S, Reuveni H, Simon-Tuval T, Oksenberg A, Tarasiuk A.. Gender differences in morbidity and health care utilization among adult obstructive sleep apnea patients. Sleep. 2007;30(9):1173–1180. doi: https://doi.org/ 10.1093/sleep/30.9.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S.. Sex influences endothelial function in sleep-disordered breathing. Sleep. 2004;27(6):1113–1120. doi: https://doi.org/ 10.1093/sleep/27.6.1113 [DOI] [PubMed] [Google Scholar]
- 53. Glidewell RN, Roby EK, Orr WC.. Is insomnia an independent predictor of obstructive sleep apnea? J Am Board Fam Med. 2012;25(1):104–110. doi: https://doi.org/ 10.3122/jabfm.2012.01.110123 [DOI] [PubMed] [Google Scholar]
- 54. Bublitz M, Adra N, Hijazi L, Shaib F, Attarian H, Bourjeily G.. A narrative review of sex and gender differences in sleep disordered breathing: gaps and opportunities. Life (Basel). 2022;12(12):2003. doi: https://doi.org/ 10.3390/life12122003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98(10):984–989. doi: https://doi.org/ 10.1016/j.rmed.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 56. Haimov I, Hanuka E, Horowitz Y.. Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6(1):32–54. doi: https://doi.org/ 10.1080/15402000701796080 [DOI] [PubMed] [Google Scholar]
- 57. Kadotani H, Kadotani T, Young T, et al. Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA. 2001;285(22):2888–2890. doi: https://doi.org/ 10.1001/jama.285.22.2888 [DOI] [PubMed] [Google Scholar]
- 58. Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G.. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: https://doi.org/ 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang T, Lin BM, Redline S, Curhan GC, Hu FB, Tworoger SS.. Type of menopause, age at menopause, and risk of developing obstructive sleep apnea in postmenopausal women. Am J Epidemiol. 2018;187(7):1370–1379. doi: https://doi.org/ 10.1093/aje/kwy011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dancey DR, Hanly PJ, Soong C, Lee B, Hoffstein V.. Impact of menopause on the prevalence and severity of sleep apnea. Chest. 2001;120(1):151–155. doi: https://doi.org/ 10.1378/chest.120.1.151 [DOI] [PubMed] [Google Scholar]
- 61. Hao W, Fu C, Dong C, et al. Age at menopause and all-cause and cause-specific dementia: a prospective analysis of the UK Biobank cohort. Hum Reprod. 2023;38(9):1746–1754. doi: https://doi.org/ 10.1093/humrep/dead130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thompson C, Legault J, Moullec G, et al. A portrait of obstructive sleep apnea risk factors in 27,210 middle-aged and older adults in the Canadian Longitudinal Study on Aging. Sci Rep. 2022;12(1):5127. doi: https://doi.org/ 10.1038/s41598-022-08164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M.. Differences between women and men in incidence rates of dementia and Alzheimer’s Disease. Journal of Alzheimer's disease : JAD. 2018;64(4):1077–1083. doi: https://doi.org/ 10.3233/JAD-180141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martins EF, Martinez D, Cortes AL, Nascimento N, Brendler J.. Exploring the STOP-BANG questionnaire for obstructive sleep apnea screening in seniors. J Clin Sleep Med. 2020;16(2):199–206. doi: https://doi.org/ 10.5664/jcsm.8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Core survey data for the Health and Retirement Study is publicly available at https://hrsdata.isr.umich.edu/data-products/public-survey-data?_gl=1*1hu7f0c*_ga*MTk5MDc2MDM5LjE2OTUzMjMxNTk.*_ga_FF28MW3MW2*MTY5NjM2NzYwMC4xLjEuMTY5NjM2NzkxMC4wLjAuMA. &_ga=2.266139215.1336083028.1696342728-199076039.1695323159.