Abstract
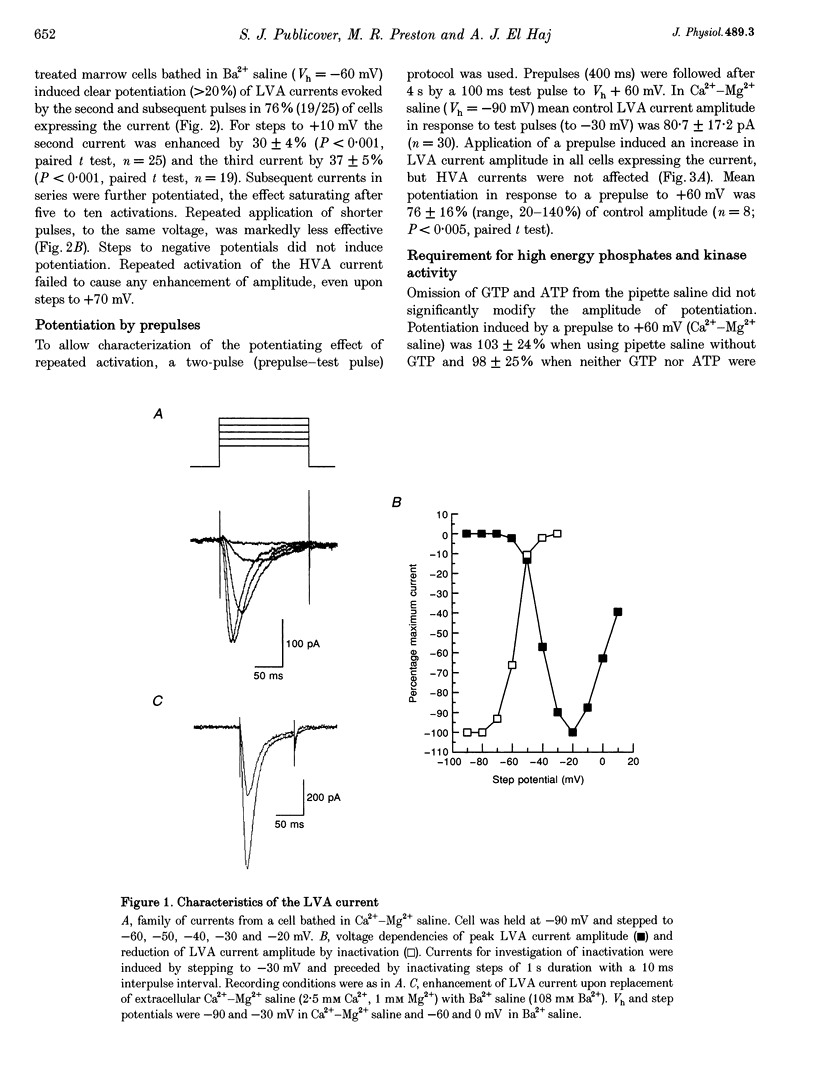
1. The whole-cell patch-clamp technique was used to study Ca2+ channel currents in stromal cells of 7-10 day dexamethasone-treated and control rat bone marrow cultures. In saline containing either 108 mM Ba2+ or a 2.5 mM Ca(2+)-1 mM Mg2+ mixture, most cells expressed both fast-inactivating, low-voltage-activated (LVA) and slow-inactivating, high-voltage-activated (HVA) currents. 2. Repeated application of 400 ms voltage steps to 60 mV above the holding potential (Vh, -90 mV in Ca(2+)-Mg2+ mixture and -60 mV in Ba2+) at a frequency > or = 0.1 Hz resulted in a potentiation of the LVA component of the 2nd and subsequent currents. 3. LVA current potentiation was examined using a two-pulse (prepulse-test pulse) method. Prepulses to Vh + 150 mV induced an 80-100% increase in the amplitude of the LVA component of Ca2+ channel currents in saline containing either Ba2+ or Ca(2+)-Mg2+. This effect was also seen in non-dexamethasone-treated cultures. 4. Potentiation was not modified by omission of ATP and GTP from the pipette saline, and was not inhibited by extracellular application of the broad spectrum kinase inhibitors H-7 or RK252-a. 5. Potentiation was dependent on the amplitude and duration of the prepulse. Using the standard protocol, the threshold for potentiation was approximately Vh + 45 mV and saturation occurred at Vh + 150-180 mV. Further increases in prepulse amplitude did not modify potentiation. With a prepulse to +10 mV (Ba2+ saline) potentiation was half-maximal with a prepulse duration of 250-300 ms duration and saturated at 750-1000 ms. 6. Peak potentiation occurred 1-2 s after the prepulse. The time for total decay of potentiation varied from 10 to 90 s. 7. Voltage dependency of prepulse-induced potentiation did not resemble that of inactivation induced by similar prepulses. 8. Current kinetics, I-V relationship and sensitivity to blockade by Ni2+ and diphenylhydantoin of prepulse-recruited current resembled those of control LVA current. 9. The amplitude of prepulse-recruited current was positively correlated with control LVA current amplitude. 10. LVA currents supported regenerative potentials under current clamp. Repeated activation reduced spike latency. 11. It is suggested that current potentiation may be recruited physiologically, possibly in association with activation of stretch-sensitive channels, causing enhanced activation of HVA Ca2+ currents.
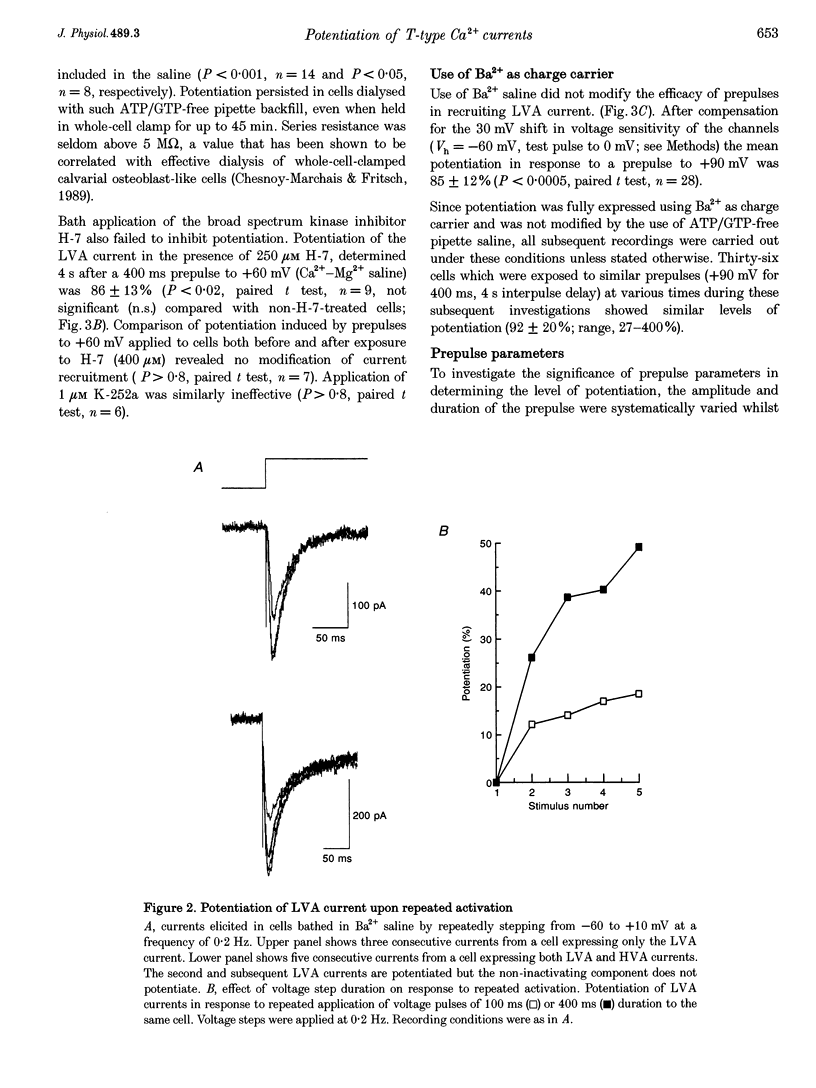
Full text
PDF


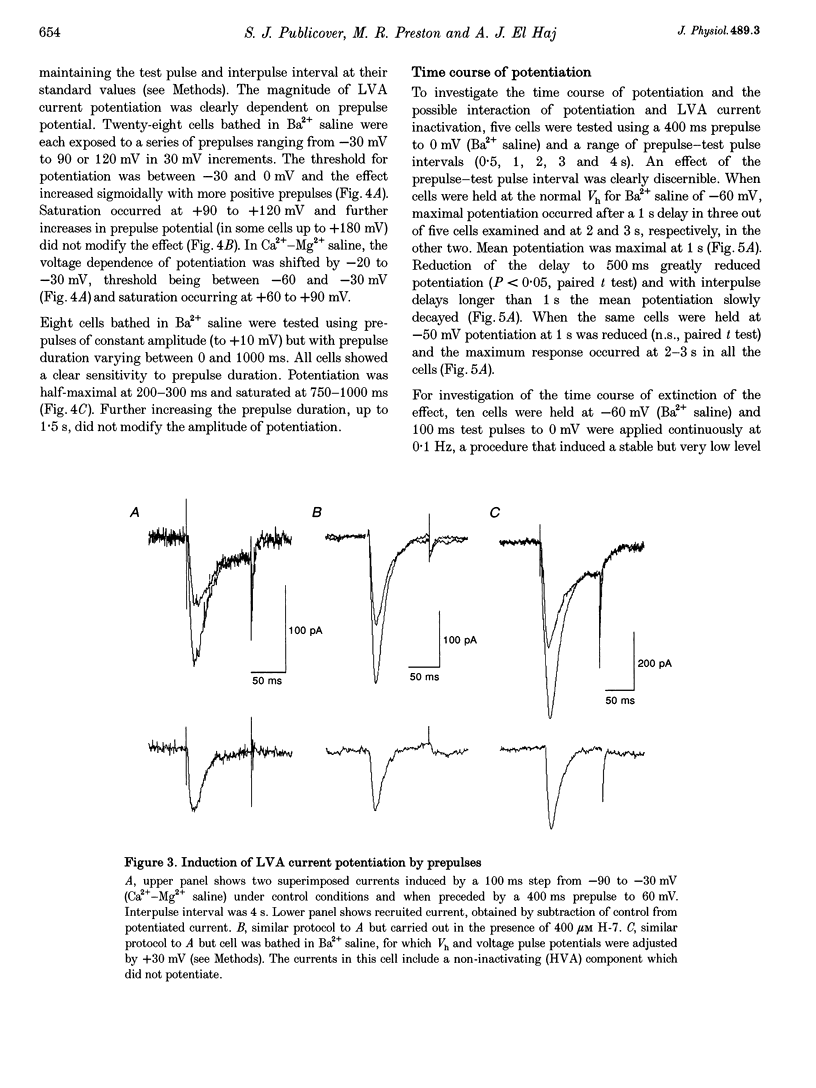
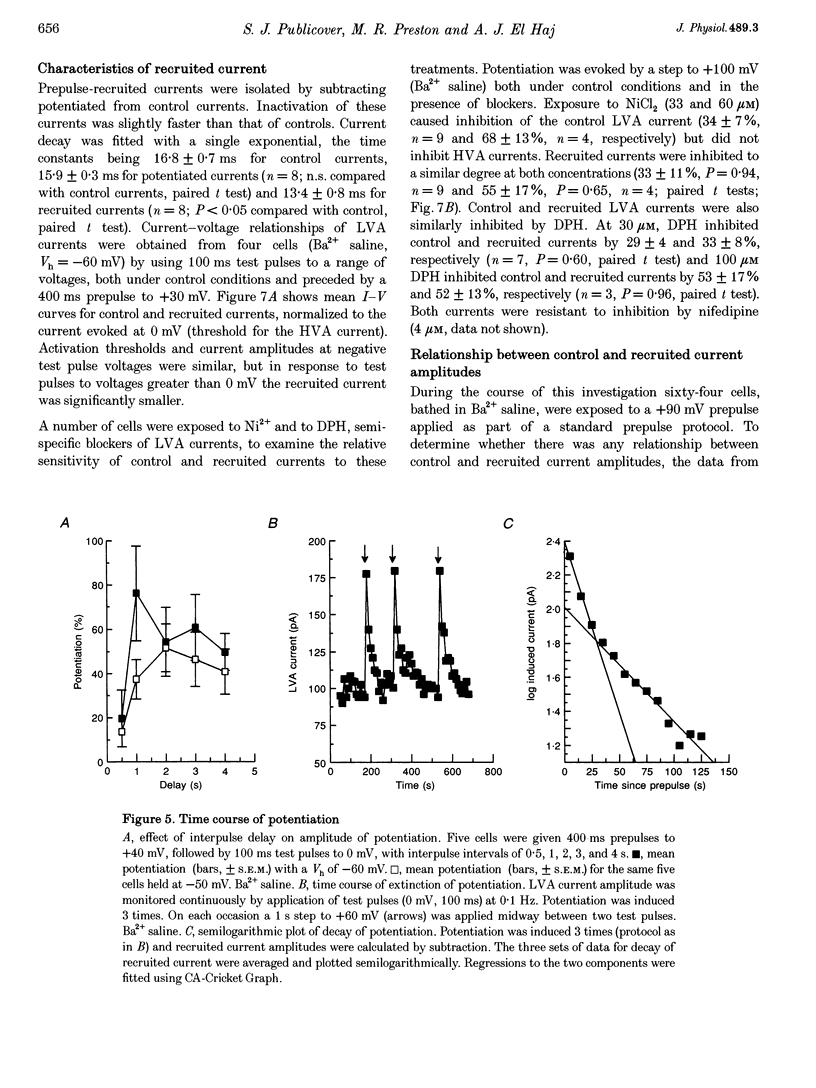
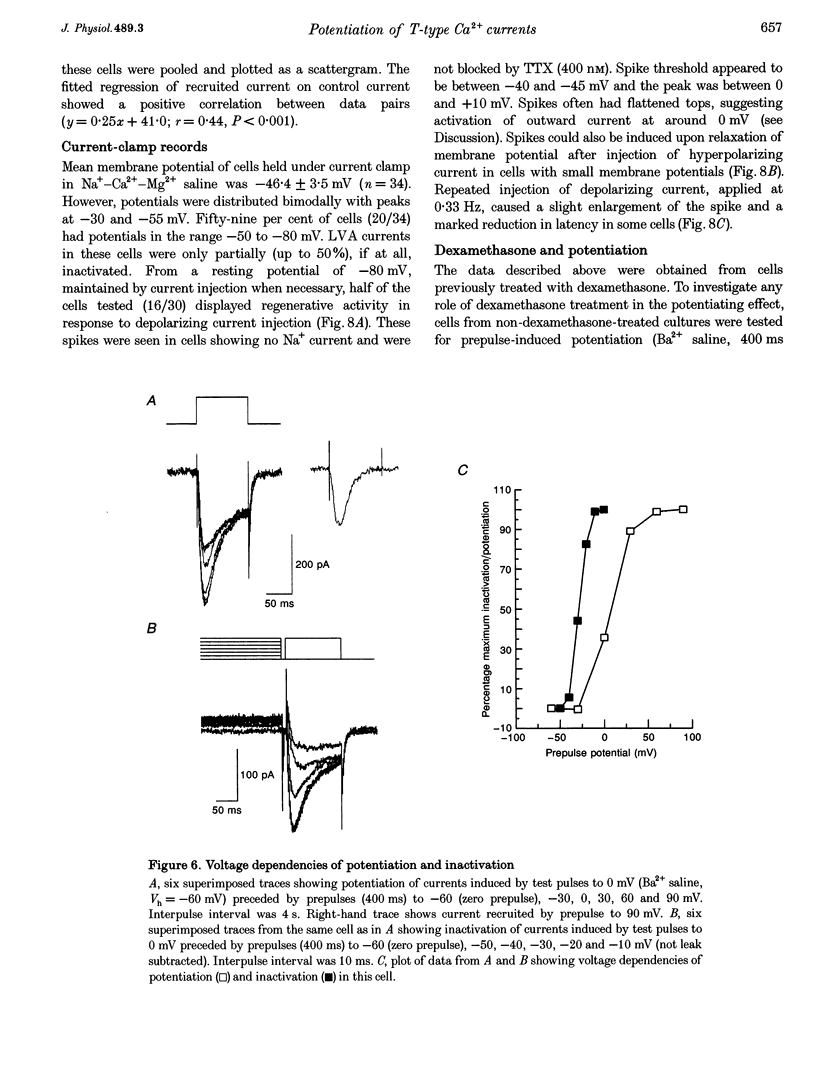
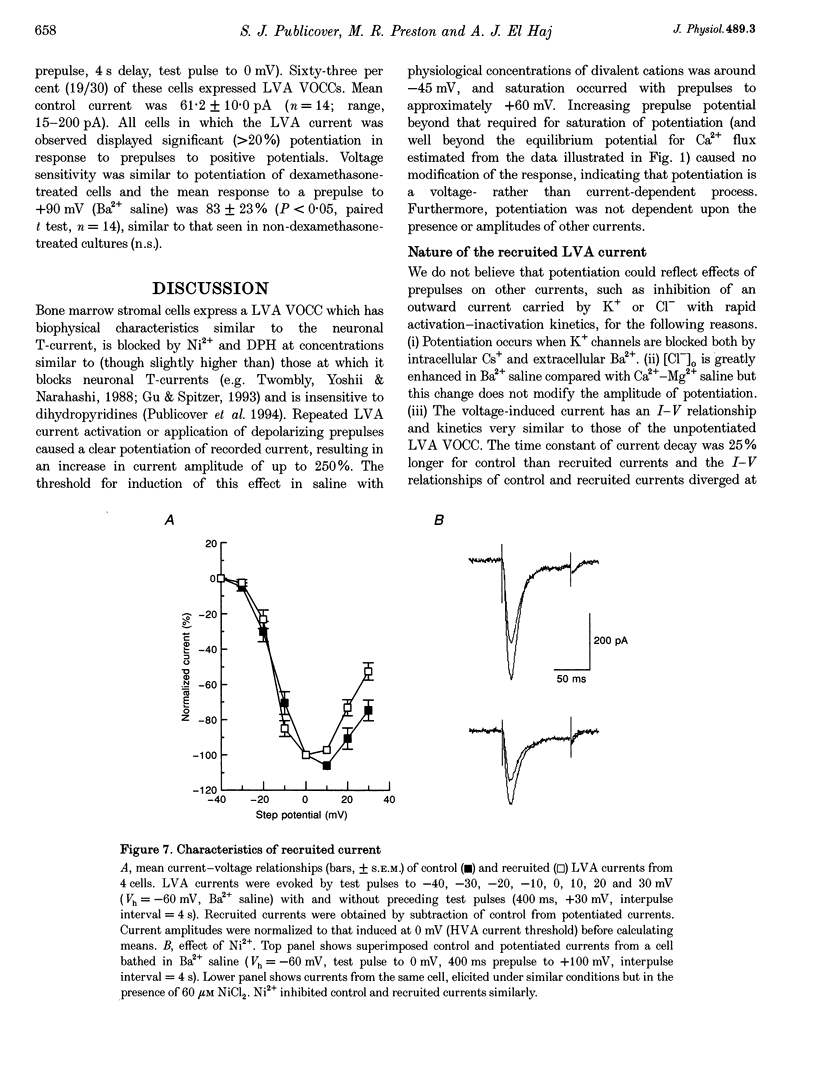
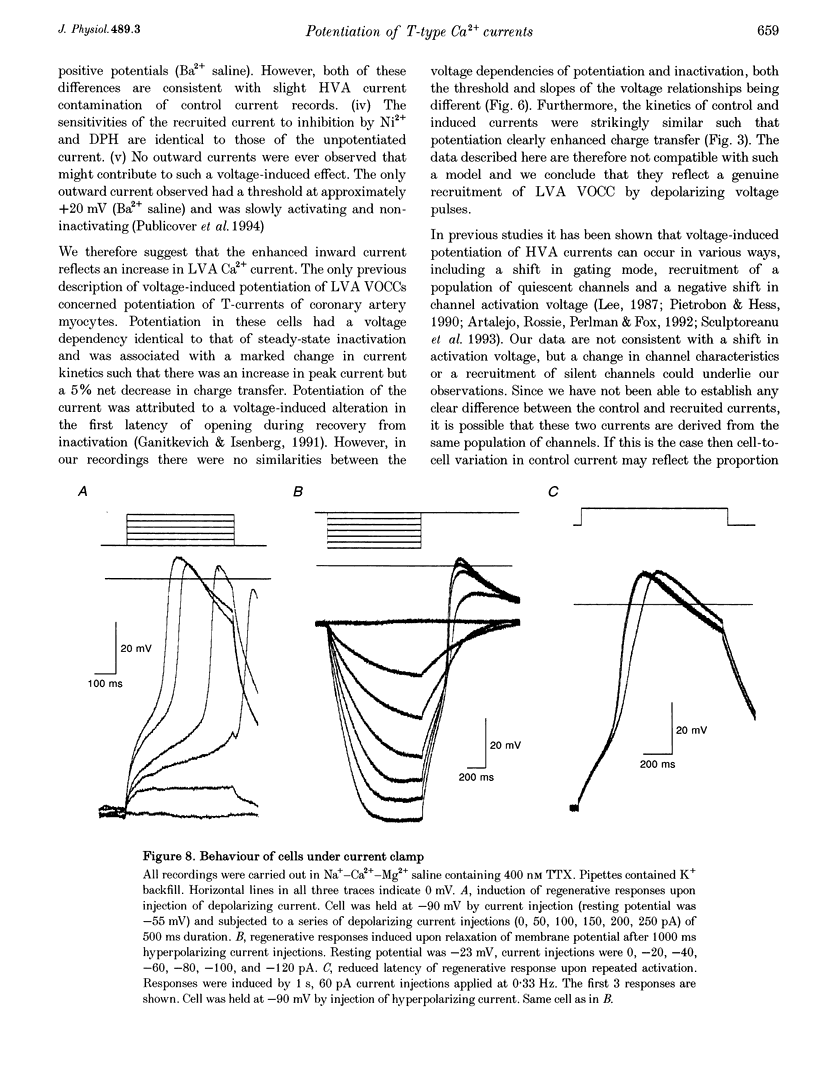


Selected References
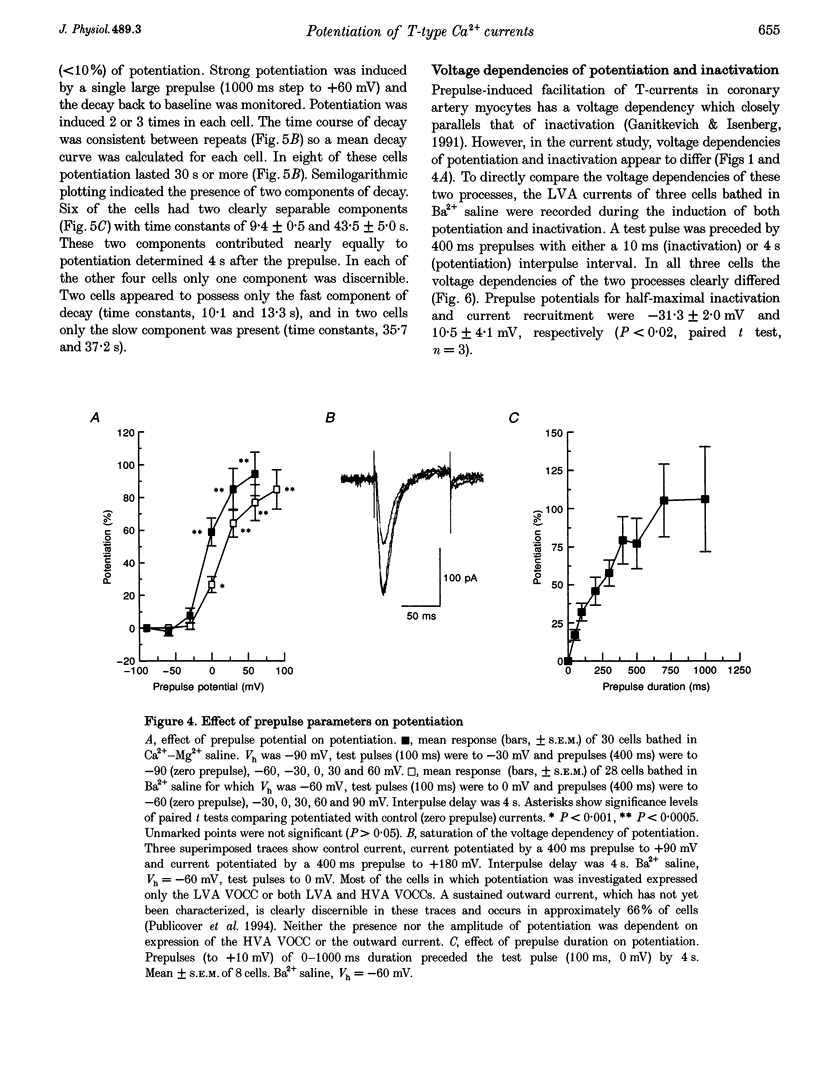
These references are in PubMed. This may not be the complete list of references from this article.
- Amagai Y., Kasai S. A voltage-dependent calcium current in mouse MC3T3-E1 osteogenic cells. Jpn J Physiol. 1989;39(5):773–777. doi: 10.2170/jjphysiol.39.773. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Dahmer M. K., Perlman R. L., Fox A. P. Two types of Ca2+ currents are found in bovine chromaffin cells: facilitation is due to the recruitment of one type. J Physiol. 1991 Jan;432:681–707. doi: 10.1113/jphysiol.1991.sp018406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., Rossie S., Perlman R. L., Fox A. P. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 1992 Jul 2;358(6381):63–66. doi: 10.1038/358063a0. [DOI] [PubMed] [Google Scholar]
- Caffrey J. M., Farach-Carson M. C. Vitamin D3 metabolites modulate dihydropyridine-sensitive calcium currents in clonal rat osteosarcoma cells. J Biol Chem. 1989 Dec 5;264(34):20265–20274. [PubMed] [Google Scholar]
- Chesnoy-Marchais D., Fritsch J. Chloride current activated by cyclic AMP and parathyroid hormone in rat osteoblasts. Pflugers Arch. 1989 Oct;415(1):104–114. doi: 10.1007/BF00373147. [DOI] [PubMed] [Google Scholar]
- Chesnoy-Marchais D., Fritsch J. Concentration-dependent modulations of potassium and calcium currents of rat osteoblastic cells by arachidonic acid. J Membr Biol. 1994 Mar;138(2):159–170. doi: 10.1007/BF00232644. [DOI] [PubMed] [Google Scholar]
- Chesnoy-Marchais D., Fritsch J. Voltage-gated sodium and calcium currents in rat osteoblasts. J Physiol. 1988 Apr;398:291–311. doi: 10.1113/jphysiol.1988.sp017043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. M. Membrane stretch activates a high-conductance K+ channel in G292 osteoblastic-like cells. J Membr Biol. 1993 Jan;131(1):81–92. doi: 10.1007/BF02258536. [DOI] [PubMed] [Google Scholar]
- Davidson R. M., Tatakis D. W., Auerbach A. L. Multiple forms of mechanosensitive ion channels in osteoblast-like cells. Pflugers Arch. 1990 Aug;416(6):646–651. doi: 10.1007/BF00370609. [DOI] [PubMed] [Google Scholar]
- Duncan R. L., Hruska K. A., Misler S. Parathyroid hormone activation of stretch-activated cation channels in osteosarcoma cells (UMR-106.01). FEBS Lett. 1992 Jul 28;307(2):219–223. doi: 10.1016/0014-5793(92)80771-8. [DOI] [PubMed] [Google Scholar]
- Duncan R., Misler S. Voltage-activated and stretch-activated Ba2+ conducting channels in an osteoblast-like cell line (UMR 106). FEBS Lett. 1989 Jul 17;251(1-2):17–21. doi: 10.1016/0014-5793(89)81420-6. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Stimulation-induced potentiation of T-type Ca2+ channel currents in myocytes from guinea-pig coronary artery. J Physiol. 1991 Nov;443:703–725. doi: 10.1113/jphysiol.1991.sp018859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk C., Grygorczyk R., Ferrier J. Osteoblastic cells have L-type calcium channels. Bone Miner. 1989 Sep;7(2):137–148. doi: 10.1016/0169-6009(89)90071-8. [DOI] [PubMed] [Google Scholar]
- Gu X., Spitzer N. C. Low-threshold Ca2+ current and its role in spontaneous elevations of intracellular Ca2+ in developing Xenopus neurons. J Neurosci. 1993 Nov;13(11):4936–4948. doi: 10.1523/JNEUROSCI.13-11-04936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggino S. E., Lajeunesse D., Wagner J. A., Snyder S. H. Bone remodeling signaled by a dihydropyridine- and phenylalkylamine-sensitive calcium channel. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2957–2960. doi: 10.1073/pnas.86.8.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasugai S., Todescan R., Jr, Nagata T., Yao K. L., Butler W. T., Sodek J. Expression of bone matrix proteins associated with mineralized tissue formation by adult rat bone marrow cells in vitro: inductive effects of dexamethasone on the osteoblastic phenotype. J Cell Physiol. 1991 Apr;147(1):111–120. doi: 10.1002/jcp.1041470115. [DOI] [PubMed] [Google Scholar]
- Lee K. S. Potentiation of the calcium-channel currents of internally perfused mammalian heart cells by repetitive depolarization. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3941–3945. doi: 10.1073/pnas.84.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatopoulos C., Sodek J., Melcher A. H. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988 Nov;254(2):317–330. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990 Aug 16;346(6285):651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- Publicover S. J., Thomas G. P., el Haj A. J. Induction of a low voltage-activated, fast-inactivating Ca2+ channel in cultured bone marrow stromal cells by dexamethasone. Calcif Tissue Int. 1994 Feb;54(2):125–132. doi: 10.1007/BF00296063. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A., Scheuer T., Catterall W. A. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993 Jul 15;364(6434):240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- Twombly D. A., Yoshii M., Narahashi T. Mechanisms of calcium channel block by phenytoin. J Pharmacol Exp Ther. 1988 Jul;246(1):189–195. [PubMed] [Google Scholar]
- Vadiakas G. P., Banes A. J. Verapamil decreases cyclic load-induced calcium incorporation in ROS 17/2.8 osteosarcoma cell cultures. Matrix. 1992 Dec;12(6):439–447. doi: 10.1016/s0934-8832(11)80088-0. [DOI] [PubMed] [Google Scholar]
- Yukihiro S., Posner G. H., Guggino S. E. Vitamin D3 analogs stimulate calcium currents in rat osteosarcoma cells. J Biol Chem. 1994 Sep 30;269(39):23889–23893. [PubMed] [Google Scholar]


