Abstract
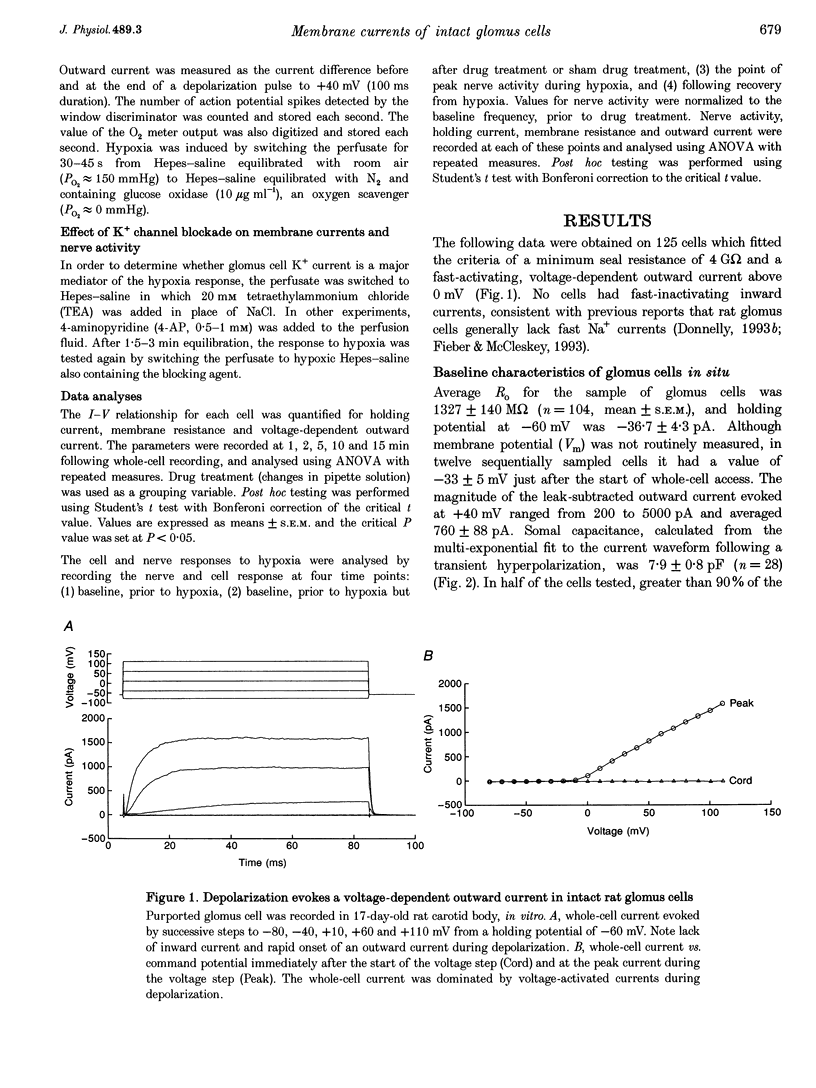
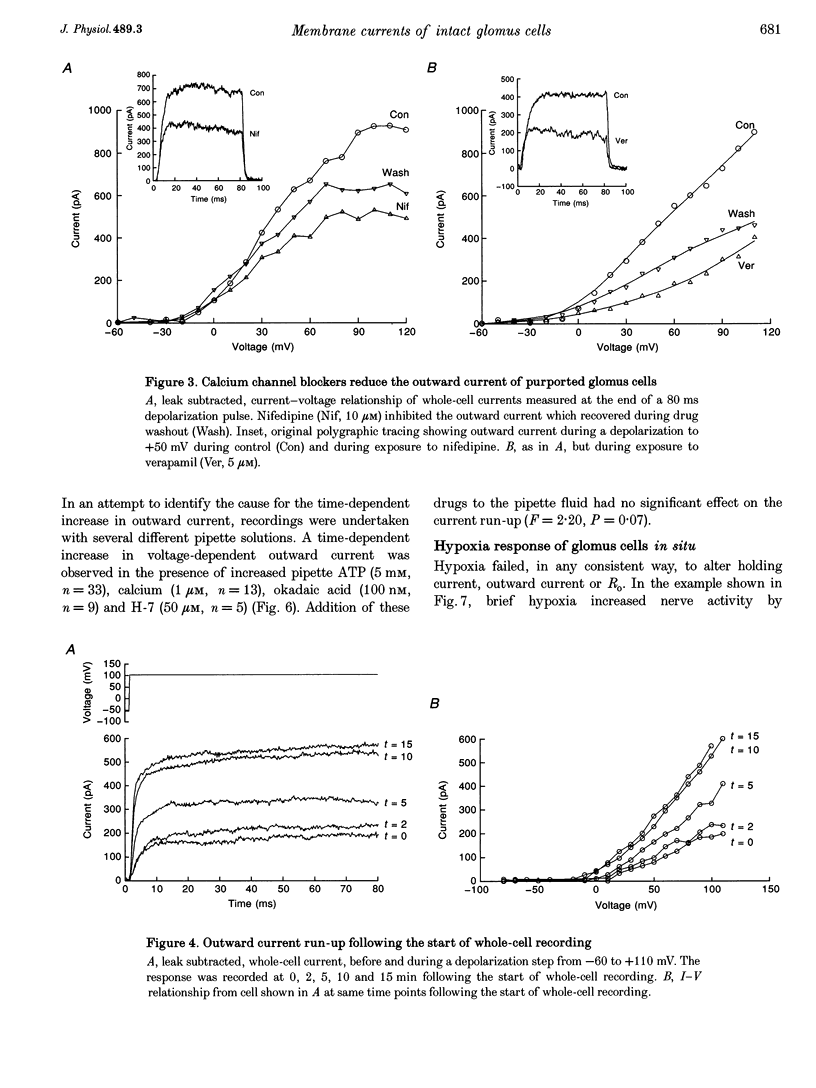
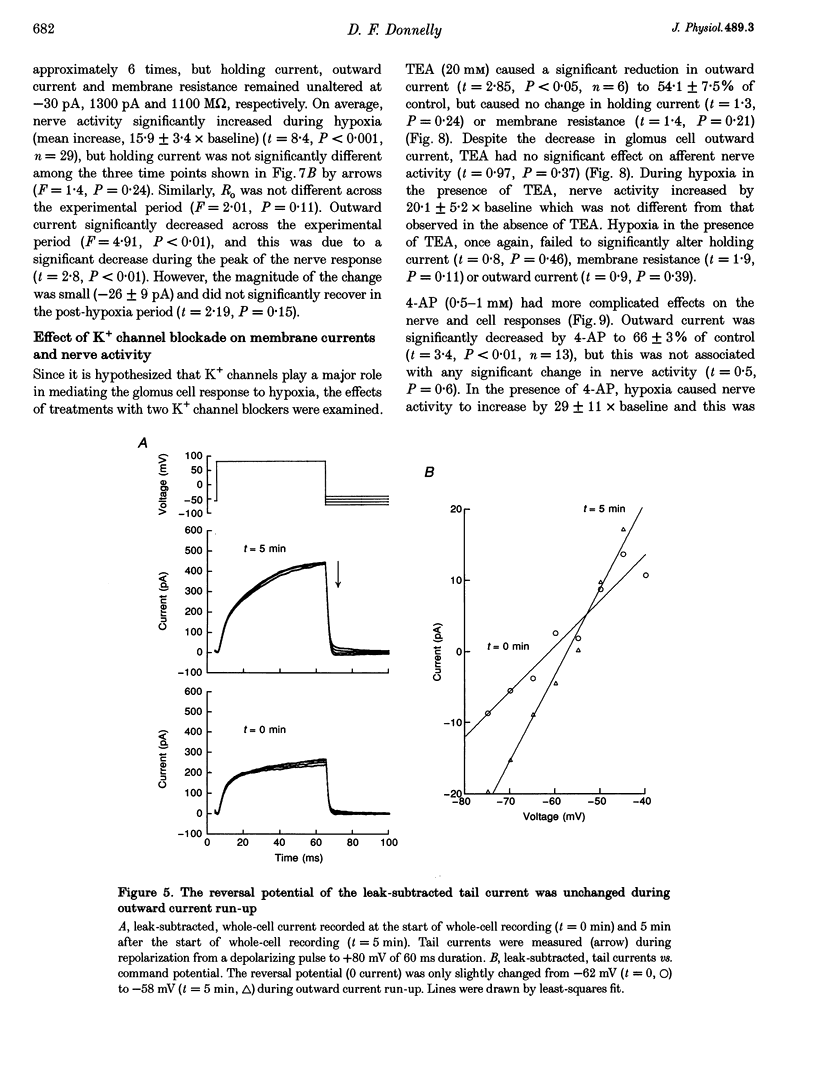
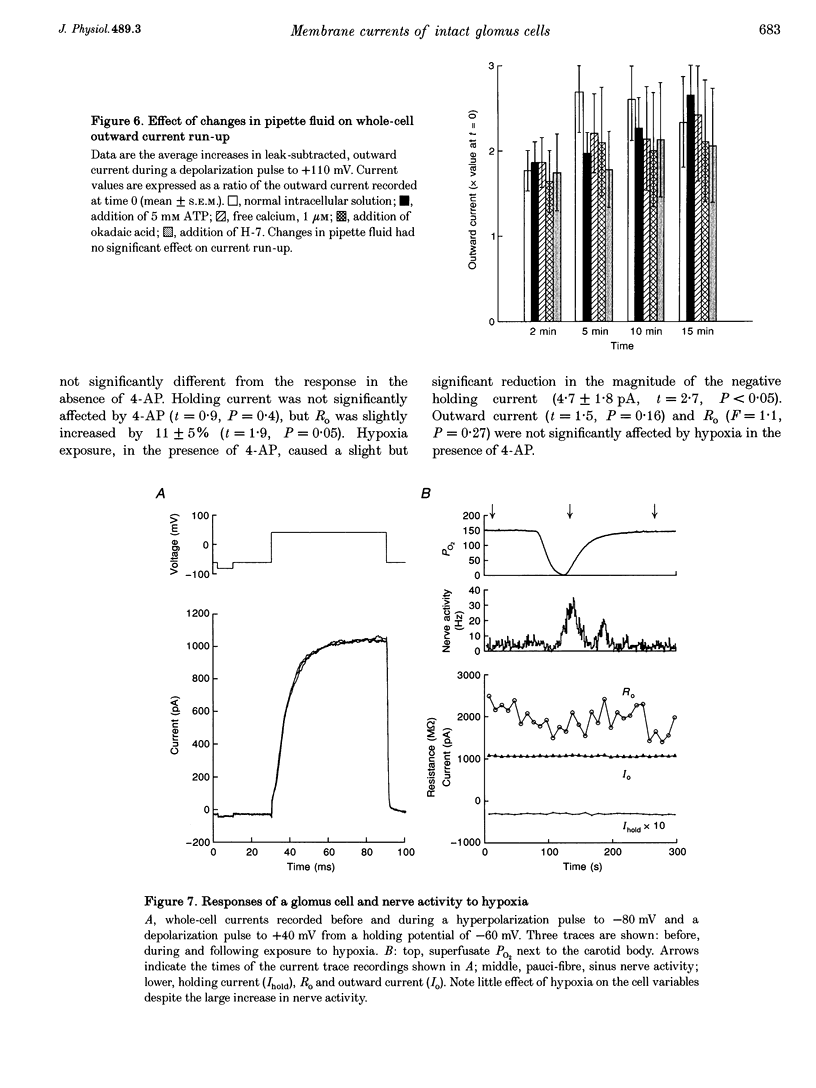
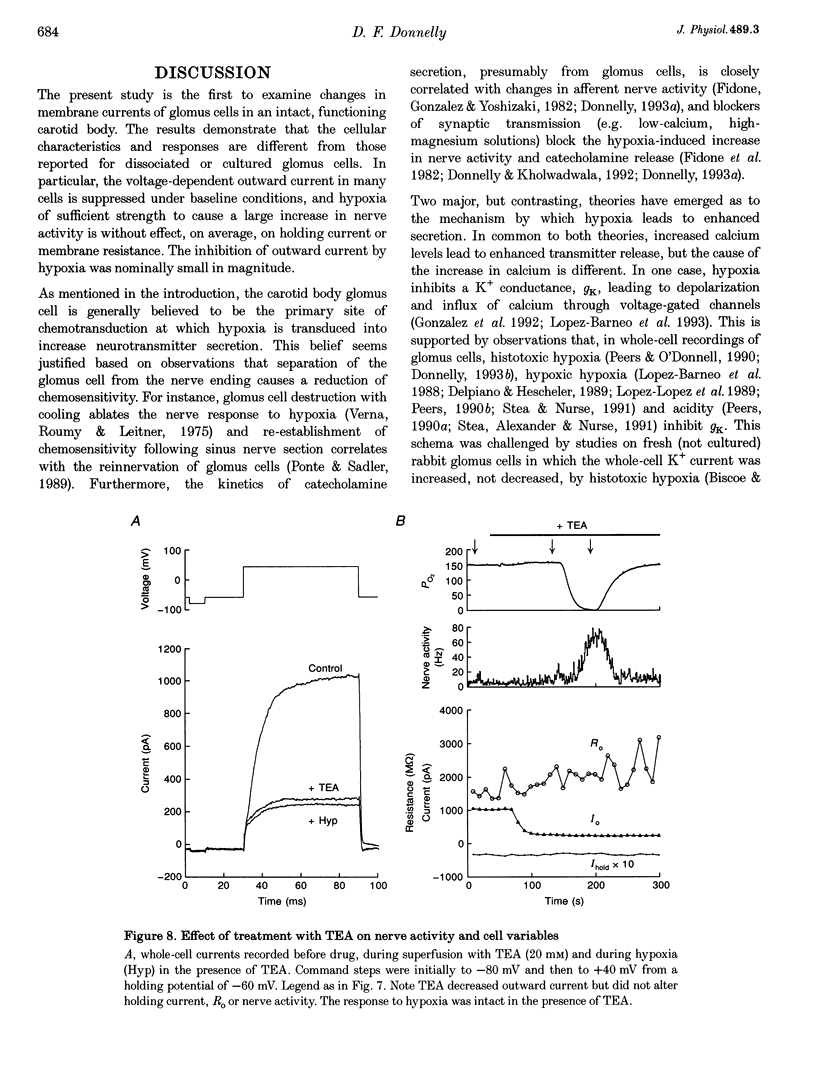
1. In order to understand better the relationship between sinus nerve chemoreceptor activity and changes in glomus cell membrane current, both were measured simultaneously in rat carotid bodies in vitro. Mean membrane resistance of intact glomus cells was 1327 +/- 140 M omega (n = 104, mean +/- S.E.M.) and membrane capacitance was 7.9 +/- 0.8 pF (n = 28). 2. Over the course of 15 min following the start of whole-cell recording, outward current increased by 169 +/- 48% (n = 19), but there was no significant change in holding current or membrane resistance. Reversal potential of the tail current was not changed over this time period. Current run-up was not affected by addition of ATP, Ca2+, okadaic acid or H-7 to the pipette fluid. 3. Brief hypoxia (30-45 s duration, 0 mmHg at nadir) caused a rapid increase in nerve activity, but, on average, no significant change in cell holding current, or resistance. Outward current slightly decreased during hypoxia but failed to recover in the post-hypoxia period. 4. Tetraethylammonium (20 mM), and 4-aminopyridine (1 mM) reduced the outward current to 54 +/- 7 and 66 +/- 3% of control, respectively, but basal nerve activity was unchanged and the nerve response to hypoxia remained intact. 5. These results suggest that hypoxia modulation of glomus cell K+ current is not the primary initiating factor in the nerve response to brief periods of hypoxia in the rat carotid body.
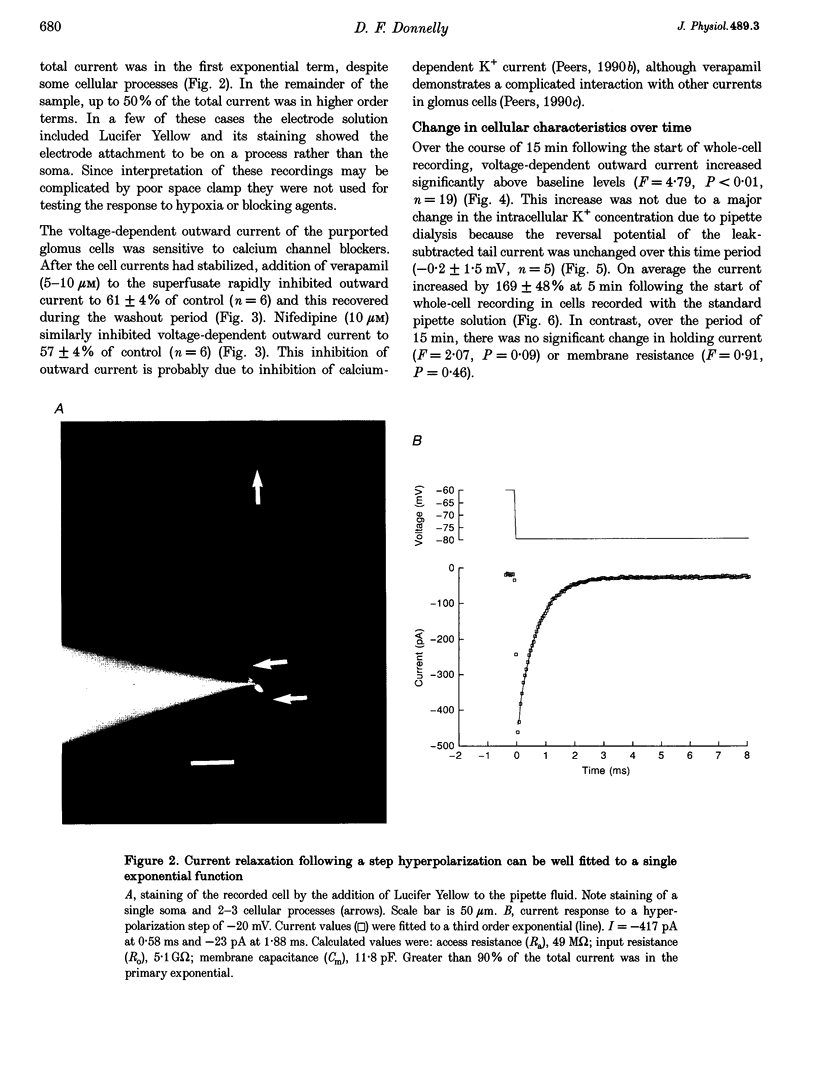
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Cellular basis of transduction in carotid chemoreceptors. Am J Physiol. 1990 Jun;258(6 Pt 1):L271–L278. doi: 10.1152/ajplung.1990.258.6.L271. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Electrophysiological responses of dissociated type I cells of the rabbit carotid body to cyanide. J Physiol. 1989 Jun;413:447–468. doi: 10.1113/jphysiol.1989.sp017663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Responses of type I cells dissociated from the rabbit carotid body to hypoxia. J Physiol. 1990 Sep;428:39–59. doi: 10.1113/jphysiol.1990.sp018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. J Physiol. 1994 Jul 1;478(Pt 1):157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994 May 1;476(3):423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpiano M. A., Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989 Jun 5;249(2):195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Donnelly D. F. Electrochemical detection of catecholamine release from rat carotid body in vitro. J Appl Physiol (1985) 1993 May;74(5):2330–2337. doi: 10.1152/jappl.1993.74.5.2330. [DOI] [PubMed] [Google Scholar]
- Donnelly D. F., Kholwadwala D. Hypoxia decreases intracellular calcium in adult rat carotid body glomus cells. J Neurophysiol. 1992 Jun;67(6):1543–1551. doi: 10.1152/jn.1992.67.6.1543. [DOI] [PubMed] [Google Scholar]
- Donnelly D. F. Response to cyanide of two types of glomoid cells in mature rat carotid body. Brain Res. 1993 Dec 10;630(1-2):157–168. doi: 10.1016/0006-8993(93)90653-5. [DOI] [PubMed] [Google Scholar]
- Doyle T. P., Donnelly D. F. Effect of Na+ and K+ channel blockade on baseline and anoxia-induced catecholamine release from rat carotid body. J Appl Physiol (1985) 1994 Dec;77(6):2606–2611. doi: 10.1152/jappl.1994.77.6.2606. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Caddy K. W., Kirby G. C., Patterson D. L., Ponte J., Biscoe T. J. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience. 1988 Jul;26(1):291–311. doi: 10.1016/0306-4522(88)90146-7. [DOI] [PubMed] [Google Scholar]
- Eden G. J., Hanson M. A. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol. 1987 Nov;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Monti-Bloch L., Baron M., Hayashida Y., Woodbury J. W. Changes in glomus cell membrane properties in response to stimulants and depressants of carotid nerve discharge. Brain Res. 1989 Jan 16;477(1-2):265–279. doi: 10.1016/0006-8993(89)91414-5. [DOI] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber L. A., McCleskey E. W. L-type calcium channels in type I cells of the rat carotid body. J Neurophysiol. 1993 Oct;70(4):1378–1384. doi: 10.1152/jn.1993.70.4.1378. [DOI] [PubMed] [Google Scholar]
- González C., Almaraz L., Obeso A., Rigual R. Oxygen and acid chemoreception in the carotid body chemoreceptors. Trends Neurosci. 1992 Apr;15(4):146–153. doi: 10.1016/0166-2236(92)90357-e. [DOI] [PubMed] [Google Scholar]
- He S. F., Wei J. Y., Eyzaguirre C. Intracellular pH and some membrane characteristics of cultured carotid body glomus cells. Brain Res. 1991 May 3;547(2):258–266. doi: 10.1016/0006-8993(91)90969-3. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Cable analysis with the whole-cell patch clamp. Theory and experiment. Biophys J. 1992 Mar;61(3):756–766. doi: 10.1016/S0006-3495(92)81880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholwadwala D., Donnelly D. F. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J Physiol. 1992;453:461–473. doi: 10.1113/jphysiol.1992.sp019239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., López-López J. R., Ureña J., González C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988 Jul 29;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov S. L., Lux H. D. Calmodulin antagonists and protein phosphatase inhibitor okadaic acid fasten the 'run-up' of high-voltage activated calcium current in rat hippocampal neurones. Neurosci Lett. 1991 Dec 9;133(2):175–178. doi: 10.1016/0304-3940(91)90563-9. [DOI] [PubMed] [Google Scholar]
- Monti-Bloch L., Abudara V., Eyzaguirre C. Electrical communication between glomus cells of the rat carotid body. Brain Res. 1993 Sep 17;622(1-2):119–131. doi: 10.1016/0006-8993(93)90810-a. [DOI] [PubMed] [Google Scholar]
- Pang L., Eyzaguirre C. Different effects of hypoxia on the membrane potential and input resistance of isolated and clustered carotid body glomus cells. Brain Res. 1992 Mar 13;575(1):167–173. doi: 10.1016/0006-8993(92)90440-k. [DOI] [PubMed] [Google Scholar]
- Peers C. Effect of lowered extracellular pH on Ca2(+)-dependent K+ currents in type I cells from the neonatal rat carotid body. J Physiol. 1990 Mar;422:381–395. doi: 10.1113/jphysiol.1990.sp017990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Effects of D600 on hypoxic suppression of K+ currents in isolated type I carotid body cells of the neonatal rat. FEBS Lett. 1990 Oct 1;271(1-2):37–40. doi: 10.1016/0014-5793(90)80366-q. [DOI] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci Lett. 1990 Nov 13;119(2):253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Peers C., O'Donnell J. Potassium currents recorded in type I carotid body cells from the neonatal rat and their modulation by chemoexcitatory agents. Brain Res. 1990 Jul 9;522(2):259–266. doi: 10.1016/0006-8993(90)91470-2. [DOI] [PubMed] [Google Scholar]
- Ponte J., Sadler C. L. Studies on the regenerated carotid sinus nerve of the rabbit. J Physiol. 1989 Mar;410:411–424. doi: 10.1113/jphysiol.1989.sp017541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata M., Fitzgerald R. S. The presence of CO2/HCO3- is essential for hypoxic chemotransduction in the in vivo perfused carotid body. Brain Res. 1991 Apr 5;545(1-2):297–300. doi: 10.1016/0006-8993(91)91301-g. [DOI] [PubMed] [Google Scholar]
- Stea A., Alexander S. A., Nurse C. A. Effects of pHi and pHe on membrane currents recorded with the perforated-patch method from cultured chemoreceptors of the rat carotid body. Brain Res. 1991 Dec 13;567(1):83–90. doi: 10.1016/0006-8993(91)91439-8. [DOI] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflugers Arch. 1991 Mar;418(1-2):93–101. doi: 10.1007/BF00370457. [DOI] [PubMed] [Google Scholar]
- Ureña J., López-López J., González C., López-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna A., Roumy M., Leitner L. M. Loss of chemoreceptive properties of the rabbit carotid body after destruction of the glomus cells. Brain Res. 1975 Dec 12;100(1):13–23. doi: 10.1016/0006-8993(75)90239-5. [DOI] [PubMed] [Google Scholar]