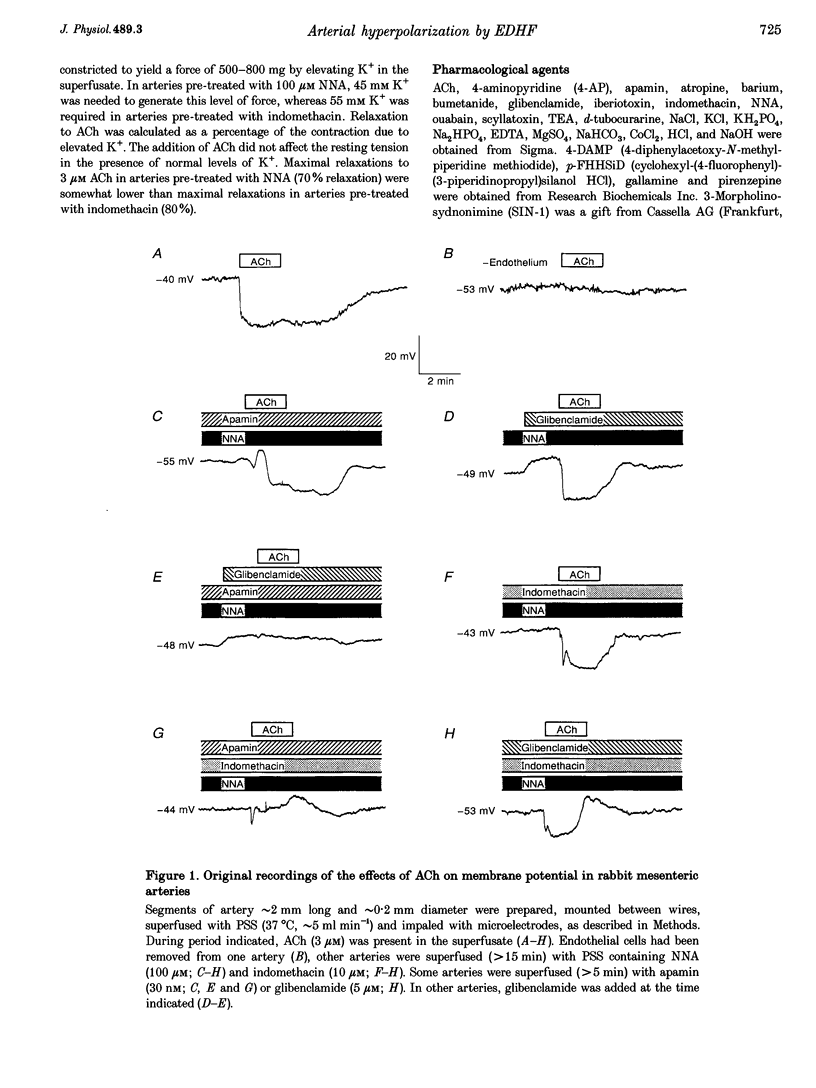
Abstract
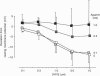
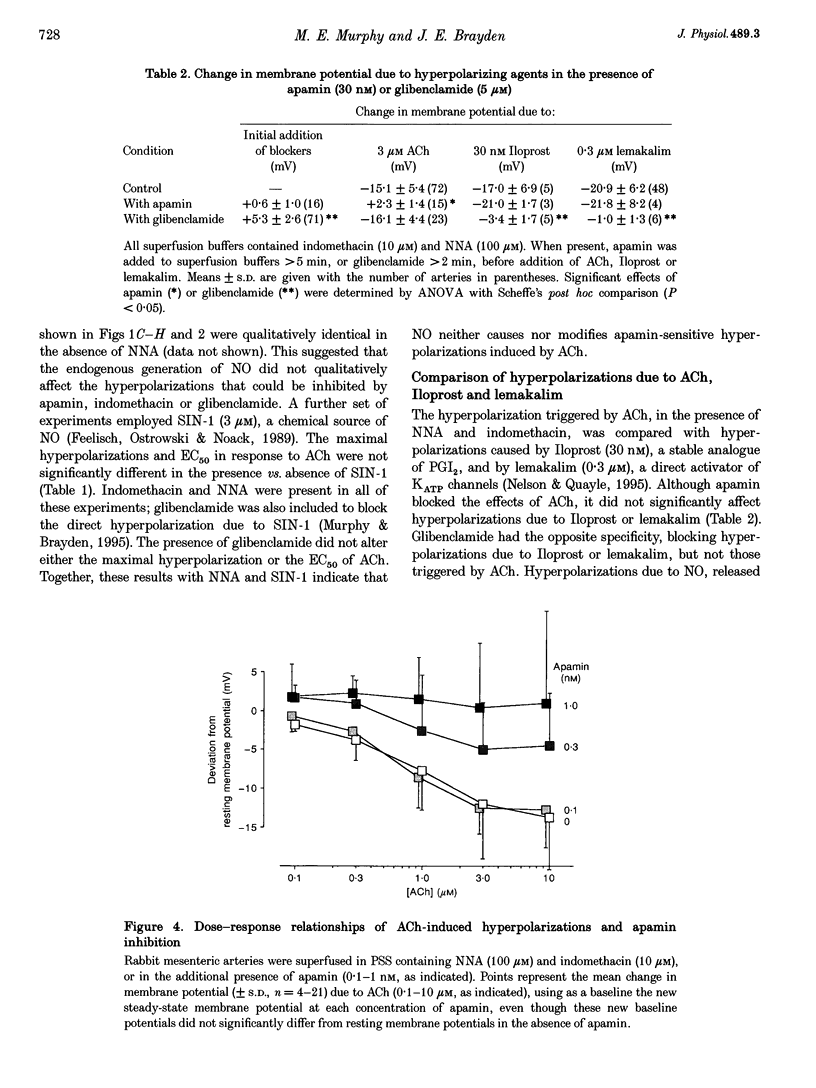
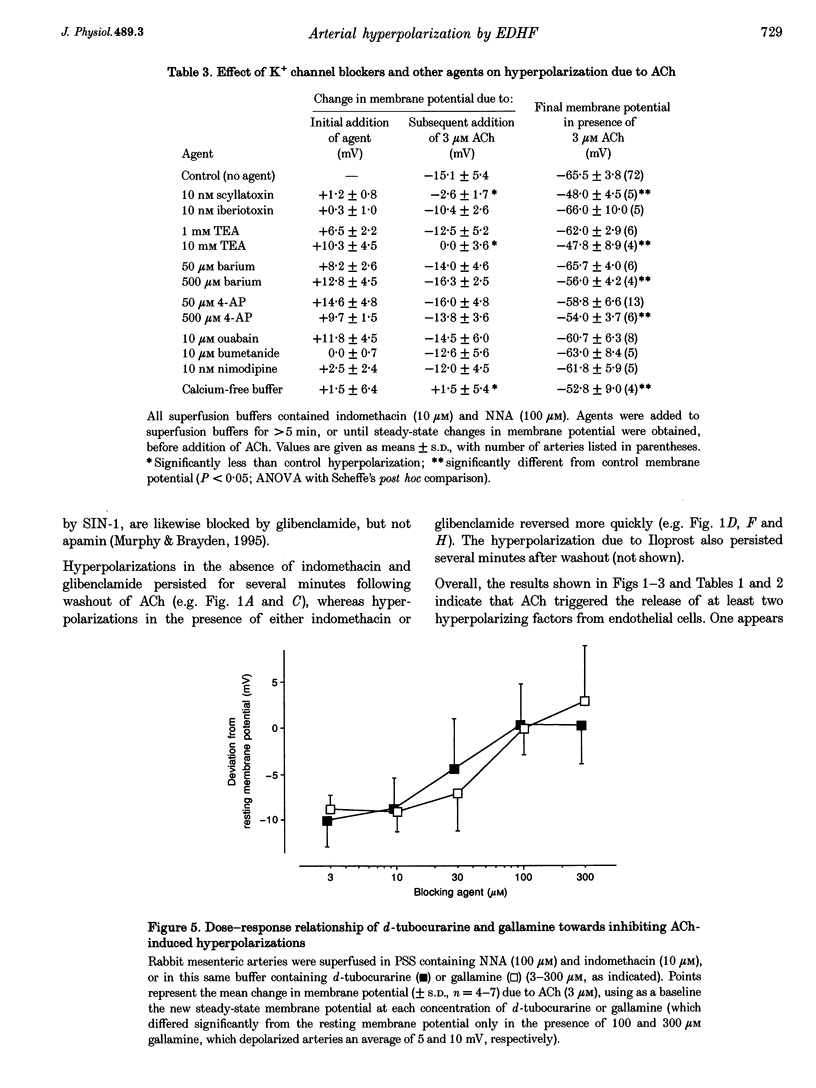
1. Vascular endothelial cells release a variety of substances which affect the membrane potential and tone of underlying vascular smooth muscle. In the presence of N omega-nitro-L-arginine to inhibit nitric oxide synthase and indomethacin to inhibit cyclo-oxygenase, acetylcholine (ACh; EC50 approximately 1 microM) elicited the release of an endothelium-derived hyperpolarizing factor (EDHF) in rabbit mesenteric arteries. 2. The hyperpolarization due to EDHF was blocked by apamin (IC50 approximately 0.3 nM), and by other inhibitors of the apamin-sensitive K+ channel (10 nM scyllatoxin, 100 microM d-tubocurarine, 300 microM gallamine) in the presence of indomethacin and N omega-nitro-L-arginine. The hyperpolarization was not blocked by glibenclamide (5 microM), iberiotoxin (10 nM), tetraethylammonium (1 mM), barium (500 microM), 4-aminopyridine (500 microM), ouabain (10 microM), bumetanide (10 microM), or nimodipine (100 nM). 3. In the presence of apamin and N omega-nitro-L-arginine, but the absence of indomethacin, ACh triggered a hyperpolarization that was blocked by glibenclamide, an inhibitor of ATP-sensitive K+ (KATP) channels. A similar glibenclamide-sensitive hyperpolarization was caused by Iloprost, a stable analogue of prostacyclin. 4. In experiments which distinguished the effects of EDHF, prostanoids and nitric oxide, hyperpolarizations and/or relaxations triggered by ACh were antagonized by muscarinic antagonists, the relative potencies (atropine approximately 4-DAMP > pirenzepine) of which indicated that the release of all three endothelium-derived factors was mediated by M3 receptors. 5. Our results suggest that ACh stimulates M3 receptors on endothelial cells, triggering the release of nitric oxide and prostanoids, which hyperpolarize underlying smooth muscle by activation of KATP channels, and the release of an EDHF, which hyperpolarizes smooth muscle through the activation of apamin-sensitive K+ (KAS) channels.
Full text
PDF

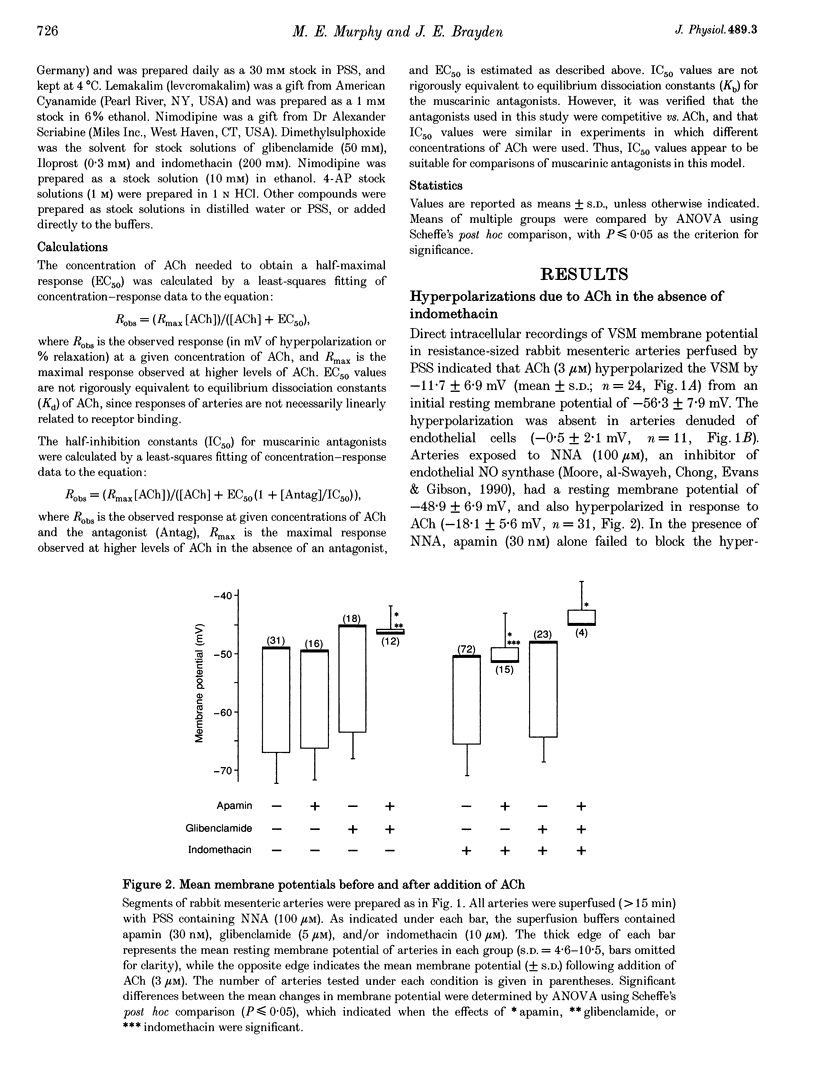
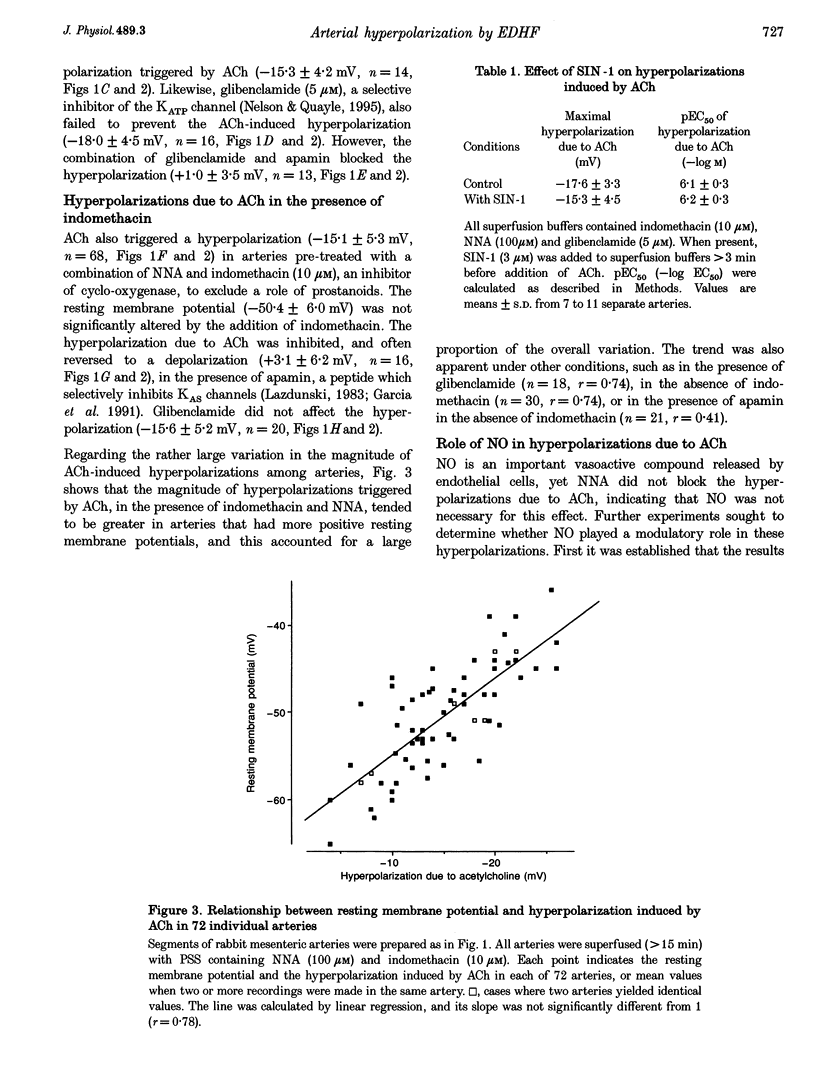




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeagbo A. S., Malik K. U. Contribution of K+ channels to arachidonic acid-induced endothelium-dependent vasodilation in rat isolated perfused mesenteric arteries. J Pharmacol Exp Ther. 1991 Aug;258(2):452–458. [PubMed] [Google Scholar]
- Adeagbo A. S., Triggle C. R. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J Cardiovasc Pharmacol. 1993 Mar;21(3):423–429. [PubMed] [Google Scholar]
- Altiere R. J., Travis D. C., Roberts J., Thompson D. C. Pharmacological characterization of muscarinic receptors mediating acetylcholine-induced contraction and relaxation in rabbit intrapulmonary arteries. J Pharmacol Exp Ther. 1994 Jul;270(1):269–276. [PubMed] [Google Scholar]
- Berweck S., Thieme H., Lepple-Wienhues A., Helbig H., Wiederholt M. Insulin-induced hyperpolarization in retinal capillary pericytes. Invest Ophthalmol Vis Sci. 1993 Nov;34(12):3402–3407. [PubMed] [Google Scholar]
- Brayden J. E. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. Am J Physiol. 1990 Sep;259(3 Pt 2):H668–H673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- Chen G. F., Cheung D. W. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ Res. 1992 Feb;70(2):257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- Chen G., Yamamoto Y., Miwa K., Suzuki H. Hyperpolarization of arterial smooth muscle induced by endothelial humoral substances. Am J Physiol. 1991 Jun;260(6 Pt 2):H1888–H1892. doi: 10.1152/ajpheart.1991.260.6.H1888. [DOI] [PubMed] [Google Scholar]
- Cook N. S., Haylett D. G. Effects of apamin, quinine and neuromuscular blockers on calcium-activated potassium channels in guinea-pig hepatocytes. J Physiol. 1985 Jan;358:373–394. doi: 10.1113/jphysiol.1985.sp015556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C. L., Palacino J. J., Najibi S., Cohen R. A. Potassium channel-mediated relaxation to acetylcholine in rabbit arteries. J Pharmacol Exp Ther. 1993 Sep;266(3):1482–1489. [PubMed] [Google Scholar]
- Eckman D. M., Frankovich J. D., Keef K. D. Comparison of the actions of acetylcholine and BRL 38227 in the guinea-pig coronary artery. Br J Pharmacol. 1992 May;106(1):9–16. doi: 10.1111/j.1476-5381.1992.tb14285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. L., Galvez A., Garcia-Calvo M., King V. F., Vazquez J., Kaczorowski G. J. Use of toxins to study potassium channels. J Bioenerg Biomembr. 1991 Aug;23(4):615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- Groschner K., Graier W. F., Kukovetz W. R. Activation of a small-conductance Ca(2+)-dependent K+ channel contributes to bradykinin-induced stimulation of nitric oxide synthesis in pig aortic endothelial cells. Biochim Biophys Acta. 1992 Oct 27;1137(2):162–170. doi: 10.1016/0167-4889(92)90198-k. [DOI] [PubMed] [Google Scholar]
- Holzmann S., Kukovetz W. R., Schmidt K. Mode of action of coronary arterial relaxation by prostacyclin. J Cyclic Nucleotide Res. 1980;6(6):451–460. [PubMed] [Google Scholar]
- Hutcheson I. R., Griffith T. M. Heterogeneous populations of K+ channels mediate EDRF release to flow but not agonists in rabbit aorta. Am J Physiol. 1994 Feb;266(2 Pt 2):H590–H596. doi: 10.1152/ajpheart.1994.266.2.H590. [DOI] [PubMed] [Google Scholar]
- Jackson W. F., König A., Dambacher T., Busse R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol. 1993 Jan;264(1 Pt 2):H238–H243. doi: 10.1152/ajpheart.1993.264.1.H238. [DOI] [PubMed] [Google Scholar]
- Jaiswal N., Lambrecht G., Mutschler E., Tacke R., Malik K. U. Pharmacological characterization of the vascular muscarinic receptors mediating relaxation and contraction in rabbit aorta. J Pharmacol Exp Ther. 1991 Sep;258(3):842–850. [PubMed] [Google Scholar]
- Jodal M., Lundgren O., Sjöqvist A. The effect of apamin on non-adrenergic, non-cholinergic vasodilator mechanisms in the intestines of the cat. J Physiol. 1983 May;338:207–219. doi: 10.1113/jphysiol.1983.sp014669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Heterogeneous distribution of muscarinic receptors in the rabbit saphenous artery. Br J Pharmacol. 1987 Nov;92(3):657–664. doi: 10.1111/j.1476-5381.1987.tb11369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P. D., Nelson M. T., Huang Y., Standen N. B. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol. 1991 Mar;260(3 Pt 2):H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- Lazdunski M. Apamin, a neurotoxin specific for one class of Ca2+-dependent K+ channels. Cell Calcium. 1983 Dec;4(5-6):421–428. doi: 10.1016/0143-4160(83)90018-0. [DOI] [PubMed] [Google Scholar]
- McPherson G. A., Angus J. A. Evidence that acetylcholine-mediated hyperpolarization of the rat small mesenteric artery does not involve the K+ channel opened by cromakalim. Br J Pharmacol. 1991 May;103(1):1184–1190. doi: 10.1111/j.1476-5381.1991.tb12321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. E., Brayden J. E. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol. 1995 Jul 1;486(Pt 1):47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Quayle J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995 Apr;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Parkington H. C., Tare M., Tonta M. A., Coleman H. A. Stretch revealed three components in the hyperpolarization of guinea-pig coronary artery in response to acetylcholine. J Physiol. 1993 Jun;465:459–476. doi: 10.1113/jphysiol.1993.sp019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane F., Garland C. J. Differential effects of acetylcholine, nitric oxide and levcromakalim on smooth muscle membrane potential and tone in the rabbit basilar artery. Br J Pharmacol. 1993 Oct;110(2):651–656. doi: 10.1111/j.1476-5381.1993.tb13861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle J. M., Bonev A. D., Brayden J. E., Nelson M. T. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994 Feb 15;475(1):9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle J. M., McCarron J. G., Brayden J. E., Nelson M. T. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol. 1993 Nov;265(5 Pt 1):C1363–C1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- Sokol P. T., Hu W., Yi L., Toral J., Chandra M., Ziai M. R. Cloning of an apamin binding protein of vascular smooth muscle. J Protein Chem. 1994 Jan;13(1):117–128. doi: 10.1007/BF01891999. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Lazdunski M. A small-conductance charybdotoxin-sensitive, apamin-resistant Ca(2+)-activated K+ channel in aortic smooth muscle cells (A7r5 line and primary culture). Pflugers Arch. 1992 Apr;420(5-6):417–423. doi: 10.1007/BF00374614. [DOI] [PubMed] [Google Scholar]
- Wadsworth J. D., Doorty K. B., Strong P. N. Comparable 30-kDa apamin binding polypeptides may fulfill equivalent roles within putative subtypes of small conductance Ca(2+)-activated K+ channels. J Biol Chem. 1994 Jul 8;269(27):18053–18061. [PubMed] [Google Scholar]