Abstract
We investigated the role of oxygen-sensing atypical guanylyl cyclase subunit Gyc89Db in the developing brain. Despite its expression in the hypoxic neuroepithelium of the larval optic lobe of Drosophila , loss-of-function mutants and ectopic expression did not alter neuroepithelial cell number or proliferation. Notably, while ectopic expression of Gyc89Db increases optic lobe volume and neuroblast numbers, our negative results suggest that these effects manifest earlier in development without persistent alteration of the neuroepithelium, through mechanisms that might be independent of neuroepithelial proliferation.
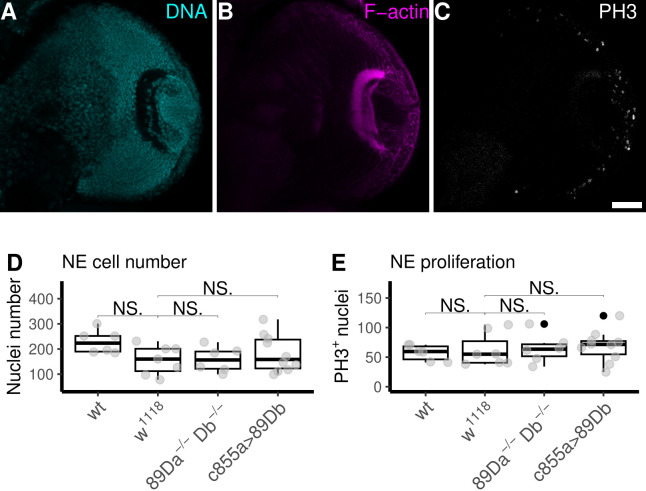
Figure 1. Assessment of Neuroepithelial Cell Number and Proliferation in Larval Brain.
(A-C) show the three fluorescent markers at a single confocal optical section across a brain hemisphere of a wild-type (wt) L3 larva. (A) DNA staining (cyan) with methyl green shows all nuclei. (B) F-actin staining with phalloidin (magenta) used for the anatomical segmentation of the neuroepithelium. (C) Immunostaining against phosphorylated histone H3 (gray) shows mitotic nuclei for determination of the number of proliferating neuroepithelial cells. (D) The numbers of neuroepithelial cells found in double mutant larvae ( 89Da -/- Db -/- ; median±SD: 156.50 ± 49.87) or in larvae ectopically expressing Gyc89Db in the neuroepithelium ( c855a>89Db ; median±SD: 158.50 ± 73.83) did not differ from the numbers found in the two control genotypes ( wt ; median±SD: 223.5 ± 46.94 and w 1118 ; median±SD: 160.00 ± 57.87). (E) Proliferating neuroepithelial cells were counted as cell nuclei stained positively for anti-PH3 (MG + /PH3 + ) within the segmented neuroepithelial volume. The numbers of proliferating cells in larvae carrying the double mutation Gyc89Da -/- Db -/- ( 89Da -/- Db -/- ) (median±SD: 63.50 ± 24.58) were not different from the numbers found in larvae expressing Gyc89Db ectopically in the neuroepithelium ( c855a> Gyc89Db ) (median±SD: 71.50 ± 26.50) or in larvae from any of the two control strains wt (median±SD: 59.50 ± 13.31) and w 1118 (median±SD: 55.00 ± 28.33) and. N=6; Mann-Whitney U-test. NE:=Neuroepithelium. Scale bar: 30 μm.
Description
The neuroepithelium (NE) of the optic lobe (OL) is the major stem cell niche in the larval brain of Drosophila melanogaster (Hofbauer and Campos-Ortega 1990). It comprises hundreds of cells arising through symmetric proliferative divisions, progressively differentiating into self-renewing neuroblasts. These neuroblasts proliferate through asymmetric cell divisions, generating ganglion mother cells, which, in turn, give rise to glia and neurons (Egger et al. 2007; Yasugi et al. 2008) . The NE is hypoxic relative to the rest of the brain (Baccino-Calace et al. 2020) and since we were not able to observe some of the signs of a typical hypoxia response (Misra et al. 2017; Baccino-Calace et al. 2020) we wondered whether NE cells were able to sense hypoxia through some alternative way.
Three oxygen-sensing atypical guanylyl cyclases (asGCs) activated by hypoxia have been described in Drosophila , namely Gyc88E, Gyc89Da, and Gyc89Db (Vermehren-Schmaedick et al. 2010; Morton 2011) . These asGCs, despite being mostly inactive individually, show enhanced activity when co-expressed, suggesting functional relevance as heterodimers, particularly in the NE (Morton et al. 2005; Morton and Vermehren 2007) .
Additionally, it is accepted that the Drosophila asGCs exist mostly as heterodimers with some functional redundancy of the heterodimers compared to the homodimers, but the fact that in vivo Gyc89Da and Gyc89Db are frequently co-expressed with Gyc88E (Morton et al. 2005) support the idea that heterodimers might be the functionally relevant conformation.
Our previous findings demonstrated that ectopic expression of Gyc89Db in the NE increased the number of neuroblasts and OL volume (Prieto et al. 2024) . Different asGC subunits exhibit unique kinetic properties, and their combinations into heterodimers lead to distinct functions (Morton et al. 2005; Morton et al. 2008) . A plausible explanation for our observations is that by inducing the expression of only one subunit, we may be shifting the natural equilibrium of dimers toward one function over the others; however, this remains to be experimentally demonstrated. To investigate whether the effect of Gyc89Db on OL volume was due to altered cell proliferation, we examined the consequences of asGC subunit loss and targeted gain-of-function on NE cell number and proliferation.
Our staining techniques included nuclear staining with methyl green to quantify total cell numbers ( Fig. 1A ), F-actin staining for anatomical segmentation of the NE ( Fig. 1B ), and anti-PH3 staining to quantify mitotic cells ( Fig. 1C ). Despite rigorous examination using confocal microscopy of brains displaying the three markers with outstanding quality (Fig.1 A-C), we found no statistically significant differences in cell number or proliferation when control samples were compared with either the contextual double loss-of-function in Gyc89Da and Gyc89Db ( Gyc89Da -/- Db -/- ) or the ectopic expression of Gyc89Db in neuroepithelial cells under the GAL4 c855a driver ( Fig. 1E ) compared to wt or w 1118 .
These negative results suggest that the previously observed increase in neuroblast numbers and OL volume caused by Gyc89Db overexpression (Prieto et al. 2024) may occur without significant alterations in the third-instar NE. Should accelerated NE proliferation have occurred, it likely took place earlier during larval development, possibly alongside compensating waves of neuroblast differentiation that prevented NE from overgrowth.
Methods
Fly strains
Flies were raised on cornmeal medium at 25°C with 12:12 h light:dark cycles, as previously described (Ferreiro et al., 2018) . The wild-type strain ( wt ) Vallecas (Izquierdo 1994) and w 1118 (BDSC #5905, Bloomington Drosophila Stock Centre, Bloomington, Indiana, USA) were used as control strains. Expression of UAS- Gyc89Db (Vermehren-Schmaedick et al. 2010) in the OL was obtained with the driver GAL4 c855a (Egger et al., 2007; BDSC stock #6990 Bloomington Drosophila Stock Centre, Bloomington, Indiana, USA). The UAS- Gyc89Db as well as the double mutant Gyc89Da -/- Db -/- (Vermehren-Schmaedick et al. 2010) (BDSC #93108) were kind gifts from Prof. David Morton (Oregon Health & Science University, Oregon, USA).
Immunostaining and image acquisition
Brains were dissected from wandering third-instar larvae (L3), fixed, and immunostained as previously described (Baccino-Calace et al. 2020) . Primary antibodies to phosphorylated H3 histone (anti-PH3, 1:200, Cell Signaling Technologies #9713) were used as a mitotic marker to assess cell proliferation. Methyl green (Sigma-Aldrich) was used to stain cell nuclei, and rhodamine-conjugated phalloidin (Sigma-Aldrich) was used to stain F-actin as previously described (Prieto et al. 2017) . Fluorescent conjugated secondary antibodies Alexa488, Cy3 and Cy5 from Thermo Fisher were used. Images were acquired with a Zeiss LSM800 Airyscan confocal microscope and processed with FIJI (Schindelin et al. 2012) . Semi-automated OL segmentation was performed with TrakEM2 (Cardona et al. 2012) . Analyses and illustrations were made using R version 4.4.0 on RStudio version 2023.06.01.
Reagents
Strains: Stocks obtained from the BDSC or kindly provided by colleagues as stated above.
Gyc89Da -/- Db -/- (w[*]; PBac{w[+mC]=RB}Gyc89Da[e01821] Mi{GFP[E.3xP3]=ET1}Gyc89Db[MB03197])
GAL4 c855a ( w[1118]; P{w[+mW.hs]=GawB}C855a )
UAS-gyc89Db ( w[*]; P{w[+mC]=UAS- Gyc89Db .V}2 )
Acknowledgments
Acknowledgments
The authors would like to thank Dr. Boris Egger for reading the manuscript critically and fruitful discussions, Dr. Maria Jose Ferreiro for her continued valuable help with fly care and advice, and the Developmental Studies Hybridoma Bank for antibodies. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Funding Statement
DP was a post-doctoral researcher at the Instituto de Investigaciones Biológicas Clemente Estable, Ministerio de Educación y Cultura, Uruguay. Grants Fondo Vaz Ferreira awarded to DP and Fondo Clemente Estable, awarded to RC and support from Agencia Nacional de Investigación e Innovación to DP and RC are acknowledged.
References
- Baccino-Calace Martin, Prieto Daniel, Cantera Rafael, Egger Boris. Compartment and cell-type specific hypoxia responses in the developing Drosophila brain . Biology Open. 2020 Aug 15;9(8) doi: 10.1242/bio.053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona Albert, Saalfeld Stephan, Schindelin Johannes, Arganda-Carreras Ignacio, Preibisch Stephan, Longair Mark, Tomancak Pavel, Hartenstein Volker, Douglas Rodney J. TrakEM2 Software for Neural Circuit Reconstruction. PLoS ONE. 2012 Jun 19;7(6):e38011–e38011. doi: 10.1371/journal.pone.0038011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger Boris, Boone Jason Q, Stevens Naomi R, Brand Andrea H, Doe Chris Q. Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. Neural Development. 2007 Jan 5;2(1) doi: 10.1186/1749-8104-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreiro María José, Pérez Coralia, Marchesano Mariana, Ruiz Santiago, Caputi Angel, Aguilera Pedro, Barrio Rosa, Cantera Rafael. Drosophila melanogaster White Mutant w1118 Undergo Retinal Degeneration. Frontiers in Neuroscience. 2018 Jan 4;11 doi: 10.3389/fnins.2017.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo Marta. Ubiquitin genes and ubiquitin protein location in polytene chromosomes of Drosophila. Chromosoma. 1994 Jun 1;103(3):193–197. doi: 10.1007/bf00368012. [DOI] [PubMed] [Google Scholar]
- Morton David B., Langlais Kristofor K., Stewart Judith A., Vermehren Anke. Comparison of the properties of the five soluble guanylyl cyclase subunits in Drosophila melanogaster. Journal of Insect Science. 2005 Apr 1;5(12):1–10. doi: 10.1673/031.005.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton David B., Vermehren Anke. Soluble guanylyl cyclases in invertebrates: Targets for NO and O2. Advances in Experimental Biology. 2007:65–82. doi: 10.1016/s1872-2423(07)01003-4. [DOI] [PMC free article] [PubMed]
- Morton David B., Stewart Judith A., Langlais Kristofor K., Clemens-Grisham Rachel A., Vermehren Anke. Synaptic transmission in neurons that express the Drosophila atypical soluble guanylyl cyclases, Gyc-89Da and Gyc-89Db, is necessary for the successful completion of larval and adult ecdysis . Journal of Experimental Biology. 2008 May 15;211(10):1645–1656. doi: 10.1242/jeb.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton David B. Behavioral responses to hypoxia and hyperoxia in Drosophila larvae. Fly. 2011 Apr 1;5(2):119–125. doi: 10.4161/fly.5.2.14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto Daniel, Aparicio Gonzalo, Sotelo‐Silveira Jose R. Cell migration analysis: A low‐cost laboratory experiment for cell and developmental biology courses using keratocytes from fish scales. Biochemistry and Molecular Biology Education. 2017 Jun 19;45(6):475–482. doi: 10.1002/bmb.21071. [DOI] [PubMed] [Google Scholar]
- Prieto D, Egger B, Cantera R. Atypical soluble guanylyl cyclases control brain size in Drosophila. MicroPubl Biol. 2024 Aug 11;2024 doi: 10.17912/micropub.biology.001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin Johannes, Arganda-Carreras Ignacio, Frise Erwin, Kaynig Verena, Longair Mark, Pietzsch Tobias, Preibisch Stephan, Rueden Curtis, Saalfeld Stephan, Schmid Benjamin, Tinevez Jean-Yves, White Daniel James, Hartenstein Volker, Eliceiri Kevin, Tomancak Pavel, Cardona Albert. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012 Jun 28;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermehren-Schmaedick Anke, Ainsley Joshua A, Johnson Wayne A, Davies Shireen-A, Morton David B. Behavioral Responses to Hypoxia in Drosophila Larvae Are Mediated by Atypical Soluble Guanylyl Cyclases. Genetics. 2010 Sep 1;186(1):183–196. doi: 10.1534/genetics.110.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasugi Tetsuo, Umetsu Daiki, Murakami Satoshi, Sato Makoto, Tabata Tetsuya. Drosophila optic lobe neuroblasts triggered by a wave of proneural gene expression that is negatively regulated by JAK/STAT . Development. 2008 Apr 15;135(8):1471–1480. doi: 10.1242/dev.019117. [DOI] [PubMed] [Google Scholar]