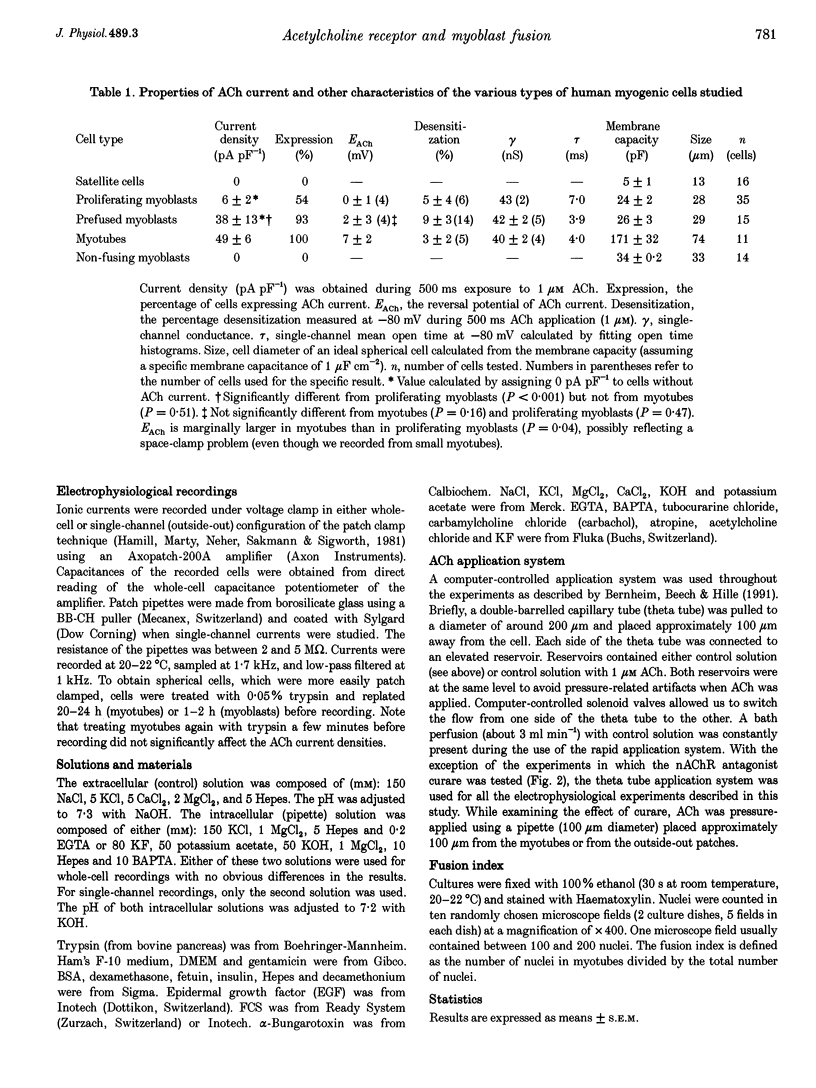
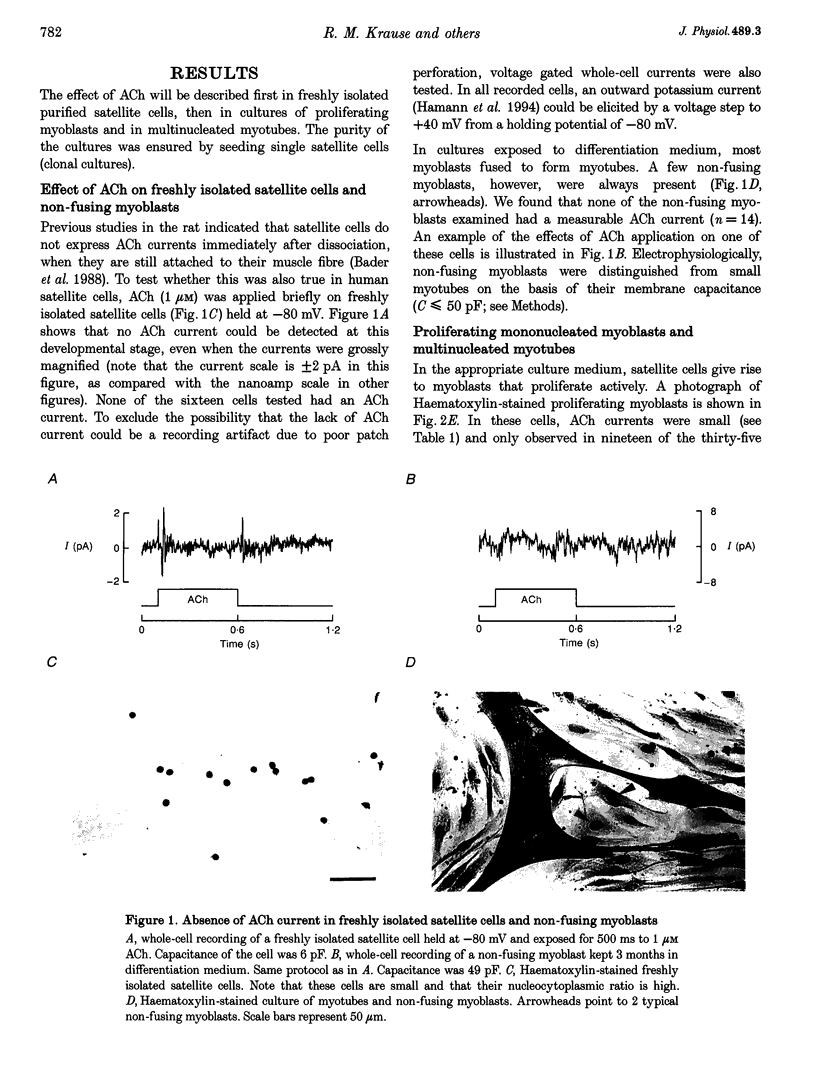
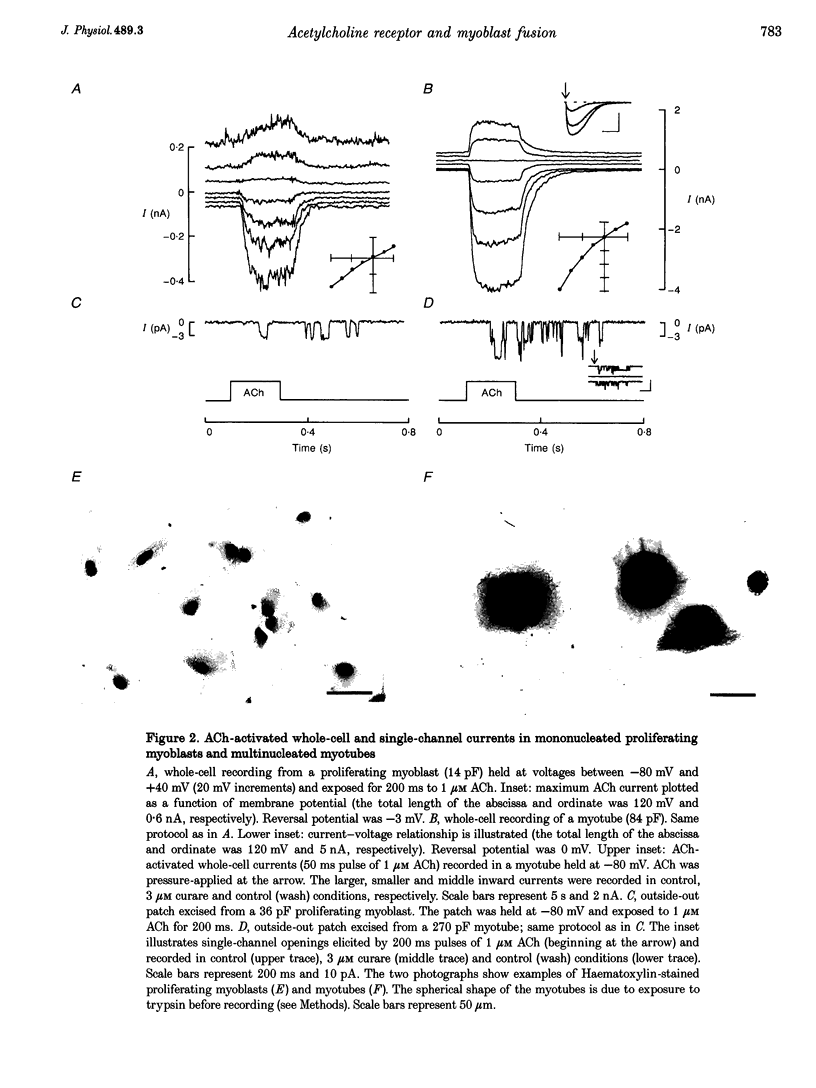
Abstract
1. Fusion of myogenic cells is important for muscle growth and repair. The aim of this study was to examine the possible involvement of nicotinic acetylcholine receptors (nAChR) in the fusion process of myoblasts derived from postnatal human satellite cells. 2. Acetylcholine-activated currents (ACh currents) were characterized in pure preparations of freshly isolated satellite cells, proliferating myoblasts, myoblasts triggered to fuse and myotubes, using whole-cell and single-channel voltage clamp recordings. Also, the effect of cholinergic agonists on myoblast fusion was tested. 3. No nAChR were observed in freshly isolated satellite cells. nAChR were first observed in proliferating myoblasts, but ACh current densities increased markedly only just before fusion. At that time most mononucleated myoblasts had ACh current densities similar to those of myotubes. ACh channels had similar properties at all stages of myoblast maturation. 4. The fraction of myoblasts that did not fuse under fusion-promoting conditions had no ACh current and thus resembled freshly isolated satellite cells. 5. The rate of myoblast fusion was increased by carbachol, an effect antagonized by alpha-bungarotoxin, curare and decamethonium, but not by atropine, indicating that nAChR were involved. Even though a prolonged exposure to carbachol led to desensitization, a residual ACh current persisted after several days of exposure to the nicotinic agonist. 6. Our observations suggest that nAChR play a role in myoblast fusion and that part of this role is mediated by the flow of ions through open ACh channels.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bertrand D., Cooper E., Mauro A. Membrane currents of rat satellite cells attached to intact skeletal muscle fibers. Neuron. 1988 May;1(3):237–240. doi: 10.1016/0896-6273(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Baroffio A., Aubry J. P., Kaelin A., Krause R. M., Hamann M., Bader C. R. Purification of human muscle satellite cells by flow cytometry. Muscle Nerve. 1993 May;16(5):498–505. doi: 10.1002/mus.880160511. [DOI] [PubMed] [Google Scholar]
- Bernheim L., Beech D. J., Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991 Jun;6(6):859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- Bernheim L., Krause R. M., Baroffio A., Hamann M., Kaelin A., Bader C. R. A voltage-dependent proton current in cultured human skeletal muscle myotubes. J Physiol. 1993 Oct;470:313–333. doi: 10.1113/jphysiol.1993.sp019860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D., Ballivet M., Rungger D. Activation and blocking of neuronal nicotinic acetylcholine receptor reconstituted in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1993–1997. doi: 10.1073/pnas.87.5.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Henderson L. Regulation of acetylcholine receptor channel function during development of skeletal muscle. Dev Biol. 1988 Sep;129(1):1–11. doi: 10.1016/0012-1606(88)90156-x. [DOI] [PubMed] [Google Scholar]
- Campion D. R. The muscle satellite cell: a review. Int Rev Cytol. 1984;87:225–251. doi: 10.1016/s0074-7696(08)62444-4. [DOI] [PubMed] [Google Scholar]
- Cossu G., Eusebi F., Grassi F., Wanke E. Acetylcholine receptor channels are present in undifferentiated satellite cells but not in embryonic myoblasts in culture. Dev Biol. 1987 Sep;123(1):43–50. doi: 10.1016/0012-1606(87)90425-8. [DOI] [PubMed] [Google Scholar]
- Cossu G., Molinaro M. Cell heterogeneity in the myogenic lineage. Curr Top Dev Biol. 1987;23:185–208. doi: 10.1016/s0070-2153(08)60625-0. [DOI] [PubMed] [Google Scholar]
- Decker E. R., Dani J. A. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci. 1990 Oct;10(10):3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Electrically induced release of acetylcholine from denervated Schwann cells. J Physiol. 1974 Mar;237(2):431–452. doi: 10.1113/jphysiol.1974.sp010490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entwistle A., Zalin R. J., Bevan S., Warner A. E. The control of chick myoblast fusion by ion channels operated by prostaglandins and acetylcholine. J Cell Biol. 1988 May;106(5):1693–1702. doi: 10.1083/jcb.106.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entwistle A., Zalin R. J., Warner A. E., Bevan S. A role for acetylcholine receptors in the fusion of chick myoblasts. J Cell Biol. 1988 May;106(5):1703–1712. doi: 10.1083/jcb.106.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi F., Molinaro M. Acetylcholine sensitivity in replicating satellite cells. Muscle Nerve. 1984 Jul-Aug;7(6):488–492. doi: 10.1002/mus.880070613. [DOI] [PubMed] [Google Scholar]
- Fambrough D., Rash J. E. Development of acetylcholine sensitivity during myogenesis. Dev Biol. 1971 Sep;26(1):55–68. doi: 10.1016/0012-1606(71)90107-2. [DOI] [PubMed] [Google Scholar]
- Giovannelli A., Grassi F., Mattei E., Mileo A. M., Eusebi F., Giovanelli A. Acetylcholine induces voltage-independent increase of cytosolic calcium in mouse myotubes. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10069–10073. doi: 10.1073/pnas.88.22.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi F., Giovannelli A., Fucile S., Eusebi F. Activation of the nicotinic acetylcholine receptor mobilizes calcium from caffeine-insensitive stores in C2C12 mouse myotubes. Pflugers Arch. 1993 Mar;422(6):591–598. doi: 10.1007/BF00374007. [DOI] [PubMed] [Google Scholar]
- Grubic Z., Komel R., Walker W. F., Miranda A. F. Myoblast fusion and innervation with rat motor nerve alter distribution of acetylcholinesterase and its mRNA in cultures of human muscle. Neuron. 1995 Feb;14(2):317–327. doi: 10.1016/0896-6273(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Gulati A. K. Pattern of skeletal muscle regeneration after reautotransplantation of regenerated muscle. J Embryol Exp Morphol. 1986 Mar;92:1–10. [PubMed] [Google Scholar]
- Hall P. A., Watt F. M. Stem cells: the generation and maintenance of cellular diversity. Development. 1989 Aug;106(4):619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- Hamann M., Chamoin M. C., Portalier P., Bernheim L., Baroffio A., Widmer H., Bader C. R., Ternaux J. P. Synthesis and release of an acetylcholine-like compound by human myoblasts and myotubes. J Physiol. 1995 Dec 15;489(Pt 3):791–803. doi: 10.1113/jphysiol.1995.sp021092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M., Widmer H., Baroffio A., Aubry J. P., Krause R. M., Kaelin A., Bader C. R. Sodium and potassium currents in freshly isolated and in proliferating human muscle satellite cells. J Physiol. 1994 Mar 1;475(2):305–317. doi: 10.1113/jphysiol.1994.sp020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hebb C. O. Acetylcholine content of the rabbit plantaris muscle after denervation. J Physiol. 1962 Sep;163(2):294–306. doi: 10.1113/jphysiol.1962.sp006975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajtha L. G. Stem cell concepts. Differentiation. 1979;14(1-2):23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Lingle C. J., Maconochie D., Steinbach J. H. Activation of skeletal muscle nicotinic acetylcholine receptors. J Membr Biol. 1992 Mar;126(3):195–217. doi: 10.1007/BF00232318. [DOI] [PubMed] [Google Scholar]
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. An analysis of acetylcholine in frog muscle by mass fragmentography. Proc R Soc Lond B Biol Sci. 1977 Jun 15;197(1128):285–297. doi: 10.1098/rspb.1977.0071. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L., Tas J. W., van der Laaken T. Neural and non-neural acetylcholine in the rat diaphragm. Proc R Soc Lond B Biol Sci. 1982 Jan 22;214(1195):153–168. doi: 10.1098/rspb.1982.0002. [DOI] [PubMed] [Google Scholar]
- Mitchell J. F., Silver A. The spontaneous release of acetylcholine from the denervated hemidiaphragm of the rat. J Physiol. 1963 Jan;165(1):117–129. doi: 10.1113/jphysiol.1963.sp007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody-Corbett F. L., Virgo N. S. Acetylcholine reduces the slow calcium current in embryonic skeletal muscle cells in culture. Pflugers Arch. 1993 Jun;424(1):25–29. doi: 10.1007/BF00375098. [DOI] [PubMed] [Google Scholar]
- Morlet K., Grounds M. D., McGeachie J. K. Muscle precursor replication after repeated regeneration of skeletal muscle in mice. Anat Embryol (Berl) 1989;180(5):471–478. doi: 10.1007/BF00305122. [DOI] [PubMed] [Google Scholar]
- Rash J. E., Fambrough D. Ultrastructural and electrophysiological correlates of cell coupling and cytoplasmic fusion during myogenesis in vitro. Dev Biol. 1973 Jan;30(1):166–186. doi: 10.1016/0012-1606(73)90055-9. [DOI] [PubMed] [Google Scholar]
- Schultz E. Changes in the satellite cells of growing muscle following denervation. Anat Rec. 1978 Feb;190(2):299–311. doi: 10.1002/ar.1091900212. [DOI] [PubMed] [Google Scholar]
- Schultz E., Jaryszak D. L. Effects of skeletal muscle regeneration on the proliferation potential of satellite cells. Mech Ageing Dev. 1985 Apr;30(1):63–72. doi: 10.1016/0047-6374(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Control of myogenesis in vitro by Ca 2 + concentration in nutritional medium. Exp Cell Res. 1969 Nov;58(1):163–167. doi: 10.1016/0014-4827(69)90127-x. [DOI] [PubMed] [Google Scholar]
- St Clair J. A., Meyer-Demarest S. D., Ham R. G. Improved medium with EGF and BSA for differentiated human skeletal muscle cells. Muscle Nerve. 1992 Jul;15(7):774–779. doi: 10.1002/mus.880150705. [DOI] [PubMed] [Google Scholar]
- Tucek S. The synthesis of acetylcholine in skeletal muscles of the rat. J Physiol. 1982 Jan;322:53–69. doi: 10.1113/jphysiol.1982.sp014022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer H., Hamann M., Baroffio A., Bijlenga P., Bader C. R. Expression of a voltage-dependent potassium current precedes fusion of human muscle satellite cells (myoblasts). J Cell Physiol. 1995 Jan;162(1):52–63. doi: 10.1002/jcp.1041620108. [DOI] [PubMed] [Google Scholar]
- Yao S. N., Kurachi K. Implanted myoblasts not only fuse with myofibers but also survive as muscle precursor cells. J Cell Sci. 1993 Aug;105(Pt 4):957–963. doi: 10.1242/jcs.105.4.957. [DOI] [PubMed] [Google Scholar]
- Young S. H., Poo M. M. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983 Oct 13;305(5935):634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]