To the Editor:
There is a link between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and increased thrombotic risk. In a population-level study from Denmark, the 30-day risks of venous thromboembolism (VTE) after confirmed infections were 0.2% for nonhospitalized patients and 1.5% for hospitalized patients (1). Others have found that pulmonary embolism (PE) is present in 14.2% of patients at hospital admission for coronavirus disease (COVID-19), increasing to 35% in critically ill patients (2, 3). It is established that male patients have a higher risk of adverse health outcomes, including death, after COVID-19 infection (4). Our aims were: 1) to describe sex differences in short- and long-term population-level risk of VTE specifically after COVID-19 infection and 2) to assess sex differences in outcomes among those with COVID-19 and VTE.
Methods
This was a retrospective population-level cohort study performed in Alberta, Canada (2021 population, 4,262,635) using secondary administrative data sources. The study was approved by the University of Calgary Health Research Ethics Board (REB20-0688) and is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology statement for observational studies (5). We included all people in Alberta with a positive polymerase chain reaction (PCR) test for COVID-19 (i.e., exposed) between April 1, 2020, and December 15, 2021. For each case, we identified two unexposed control patients with a negative COVID-19 PCR test result and no subsequent positive results in the observation period (Figure 1A). Unexposed patients were matched for age (±2 y), sex, and rural versus urban residence; matching was chosen for the latter because there is less access to certain diagnostic tests for VTE (i.e., computed tomography) in rural hospitals in Alberta.
Figure 1.
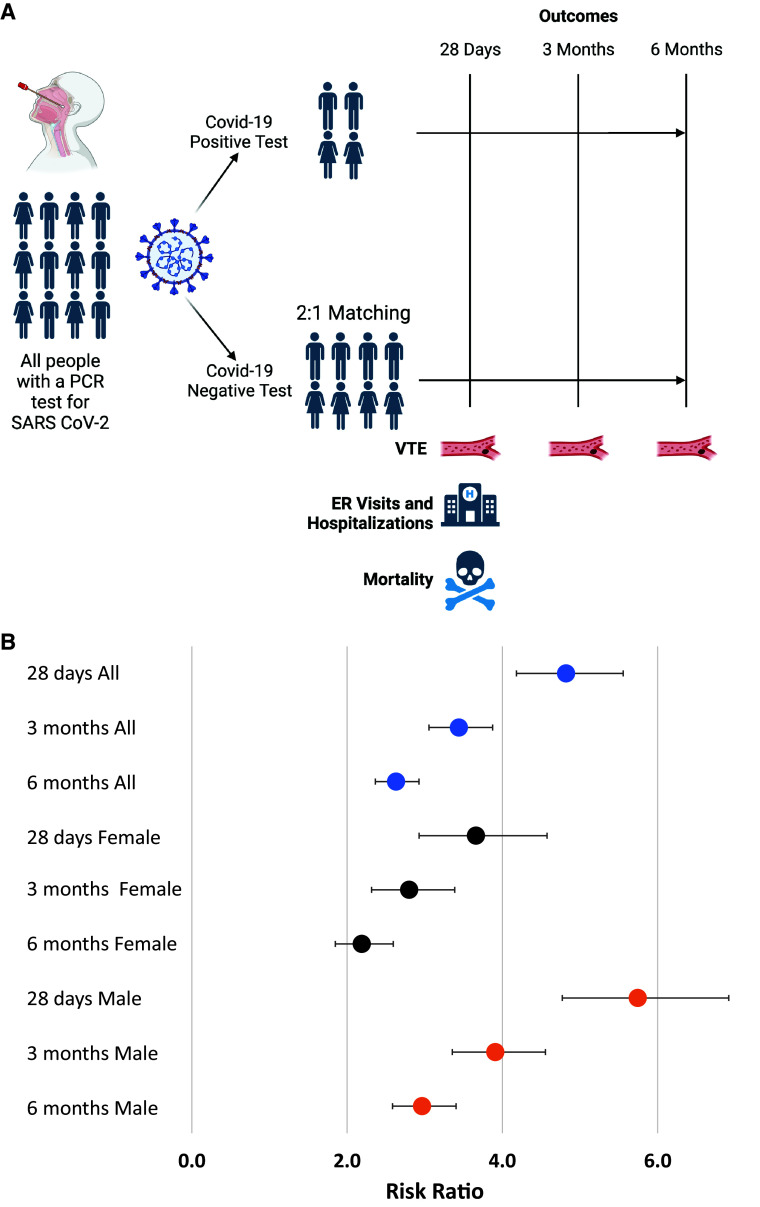
(A) Study design. (B) Risk ratios and 95% confidence intervals for venous thromboembolism at 28 days and 3 and 6 months in people with coronavirus disease (COVID-19) compared with people without COVID-19, stratified by sex. ER = emergency room; PCR = polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; VTE = venous thromboembolism.
The primary outcome was the first VTE event based on International Classification of Diseases, 10th Revision (Canadian modification) codes associated with healthcare visits on or after the index COVID-19 test date for deep vein thrombosis (DVT; codes I80.1-3, 8, 9; I82.8, 9; O22.3, 9; O87.1) or PE (codes I26.0, 9) plus at least one imaging code within 14 days for leg ultrasonography, computed tomography of the chest, ventilation-perfusion scan, or echocardiography. This approach improves the sensitivity and specificity compared with administrative codes alone (6). Secondary outcomes included emergency department (ED) visits, hospitalization, and all-cause mortality after the index COVID-19 test date. We calculated overall and sex-disaggregated risk ratios for VTE, PE, and DVT at 28 days, 3 months, and 6 months. We used multivariable negative binomial regression to determine factors associated with 28-day ED visits and hospitalizations and multivariable logistic regression for factors associated with 28-day mortality. Covariates included in the multivariable models included the exposure of interest (i.e., COVID-19) and known prognostic factors for mortality and hospitalization after COVID-19 infection: age, male sex, VTE, and comorbidities (7). We chose the Elixhauser comorbidity index as a covariate to quantify comorbidities because it contains a greater number of conditions (n = 31) than the Charlson comorbidity index (n = 17), which was also available and because the Elixhauser index was more strongly associated with outcomes after COVID-19 infection (8). The Elixhauser index also contains certain comorbidities, such as obesity, that are associated with adverse outcomes after COVID-19 and are not present in the Charlson index (9). Models were also adjusted for treatment with anticoagulant because this has been associated with mortality and hospitalizations after COVID-19 infection (10).
Results
During the observation period, there were 255,037 exposed individuals with a positive PCR COVID-19 test and 509,876 unexposed individuals (254,839 exposed individuals had 2:1 unexposed control matching, and 198 had only one available unexposed matched control). The mean age was 42 years ± 16.8 (standard deviation), and 49.6% were male (Table 1). There were 921 VTE events after COVID-19 infection and 954 VTE events in the unexposed group (0.4% vs. 0.2%; P < 0.001). Among all patients who had a VTE event following a COVID-19 test, those who tested positive for COVID-19 were more likely to be male (64.4% vs. 54.9%; P < 0.001) and were younger (mean age, 58.5 ± 15.5 vs. 61.2 ± 16.8 yr; P < 0.001). The risk ratios for VTE at 28 days, 3 months, and 6 months in the overall cohort and stratified by sex are shown in Figure 1B, with a higher VTE risk among COVID-19–positive patients at all time points compared with those without a positive COVID-19 test. There was higher risk among male individuals (P < 0.001 for interaction) at all time points. Results were similar when PE and DVT were considered separately (Table 2). A VTE diagnosis was associated with higher 28-day mortality rates among all COVID-19–positive patients (adjusted odds ratio [aOR], 8.70; 95% confidence interval [CI], 6.41–11.82).
Table 1.
Characteristics and outcomes of individuals with a positive COVID-19 test (exposed) and matched control individuals with a negative COVID-19 test (unexposed)
| All |
Female |
Male |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 Exposed (n = 255,037) | COVID-19 Unexposed (n = 509,876) | Total (N = 764,913) | P Value | COVID-19 Exposed (n = 128,607) | COVID-19 Unexposed (n = 257,133) | Total (n = 385,740) | P Value | COVID-19 Exposed (n = 126,337) | COVID-19 Unexposed (n = 252,577) | Total (n = 378,914) | P Value | |
| Age, yr | 42.1 ± 16.8 | 42.2 ± 16.7 | 42.2 ± 16.8 | 0.90 | 42.3 ± 17.1 | 42.3 ± 17.1 | 42.3 ± 17.1 | 0.94 | 42.0 ± 16.4 | 42.0 ± 16.4 | 42.0 ± 16.4 | 0.93 |
| Male sex | 126,337 (49.6%) | 252,577 (49.6%) | 378,914 (49.6%) | 0.99 | — | — | — | — | — | — | — | — |
| Elixhauser index | 0.3 ± 4.7 | 0.2 ± 4.8 | 0.2 ± 4.7 | 0.002 | 0.04 ± 4.6 | −0.01 ± 4.7 | 0.01 ± 4.7 | 0.004 | 0.5 ± 4.7 | 0.4 ± 4.9 | 0.5 ± 4.8 | 0.14 |
| Rural | 36,736 (14.4%) | 73,592 (14.4%) | 110,328 (14.4%) | 0.73 | 18,989 (14.8%) | 38,037 (14.8%) | 57,026 (14.8%) | 0.82 | 17,728 (14.0%) | 35,530 (14.1%) | 53,258 (14.1%) | 0.77 |
| VTE | 921 (0.4%) | 954 (0.2%) | 1,875 (0.2%) | <0.0001 | 328 (0.3%) | 430 (0.2%) | 758 (0.2%) | <0.0001 | 593 (0.5%) | 524 (0.2%) | 1,117 (0.3%) | <0.0001 |
| Days from COVID-19 test date to VTE diagnosis date | 10 (4 to 19) | 6 (−1 to 57) | 9 (0 to 28) | 0.01 | 10 (3 to 22) | 5 (−1 to 53) | 8 (0 to 34) | 0.002 | 10 (4 to 17) | 6 (−1 to 59) | 9 (1 to 26) | 0.54 |
| Died ≤28 d from COVID-19 test | 2,829 (1.1%) | 2,168 (0.4%) | 4,997 (0.7%) | <0.0001 | 1,203 (0.9%) | 879 (0.3%) | 2,082 (0.5%) | <0.0001 | 1,626 (1.3%) | 1,289 (0.5%) | 2,915 (0.8%) | <0.0001 |
| ED visits ≤28 d from COVID-19 test | 0.22 ± 0.6 | 0.16 ± 0.6 | 0.18 ± 0.6 | <0.0001 | 0.2 ± 0.6 | 0.2 ± 0.5 | 0.2 ± 0.6 | <0.0001 | 0.2 ± 0.6 | 0.2 ± 0.7 | 0.2 ± 0.6 | <0.0001 |
| Hospitalization ≤28 d from COVID-19 test | 0.07 ± 0.29 | 0.05 ± 0.26 | 0.06 ± 0.27 | <0.0001 | 0.1 ± 0.3 | 0.0 ± 0.2 | 0.1 ± 0.3 | <0.0001 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | <0.0001 |
| Received an anticoagulant | 2,321 (0.9%) | 4,097 (0.8%) | 6,418 (0.8%) | <0.0001 | 1,081 (0.8%) | 1,942 (0.8%) | 3,023 (0.8%) | 0.005 | 1,240 (1.0%) | 2,155 (0.9%) | 3,395 (0.9%) | <0.0001 |
Definition of abbreviations: COVID-19 = coronavirus disease; ED = emergency department; VTE = venous thromboembolism.
Data are presented as mean ± standard deviation or median (interquartile range) as applicable. Characteristics of individuals with COVID-19 and matched control individuals without COVID-19 and the VTE outcomes in 4 weeks, 3 months, and 6 months after COVID-19 or index date.
Table 2.
RRs and 95% CIs for DVT and PE at 1, 3, and 6 months in people with COVID-19 compared with people without COVID-19, stratified by sex
| COVID test | VTE |
DVT |
PE |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events, n | Total, n | Risk ratio (95%CI) | Events, n | Total, n | Risk ratio (95%CI) | Events, n | Total, n | Risk ratio (95%CI) | ||
| Both sex 4 weeks | Negative | 269 | 509,351 | 4.82 (4.18, 5.56)* |
130 | 509,621 | 2.65 (2.06, 3.4)* |
159 | 509,391 | 5.51 (4.61, 6.61)* |
| Positive | 649 | 254,807 | 130 | 254,913 | 546 | 254,827 | ||||
| 3 months | Negative | 430 | 509,351 | 3.44 (3.05, 3.87)* |
205 | 509,621 | 2.23 (1.81, 2.75)* |
257 | 509,391 | 3.76 (3.24, 4.36)* |
| Positive | 740 | 254,807 | 173 | 254,913 | 602 | 254,827 | ||||
| 6 months | Negative | 595 | 509,351 | 2.63 (2.37, 2.93)* |
285 | 509,621 | 1.75 (1.45, 2.12)* |
359 | 509,391 | 2.83 (2.48, 3.23)* |
| Positive | 784 | 254,807 | 189 | 254,913 | 633 | 254,827 | ||||
| Female 4 weeks | Negative | 119 | 256,959 | 3.66 (2.93, 4.58)* |
58 | 257,090 | 2.23 (1.5, 3.31)* |
68 | 256,975 | 4.67 (3.51, 6.27)* |
| Positive | 218 | 128,560 | 51 | 128,596 | 177 | 128,566 | ||||
| 3 months | Negative | 182 | 256,959 | 2.8 (2.32, 3.39)* |
89 | 257,090 | 2.03 (1.46, 2.8)* |
104 | 256,975 | 3.38 (2.65, 4.33)* |
| Positive | 255 | 128,560 | 71 | 128,596 | 196 | 128,566 | ||||
| 6 months | Negative | 256 | 256,959 | 2.19 (1.85, 2.60)* |
127 | 257,090 | 1.6 (1.19, 2.13)* |
152 | 256,975 | 2.53 (2.04, 3.13)* |
| Positive | 281 | 128,560 | 80 | 128,596 | 214 | 128,566 | ||||
| Male 4 weeks | Negative | 150 | 252,392 | 5.74 (4.77, 6.92)* |
72 | 252,531 | 3.01 (2.16, 4.21)* |
91 | 252,416 | 5.98 (4.74, 7.61)* |
| Positive | 431 | 126,247 | 79 | 126,317 | 369 | 126,261 | ||||
| 3 months | Negative | 248 | 252,392 | 3.91 (3.36, 4.56)* |
116 | 252,531 | 2.42 (1.83, 3.18)* |
153 | 252,416 | 3.92 (3.24, 4.75)* |
| Positive | 485 | 126,247 | 102 | 126,317 | 406 | 126,261 | ||||
| 6 months | Negative | 339 | 252,392 | 2.97 (2.59, 3.40)* |
158 | 252,531 | 1.9 (1.47, 2.43)* |
207 | 252,416 | 2.99 (2.52, 3.54)* |
| Positive | 503 | 126,247 | 109 | 126,317 | 419 | 126,261 | ||||
Definition of abbreviations: CI = confidence interval; DVT = deep vein thrombosis; PE = pulmonary embolism; RR = risk ratio; VTE = venous thromboembolism.
The total number of VTE cases is 1,379, including 474 DVT cases and 992 PE cases, with 147 cases overlapping. 496 VTE cases were excluded from this analysis due to diagnostic tests for VTE being performed prior to COVID-19 testing.
p < 0.001
Male sex was also associated with higher rates of ED visits (adjusted incidence rate ratio, 1.01; 95% CI, 1.001–1.03) and hospitalizations (adjusted incidence rate ratio, 1.19; 95% CI, 1.16–1.21) within 28 days of COVID-19 testing after adjusting for COVID-19 test positivity, age, anticoagulant prescription, Elixhauser comorbidity index, and presence of VTE. The 28-day all-cause mortality rate was also higher among male patients (aOR, 1.57; 95% CI, 1.48–1.67). Conversely, among all patients with VTE events (n = 1,875) following a COVID-19 test, the risk of death within 28 days was not increased for male patients (aOR, 1.01; 95% CI, 0.71–1.45).
Discussion
In this large population-based study of patients who were tested for COVID-19, the risk of VTE was higher among COVID-19–positive patients as long as 6 months after PCR testing, with disproportionately higher risks among male patients compared with female patients for VTE, DVT, and PE. Male patients also had higher use of health care, including ED visits and hospitalizations, after COVID-19. VTE was associated with an increased short-term risk of death among COVID-19–positive individuals, but male sex was not a risk factor for death after VTE after accounting for COVID-19 positivity and other confounders.
This is one of the largest population-based studies to explore sex differences in VTE risk related to COVID-19 and to assess the association between sex and outcomes after COVID-19. We confirmed a higher VTE risk in male patients that was previously observed in short-term follow-up studies of hospitalized patients with COVID-19 (11). Population-based studies in Sweden and the United Kingdom also found increased VTE persisting beyond 6 months and noted a higher risk of VTE after COVID-19 in male patients compared with female patients (12, 13). We extend these previous studies to show higher all-cause mortality rates and healthcare resource use for male patients. Male patients had higher short-term mortality rates after COVID-19 testing, regardless of test positivity and adjusting for the presence of VTE and other confounders. However, when considering all patients with a VTE event, male sex was not associated with higher adjusted risk of death, suggesting that the higher risk of short-term mortality after COVID-19 is not driven by fatal VTE events but by other factors.
Strengths of this study include the large population-based sample and matching for age, sex, and geographic location in the province of Alberta, the latter of which could have influenced access to COVID-19 testing and imaging confirmation of VTE. Limitations of this analysis include the retrospective design and use of administrative data rather than patient-level data from electronic medical records, even though this approach has been previously shown to be accurate (6). Overall, this study demonstrates sex disparities in short- and long-term VTE risk after COVID-19 and worse outcomes among male patients, which may inform clinical decisions and follow-up pathways after COVID-19 infection.
Footnotes
Author Contributions: Study design: J.W., M.K.S., R.D., M.P.S., L.J.S., E.R.-M., J.L., and G.Y.L. Data acquisition: C.W, Z.Z., A.G.D’S., E.R.-M., and J.L. Data analysis: C.W., Z.Z., and A.G.D’S. Draft manuscript writing: J.W. Critical revisions: J.W., C.W., M.K.S., R.D., M.P.S., L.J.S., Z.Z., A.G.D’S., E.R.-M., J.L., and G.Y.L.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Dalager-Pedersen M, Lund LC, Mariager T, Winther R, Hellfritzsch M, Larsen TB, et al. Venous thromboembolism and major bleeding in patients with coronavirus disease 2019 (COVID-19): a nationwide, population-based cohort study. Clin Infect Dis . 2021;73:2283–2293. doi: 10.1093/cid/ciab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jevnikar M, Sanchez O, Chocron R, Andronikof M, Raphael M, Meyrignac O, et al. Prevalence of pulmonary embolism in patients with COVID-19 at the time of hospital admission. Eur Respir J . 2021;58:2100116. doi: 10.1183/13993003.00116-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res . 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol . 2020;20:442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet . 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 6. Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. The validity of ICD codes coupled with imaging procedure codes for identifying acute venous thromboembolism using administrative data. Vasc Med . 2015;20:364–368. doi: 10.1177/1358863X15573839. [DOI] [PubMed] [Google Scholar]
- 7. Semenzato L, Botton J, Drouin J, Cuenot F, Dray-Spira R, Weill A, et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health Eur . 2021;8:100158. doi: 10.1016/j.lanepe.2021.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monterde D, Carot-Sans G, Cainzos-Achirica M, Abilleira S, Coca M, Vela E, et al. Performance of three measures of comorbidity in predicting critical COVID-19: a retrospective analysis of 4607 hospitalized patients. Risk Manag Healthc Policy. 2021;14:4729–4737. doi: 10.2147/RMHP.S326132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh R, Rathore SS, Khan H, Karale S, Chawla Y, Iqbal K, et al. Association of obesity with COVID-19 severity and mortality: an updated systemic review, meta-analysis, and meta-regression. Front Endocrinol (Lausanne) . 2022;13:780872. doi: 10.3389/fendo.2022.780872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rivera-Caravaca JM, Buckley BJR, Harrison SL, Fazio-Eynullayeva E, Underhill P, Marín F, et al. Direct-acting oral anticoagulants use prior to COVID-19 diagnosis and associations with 30-day clinical outcomes. Thromb Res . 2021;205:1–7. doi: 10.1016/j.thromres.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ilyas S, Henkin S, Martinez-Camblor P, Suckow BD, Beach JM, Stone DH, et al. Sex-, race- and ethnicity-based differences in thromboembolic events among adults hospitalized with COVID-19. J Am Heart Assoc . 2021;10:e022829. doi: 10.1161/JAHA.121.022829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knight R, Walker V, Ip S, Cooper JA, Bolton T, Keene S, et al. CVD-COVID-UK/COVID-IMPACT Consortium and the Longitudinal Health and Wellbeing COVID-19 National Core Study Association of COVID-19 with major arterial and venous thrombotic diseases: a population-wide cohort study of 48 million adults in England and Wales. Circulation . 2022;146:892–906. doi: 10.1161/CIRCULATIONAHA.122.060785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katsoularis I, Fonseca-Rodríguez O, Farrington P, Jerndal H, Lundevaller EH, Sund M, et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. BMJ . 2022;377:e069590. doi: 10.1136/bmj-2021-069590. [DOI] [PMC free article] [PubMed] [Google Scholar]