Abstract
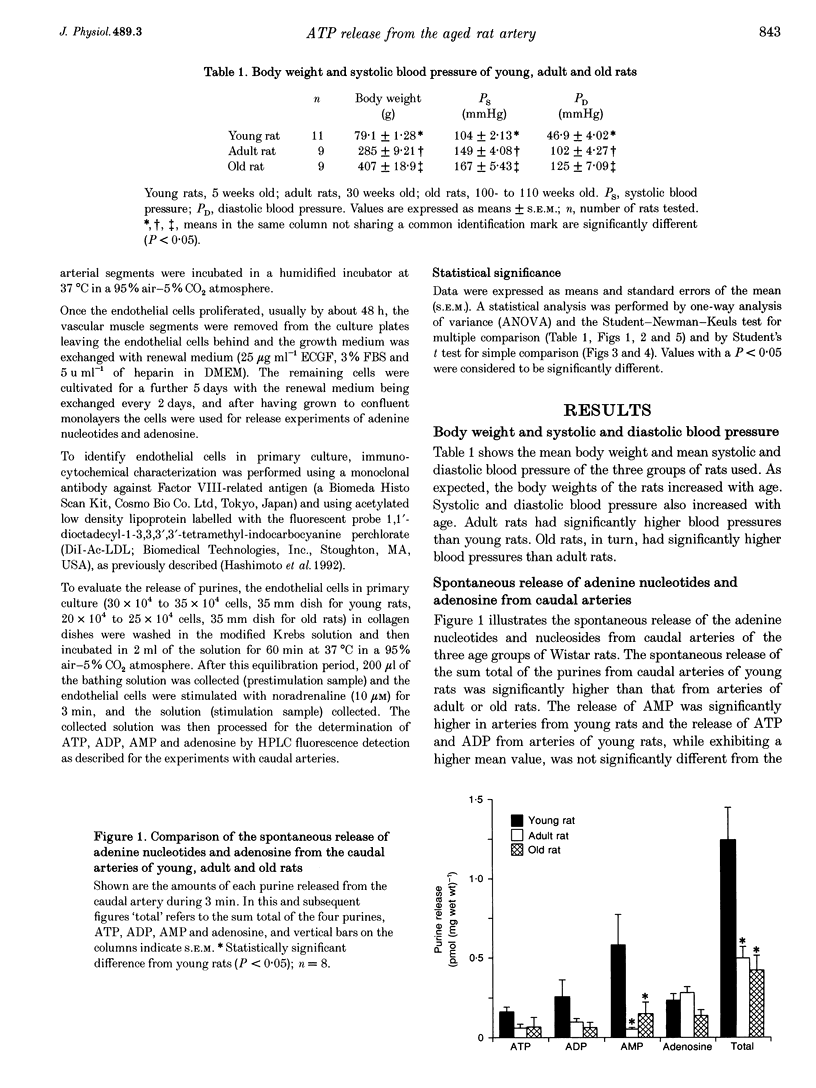
1. The spontaneous and alpha-adrenoceptor-induced release of ATP, ADP, AMP and adenosine were determined from arterial segments and from isolated endothelial cells from caudal arteries of young (5-week-old), adult (30-week-old) and old (100- to 110-week-old) Wistar rats. 2. The spontaneous (non-evoked) release of the sum total of the four purines was significantly greater from artery segments of young rats than from adult and old rats. 3. The release of the adenine nucleotides and adenosine induced by methoxamine (10 microM), an alpha 1-adrenoceptor agonist, was greater from artery segments from young rats than from old rats. 4. The spontaneous release of the sum total of the four purines was significantly greater from endothelial cells prepared from caudal arteries of young rats than of old rats. 5. The noradrenaline (10 microM)-induced release of the sum total of the four purines was significantly greater from endothelial cells prepared from caudal arteries of young rats than of old rats. 6. The levels of adenine nucleotides and adenosine, determined in plasma from anaesthetized rats, were significantly higher in young rats compared with adult and old rats. 7. These findings suggest that the release of ATP from the vascular endothelial cells is reduced with advancing age.
Full text
PDF

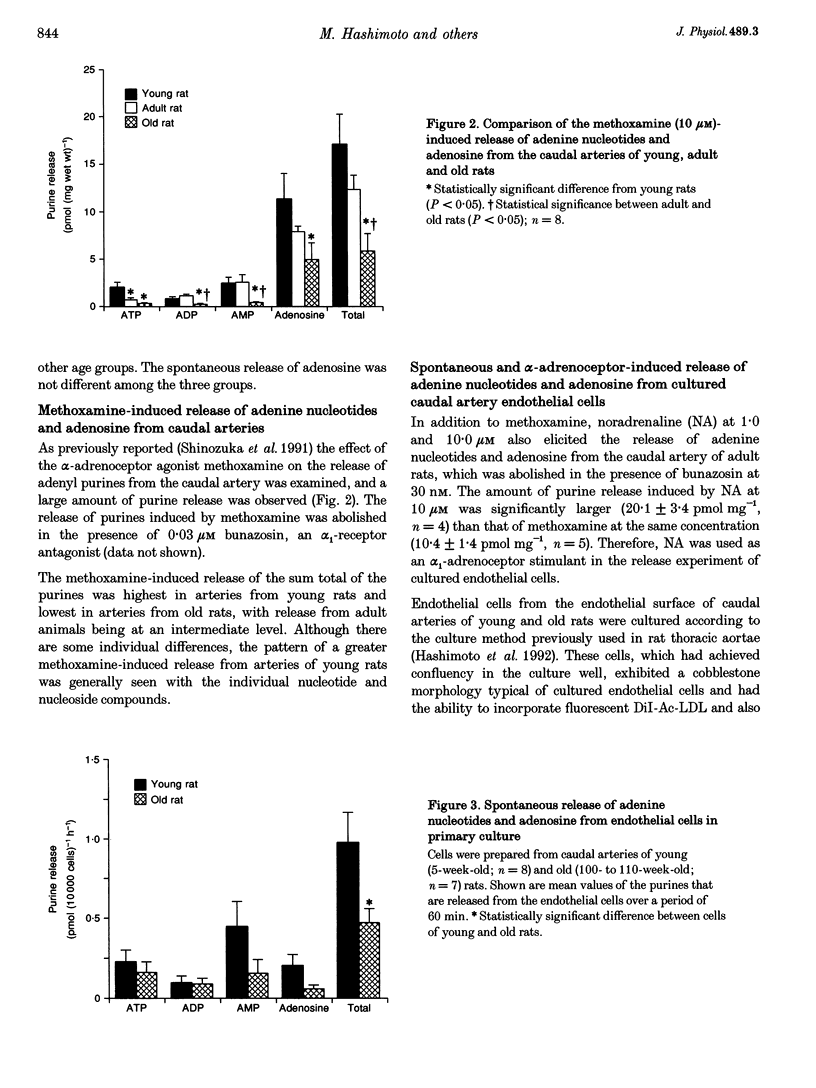



Selected References
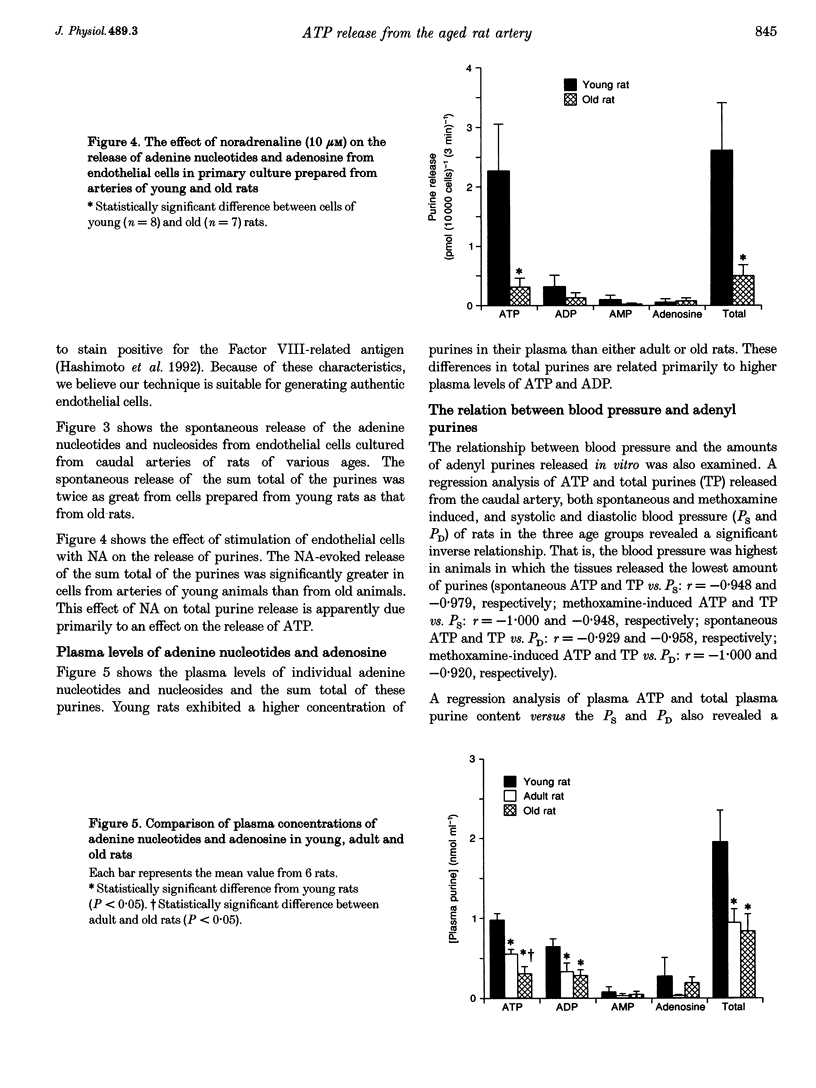
These references are in PubMed. This may not be the complete list of references from this article.
- Bodin P., Bailey D., Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991 May;103(1):1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Local control of blood pressure by purines. Blood Vessels. 1987;24(3):156–160. doi: 10.1159/000158691. [DOI] [PubMed] [Google Scholar]
- Chinellato A., Pandolfo L., Ragazzi E., Zambonin M. R., Froldi G., De Biasi M., Caparrotta L., Fassina G. Effect of age on rabbit aortic responses to relaxant endothelium-dependent and endothelium-independent agents. Blood Vessels. 1991;28(5):358–365. doi: 10.1159/000158882. [DOI] [PubMed] [Google Scholar]
- Dalziel H. H., Westfall D. P. Receptors for adenine nucleotides and nucleosides: subclassification, distribution, and molecular characterization. Pharmacol Rev. 1994 Dec;46(4):449–466. [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J Physiol. 1981 Jul;316:347–355. doi: 10.1113/jphysiol.1981.sp013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. R. Cardiovascular responses in ageing: a review. Pharmacol Rev. 1990 Jun;42(2):103–125. [PubMed] [Google Scholar]
- Finelli C., Aussedat J., Ray A., Lortet S., Lavanchy N., Guarnieri C., Caldarera C. M., Rossi A. Effect of age on phosphorylated compounds and mechanical activity of isolated rat heart: a 31P-NMR study. Cardiovasc Res. 1993 Nov;27(11):1978–1982. doi: 10.1093/cvr/27.11.1978. [DOI] [PubMed] [Google Scholar]
- Fouda A. K., Atkinson J. Sensitivity to noradrenaline and electrical stimulation decreases with age in the rat tail artery. Naunyn Schmiedebergs Arch Pharmacol. 1986 Sep;334(1):37–39. doi: 10.1007/BF00498737. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Hashimoto M., Hara T., Honda M., Ishinaga Y., Moriyama K., Masumura S. Metabolic aspects of endothelial cells cultured from rat aortae. Artery. 1992;19(5):284–296. [PubMed] [Google Scholar]
- Koga T., Takata Y., Kobayashi K., Fujii K., Nagao T., Fujishima M. Age-related changes in P2-purinergic receptors on vascular smooth muscle and endothelium. Hypertension. 1992 Mar;19(3):286–289. doi: 10.1161/01.hyp.19.3.286. [DOI] [PubMed] [Google Scholar]
- Milner P., Bodin P., Loesch A., Burnstock G. Increased shear stress leads to differential release of endothelin and ATP from isolated endothelial cells from 4- and 12-month-old male rabbit aorta. J Vasc Res. 1992 Nov-Dec;29(6):420–425. doi: 10.1159/000158960. [DOI] [PubMed] [Google Scholar]
- Mohri K., Takeuchi K., Shinozuka K., Bjur R. A., Westfall D. P. Simultaneous determination of nerve-induced adenine nucleotides and nucleosides released from rabbit pulmonary artery. Anal Biochem. 1993 May 1;210(2):262–267. doi: 10.1006/abio.1993.1194. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moritoki H., Matsugi T., Takase H., Ueda H., Tanioka A. Evidence for the involvement of cyclic GMP in adenosine-induced, age-dependent vasodilatation. Br J Pharmacol. 1990 Jul;100(3):569–575. doi: 10.1111/j.1476-5381.1990.tb15848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshita M., Kigoshi S., Muramatsu I. Pharmacological characterization of two distinct alpha 1-adrenoceptor subtypes in rabbit thoracic aorta. Br J Pharmacol. 1993 Apr;108(4):1071–1076. doi: 10.1111/j.1476-5381.1993.tb13507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22 (Suppl 4):S1–14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- Sedaa K. O., Bjur R. A., Shinozuka K., Westfall D. P. Nerve and drug-induced release of adenine nucleosides and nucleotides from rabbit aorta. J Pharmacol Exp Ther. 1990 Mar;252(3):1060–1067. [PubMed] [Google Scholar]
- Shimokawa H., Vanhoutte P. M. Dietary omega 3 fatty acids and endothelium-dependent relaxations in porcine coronary arteries. Am J Physiol. 1989 Apr;256(4 Pt 2):H968–H973. doi: 10.1152/ajpheart.1989.256.4.H968. [DOI] [PubMed] [Google Scholar]
- Shinozuka K., Bjur R. A., Westfall D. P. Characterization of prejunctional purinoceptors on adrenergic nerves of the rat caudal artery. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):221–227. doi: 10.1007/BF00173391. [DOI] [PubMed] [Google Scholar]
- Shinozuka K., Hashimoto M., Masumura S., Bjur R. A., Westfall D. P., Hattori K. In vitro studies of release of adenine nucleotides and adenosine from rat vascular endothelium in response to alpha 1-adrenoceptor stimulation. Br J Pharmacol. 1994 Dec;113(4):1203–1208. doi: 10.1111/j.1476-5381.1994.tb17125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozuka K., Kitagawa S., Kunitomo M., Yamaguchi Y., Tanabe Y., Fujiwara M., Hattori K. Release of endogenous ATP from the caudal artery in rats with arteriosclerosis. Eur J Pharmacol. 1994 Nov 1;292(1):115–118. doi: 10.1016/0926-6917(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Shinozuka K., Kunitomo M., Hattori K., Bjur R. A., Westfall D. P. Differences in purinoceptor modulation of norepinephrine release between caudal arteries of normotensive and hypertensive rats. J Pharmacol Exp Ther. 1995 Mar;272(3):1193–1198. [PubMed] [Google Scholar]
- Shinozuka K., Sedaa K. O., Bjur R. A., Westfall D. P. Participation by purines in the modulation of norepinephrine release by methoxamine. Eur J Pharmacol. 1991 Jan 17;192(3):431–434. doi: 10.1016/0014-2999(91)90236-j. [DOI] [PubMed] [Google Scholar]
- Slakey L. L., Gordon E. L., Pearson J. D. A comparison of ectonucleotidase activities on vascular endothelial and smooth muscle cells. Ann N Y Acad Sci. 1990;603:366–379. doi: 10.1111/j.1749-6632.1990.tb37686.x. [DOI] [PubMed] [Google Scholar]
- Tummino P. J., Gafni A. A comparative study of succinate-supported respiration and ATP/ADP translocation in liver mitochondria from adult and old rats. Mech Ageing Dev. 1991 Jun 14;59(1-2):177–188. doi: 10.1016/0047-6374(91)90083-c. [DOI] [PubMed] [Google Scholar]
- Uchida K., Nomura Y., Kadowaki M., Takase H., Takano K., Takeuchi N. Age-related changes in cholesterol and bile acid metabolism in rats. J Lipid Res. 1978 Jul;19(5):544–552. [PubMed] [Google Scholar]
- Westfall D. P., Sedaa K. O., Shinozuka K., Bjur R. A., Buxton I. L. ATP as a cotransmitter. Ann N Y Acad Sci. 1990;603:300–310. doi: 10.1111/j.1749-6632.1990.tb37681.x. [DOI] [PubMed] [Google Scholar]
- Westfall D. P., Sedaa K., Bjur R. A. Release of endogenous ATP from rat caudal artery. Blood Vessels. 1987;24(3):125–127. doi: 10.1159/000158684. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Ozaki K., Suketa Y. Alteration of glucose consumption and adenosine triphosphate content in bone tissue of rats with different ages: the stimulatory effect of zinc. Chem Pharm Bull (Tokyo) 1990 Jun;38(6):1660–1662. doi: 10.1248/cpb.38.1660. [DOI] [PubMed] [Google Scholar]
- Yang S., Cheek D. J., Westfall D. P., Buxton I. L. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994 Mar;74(3):401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- de la Cruz J., Burón I., Roncero I. Morphological and functional studies during aging at mitochondrial level. Action of drugs. Int J Biochem. 1990;22(7):729–735. doi: 10.1016/0020-711x(90)90008-q. [DOI] [PubMed] [Google Scholar]


