Abstract
This investigation seeks to scrutinize the relationships between body composition metrics and the clinical outcomes observed in patients with cholangiocarcinoma (CCA). A comprehensive exploration was conducted across three prominent online databases: Embase, PubMed, and the Cochrane Library. This endeavor spanned the entirety of each database up to the cutoff date of September 29, 2023. To evaluate the quality of the included studies, the Newcastle-Ottawa scale was employed. This comprehensive analysis included a total of 26 articles with a combined patient cohort of 4398 individuals. The results demonstrated that CCA patients with low skeletal muscle index (SMI) had significantly inferior OS (HR: 1.93, p < 0.001) and RFS (HR: 2.02, p < 0.001), as well as a higher incidence of postoperative complications (OR: 1.69, 95% CI: 1.20–2.38, p < 0.001) compared to those with high SMI. The presence of sarcopenia in CCA patients was significantly related to poorer OS (HR: 1.96, p < 0.001) and RFS (HR: 2.05, p < 0.001), and a higher rate of postoperative complications (OR: 1.39, p = 0.049) in comparison to those without sarcopenia. Moreover, lower psoas muscle index (PMI) and myosteatosis were associated with shorter OS (PMI, HR: 1.56, p < 0.001; myosteatosis, HR: 1.49, p = 0.001) and RFS (PMI, HR: 2.16, p < 0.001; myosteatosis, HR: 1.35, p = 0.023). Our findings highlight incorporating body composition screening into clinical practice can help develop treatment strategies and optimize perioperative care, potentially improving patient outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12937-024-01037-w.
Keywords: Body composition, Sarcopenia, Skeletal muscle index, Psoas muscle index, Myosteatosis, Cholangiocarcinoma
Introduction
Cholangiocarcinoma (CCA) represents a profoundly lethal primary hepatic malignancy that arises from the biliary epithelium. CCA can be categorized into three primary subtypes distinguished by their anatomical origins: intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA) [1]. Although CCA is relatively infrequent, the occurrence of CCA, notably the iCCA subtype, has demonstrated a consistent rise over the course of the past forty years [1]. Despite recent advancements in our comprehension of CCA biology and the identification of therapeutic targets, there has been limited improvement in patient prognosis [2]. The median duration of overall survival (OS) typically falls within the range of 11.7 to 13 months, and the anticipated 5-year survival rate hovers at approximately 20% [3, 4].
Given the lack of early symptoms and reliable diagnostic markers, patients frequently seek medical attention when their condition has already reached an advanced stage, thereby severely constraining the available therapeutic interventions [1, 2]. Surgical intervention stands as the exclusive, potentially curative approach for individuals afflicted with CCA [2]. Consequently, the principal role of chemotherapy options and complementary treatments revolves around the extension of survival in cases where operative procedures are not feasible. Therefore, the identification of poor prognostic markers and timely intervention in CCA patients assume paramount importance in improving the prognosis of this patient population.
The loss of muscle mass, commonly referred to as sarcopenia, represents a fundamental characteristic of individuals experiencing malnutrition. This condition is frequently observed among elderly cancer patients and is attributed to an imbalance between inadequate dietary intake and the heightened metabolic demands of the tumor [5]. Various methodologies have been employed to assess sarcopenia, with the skeletal muscle index (SMI) or psoas muscle index (PMI) emerging as a widely utilized technique for its measurement. SMI and PMI are calculated by determining the total muscle area and psoas muscle area, respectively, as visualized on a computed tomography (CT) scan at the level of the third lumbar vertebra, and dividing it by the square of the height.
Indeed, sarcopenia has been identified as a potentially adverse prognostic factor in post-surgery patients with various malignancies, including esophageal, gastric, colon, pancreatic, and liver cancer [6–9]. However, it is noteworthy that no systematic review has been conducted on the implications of body composition or sarcopenia in patients with CCA. Unlike other types of cancer, CCA poses distinct challenges in its management due to its aggressive nature and complex anatomical considerations [10–12].
To address this gap, the present systematic review aims to answer the following research question: How do pre-treatment body composition parameters or sarcopenia influence the prognosis of CCA patients? This question is critical, as understanding the prognostic value of body composition parameters or sarcopenia could inform treatment decisions and patient management strategies, particularly in identifying high-risk patients who may benefit from more intensive perioperative care or rehabilitation interventions.
The need for a systematic review arises from the lack of a comprehensive synthesis of current evidence. While body composition parameters, or sarcopenia, have been studied as a prognostic factor in several cancer types, no review has yet focused on its impact in CCA patients, despite the unique challenges this malignancy presents. By systematically reviewing the available literature, this study aims to fill this gap and provide a clearer understanding of whether body composition parameters or sarcopenia influence long-term outcomes in CCA patients, which could have important implications for clinical practice.
Methods
Search strategy
Starting on 29th, September, 2023, we initiated a computerized search of bibliographic databases, which included EMBASE, PubMed, and the Cochrane Library. Our search employed specific terms, such as “Biliary Tract Neoplasms” [Mesh], “Bile Duct Neoplasms” [Mesh], “Cholangiocarcinoma” [Mesh], “Body Composition” [Mesh], “Skeletal muscle index (SMI)”, “Psoas muscle index (PMI)”, “Sarcopenia”, “Skeletal muscle density (SMD)”, “Myosteatosis”, “Visceral adipose index (VAI)”, “Intramuscular adipose index (IMAI)”, “Total adipose index (TAI)”, and “Subcutaneous adipose index (SAI)”. This extensive search encompassed “all fields” in these databases and was limited to human studies published in the English language. For a more detailed description of our search strategy, please refer to supplementary material 1. Furthermore, we conducted additional searches for grey literature on Google Scholar and manually scrutinized the reference lists of eligible studies. Following the guidance of the Cochrane collaboration, the results from both manual and electronic sources were integrated into the Covidence software for effective data management.
Inclusion and exclusion criteria
We established the following inclusion criteria for the selection of articles: (i) Studies focused on CCA patients; (ii) Evaluation of the prognostic significance of baseline body composition parameters (assessed before the initiation of therapy); (iii) Reporting of at least one of the following outcomes: OS, recurrence-free survival (RFS), or postoperative major complications (Clavien grade ≥ 3/3b). These outcomes were chosen because OS and RFS are widely accepted measures of long-term prognosis in oncology, while major postoperative complications significantly influence short-term recovery and overall patient outcomes. In contrast, articles were excluded if they met the following criteria: (i) Studies of other designs, such as animal studies, reviews, case reports, or conference abstracts; (ii) Studies where neither the text nor the published data provided the necessary information to calculate hazard ratios (HR) or odds ratios (OR) for the specified outcomes. In cases where multiple studies featured overlapping patient populations, we favored the selection of articles that presented comprehensive data and followed rigorous methodology [13].
Data extraction and quality assessment
During the process of data extraction, we systematically gathered essential information, which encompassed details such as the author’s name, publication year, study region, study duration, study design, demographic characteristics (including sample size, age, and sex), cancer type, treatment, reported outcomes, and specifics regarding body composition assessment, such as the methodology, site of measurement, analytical software employed, and cut-off points for analysis. HR, OR, and their corresponding 95% confidence intervals (CIs) were predominantly extracted from multivariate analysis. In cases where these statistics were not available, we resorted to univariate analysis or utilized Engauge Digitizer to extract data from survival analysis charts. To gauge the quality of observational studies, we applied the Newcastle-Ottawa Scale (NOS) score, which allocated up to nine points based on quality-related criteria within the domains of patient selection, study comparability, and study endpoints. Studies scoring higher than six points were regarded as high-quality literature. It is important to note that all stages of this process, spanning literature retrieval, screening, data extraction, and quality assessment, were carried out meticulously and independently by three researchers. In cases of disagreement or dispute, these were duly referred to the senior author for resolution.
Statistical methods
We performed the statistical analysis using Stata 15.0 software. The results were visually represented through forest plots. Heterogeneity was evaluated using Cochran’s Q test and I2 statistics. Significantly high heterogeneity was characterized by a p-value < 0.1 or an I2 value > 50%. In cases of pronounced heterogeneity, we employed a random-effects model using the DerSimonian-Laird method. Conversely, in the absence of substantial heterogeneity, we applied a fixed-effect model with the Inverse Variance method. To assess the potential for publication bias, we utilized Egger’s test [14] and Begg’s test [15]. Sensitivity analysis was carried out by systematically excluding each study to assess the resilience of the findings. Subgroup analysis was also conducted, taking into account factors such as Cox regression analysis, cancer type, and treatment methods.
Results
Search results and included studies
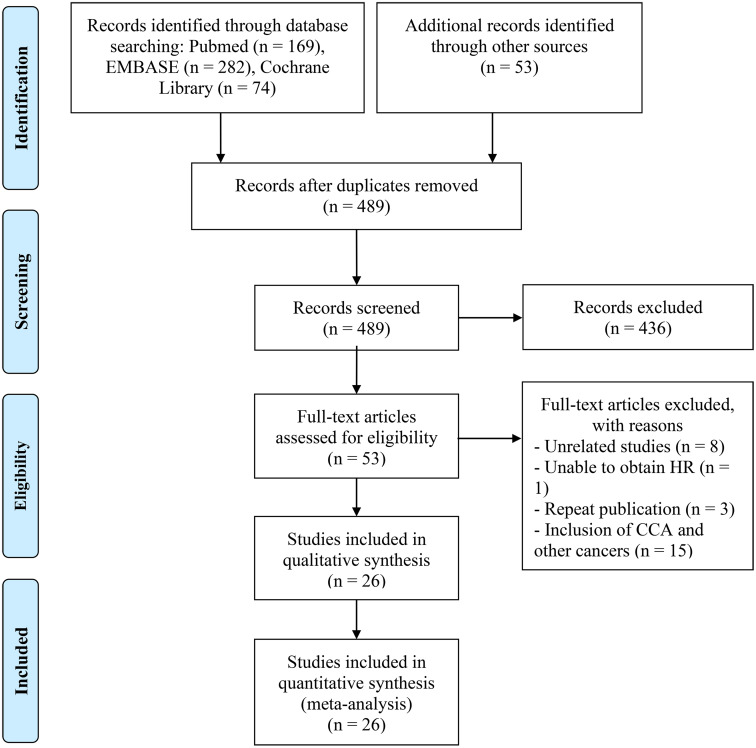
Figure 1 visually presents the outcomes as depicted in the PRISMA flow diagram. Initially, a total of 578 articles were identified via database searches and manual searches. Following the elimination of duplicate entries, 489 distinct articles were identified. Subsequently, a meticulous review of the titles and abstracts led to the exclusion of 436 articles that were deemed ineligible. Of the remaining pool, 53 articles were subjected to a comprehensive full-text review, ultimately including 26 articles (28 studies) that met established criteria for analysis [16–41].
Fig. 1.
The flow diagram of identifying eligible studies
Study characteristics
The main characteristics of the studies included in this analysis are summarized in Table 1. A total of 4,398 patients, with 57.34% of them being males, were included in the study. The mean or median age of the patients ranged from 56.4 to 72.0 years, and the sample sizes varied from 41 to 460 individuals. Regarding the geographical distribution of the studies, twelve were conducted in Japan, six in China, five in Germany, and two in Korea. Additionally, there was one cohort each from France, the Netherlands, and Rotterdam. These cohorts exhibited diversity in terms of the specific CCA types they enrolled, with thirteen cohorts focused on patients with iCCA, ten on pCCA, three on dCCA, and one on CCA. 24 cohorts of patients underwent surgical treatment. Body composition was measured on CT scans at the level of the third lumbar vertebra in all studies. Furthermore, the 28 cohorts received NOS scores ranging from 6 to 8, underscoring a minimal likelihood of bias (Table 1).
Table 1.
Main characteristics of the studies included
| Study | Study region | Study period | Study design | Sample size | Age | Male/female | Site | Treatment | Body compositions and outcomes | Methods, site, and software | Cut-off | NOS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coelen et al. [16] | Nether-lands | 1998–2013 | R | 100 | - | 64/36 | PHC | Surgery | SMI (OS, PMC, Liver failure) | CT, L3, OsiriX Version 5.8 | < 46.8 cm2/m2 in males and < 39.1 cm2/m2 in females | 7 | |
| Zhou et al. [17] | China | 01/2000-08/2014 | R | 67 | 61 (47–81)c | 22/45 | ICC | Surgery | SMI (OS, RFS, PMC) | CT, L3 | < 43.8 cm2/m2 in males and < 41.1 cm2/m2 in females | 6 | |
| Okumura et al. [18] | Japan | 01/2004-04/2015 | R | 109 | 68 (61–73)c | 67/42 | ICC | Surgery | SMI (OS, RFS, PMC), SMD (OS, RFS) | CT, L3, Aquarius iNtuition Server | SMI: < 52.5 cm2/m2 in males and < 41.2 cm2/m2 in females; SMD: < 38.3 HU in males and < 31.0 HU in females | 7 | |
| Umetsu et al. [19] | Japan | 02/2008-03/2015 | R | 65 | 72 (31–81)b | 47/18 | DCC | Surgery | PMI (OS, RFS, PMC) | CT, L3 | < 5.9 cm2/m2 in males and < 3.5 cm2/m2 in females | 7 | |
| Yugawa et al. [23] | Japan | 05/2000-10/2017 | R | 61 | - | 42/19 | ICC | Surgery | PMA (OS, RFS) | CT, L3 | < 34.6 cm2 in males and < 18.1 cm2 in females | 6 | |
| Hahn et al. [20] | Germany | 01/1997-01/2018 | R | 361 | 64.8 (56–72)c | 206/155 | ICC | Surgical or non-surgical treatment | PMI (OS) | CT, L3 | < 5.7 cm2/m2 in males and < 5.1 cm2/m2 in females | 8 | |
| Kitano et al. [21] | Japan | 04/2005-12/2014 | R | 110 | - | 75/35 | PHC, DCC | Surgery | SMI (OS), SAI (OS), VAI (OS) | CT, L3, SYNAPSE VINCENT software | SMI: < 43 cm2/m2 in male and < 41 cm2/m2 in female in patients with a BMI < 25 kg/m2, < 53 cm2/m2 in male in patients with a BMI ≥ 25 kg/m2; SAI: < 82 cm2; VAI: < 153 cm2 | 7 | |
| Van et al. [22] | Rotterdam | 2002–2014 | R | 233 | 66 (57–74)c | 140/93 | PHC | Surgical or non-surgical treatment | SMI (OS), SMD (OS) | CT, L3 | SMI: < 46.8 cm2/m2 in males and < 39.1 cm2/m2 in females; SMD: median value | 7 | |
| Deng et al. [28] | China | 08/2012-10/2019 | P | 121 | 65 (40–87)c | 52/69 | ICC | Surgery | PMI (OS, RFS) | CT, L3 | < 8.6 cm2/m2 in males and < 6.0 cm2/m2 in females | 6 | |
| Jördens et al. [25] | Germany | 2011–2021 | R | 75 | 70 (30–87)b | 40/35 | CCA | Non-surgical treatment | SMI (OS), PMI (OS), SMD (OS) | CT, L3, 3D Slicer | SMI: < 72 cm2/m2; PMI: < 6.3 cm2/m2; SMD: < 30.5 HU | 6 | |
| Li et al. [26](D) | China | 01/2009-05/2017 | R | 460 | 58 (49–64)c | 223/237 | ICC | Surgery | SMI (OS, RFS) | CT, L3, Mimics | < 42.6 cm2/m2 in males and < 37.8 cm2/m2 in females | 7 | |
| Li et al. [26](V) | 153 | 59 (50–67)c | 87/66 | ||||||||||
| Zhang et al. [28] | China | 01/2016-03/2019 | R | 104 | 56.4 ± 22.9a | 56/48 | PHC | Non-surgical treatment | SMI (OS, PMC) | CT, L3, MAGNETOM Skyra | < 47.0 cm2/m2 in males and < 35.1 cm2/m2 in females | 6 | |
| Lee et al. [29] | Korea | 01/2006-12/2016 | P | 328 | - | 220/108 | PHC | Surgery | SMI (Liver failure) | CT, L3, MATLAB version R2010a | < 50.2 cm2/m2 in males and < 38.6 cm2/m2 in females | 7 | |
| Lurje et al. [30](i) | Germany | 05/2010-12/2019 | R | 86 | 65 ± 11.4a | 49/37 | ICC | Surgery | SMI (OS, PMC), SMD (OS) | CT, L3, 3D Slicer software | SMI: < 43 cm2/m2 in male and < 41 cm2/m2 in female in patients with a BMI < 25 kg/m2, < 53 cm2/m2 in male in patients with a BMI ≥ 25 kg/m2; SMD: < 41 HU in patients with a BMI < 25 kg/m2, < 33 HU in patients with a BMI ≥ 25 kg/m2 | 7 | |
| Lurje et al. [30](p) | 103 | 66 ± 10.4a | 32/71 | PHC | |||||||||
| Umezawa et al. [31] | Japan | 01/2006-04/2020 | R | 88 | - | 65/23 | DCC | Surgery | PMI (PMC) | CT, L3 | < 5.9 cm2/m2 in males and < 4.0 cm2/m2 in females | 6 | |
| Tamura et al. [27] | Japan | 09/2002-12/2017 | R | 111 | 72 (39–85)b | 86/25 | DCC | Surgery | SMI (OS) | CT, L3, SYNAPSE VINCENT software | < 55 cm2/m2 in males and < 36 cm2/m2 in females | 6 | |
| Watanabe et al. [32] | Japan | 08/2007-08/2021 | R | 58 | - | 42/16 | PHC | Surgery | PMI (OS) | CT, L3 | < 6.4 cm2/m2 in males and < 3.9 cm2/m2 in females | 7 | |
| Taniai et al. [40] | Japan | 01/2007-12/2019 | R | 41 | 63 (55–68)c | 21/20 | ICC | Surgery | PMA (OS) | CT, L3 | < 31.5 cm2 in males and < 14.9 cm2 in females | 7 | |
| Miki et al. [39] | Japan | 01/2008-06/2022 | R | 71 | 68.3 ± 8.6a | 46/25 | ICC | Surgery | PMI (OS, RFS), SMD (OS, RFS) | CT, L3 | PMI: < 6.4 cm2/m2 in males and < 3.9 cm2/m2 in females; SMD: -0.0215 | 6 | |
| Lacaze et al. [37] | France | 01/2004-11/2016 | R | 91 | - | - | ICC | Surgery | SMI (OS), SAI (OS), VAI (OS) | CT, L3, ImageJ® software | SMI: < 52.4 cm2/m2 in males and < 38.5 cm2/m2 in females; SAI: < 50 cm²/m²; VAI: < 50 cm²/m² | 7 | |
| Lurje et al. [38] | Germany | 03/2010-12/2020 | R | 173 | 65 (23–83)b | 86/87 | ICC | Surgery | SMI (PMC), SMD (OS) | CT, L3, semi-automatically with 3D Slicer | SMI: < 52.4 cm2/m2 in males and < 38.5 cm2/m2 in females; SMD: < 41 HU in patients with a BMI < 25 kg/m2, < 33 HU in patients with a BMI ≥ 25 kg/m2 | 8 | |
| Jung et al. [36] | Korea | 11/2005-06/2022 | R | 317 | 65.6 ± 9.0a | 202/115 | PHC | Surgery | PMI (PMC) | CT, L3, Aquarius iNtuition Viewer | < 6.7 cm2/m2 in males and < 3.4 cm2/m2 in females | 8 | |
| Hayashi et al. [35] | Japan | 01/2013-12/2019 | R | 89 | - | 55/34 | PHC | Surgery | PMI (OS, RFS) | CT, L3, SYNAPSE VINCENT software | < 6.4 cm2/m2 in males and < 3.9 cm2/m2 in females | 7 | |
| Asai et al. [34] | Japan | 01/2008-12/2018 | R | 456 | 69d | 308/148 | PHC | Surgery | PMI (OS, PMC), SMD (OS) | CT, L3, | SMI: < 6.4 cm2/m2 in males and < 4.7 cm2/m2 in females; SMD: < 40.6 HU in male and < 35.4 HU in females | 8 | |
| Yasuta et al. [33] | Japan | 05/2006-06/2017 | R | 65 | 70 (39–81)b | 38/27 | PHC | Surgery | SMI (OS, RFS) | CT, L3, SYNAPSE VINCENT software | < 43 cm2/m2 in male and < 41 cm2/m2 in female in patients with a BMI < 25 kg/m2, < 53 cm2/m2 in male in patients with a BMI ≥ 25 kg/m2 | 6 | |
| Zhao et al. [41] | China | 07/2015-07/2021 | R | 302 | 63 (57–69)c | 151/151 | ICC | Surgery | SMI (OS, RFS, PMC), SMD (OS, PFS) | CT, L3, Image J software | SMI: < 53.5 cm2/m2 in males and < 39.9 cm2/m2 in females; SMD: < 41 HU in patients with BMI < 25 kg/m2 and < 33 HU in patients with BMI ≥ 25 kg/m2 | 8 |
R, retrospective study; P, prospective study; CCA, cholangiocarcinoma, PHC, perihilar cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; DCC, distal cholangiocarcinoma; PMC, postoperative major complications; SMI, skeletal muscle index; SMD, skeletal muscle density; PMI, psoas muscle index; SAI, subcutaneous adipose index; VAI, visceral adipose index; OS, overall survival; RFS, relapse-free survival; L3, third lumbar vertebra
aMean ± standard deviation; bmedians with ranges; cmedian and interquartile range; dmedians
Association of baseline skeletal muscle index with overall and recurrence-free survival
The effect of pre-treatment SMI levels on OS and RFS in patients with CCA has been investigated in 15 studies with 2169 patients and six studies with 1156 patients, respectively. Our study found that patients with low SMI had significantly worse OS (HR: 1.93, 95% CI: 1.55–2.40, p < 0.001, Fig. 2A) and RFS (HR: 2.02, 95% CI: 1.73–2.36, p < 0.001, Fig. 2B) than those with high SMI. A random-effects model was used for OS since the Cochran’s Q test and I2 statistics showed significant heterogeneity (I2 = 61.6%, p = 0.001). In contrast, a fixed-effects model was used for RFS as no significant heterogeneity (I2 = 35.0%, p = 0.174) existed, suggesting methodological homogeneity between the studies.
Fig. 2.
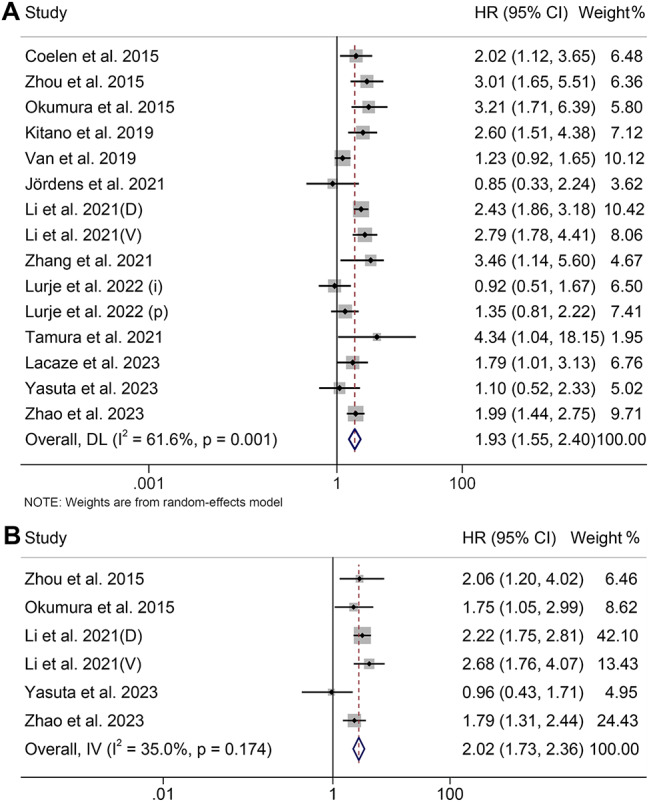
Forest plots of the relationship between skeletal muscle index and overall survival (A) and recurrence-free survival (B). HR, hazard ratio; CI, confidence interval
Baseline skeletal muscle index and postoperative complications
The analysis of the influence of a low SMI on the development of postoperative major complications is depicted in Figure S1A. We considered the heterogeneity to be low based on the value of I2 (I2 = 9.7, p = 0.355), so the fixed-effects model was applied. The results revealed that the pooled OR was 1.69 (95% CI: 1.20–2.38), indicating a markedly higher risk of postoperative major complications for patients with low SMI compared to those with high SMI. Besides, two studies have explored the relationship between baseline SMI and liver failure (International Study Group of Liver Surgery, grade ≥ B), and the pooled results showed that low SMI does not lead to a higher incidence of liver failure (OR: 1.64, 95% CI: 0.58–4.60, p = 0.351, Figure S1B).
Association of baseline psoas muscle index with prognosis and postoperative major complications
A total of eight studies, involving 1296 patients, were included in this analysis, examining the impact of baseline PMI levels on OS or RFS in CCA patients. There was no significant heterogeneity among studies (OS: I2 = 37.8%, p = 0.128; RFS: I2 = 0, p = 0.407), and thus, a fixed-effect model was used. Our findings demonstrated that patients with low PMI had significantly inferior OS (HR: 1.56, 95% CI: 1.33–1.84, p < 0.001, Fig. 3A) and PFS (HR: 2.16, 95% CI: 1.55–3.03, p < 0.001, Fig. 3B) compared to those with high PMI.
Fig. 3.
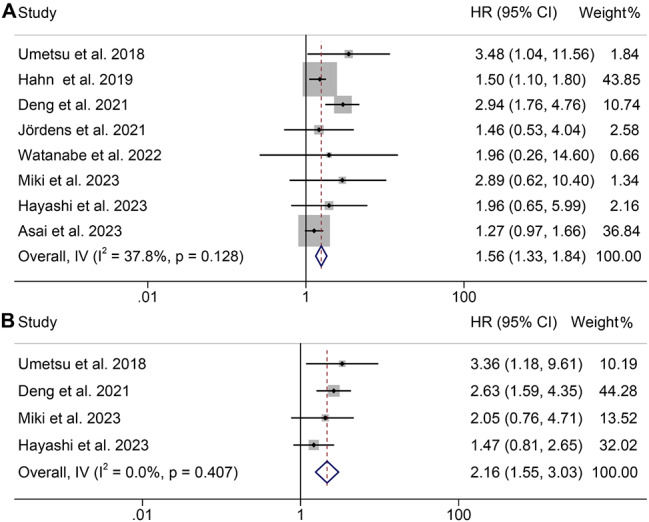
Forest plots of the relationship between psoas muscle index and overall survival (A) and recurrence-free survival (B). HR, hazard ratio; CI, confidence interval
We also analyzed the relationship between PMI levels and postoperative major complications in CCA patients using four studies with 926 patients. Notably, there was significant heterogeneity among the included studies (I2 = 71.1%, p = 0.016), so a random-effects model was employed. The results showed that the incidence of major complications in patients with low PMI was not significantly different from that in patients with high PMI (OR: 1.02, 95% CI: 0.55–1.87, p = 0.961, Figure S2).
Baseline myosteatosis and overall and recurrence-free survival
This analysis included a total of nine studies, encompassing 1608 patients, which investigated the impact of myosteatosis on OS or RFS in CCA patients. It’s important to note that there was significant heterogeneity among the OS studies (I2 = 51.5%, p = 0.036), leading to the use of a random effects model. Conversely, the RFS studies exhibited the opposite pattern, allowing for the use of a fixed effects model. Our findings indicate that patients with myosteatosis had notably poorer OS (HR: 1.49, 95% CI: 1.17–1.88, p = 0.001, Fig. 4A) and RFS (HR: 1.35, 95% CI: 1.04–1.75, p = 0.023, Fig. 4B) compared to those without myosteatosis.
Fig. 4.
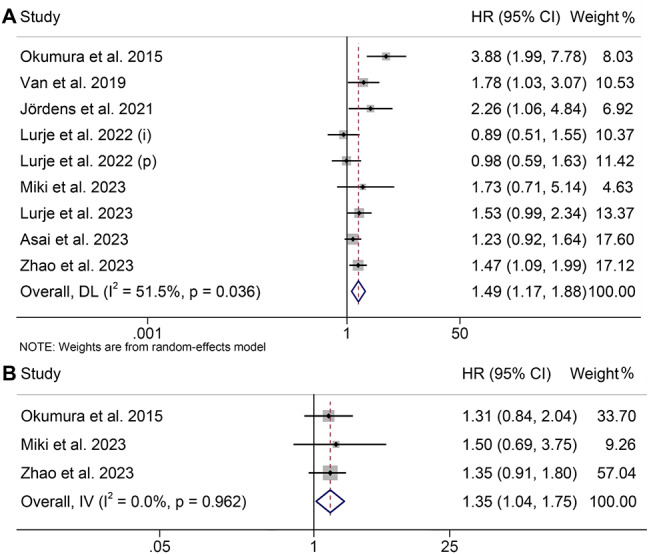
Funnel plots of the relationship between myosteatosis and overall survival (A) and recurrence-free survival (B). HR, hazard ratio; CI, confidence interval
Pre-treatment subcutaneous adipose index, visceral adipose index, and overall survival
Two studies involving 201 patients examined the predictive roles of SAI and VAI on the prognosis of CCA patients. We found no correlation between the levels of SAI (I2 = 0, p = 0.361, HR: 0.90, 95% CI: 0.63–1.28, p = 0.560, Figure S3A) and VAI (I2 = 92.2%, p < 0.001, HR: 1.35, 95% CI: 0.40–4.54, p = 0.627, Figure S3B) and the OS of CCA patients.
Relationship of sarcopenia levels with prognosis and postoperative major complications
14 studies (2094 patients) used SMI, eight studies used PMI (1296 patients), and two studies used PMA (102 patients) to diagnose sarcopenia. 24 studies with 3492 patients investigated the association between sarcopenia and OS, while 11 studies with 1261 patients explored the RFS. Sarcopenia was shown to be an unfavorable predictor, with the pooled HR for OS being 1.96 (95% CI: 1.65–2.31) and RFS being 2.05 (95% CI: 1.79–2.36) compared with those patients without sarcopenia (Fig. 5A and B).
Fig. 5.
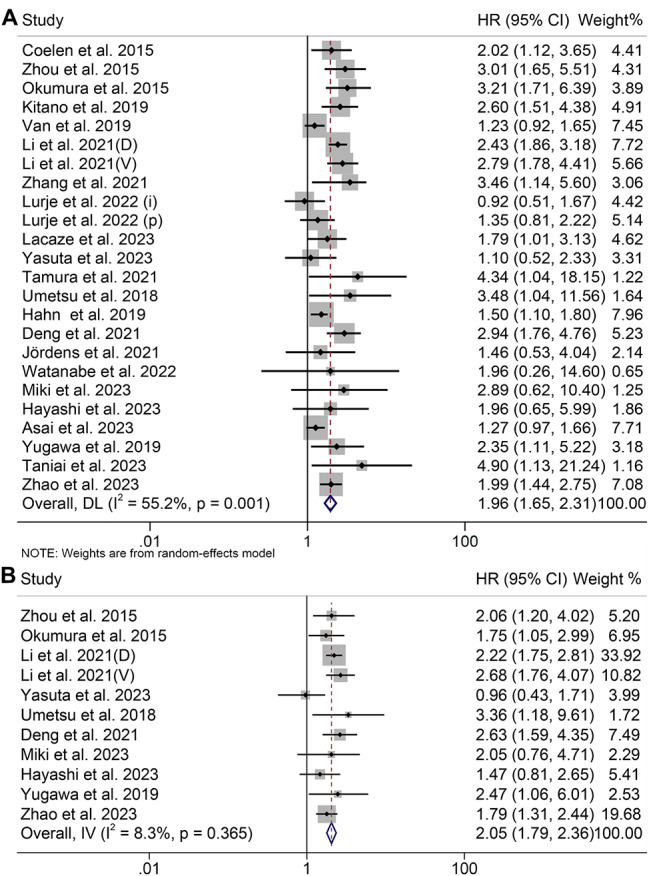
Funnel plots of the relationship between sarcopenia and overall survival (A) and recurrence-free survival (B). HR, hazard ratio; CI, confidence interval
We also investigated the relationship between sarcopenia and postoperative major complications in CCA patients using data from 11 studies with 1970 patients. CCA patients with sarcopenia had a higher probability of postoperative major complications (OR: 1.39, 95% CI: 1.00-1.94, p = 0.049, Figure S4).
Subgroup analysis
Subgroup analysis was performed according to the Cox regression analysis, cancer type, and treatment (Tables 2 and 3). The results of both univariate and multivariate analysis consistently supported the prediction of OS and RFS by SMI, PMI, and sarcopenia (Tables 2 and 3), confirming the reliability of our findings. Subgroup analysis by cancer types revealed that SMI, PMI, and sarcopenia significantly predicted worse OS in patients with ICC and PHC, while these factors also significantly predicted worse RFS in patients with ICC (Tables 2 and 3). Upon analyzing subgroups based on treatment, we confirmed that SMI, PMI, and sarcopenia were linked to poorer OS and RFS in CCA patients who underwent surgery (Tables 2 and 3).
Table 2.
Subgroup analysis of the association between baseline body composition and the outcomes for cholangiocarcinoma
| Variable | Included studies | Test of association | Test of heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | Modal | I2 | p-value | |||
| Skeletal muscle index (OS) | ||||||||
| Cox regression analysis | ||||||||
| Multivariate analysis | 8 | 2.36 | 1.85–3.01 | p < 0.001 | R | 19.9% | p = 0.272 | |
| Univariate analysis | 7 | 1.59 | 1.15–2.20 | p = 0.005 | R | 73.3% | p = 0.001 | |
| Cancer type | ||||||||
| ICC | 7 | 2.17 | 1.68–2.80 | p < 0.001 | R | 54.7% | p = 0.039 | |
| PHC | 5 | 1.54 | 1.11–2.16 | p = 0.011 | R | 47.3% | p = 0.108 | |
| Other | 3 | 2.02 | 0.87–4.68 | p = 0.100 | R | 59.6% | p = 0.084 | |
| Treatment | ||||||||
| Surgery | 13 | 2.10 | 1.71–2.58 | p < 0.001 | R | 46.6% | p = 0.033 | |
| Other | 2 | 1.19 | 0.90–1.58 | p = 0.216 | R | 0 | p = 0.477 | |
| Skeletal muscle index (RFS) | ||||||||
| Cox regression analysis | ||||||||
| Multivariate analysis | 3 | 1.82 | 1.43–2.33 | p = 0.002 | R | 0 | p = 0.908 | |
| Univariate analysis | 3 | 1.97 | 1.28–2.26 | p = 0.002 | R | 68.6% | p = 0.041 | |
| Cancer type | ||||||||
| ICC | 5 | 2.10 | 1.79–2.46 | p < 0.001 | F | 0 | p = 0.560 | |
| PHC | 1 | 0.96 | 0.48–1.91 | p = 0.908 | - | - | - | |
| Treatment | ||||||||
| Surgery | 6 | 2.02 | 1.73–2.36 | p < 0.001 | F | 35.0% | p = 0.174 | |
| Psoas muscle index (OS) | ||||||||
| Cox regression analysis | ||||||||
| Multivariate analysis | 6 | 1.68 | 1.26–2.24 | p < 0.001 | R | 47.2% | p = 0.092 | |
| Univariate analysis | 2 | 2.99 | 1.07–8.40 | p = 0.037 | R | 0 | p = 0.632 | |
| Cancer type | ||||||||
| ICC | 3 | 2.10 | 1.20–3.67 | p = 0.009 | R | 65.7% | p = 0.046 | |
| PHC | 3 | 1.31 | 1.01–1.70 | p = 0.041 | R | 0 | p = 0.701 | |
| Other | 2 | 2.12 | 0.92–4.91 | p = 0.080 | R | 14.0% | p = 0.281 | |
| Treatment | ||||||||
| Surgery | 6 | 2.09 | 1.28–3.39 | p = 0.001 | R | 54.6% | p = 0.051 | |
| Other | 2 | 1.50 | 1.18–1.90 | p = 0.003 | R | 0 | p = 0.963 | |
| Psoas muscle index (RFS) | ||||||||
| Cox regression analysis | ||||||||
| Multivariate analysis | 1 | 2.63 | 1.59–4.35 | p < 0.001 | F | - | - | |
| Univariate analysis | 3 | 1.85 | 1.18–2.90 | p = 0.007 | F | 0 | p = 0.393 | |
| Cancer type | ||||||||
| ICC | 2 | 2.48 | 1.60–3.86 | p < 0.001 | F | 0 | p = 0.639 | |
| Other | 2 | 1.79 | 1.07–3.01 | p = 0.026 | F | 44.7% | p = 0.179 | |
| Treatment | ||||||||
| Surgery | 4 | 2.16 | 1.55–3.03 | p < 0.001 | F | 0 | p = 0.407 | |
PHC, perihilar cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; HR, hazard ratio; CL, confidence interval; OS, overall survival; RFS, recurrence-free survival; R, random-effect model; F, fixed-effect model
Table 3.
Subgroup analysis of the association between Sarcopenia and the outcomes for cholangiocarcinoma
| Variable | Included studies | Test of association | Test of heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | Modal | I2 | p-value | |||
| Skeletal muscle index (OS) | ||||||||
| Cox regression analysis | ||||||||
| Multivariate analysis | 8 | 2.36 | 1.85–3.01 | p < 0.001 | R | 19.9% | p = 0.272 | |
| Univariate analysis | 7 | 1.59 | 1.15–2.20 | p = 0.005 | R | 73.3% | p = 0.001 | |
| Cancer type | ||||||||
| ICC | 7 | 2.17 | 1.68–2.80 | p < 0.001 | R | 54.7% | p = 0.039 | |
| PHC | 5 | 1.54 | 1.11–2.16 | p = 0.011 | R | 47.3% | p = 0.108 | |
| Other | 3 | 2.02 | 0.87–4.68 | p = 0.100 | R | 59.6% | p = 0.084 | |
| Treatment | ||||||||
| Surgery | 13 | 2.10 | 1.71–2.58 | p < 0.001 | R | 46.6% | p = 0.033 | |
| Other | 2 | 1.19 | 0.90–1.58 | p = 0.216 | R | 0 | p = 0.477 | |
| Skeletal muscle index (RFS) | ||||||||
| Cox regression analysis | ||||||||
| Multivariate analysis | 3 | 1.82 | 1.43–2.33 | p = 0.002 | R | 0 | p = 0.908 | |
| Univariate analysis | 3 | 1.97 | 1.28–2.26 | p = 0.002 | R | 68.6% | p = 0.041 | |
| Cancer type | ||||||||
| ICC | 5 | 2.10 | 1.79–2.46 | p < 0.001 | F | 0 | p = 0.560 | |
| PHC | 1 | 0.96 | 0.48–1.91 | p = 0.908 | - | - | - | |
| Treatment | ||||||||
| Surgery | 6 | 2.02 | 1.73–2.36 | p < 0.001 | F | 35.0% | p = 0.174 | |
| Psoas muscle index (OS) | ||||||||
| Cox regression analysis | ||||||||
| Multivariate analysis | 6 | 1.68 | 1.26–2.24 | p < 0.001 | R | 47.2% | p = 0.092 | |
| Univariate analysis | 2 | 2.99 | 1.07–8.40 | p = 0.037 | R | 0 | p = 0.632 | |
| Cancer type | ||||||||
| ICC | 3 | 2.10 | 1.20–3.67 | p = 0.009 | R | 65.7% | p = 0.046 | |
| PHC | 3 | 1.31 | 1.01–1.70 | p = 0.041 | R | 0 | p = 0.701 | |
| Other | 2 | 2.12 | 0.92–4.91 | p = 0.080 | R | 14.0% | p = 0.281 | |
| Treatment | ||||||||
| Surgery | 6 | 2.09 | 1.28–3.39 | p = 0.001 | R | 54.6% | p = 0.051 | |
| Other | 2 | 1.50 | 1.18–1.90 | p = 0.003 | R | 0 | p = 0.963 | |
| Psoas muscle index (RFS) | ||||||||
| Cox regression analysis | ||||||||
| Multivariate analysis | 1 | 2.63 | 1.59–4.35 | p < 0.001 | F | - | - | |
| Univariate analysis | 3 | 1.85 | 1.18–2.90 | p = 0.007 | F | 0 | p = 0.393 | |
| Cancer type | ||||||||
| ICC | 2 | 2.48 | 1.60–3.86 | p < 0.001 | F | 0 | p = 0.639 | |
| Other | 2 | 1.79 | 1.07–3.01 | p = 0.026 | F | 44.7% | p = 0.179 | |
| Treatment | ||||||||
| Surgery | 4 | 2.16 | 1.55–3.03 | p < 0.001 | F | 0 | p = 0.407 | |
PHC, perihilar cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; HR, hazard ratio; CL, confidence interval; OS, overall survival; RFS, recurrence-free survival; R, random-effect model; F, fixed-effect model
Sensitivity analysis and publication bias
Sensitivity analysis and publication bias were first used to assess the robustness of the relationship between SMI and survival outcomes. Excluding individual studies did not significantly impact the pooled HR for OS, which ranged from 1.88 (95% CI: 1.48–2.38, after excluding Li et al. [26](D)) to 2.03 (95% CI: 1.65–2.51, after excluding Van et al. 2019, Figure S5A), and for RFS, which ranged from 1.89 (95% CI: 1.54–2.31, after excluding Li et al. [26](D)) to 2.10 (95% CI: 1.76–2.51, after excluding Zhao et al. 41, Figure S5B). Begg’s and Egger’s tests did not show significant publication bias for OS (Egger’s test: p = 0.823, Begg’s test: p = 0.767) and RFS (Egger’s test: p = 0.307, Begg’s test: p = 0.452).
Similarly, sensitivity analysis and publication bias have been used to explore the stability and reliability of the relationship between PMI and sarcopenia and OS and RFS. The results demonstrated that the survival outcome of the primary analysis was not impacted by removing any single study (Figure S5C and S5D , Figure S6A and S6B). The Egger’s and Begg’s tests confirmed the absence of significant publication bias in OS (PMI, Egger’s test: p = 0.146, Begg’s test: p = 0.386; sarcopenia, Egger’s test: p = 0.164, Begg’s test: p = 0.535) and RFS (PMI, Egger’s test: p = 0.809, Begg’s test: p = 1.000; sarcopenia, Egger’s test: p = 0.730, Begg’s test: p = 0.755). As evident from the above, our results are both stable and reliable.
Discussion
In our study, we included 28 studies with a total of 4398 patients with CCA. According to the results, forest plots clearly showed that low SMI, low PMI, myosteatosis, and sarcopenia could significantly predict worse OS and DFS. Low SMI and sarcopenia were also shown to be risk factors for postoperative major complications.
The etiologies driving the progression of skeletal muscle loss in advanced cancer patients are intricate [42]. While in healthy adults, muscle loss is primarily attributed to the natural aging process, with muscle mass diminishing at a rate of approximately 1% per annum [43], the acceleration of muscle loss is notably more pronounced when an underlying illness, particularly malignancy, is present [43]. Building upon this knowledge, our current investigation reveals that SMI and sarcopenia exhibit robust potential as a simplified indicator of unfavorable prognostic outcomes in CCA patients facing competing mortality risks. In these individuals, reduced muscle mass not only signifies the effects of aging but also represents the advancement of disease, with the latter’s significance remaining independent of age-related skeletal muscle loss [42, 44]. Typically, the decline in muscle mass arises from constraints in nutrient intake and availability. Principally, both compromised dietary intake and impaired nutrient uptake fail to maintain metabolic equilibrium [45, 46], while cancer-related physical inactivity further exacerbates the deterioration in muscle quality and morphology [47, 48]. Consequently, the assessment of low SMI or PMI may hold the potential to offer comprehensive insights for treatment strategies aimed at extending the survival of such patients, although further investigations are necessary to validate its clinical utility.
The SMI is regarded as the established gold standard for quantifying muscle mass in scientific investigations in alignment with the guidelines set forth by the European Working Group on Sarcopenia in Older People (EWGSOP) [42]. Nevertheless, alternate methods such as bioelectric impedance analysis (BIA) and dual-energy X-ray absorptiometry (DXA) are also recognized as conventional approaches for evaluating muscle mass. BIA entails the application of a low-intensity alternating electrical current through the body. Since this current is conducted primarily through bodily water, impedance exhibits an inverse relationship with total body water, thereby enabling the computation of total muscle mass. On the other hand, DXA measures the differential attenuation of X-rays at two distinct energy levels as they traverse the body. This method delineates a three-part model of body composition encompassing fat, bone mineral content, and lean tissue [49]. However, a previous investigation revealed that BIA tended to underestimate muscle mass when compared to DXA measurements [50]. Hence, our current investigation opted to gauge muscle mass through the SMI and PMI, which obviated the need for any additional radiation exposure. This is in stark contrast to DXA, as computed tomography (CT) scans were regularly performed as part of the preoperative assessment and cancer staging process.
Some potential rationales underpin the link between sarcopenia and an adverse prognosis in CCA patients. To begin, tumors exhibiting heightened metabolic activity, often associated with a more aggressive phenotype, may contribute to the development of sarcopenia [51]. Furthermore, the recognition of skeletal muscle and adipose tissue as secretory organs has expanded our understanding, with a myriad of cytokines and peptides, categorized as myokines, emanating from skeletal muscle [52]. These myokines include interleukin (IL)-6, insulin-like growth factor-1, and IL-15. In parallel, adipose tissue releases a repertoire of adipocytokines, known as adipokines, among which adiponectin and leptin feature prominently [53]. Recent research has highlighted the influence of myokines and adipokines on the immune system, particularly on natural killer cells—essential players in the defense against intracellular infections and cancer [54]. For example, IL-15 not only restrains fat deposition and insulin resistance but also plays a pivotal role in the maturation and viability of natural killer cells [55]. A decline in muscle mass is anticipated to result in reduced IL-15 production, thus compromising immune function. Conversely, an expansion of adipose tissue leads to an upsurge in pro-inflammatory molecules, including leptin, tumor necrosis factor-alpha, and IL-6, which, in turn, hinder the activity of natural killer cells [54]. Additional mechanisms proposed encompass diminished mitochondrial oxidative capacity within muscle tissue [56] and the adipogenic transformation of muscle satellite cells [57]. This intricate interplay between muscle and adipose tissue, mediated by myokines and adipokines, coupled with the intricate interrelations of immunity and inflammation, has more recently emerged as the central mechanism through which sarcopenia exerts its impact on patient survival [58]. Thus, such theories as the above support our finding that low SMI or sarcopenia is an influential factor in the poor prognosis of CCA.
Several constraints necessitate acknowledgment within the context of this analysis. To commence, it is imperative to recognize the paucity of studies that have specifically examined myosteatosis, SAI, and VAI in the context of CCA, warranting further confirmation to elucidate their implications for CCA outcomes. It is crucial to note the variability in cut-off values employed for identical diagnostic parameters across different investigations. Additionally, other potential sources of bias and confounding factors, such as patient heterogeneity (in terms of cancer stage, treatments received, and comorbidities), variations in imaging techniques used to assess body composition, and retrospective study designs, may have influenced the results.
To furnish more robust and dependable insights, it is essential that future studies incorporate several key design improvements. First, large-scale, prospective, multicenter studies should be conducted to ensure greater generalizability of findings, with standardized diagnostic criteria for myosteatosis, SAI, and VAI. Second, the use of advanced imaging techniques, such as MRI or CT scans, standardized across different institutions, would enable more consistent body composition assessments. Third, patient cohorts should be stratified by cancer stage, treatment type, and other clinical variables to mitigate the impact of confounders. Lastly, efforts should be made to explore the mechanistic pathways linking body composition changes to CCA outcomes, which could further inform personalized treatment strategies.
Conclusion
In conclusion, low SMI and sarcopenia were found to be strongly associated with shorter survival and higher rates of postoperative complications in CCA patients. These findings underscore the importance of incorporating sarcopenia assessments into the routine clinical evaluation of CCA patients. Early identification of sarcopenia could enable healthcare providers to tailor treatment strategies more effectively, including preemptive nutritional interventions and personalized management plans to mitigate adverse outcomes. Moreover, our study highlights the need for further research to validate these findings and explore the potential benefits of integrating sarcopenia-targeted therapies into CCA treatment regimens. By adopting these practices, clinicians can improve patient outcomes and optimize perioperative care.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
ZL, WK, LR, CC, YF, and WW conceived and designed the study. ZL, WK, LR, and KT were responsible for the collection and assembly of data, data analysis, and interpretation. ZL, WK, and LR were involved in writing the manuscript. ZL, WK, LR, CC, YF, and WW revised the manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 82172855, 82370654).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lilong Zhang, Kunpeng Wang and Rongqiang Liu contributed equally to this work.
Contributor Information
Feng Yao, Email: rm000751@whu.edu.cn.
Weixing Wang, Email: wangwx@whu.edu.cn.
References
- 1.Blumenthal G. M., Pazdur R. Approvals in 2016: the march of the checkpoint inhibitors. Nat Rev Clin Oncol, 2017;14(3):131–2. [DOI] [PubMed]
- 2.Banales J. M., Marin J. J. G., Lamarca A., Rodrigues P. M., Khan S. A., Roberts L. R., Cardinale V., Carpino G., Andersen J. B., Braconi C., Calvisi D. F., Perugorria M. J., Fabris L., Boulter L., Macias R. I. R., Gaudio E., Alvaro D., Gradilone S. A., Strazzabosco M., Marzioni M., Coulouarn C., Fouassier L., Raggi C., Invernizzi P., Mertens J. C., Moncsek A., Rizvi S., Heimbach J., Koerkamp B. G., Bruix J., Forner A., Bridgewater J., Valle J. W., Gores G. J. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol, 2020;17(9):557–88. [DOI] [PMC free article] [PubMed]
- 3.Brindley P. J., Bachini M., Ilyas S. I., Khan S. A., Loukas A., Sirica A. E., Teh B. T., Wongkham S., Gores G. J. Cholangiocarcinoma. Nat Rev Dis Primers, 2021;7(1):65. [DOI] [PMC free article] [PubMed]
- 4.Valle J. W., Vogel A., Denlinger C. S., He A. R., Bai L. Y., Orlova R., Van Cutsem E., Adeva J., Chen L. T., Obermannova R., Ettrich T. J., Chen J. S., Wasan H., Girvan A. C., Zhang W., Liu J., Tang C., Ebert P. J., Aggarwal A., Mcneely S. C., Moser B. A., Oliveira J. M., Carlesi R., Walgren R. A., Oh D. Y. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: a randomised, double-blind, multicentre, phase 2 study. Lancet Oncol. 2021;22(10):1468–82. [DOI] [PubMed] [Google Scholar]
- 5.Muscaritoli M., Anker S. D., Argilés J., Aversa Z., Bauer J. M., Biolo G., Boirie Y., Bosaeus I., Cederholm T., Costelli P., Fearon K. C., Laviano A., Maggio M., Rossi Fanelli F., Schneider S. M., Schols A., Sieber C. C. Consensus definition of Sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest groups (SIG) cachexia-anorexia in chronic wasting diseases and nutrition in geriatrics. Clin Nutr. 2010;29(2):154–9. [DOI] [PubMed] [Google Scholar]
- 6.Levolger S., Van Vugt J. L., De Bruin R. W., Jn I. Jzermans. Systematic review of Sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102(12):1448–58. [DOI] [PubMed] [Google Scholar]
- 7.Jogiat U. M., Bédard E. L. R., Sasewich H., Turner S. R., Eurich D. T., Filafilo H., Baracos V. Sarcopenia reduces overall survival in unresectable oesophageal cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(6):2630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamarajah S. K., Bundred J., Tan B. H. L. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer, 2019;22(1):10–22. [DOI] [PubMed]
- 9.Bundred J., Kamarajah S. K., Roberts K. J. Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford), 2019;21(12):1603–12. [DOI] [PubMed]
- 10.Gamboa A. C., Maithel S. K. The Landmark Series: Gallbladder Cancer. Ann Surg Oncol, 2020;27(8):2846–58. [DOI] [PubMed]
- 11.Cloyd J. M., Ejaz A., Pawlik T. M. The Landmark Series: Intrahepatic Cholangiocarcinoma. Ann Surg Oncol, 2020;27(8):2859–65. [DOI] [PubMed]
- 12.Soares K. C., Jarnagin W. R. The Landmark Series: Hilar Cholangiocarcinoma. Ann Surg Oncol. 2021;28(8):4158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Kuang T., Chai D., Deng W., Wang P., Wang W. The Use of Antibiotics during Immune checkpoint inhibitor treatment is Associated with Lower Survival in Advanced Esophagogastric Cancer. Int Immunopharmacol, 2023;119:110200. [DOI] [PubMed]
- 14.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begg C. B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics, 1994;50(4):1088–101. [PubMed]
- 16.Coelen R. J., Wiggers J. K., Nio C. Y., Besselink M. G., Busch O. R., Gouma D. J., Van Gulik T. M. Preoperative computed tomography assessment of skeletal muscle mass is valuable in predicting outcomes following hepatectomy for perihilar cholangiocarcinoma. HPB (Oxford), 2015;17(6):520–8. [DOI] [PMC free article] [PubMed]
- 17.Zhou G., Bao H., Zeng Q., HU W., Zhang Q. Sarcopenia as a prognostic factor in hepatolithiasis-associated intrahepatic cholangiocarcinoma patients following hepatectomy: a retrospective study. Int J Clin Exp Med, 2015;8(10):18245–54. [PMC free article] [PubMed]
- 18.Okumura S., Kaido T., Hamaguchi Y., Kobayashi A., Shirai H., Fujimoto Y., Iida T., Yagi S., Taura K., Hatano E., Okajima H., Uemoto S. Impact of Skeletal Muscle Mass, muscle quality, and visceral adiposity on outcomes following resection of Intrahepatic Cholangiocarcinoma. Ann Surg Oncol, 2017;24(4):1037–45. [DOI] [PubMed]
- 19.Umetsu S., Wakiya T., Ishido K., Kudo D., Kimura N., Miura T., Toyoki Y., Hakamada K. Effect of Sarcopenia on the outcomes after pancreaticoduodenectomy for distal cholangiocarcinoma. ANZ J Surg. 2018;88(9):E654–8. [DOI] [PubMed] [Google Scholar]
- 20.Hahn F., Müller L., Stöhr F., Mähringer-Kunz A., Schotten S., Düber C., Bartsch F., Lang H., Galle P. R., Weinmann A., Kloeckner R. The role of Sarcopenia in patients with intrahepatic cholangiocarcinoma: prognostic marker or hyped parameter?. Liver Int. 2019;39(7):1307–14. [DOI] [PubMed] [Google Scholar]
- 21.Kitano Y., Yamashita Y. I., Saito Y., Nakagawa S., Okabe H., Imai K., Komohara Y., Miyamoto Y., Chikamoto A., Ishiko T., Baba H. Sarcopenia affects systemic and local Immune System and impacts Postoperative Outcome in patients with extrahepatic cholangiocarcinoma. World J Surg. 2019;43(9):2271–80. [DOI] [PubMed] [Google Scholar]
- 22.Van Vugt J. L. A., Gaspersz M. P., Vugts J., Buettner S., Levolger S., De Bruin R. W. F., Polak W. G., De Jonge J., Willemssen Feja, Groot Koerkamp B., Jnm I. Jzermans. Low skeletal muscle density is Associated with early death in patients with Perihilar Cholangiocarcinoma regardless of subsequent treatment. Dig Surg. 2019;36(2):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yugawa K., Itoh S., Kurihara T., Yoshiya S., Mano Y., Takeishi K., Harada N., Ikegami T., Soejima Y., Mori M., Yoshizumi T. Skeletal muscle mass predicts the prognosis of patients with intrahepatic cholangiocarcinoma. Am J Surg, 2019;218(5):952–8. [DOI] [PubMed]
- 24.Deng L., Wang Y., Zhao J., Tong Y., Zhang S., Jin C., Chen K., Bao W., Yu Z., Chen G. The prognostic value of Sarcopenia combined with hepatolithiasis in intrahepatic cholangiocarcinoma patients after surgery: a prospective cohort study. Eur J Surg Oncol. 2021;47(3 Pt B):603–12. [DOI] [PubMed] [Google Scholar]
- 25.Jördens M. S., Wittig L., Heinrichs L., Keitel V., Schulze-Hagen M., Antoch G., Knoefel W. T., Fluegen G., Luedde T., Loberg C., Roderburg C., Loosen S. H. Sarcopenia and myosteatosis as prognostic markers in patients with advanced cholangiocarcinoma undergoing palliative treatment. J Clin Med, 2021; 10(19). [DOI] [PMC free article] [PubMed]
- 26.Li H., Dai J., Lan T., Liu H., Wang J., Cai B., Xu L., Yuan K., Wang G., Wu H. Combination of albumin-globulin score and skeletal muscle index predicts long-term outcomes of intrahepatic cholangiocarcinoma patients after curative resection. Clin Nutr. 2021;40(6):3891–900. [DOI] [PubMed] [Google Scholar]
- 27.Tamura S., Ashida R., Sugiura T., Okamura Y., Ito T., Yamamoto Y., Ohgi K., Uesaka K. The prognostic impact of skeletal muscle status and bone mineral density for resected distal cholangiocarcinoma. Clin Nutr. 2021;40(5):3552–8. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J. X., Ding Y., Yan H. T., Zhou C. G., Liu J., Liu S., Zu Q. Q., Shi H. B. Skeletal-muscle index predicts survival after percutaneous transhepatic biliary drainage for obstructive jaundice due to perihilar cholangiocarcinoma. Surg Endosc, 2021;35(11):6073–80. [DOI] [PubMed]
- 29.Lee O., Shin Y. C., Ryu Y., Shin S. H., Heo J. S., Lim C. S., Han I. W. Adverse effects of sarcopenic obesity on postoperative complications after major hepatectomy in patients with hilar cholangiocarcinoma. J Clin Med, 2022;11(7). [DOI] [PMC free article] [PubMed]
- 30.Lurje I., Czigany Z., Eischet S., Bednarsch J., Ulmer T. F., Isfort P., Strnad P., Trautwein C., Tacke F., Neumann U. P., Lurje G. The prognostic impact of preoperative body composition in perihilar and intrahepatic cholangiocarcinoma. Hepatol Commun, 2022;6(9):2400–17. [DOI] [PMC free article] [PubMed]
- 31.Umezawa S., Kobayashi S., Otsubo T. Low preoperative psoas muscle mass index is a risk factor for distal cholangiocarcinoma recurrence after pancreatoduodenectomy: a retrospective analysis. World J Surg Oncol, 2022;20(1):176. [DOI] [PMC free article] [PubMed]
- 32.Watanabe J., Miki A., Sakuma Y., Shimodaira K., Aoki Y., Meguro Y., Morishima K., Endo K., Sasanuma H., Lefor A. K., Teratani T., Fukushima N., Kitayama J., Sata N. Preoperative osteopenia is associated with significantly shorter survival in patients with perihilar cholangiocarcinoma. Cancers (Basel), 2022;14(9). [DOI] [PMC free article] [PubMed]
- 33.Yasuta S., Sugimoto M., Kudo M., Kobayashi S., Takahashi S., Konishi M., Gotohda N. Early postoperative decrease of skeletal muscle mass predicts recurrence and poor survival after surgical resection for perihilar cholangiocarcinoma. BMC Cancer. 2022;22(1):1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asai Y., Yamaguchi J., Mizuno T., Onoe S., Watanabe N., Igami T., Uehara K., Yokoyama Y., Ebata T. Impact of preoperative muscle mass and quality on surgical outcomes in patients undergoing major hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2023;30(2):202–11. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi K., Abe Y., Kitago M., Yagi H., Hasegawa Y., Hori S., Tanaka M., Nakano Y., Kitagawa Y. Prognostic impact of preoperative skeletal muscle change from diagnosis to surgery in patients with perihilar cholangiocarcinoma. Ann Gastroenterol Surg, 2023;7(3):523–32. [DOI] [PMC free article] [PubMed]
- 36.Jung H. E., Han D. H., Koo B. N., Kim J. Effect of Sarcopenia on postoperative ICU admission and length of stay after hepatic resection for Klatskin tumor. Front Oncol, 2023;13:1136376. [DOI] [PMC free article] [PubMed]
- 37.Lacaze L., Bergeat D., Rousseau C., Sulpice L., Val-Laillet D., Thibault R., Boudjema K. High Visceral Fat is Associated with a worse survival after liver resection for Intrahepatic Cholangiocarcinoma. Nutr Cancer. 2023;75(1):339–48. [DOI] [PubMed] [Google Scholar]
- 38.Lurje I., Uluk D., Pavicevic S., Phan M. D., Eurich D., Fehrenbach U., Geisel D., Auer T. A., Pelzer U., Modest D. P., Raschzok N., Sauer I. M., Schöning W., Tacke F., Pratschke J., Lurje G. Body composition is associated with disease aetiology and prognosis in patients undergoing resection of intrahepatic cholangiocarcinoma. Cancer Med, 2023. [DOI] [PMC free article] [PubMed]
- 39.Miki A., Sakuma Y., Watanabe J., Endo K., Sasanuma H., Teratani T., Lefor A. K., Kitayama J., Sata N. Osteopenia Is Associated with shorter survival in patients with intrahepatic cholangiocarcinoma. Curr Oncol, 2023;30(2):1860–8. [DOI] [PMC free article] [PubMed]
- 40.Taniai T., Haruki K., Yanagaki M., Igarashi Y., Furukawa K., Onda S., Yasuda J., Matsumoto M., Tsunematsu M., Ikegami T. Osteosarcopenia predicts poor prognosis for patients with intrahepatic cholangiocarcinoma after hepatic resection. Surg Today, 2023;53(1):82–9. [DOI] [PubMed]
- 41.Zhao Z, Bo Z, Ye N, Dong Y, Xu Y, Wang B, et al. Impact of sarcopenia on postoperative outcomes after hepatectomy in older patients with intrahepatic cholangiocarcinoma: A multicentre cohort study. Liver Int. 2024;44(1):155–68. 10.1111/liv.15757. [DOI] [PubMed]
- 42.Cruz-Jentoft A. J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A. A., Schneider S. M., Sieber C. C., Topinkova E., Vandewoude M., Visser M., Zamboni M. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishikawa H., Shiraki M., Hiramatsu A., Moriya K., Hino K., Nishiguchi S. Japan society of hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res, 2016;46(10):951–63. [DOI] [PubMed]
- 44.Chakedis J., Spolverato G., Beal E. W., Woelfel I., Bagante F., Merath K., Sun S. H., Chafitz A., Galo J., Dillhoff M., Cloyd J., Pawlik T. M. Pre-operative sarcopenia identifies patients at risk for poor survival after resection of biliary tract cancers. J Gastrointest Surg, 2018;22(10):1697–708. [DOI] [PubMed]
- 45.Clugston A., Paterson H. M., Yuill K., Garden O. J., Parks R. W. Nutritional risk index predicts a high-risk population in patients with obstructive jaundice. Clin Nutr, 2006;25(6):949–54. [DOI] [PubMed]
- 46.Gong Q., Zhu P., Zhang B., Shu C., Ding Z., Wu J., Zhang B., Chen X. P. Safety and efficacy of n-3 fatty acid-based parenteral nutrition in patients with obstructive jaundice: a propensity-matched study. Eur J Clin Nutr, 2018;72(8):1159–66. [DOI] [PMC free article] [PubMed]
- 47.Distefano G., Standley R. A., Zhang X., Carnero E. A., Yi F., Cornnell H. H., Coen P. M. Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J Cachexia Sarcopenia Muscle, 2018;9(2):279–94. [DOI] [PMC free article] [PubMed]
- 48.Blair C. K., Robien K., Inoue-Choi M., Rahn W., Lazovich D. Physical inactivity and risk of poor quality of life among elderly cancer survivors compared to women without cancer: the Iowa women’s Health study. J Cancer Surviv. 2016;10(1):103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubbieri G., Mossello E., Di Bari M. Techniques for the diagnosis of sarcopenia. Clin Cases Min Bone Metab, 2014;11(3):181–4. [PMC free article] [PubMed]
- 50.Kim M., Kim H. Accuracy of segmental multi-frequency bioelectrical impedance analysis for assessing whole-body and appendicular fat mass and lean soft tissue mass in frail women aged 75 years and older. Eur J Clin Nutr, 2013;67(4):395–400. [DOI] [PubMed]
- 51.Dodson S., Baracos V. E., Jatoi A., Evans W. J., Cella D., Dalton J. T., Steiner M. S. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011;62:265–79. [DOI] [PubMed]
- 52.Suriano F., Van Hul M., Cani P. D. Gut microbiota and regulation of myokine-adipokine function. Curr Opin Pharmacol. 2020;52:9–17. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen B. K., Febbraio M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–65. [DOI] [PubMed]
- 54.Lutz C. T., Quinn L. S. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging. 2012;4(8):535–46. [DOI] [PMC free article] [PubMed]
- 55.Marks-Konczalik J., Dubois S., Losi J. M., Sabzevari H., Yamada N., Feigenbaum L., Waldmann T. A., Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97(21):11445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Julienne C. M., Dumas J. F., Goupille C., Pinault M., Berri C., Collin A., Tesseraud S., Couet C., Servais S. Cancer cachexia is associated with a decrease in skeletal muscle mitochondrial oxidative capacities without alteration of ATP production efficiency. J Cachexia Sarcopenia Muscle. 2012;3(4):265–75. [DOI] [PMC free article] [PubMed]
- 57.Vettor R., Milan G., Franzin C., Sanna M., De Coppi P., Rizzuto R., Federspil G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297(5):E987–98. [DOI] [PubMed] [Google Scholar]
- 58.Tilg H., Moschen A. R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–83. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.