Abstract
Objective
The identification of risk factors is crucial for the clinical prevention and diagnosis of necrotizing enterocolitis (NEC). Monochorionic twins (MCT), due to the high genetic homogeneity, provided a valuable model for investigating the risk factors of various diseases. This study aimed to explore the risk factors for NEC using MCT.
Methods
A retrospective review was conducted on the medical records of monochorionic twins (MCT) treated at Guangzhou Women and Children’s Medical Center from January 2012 to March 2023. We compared perinatal condition, feeding and preceding condition between MCT pairs with NEC (NEC MCT) and without NEC(No NEC MCT).Logistic regression analysis was utilized to identify independent risk factors.
Result
In 85 pairs of monochorionic twins (MCT), NEC occurred in one twin in 78.8% of cases, whereas both twins were affected in 21.2% of cases. In the final cohort of 60 pairs of MCT, several parameters were found to differ significantly between NEC MCT group and No NEC MCT group. Compared to No NEC MCT group, the incidence of umbilical cord abnormalities was significantly higher in the NEC MCT group (25% vs. 8.3%, P = 0.014). Meanwhile, NEC MCT group showed higher prevalence of SGA infants (48.3% vs. 21.7%, P = 0.002) and sFGR (38.3% vs. 6.7%, P = 0.000). Furthermore, TTTs (13.3% vs. 3.3%, P = 0.027) and septicemia (25% vs. 5%, P = 0.002) were more common in NEC MCT group. In a multivariable logistic regression model, sFGR (OR 6.81,95%CI 2.1–21.9, p = 0.001) was eventually output as an independent risk factor.
Conclusion
Non-genetic factors play a predominant role in the pathogenesis of NEC. Umbilical cord abnormalities, SGA, sFGR, TTTs and septicemia significantly increase the risk of NEC. sFGR is an independent risk factor of NEC.
Keywords: Necrotizing enterocolitis, Monochorionic, Twins, Risk factor
Introduction
Necrotizing enterocolitis (NEC) is a leading cause of neonatal death, especially among premature infants [1, 2] Despite enhanced pathophysiological understanding and advancements in neonatal intensive care, the overall mortality ratio for this condition maintains high, ranging from 25–40% [3, 4]. Worsening, long-term complications, such as neurodevelopmental deficits, short bowel syndromeand pulmonary pathologies, dramatically impair quality life of the survivor [5–7]. Identifying risk factors is crucial for developing early prevention and treatment strategies for NEC. Nonetheless, there exists a divergence of opinions among clinicians and experts regarding the relative significance of different risk factors. Monochorionic twins (MCT) develop from a single fertilized egg and share the same intrauterine environment [8]. Moreover, the highly homogeneous genetic background of MCT makes them a valuable cohort for investigating the influence of genetic and environmental factors on disease pathogenesis. HACK, et al. [9] reported an incidence rate of 3.8% for NEC MCT in twins hospitalized in the neonatal intensive care unit (NICU). Among MCT twins, those with NEC and without NEC represent a unique aspect to explore the etiological aspects of this condition. However, there is limited research on MCT associated with NEC. This study aimed to explore the risk factors of NEC using MCT.
Patients and methods
This case-control matching study was conducted at Guangzhou Women and Children’s Medical Center, the largest tertiary medical center in southern China. The twin pairs would be included: (1) one or both of them developed NEC(≥ Bell’s stage II); (2) born and treated at our center from January 2012 to March 2023. NEC(≥ Bell’s stage II) was diagnosed by Bell’s criteria according to clinical manifestations(bloody stool, etc.) and imaging findings (pneumatosis intestinalis, portal venous gas, pneumoperitoneum, etc.) [4, 10]. The NEC twin pairs would be excluded: (1) Identified as Dichorionic twins with NEC; (2) Chorionic nature could not be determined; (3) Both twins develop NEC; (4) Combined with congenital intestinal anomalies; (5) Death before NEC; (6) Missing medical records. The study was approved by Medical Ethics Committee of Guangzhou Women and Children’s Medical Center and strictly observed the declaration of Helsinki.
Regarding feeding, our center maintains a consistent neonatal feeding strategy. Newborns are primarily fed breast milk, either directly from the mother or from a donor. If breast milk is insufficient and donor milk is refused, preterm infants are given preterm formula. Based on birth weight, initial milk volumes vary. Neonates with smaller birth weights receive less milk and take longer to reach full enteral feeding. (1) Minimal Enteral Nutrition (MEN): At the start of enteral feeding, a small amount of breast milk or formula (usually 0.5-1 ml/kg/h) is given to stimulate gut maturation and peristalsis [11]. (2) Incrementally increase feeding volumes: Adjust daily based on the preterm infant’s tolerance, typically increasing by 10–20 ml/kg each time. Monitor the infant’s gastrointestinal function closely, including gastric residuals, vomiting, abdominal distension and stool characteristics [12]. When the feeding volume reaches 60 ml/kg/day, half-strength fortified breast milk or preterm formula (81 kcal/100 ml) can be used. When the feeding volume reaches 100 ml/kg/day, full-strength fortified breast milk can be introduced [13–15]. The main nutritional management objectives are as follows [16]: (1) Full enteral feeding volume: 150–180 ml/kg/day; (2) Caloric intake target: approximately 120 kcal/kg/day.
Data collection
We gathered perinatal characteristics, including maternal, obstetrical, and neonatal factors: unequally shared placenta, umbilical cord abnormalities, small for gestational age (SGA), selective fetal growth restriction(sFGR), TTTs, delivery room resuscitation, gestational age at birth, Apgar scores, birth weight and sex. Additionally, we collected the characteristics of feeding strategies, including day of the first feed, type of nutrition (breast milk, formula, breast milk fortified or fasting), the volume of the first feed, and feeding intolerance (FI) before the onset of NEC. Furthermore, we documented preceding condition of NEC: neonatal respiratory distress syndrome (NRDS), severe anemia, septicemia, congenital heart disease (CHD), pulmonary hypertension, intermittent hypoxemia.
Definition
(1) Small for gestational age (SGA) was operationally defined as birth weight below the 10th percentile for the corresponding gestational age [17–19]; (2) Septicemia was defined as systemic signs of infection, deterioration of clinical condition with the presence of positive blood or cerebrospinal fluid cultures in laboratory tests [20–22]. Only Septicemia was detected before the diagnosis of NEC was included in this study; (3) Umbilical cord abnormalities were defined as any deviations from the normal structure, function, or position of the umbilical cord during pregnancy and diagnosed based on findings from ultrasound examinations [23–25]. The umbilical cord can be divided into [25]: 1) Abnormal length; 2) Cord insertions site abnormalities; 3) Cystic abnormalities; 4) Cord hematomas; 5) Solid or complex malformations; 6) Knots; 7) Nuchal cord; 8) Vascular anomalies; 9) Funic presentation and prolapse cord; (4) Selective fetal growth restriction(sFGR) in MCT needs to meet at least two of the following standards: 1) EFW < 10th percentile for one twin; 2) AC < 10th percentile for one twin; 3) EFW discordance between twins ≥ 25%; 4) Umbilical artery pulsatility index of the smaller twin > 95th percentile [26–29]; (5) Twin-to-Twin Transfusion Syndrome (TTTs) was characterized by the presence of blood flow via arteriovenous anastomoses in both directions, serving as a prerequisite for the development of TTTs. In this syndrome, one fetus acts as the donor, while the other assumes the role of the recipient in the event of an imbalance [30–32]. Ultrasound finding appear severe amniotic fluid discordance between the twins’ amniotic sacs. The recipient fetus shows increasing polyhydramnios due to fetal polyuria defined as the deepest vertical pocket of ≥ 8 cm depth before 20 weeks and ≥ 10 cm after 20 weeks of gestation. The donor shows oligo- or anhydramnios with the deepest vertical pocket ≤ 2 cm and is stuck within its membranes to the uterine wall or placenta by the excessive polyhydramnios of the recipient [33]; (6) Feeding intolerance (FI) manifested as gastrointestinal symptoms [34, 35], including a notably large gastric residual volume (> 50% of the previous feeding), vomiting, abdominal distension, bloody stool, visible bowel loops and diarrhea; (7) Unequally shared placenta was defined as one of twin receiving less than 60% of the blood from the placenta, according to the respective area of placental surfaces measured postnatally [23]; (8) Neonatal respiratory distress syndrome (NRDS) was defined as a condition of pulmonary insufficiency that in its natural course commences at or shortly after birth and increases in severity over the first 2 days of life. The diagnostic indicator included [36]: 1) chest X-ray with a classical ‘‘ground glass’’ appearance and air bronchograms; 2) PaO2 < 50 mmHg (< 6.6 kPa) in room air; 3) central cyanosis in room air or need for supplemental oxygen to maintain PaO2 > 50 mmHg (> 6.6 kPa) as well as classical chest X-ray appearances; (9) Intermittent hypoxemia was defined as a condition where blood oxygen saturation periodically dropped below 85–90% and subsequently returned to normal levels [37]; (10) Severe anemia was defined as hemoglobin less than 60 g/L [38]; (11) Pulmonary hypertension was diagnosed [39]: 1) partial pressure of oxygen in arterial blood (PaO2) < 55 mmHg despite fraction of inspired oxygen (FiO2) of 1.0; 2) a preductal to postductal oxygen gradient greater than 20 mmHg; 3) extrapulmonary right-to-left shunting at the ductal or atrial level in the absence of severe pulmonary parenchymal disease and tricuspid regurgitation; 4) pulmonary arterial pressure (PAP) greater than 25 to 30 mmHg in cardiac catheterization; (12) Congenital heart disease (CHD) was defined as a range of structural abnormalities of the heart and the large blood vessels present from birth. In our study, the isolated patent ductus arteriosus and patent foramen ovale were not included in the scope of congenital heart disease [40, 41].
Statistical analysis
Statistical analyses were conducted using SPSS 25.0 Statistics for Windows software. Comparisons for quantitative variables were made using Student’s t-test or the Wilcoxon test, depending on which was appropriate after checking for normality using the Kolmogorov-Smirnoy test. Continuous variables following a normal distribution were presented as mean ± SD. For continuous variables that did not adhere to a normal distribution, data were expressed as quartiles (M, Q1, Q3). Associations between qualitative variables were analyzed using Pearson’s chi-squared test. Variables found to have a p value < 0.05 in the univariate analysis would be put into the stepwise logistic regression. The output value with a significance level of p < 0.05 was independent risk factors eventually.
Results
From January 2012 to March 2023, our hospital recorded a total of 114,642 cases of newborns. Among these, there were 16,374(14.28%) cases of twin births, 5826(5.08%) cases of monochorionic twin births, and 1547(1.37%) cases of NEC.
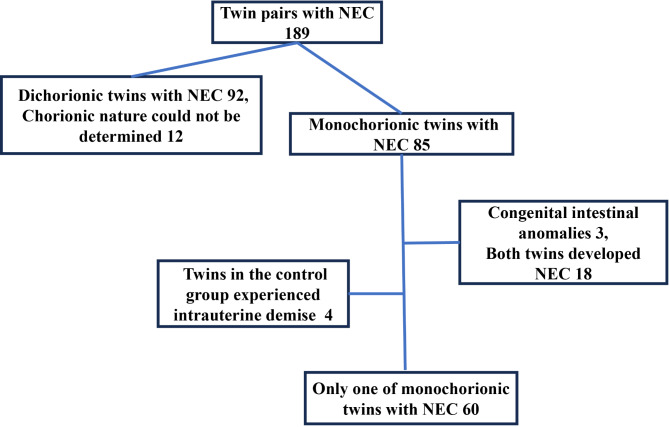
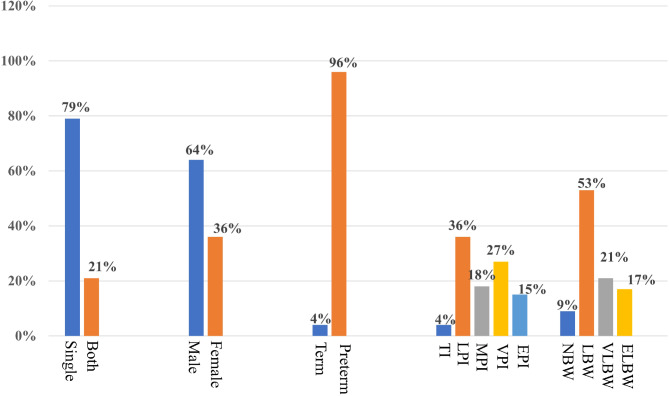
A total of 189 twin pairs with NEC were born and subsequently admitted to the study institution between January 2012 and March 2023. Among these, 92 twin pairs were diagnosed with dichorionic twins with necrotizing enterocolitis (DCT-NEC). Due to the lack of records, the 12 pairs of twins could not be confirmed as monochorionic or dichorionic twins and were therefore excluded. Of the 85 pairs of monochorionic twins, 18 pairs all had NEC, 3 pairs had congenital intestinal malformations, and 4 pairs had intrauterine death, which were excluded. Eventually, 60 MCT pairs were included in the study (Fig. 1). In 85 pairs of MCT with NEC(Fig. 2), the GA at birth for infants ranged from 26+ 6 to 37+ 2 weeks. The detailed distribution of GA included the following categories: term infants (6, 3.5%), late preterm infants (62, 36.5%), moderate preterm infants (30, 17.6%), very preterm infants (46, 27%), and extremely preterm infants (26, 15.3%). Regarding birth weights among the NEC MCT cases, the distribution was as follows: normal birth weight infants (16, 9.4%), low birth weight infants (90, 52.9%), very low birth weight infants (36, 21.2%), and extremely low birth weight infants (28, 16.5%).
Fig. 1.
Flowchart in selection of twin pairs with NEC
Fig. 2.
Demographic information of MCT with NEC. Single: NEC occurred in one case of MCT; Both: NEC occurred in both cases of MCT; TI: term infants; LPI: late preterm infants; MPI: moderate preterm infants; VPI: very preterm infants; EPI: extremely preterm infants; NBW: normal birth weight infants; LBW: low birth weight infants; VLBW: very low birth weight infants; ELBW: extremely low birth weight infants
In the NEC MCT group, the diagnosis time was 13(5.0–22.0) days. The perinatal and delivery characteristics of the two groups are summarized in Table 1. No statistically significant differences were observed between the groups in terms of birth order, birth weight, Apgar scores, the presence of Unequally shared placenta, or the need for delivery room resuscitation. NEC MCT cases exhibited a higher incidence of umbilical cord abnormalities (25% vs. 8.3%, p = 0.014), a greater proportion of SGA infants (48.3% vs. 21.7%, p = 0.002), a higher prevalence of sFGR (38.3% vs. 6.7%, p = 0.000), and a higher prevalence of blood donor (13.3% vs. 3.3%, p = 0.027).
Table 1.
Comparison of perinatal condition between NEC MCT group and no NEC MCT group
| NEC MCT G’roup (n = 60) |
No NEC MCT group (n = 60) |
P-value | |
|---|---|---|---|
| Smaller twin, n (%) | 32(53.3%) | 28(46.7%) | 0.465 |
| Birth weight, n (%) | 0.51 | ||
| ELBW | 9(15%) | 6(10%) | |
| VLBW | 12(20%) | 8(13.3%) | |
| LBW | 34(56.7%) | 38(63.3%) | |
| NBW | 5(8.3%) | 8(13.3%) | |
| BW, g | 1743.2 ± 568.7 | 1884.1 ± 468.1 | 0.184 |
| Apgar scores [IQR] | |||
| 1-minute | 9(8.0 ~ 9.0) | 9(8.0 ~ 9.0) | 0.648 |
| 5-minute | 9(9.0 ~ 9.0) | 9(9.0 ~ 10.0) | 0.292 |
| 10-minute | 9(9.0 ~ 9.0) | 9(9.0 ~ 10.0) | 0.328 |
| Unequally shared placenta | 4(6.7%) | 3(5%) | 0.697 |
| Umbilical cord Abnormalities, n(%) | 15(25%) | 5(8.3%) | 0.014 |
| SGA, n (%) | 29(48.3%) | 13(21.7%) | 0.002 |
| sFGR, n (%) | 23(38.3%) | 4(6.7%) | 0.000 |
| TTTs, n (%) | 0.027 | ||
| Blood donor | 8(13.3%) | 2(3.3%) | |
| Blood recipient | 2(3.3%) | 8(13.3%) | |
| Delivery room Resuscitation, n (%) | 20(33.3%) | 16(26.7%) | 0.426 |
LBW: extremely low birth weight; VLBW: very low birth weight; LBW: low birth weight; NBW: normal birth weight; BW: birth weight; SGA: small-for-gestational-age; sFGR: selective fetal growth restriction; TTTs: twin transfusion syndrome
The feeding condition between NEC MCT group and No NEC MCT group is summarized in Table 2. In 120 cases of MCT newborns, no neonates were fed raw cow’s milk. 4 cases received donor milk due to insufficient breast milk supply, and 1 case developed necrotizing enterocolitis (NEC). 13 cases received fortified breast milk and 5 cases developed NEC. Among the MCT, 2 MCT pairs received fortified breast milk, and one of MCT pairs developed NEC. In the NEC MCT group, the feeding volume at the onset of NEC increased to 113.9 ± 68.2 ml/kg/day, whereas in the No NEC MCT group, it was 104.6 ± 62.7 ml/kg/day. The time to reach full enteral feeding was 10(8.0–17.0) days in the NEC MCT group, compared to 11(8.0–20.0) days in the No NEC MCT group. During the process of increasing feeding volume, 26 cases exhibited feeding intolerance. Among these, 16 cases were observed in the NEC MCT group.
Table 2.
Comparison of feeding condition between NEC MCT group and no NEC MCT group
| NEC MCT group(n = 60) | No NEC MCT group(n = 60) | P-value | |
|---|---|---|---|
| Day of first feeds/d | 1(1.0 ~ 1.0) | 1(1.0 ~ 1.0) | 0.763 |
| Volume of first fed/ml/kg.d | 18(13.4 ~ 21.1) | 16.1(12.4 ~ 20.0) | 0.540 |
| Day of full feeding/d | 10(8.0 ~ 17.0) | 11(8.0 ~ 20.0) | 0.340 |
| Feeding increment ml/kg/d | 113.9 ± 68.2 | 104.6 ± 62.7 | 0.526 |
| Feeding, n (%) | 0.622 | ||
| Breastfeeding | 12(20%) | 15(25%) | |
| Preterm formula | (38.3%) | 20(33.3%) | |
| Regular formula | 1(1.7%) | 1(1.7%) | |
| Combination | 22(36.7%) | 24(40%) | |
| Fasted | 2(3.3%) | 0(0) | |
| Breast milk fortified | 5(8.3%) | 8(13.3%) | 0.378 |
| FI | 16(26.7%) | 10(16.7%) | 0.184 |
FI: feeding intolerance
The incidence of preceding condition was shown in the Table 3. It is notable that the number of infants diagnosed with septicemia was significantly higher in the NEC MCT group (25% vs5%, p = 0.002). Severe anemia, congenital heart disease (CHD), pulmonary hypertension, and intermittent hypoxemia, showed no discernible differences between the NEC group and the control group.
Table 3.
Comparison of preceding condition between NEC MCT group and no NEC MCT group
| NEC MCT group(n = 60) | No NEC MCT group(n = 60) |
P-value | |
|---|---|---|---|
| NRDS, n (%) | 28(46.7%) | 23(38.3%) | 0.356 |
| Severe anemia, n (%) | 13(21.7%) | 12(20%) | 0.822 |
| Septicemia, n (%) | 15(25%) | 3(5%) | 0.002 |
| CHD, n (%) | 8(13.3%) | 3(5%) | 0.114 |
| Pulmonary hypertension, n (%) | 4(6.7%) | 4(6.7%) | 1.000 |
| Intermittent hypoxemia, n (%) | 8(13.3%) | 11(18.3%) | 0.453 |
NRDS: neonatal respiratory distress syndrome; CHD: congenital heart disease
In the logistic regression analysis, sFGR (OR 6.8,95%CI 2.117–21.911, p = 0.001) was eventually output as a significant independent risk factor for NEC (Table 4).
Table 4.
Variable output from logistic regression analysis
| Variable | OR (95% CI of OR) | P-value |
|---|---|---|
| sFGR | 6.81(2.1–21.9) | 0.001 |
sFGR: selective fetal growth restriction
Discussion
While clinical features and risk factors of NEC have been extensively documented in the literature, there is a paucity of studies specifically addressing twin NEC [42–44], with most existing reports being limited to case studies involving MCT and often associated with TTTs [42, 43]. To the best of our knowledge, this is the first and largest study on NEC MCT in the South China region, which will help us to more accurately and deeply identify the incidence and risk factors for NEC. In our study, a total of 85 cases of NEC MCT were examined. Among them, 18 cases (21.2%) exhibited simultaneous development of NEC in both monochorionic twins, while 67 cases (78.8%) involved only one twin being affected. These findings suggest that non-genetic factors play a more prominent role in the pathogenesis of NEC in MCT compared to genetic factors. Recently, Rebai et al. [45] reviewed and analyzed 17 cases of NEC MCT and 17 cases of No NEC MCT in a single center. It’s found that in the NEC MCT group, birth weight was significantly lower. However, TTTs and SGA did not show significant differences when comparing the NEC MCT group with the no NEC MCT group which may be influenced by the limited number of patients. Through the analysis of 60 cases of NEC MCT and 60 cases of No NEC MCT, we found that umbilical cord abnormalities, SGA, sFGR, TTTs, and septicemia were risk factors for NEC MCT. sFGR can be considered an independent risk factor of NEC.
As neonatal intensive care medicine and perinatal care have advanced, the survival rates of SGA infants have improved progressively. Our analysis revealed a significantly higher proportion of SGA infants in the MCT NEC group compared to the control group (48.3% vs. 21.7%, p < 0.05), underscoring the association between SGA status and the occurrence of NEC in MCT. A cohort study conducted by Ree et al. [46], which included 475 SGA neonates, reported that the incidence of NEC in SGA infants was 2.6 times higher than that in appropriately sized infants for their GA. Similarly, a study by Boghossian et al. [47]. also found that SGA increased the risk of NEC and with the gestational age increasing, the risk of NEC may increases further.
The umbilical cord, encompassing two umbilical arteries and a vein, serves as the sole conduit providing vital life support to fetuses. Normal development umbilical cord can enhance resistance to torsion, stretching and compression while allowing for unimpeded fetal movements. The two umbilical arteries and their associated anastomoses play a crucial role in ensuring the equitable distribution of blood to the various lobes of the placenta [48]. However, when these favorable mechanisms within the umbilical cord are compromised, the developing fetus becomes vulnerable to various risks. Consequently, umbilical cord abnormalities are associated not only with intrapartum fetal heart rate (FHR) irregularities, low Apgar scores, and neonatal mortality but also with fetal growth restriction, preterm labor, and fetal demise [49]. It is notable that umbilical cord abnormalities are frequently overlooked as risk factors for the development of NEC [50, 51]. Kamoji et al. [52]. found that antenatal umbilical cord abnormalities, leading to inadequate blood perfusion, were high-risk factors for the development of NEC. In our study, we identified 15 cases (25%) of NEC MCT characterized by umbilical cord developmental abnormalities during the fetal period. Among these cases, 10 exhibited abnormalities in cord attachment, including one instance with a single umbilical artery (SUA), while 5 cases presented with a thin umbilical cord.
sFGR and TTTs are complex and severe complications that specifically affect monochorionic twins (MCT), with estimated incidence rates of 8–15% in such pregnancies [8, 53]. A cohort study by Weisz et al. revealed that among MCT neonates, those with type III sFGR exhibited a significantly higher risk of developing NEC compared to those without sFGR [54]. Furthermore, our study findings underscored distinct distributions in the proportions of TTTs donors and recipients among MCT neonates with or without NEC (p < 0.05), suggesting a potential association between TTTs and the development of NEC. The underlying mechanisms may involve blood donors experiencing insufficient circulation, leading to compromised gastrointestinal and renal perfusion. This, in turn, triggers activation of the renin-angiotensin-aldosterone system (RAAS), resulting in peripheral vasoconstriction and exacerbating intestinal ischemia and hypoxia. Concurrently, the blood-receiving fetus may suffer from congestive heart failure due to circulatory overload, leading to gastrointestinal stasis. This condition impairs oxygen exchange and toxin metabolism, potentially serving as a causative factor for the development of intestinal lesions in the affected fetus [32, 55].
Gagliardi et al. [56] conducted a multi-center study involving 2035 samples, where they identified late-onset sepsis as an independent risk factor for the development of NEC in very low birth weight (VLBW) infants. Their findings revealed that late-onset sepsis occurred at a rate 5.4 times higher in the NEC population than in the non-NEC population (odds ratio [OR] = 5.38, p < 0.001). Likewise, Lambert et al. reported a similar association between early-onset sepsis and NEC in term and near-term infants (GA > 36 weeks) [57]. In our study of 15 cases of MCT diagnosed with sepsis before the development of NEC, we observed that this cohort included 10 cases of late-onset sepsis and 5 cases of early-onset sepsis. This distribution differed significantly when compared to non-NEC newborns (p = 0.002).
The main limitations of this study are its retrospective design and the limited number of patients. However, compared to previous studies, we included a sufficient number of MCT to explore the risk factors for NEC. Additionally, we conducted a comprehensive analysis of NEC MCT, considering perinatal condition, feeding practice and preceding condition. These explorations provided significant indicators and conclusions, underscoring the importance of non-genetic factors such as fetal growth and infection in the occurrence of NEC. Large-sample and multicenter trials are needed to further investigate the risk factors for NEC by MCT.
Conclusions
Non-genetic factors play a predominant role in the pathogenesis of NEC. Umbilical cord abnormalities, small for gestational age, selective fetal growth restriction, twin transfusion syndrome, and septicemia significantly increased the risk of NEC. sFGR is an independent risk factor of NEC. The identification of these risk factors in MCT will contribute to reinforce perinatal management strategies and aid in the prevention of NEC progression.
Acknowledgements
Not applicable.
Abbreviations
- NEC
Necrotizing enterocolitis
- MCT
Monochorionic twins
- DCT
Dichorionic twins
- AGA
Appropriate for gestational age
- SGA
Small-for-gestational-age
- sFGR
Selective fetal growth restriction
- TTTs
Twin transfusion syndrome
- SUA
Single umbilical artery
- FI
Feeding intolerance
- NRDS
Neonatal respiratory distress syndrome
- CHD
Congenital Heart Disease
- TI
Term infant(GA ≥ 37 W)
- LPI
Late preterm infant(34 ≤ GA < 37 W)
- MPI
Moderate preterm infant(32 ≤ GA < 34 W)
- VPI
Very preterm infant(28 ≤ GA < 32 W )
- EPI
Extremely preterm infant (GA < 28 W )
- NBW
Normal birth weight (BW ≥ 2500 g)
- LBW
Low birth weight (1500 g ≤ BW < 2500 g)
- VLBW
Very low birth weight (1000 g ≤ BW < 1500 g)
- ELBW
Extremely low birth weight (BW < 1000 g)
Author contributions
Qiuming He and Wei Zhong conceptualized and designed this study. Pengjian Zou, Lili Wu and Wenhai Fang jointly collected the original data of the study subjects. Pengjian Zou, Qiuming He and Huimin Xia set up the research method of the article. Wenhai Fang and Pengjian Zou drafted the initial version of this paper, Wenhai Fang and Lili Wu carried out the initial analyses. Juan He, Wei Zhong coordinated and supervised data collection. Huimin Xia and Qiuming He conducted formal analysis, critically reviewed the writing and edited of this paper.
Funding
This study is supported by Guangzhou Science and Technology Plan Project (2024A03J1171).
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The research ethics committee of Guangzhou Women and Children’s Medical Center approved the study. Considering the retrospective nature of this study, ethics committee of Guangzhou Women and Children’s Medical Center approved the waiver of parents’ written consent. The waiver will not adversely affect the rights and welfare of the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pengjian Zou and Wenhai Fang contributed equally to this work and share the 1st authorship.
Huimin Xia, Wei Zhong, Qiuming He these corresponding authors contributed equally to this work.
References
- 1.Saroha V, Josephson CD, Patel RM. Epidemiology of necrotizing enterocolitis: New considerations regarding the influence of Red Blood Cell transfusions and Anemia. Clin Perinatol. 2019;46(1):101–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rich BS, Dolgin SE. Necrotizing enterocolitis. Pediatr Rev. 2017;38(12):552–9. [DOI] [PubMed] [Google Scholar]
- 3.Eaton S, Rees CM, Hall NJ. Current research on the Epidemiology, Pathogenesis, and management of necrotizing enterocolitis. Neonatology. 2017;111(4):423–30. [DOI] [PubMed] [Google Scholar]
- 4.Jones IH, Hall NJ. Contemporary Outcomes for Infants with necrotizing Enterocolitis-A systematic review. J Pediatr. 2020;220:86–e923. [DOI] [PubMed] [Google Scholar]
- 5.Berken JA, Chang J. Neurologic consequences of neonatal necrotizing enterocolitis. Dev Neurosci. 2022;44(4–5):295–308. [DOI] [PubMed] [Google Scholar]
- 6.Baillie CT, Kenny SE, Rintala RJ, Booth JM, Lloyd DA. Long-term outcome and colonic motility after the Duhamel procedure for Hirschsprung’s disease. J Pediatr Surg. 1999;34(2):325–9. [DOI] [PubMed] [Google Scholar]
- 7.Pennington EC, Javid PJ, Sullins V, Mueller C, Hunter CJ. Ethical dilemmas in the management of infants with necrotizing enterocolitis totalis. J Pediatr Surg. 2022;57(3):329–34. [DOI] [PubMed] [Google Scholar]
- 8.Cheong-See F, Schuit E, Arroyo-Manzano D, Khalil A, Barrett J, Joseph KS, et al. Prospective risk of stillbirth and neonatal complications in twin pregnancies: systematic review and meta-analysis. BMJ. 2016;354:i4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hack KE, Derks JB, Elias SG, Franx A, Roos EJ, Voerman SK, et al. Increased perinatal mortality and morbidity in monochorionic versus dichorionic twin pregnancies: clinical implications of a large Dutch cohort study. BJOG. 2008;115(1):58–67. [DOI] [PubMed] [Google Scholar]
- 10.Neu J. Necrotizing enterocolitis: the future. Neonatology. 2020;117(2):240–4. [DOI] [PubMed] [Google Scholar]
- 11.Bombell S, McGuire W. Early trophic feeding for very low birth weight infants. Cochrane Database Syst Rev. 2009(3):CD000504. [DOI] [PubMed]
- 12.Lapillonne A, O’Connor DL, Wang D, Rigo J. Nutritional recommendations for the late-preterm infant and the preterm infant after hospital discharge. J Pediatr. 2013;162(3 Suppl):S90–100. [DOI] [PubMed] [Google Scholar]
- 13.Dutta S, Singh B, Chessell L, Wilson J, Janes M, McDonald K, et al. Guidelines for feeding very low birth weight infants. Nutrients. 2015;7(1):423–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berseth CL, Van Aerde JE, Gross S, Stolz SI, Harris CL, Hansen JW. Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier. Pediatrics. 2004;114(6):e699–706. [DOI] [PubMed] [Google Scholar]
- 15.Rogers SP, Hicks PD, Hamzo M, Veit LE, Abrams SA. Continuous feedings of fortified human milk lead to nutrient losses of fat, calcium and phosphorous. Nutrients. 2010;2(3):230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50(1):85–91. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Martinez S, Papageorghiou AT, Staines-Urias E, Villar J, Gonzalez De Aguero R, Oros D. Clinical impact of Doppler reference charts on management of small-for-gestational-age fetuses: need for standardization. Ultrasound Obstet Gynecol. 2020;56(2):166–72. [DOI] [PubMed] [Google Scholar]
- 18.Lees CC, Romero R, Stampalija T, Dall’Asta A, DeVore GA, Prefumo F, et al. Clinical opinion: the diagnosis and management of suspected fetal growth restriction: an evidence-based approach. Am J Obstet Gynecol. 2022;226(3):366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cutfield W, Ayyavoo A. The auxological and metabolic consequences for children born small for gestational age. Indian J Pediatr. 2021;88(12):1235–40. [DOI] [PubMed] [Google Scholar]
- 20.Bethou A, Bhat BV. Neonatal Sepsis-newer insights. Indian J Pediatr. 2022;89(3):267–73. [DOI] [PubMed] [Google Scholar]
- 21.Shane AL, Sanchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–80. [DOI] [PubMed] [Google Scholar]
- 22.Procianoy RS, Silveira RC. The challenges of neonatal sepsis management. J Pediatr (Rio J). 2020;96(1):80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fick AL, Feldstein VA, Norton ME, Wassel Fyr C, Caughey AB, Machin GA. Unequal placental sharing and birth weight discordance in monochorionic diamniotic twins. Am J Obstet Gynecol. 2006;195(1):178–83. [DOI] [PubMed] [Google Scholar]
- 24.Hayes DJL, Warland J, Parast MM, Bendon RW, Hasegawa J, Banks J, et al. Umbilical cord characteristics and their association with adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS ONE. 2020;15(9):e0239630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krzyzanowski A, Kwiatek M, Geca T, Stupak A, Kwasniewska A. Modern Ultrasonography of the umbilical cord: prenatal diagnosis of Umbilical Cord Abnormalities and assessement of fetal wellbeing. Med Sci Monit. 2019;25:3170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalil A, Beune I, Hecher K, Wynia K, Ganzevoort W, Reed K, et al. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: a Delphi procedure. Ultrasound Obstet Gynecol. 2019;53(1):47–54. [DOI] [PubMed] [Google Scholar]
- 27.D’Antonio F, Prasad S, Masciullo L, Eltaweel N, Khalil A. Selective fetal growth restriction in dichorionic diamniotic twin pregnancy: systematic review and meta-analysis of pregnancy and perinatal outcomes. Ultrasound Obstet Gynecol. 2024;63(2):164–72. [DOI] [PubMed] [Google Scholar]
- 28.Ochsenbein-Kolble N. Twin pregnancies. Ultraschall Med. 2021;42(3):246–69. [DOI] [PubMed] [Google Scholar]
- 29.Townsend R, Khalil A. Fetal growth restriction in twins. Best Pract Res Clin Obstet Gynaecol. 2018;49:79–88. [DOI] [PubMed] [Google Scholar]
- 30.D’Antonio F, Marinceu D, Prasad S, Eltaweel N, Khalil A. Outcome following laser surgery of twin-twin transfusion syndrome complicated by selective fetal growth restriction: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2023;62(3):320–7. [DOI] [PubMed] [Google Scholar]
- 31.Bamberg C, Hecher K. Update on twin-to-twin transfusion syndrome. Best Pract Res Clin Obstet Gynaecol. 2019;58:55–65. [DOI] [PubMed] [Google Scholar]
- 32.Hecher K, Gardiner HM, Diemert A, Bartmann P. Long-term outcomes for monochorionic twins after laser therapy in twin-to-twin transfusion syndrome. Lancet Child Adolesc Health. 2018;2(7):525–35. [DOI] [PubMed] [Google Scholar]
- 33.Bamberg C, Hecher K. Twin-to-twin transfusion syndrome: controversies in the diagnosis and management. Best Pract Res Clin Obstet Gynaecol. 2022;84:143–54. [DOI] [PubMed] [Google Scholar]
- 34.Moussa R, Khashana A, Kamel N, Elsharqawy SE. Fecal calprotectin levels in preterm infants with and without feeding intolerance. J Pediatr (Rio J). 2016;92(5):486–92. [DOI] [PubMed] [Google Scholar]
- 35.Surmeli-Onay O, Korkmaz A, Yigit S, Yurdakok M. Feeding intolerance in preterm infants fed with powdered or liquid formula: a randomized controlled, double-blind, pilot study. Eur J Pediatr. 2013;172(4):529–36. [DOI] [PubMed] [Google Scholar]
- 36.Sweet D, Bevilacqua G, Carnielli V, Greisen G, Plavka R, Saugstad OD, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome. J Perinat Med. 2007;35(3):175–86. [DOI] [PubMed] [Google Scholar]
- 37.Di Fiore JM, Martin RJ, Raffay TM. Intermittent hypoxemia and bronchopulmonary dysplasia: manifestations of immature respiratory control and the Preterm Lung. Am J Respir Crit Care Med. 2021;204(10):1126–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao E, Ye D, Long G, Hu Y, Fu Q, Yuan T, et al. Severe neonatal anemia affected by massive fetomaternal hemorrhage: a single-center retrospective observational study. J Matern Fetal Neonatal Med. 2022;35(20):3972–8. [DOI] [PubMed] [Google Scholar]
- 39.Kelly LE, Ohlsson A, Shah PS. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst Rev. 2017;8(8):CD005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Heide M, Mebius MJ, Bos AF, Roofthooft MTR, Berger RMF, Hulscher JBF, et al. Hypoxic/ischemic hits predispose to necrotizing enterocolitis in (near) term infants with congenital heart disease: a case control study. BMC Pediatr. 2020;20(1):553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelleher ST, McMahon CJ, James A. Necrotizing enterocolitis in children with congenital heart disease: a Literature Review. Pediatr Cardiol. 2021;42(8):1688–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Detlefsen B, Boemers TM, Schimke C. Necrotizing enterocolitis in premature twins with twin-to-twin transfusion syndrome. Eur J Pediatr Surg. 2008;18(1):50–2. [DOI] [PubMed] [Google Scholar]
- 43.Saura L, Munoz ME, Castanon M, Eixarch E, Corradini M, Aguilar C, et al. Intestinal complications after antenatal fetoscopic laser ablation in twin-to-twin transfusion syndrome. J Pediatr Surg. 2010;45(1):E5–8. [DOI] [PubMed] [Google Scholar]
- 44.Squires LS. A case study of recipient twin surviving complications of twin-to-twin transfusion syndrome. Nurs Womens Health. 2013;17(5):390–8. [DOI] [PubMed] [Google Scholar]
- 45.Rebai N, Lopriore E, Bekker V, Slaghekke F, Schoenaker MHD, Groene SG. Necrotizing enterocolitis in monochorionic twins: insights from an identical twin model. Early Hum Dev. 2024;194:106052. [DOI] [PubMed] [Google Scholar]
- 46.Ree IM, Smits-Wintjens VE, Rijntjes-Jacobs EG, Pelsma IC, Steggerda SJ, Walther FJ, et al. Necrotizing enterocolitis in small-for-gestational-age neonates: a matched case-control study. Neonatology. 2014;105(1):74–8. [DOI] [PubMed] [Google Scholar]
- 47.Boghossian NS, Geraci M, Edwards EM, Horbar JD. Morbidity and Mortality in Small for Gestational Age Infants at 22 to 29 Weeks’ Gestation. Pediatrics. 2018;141(2). [DOI] [PubMed]
- 48.Hasegawa J. Ultrasound screening of umbilical cord abnormalities and delivery management. Placenta. 2018;62:66–78. [DOI] [PubMed] [Google Scholar]
- 49.Alfirevic Z, Stampalija T, Dowswell T. Fetal and umbilical doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev. 2017;6(6):CD007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machin GA, Ackerman J, Gilbert-Barness E. Abnormal umbilical cord coiling is associated with adverse perinatal outcomes. Pediatr Dev Pathol. 2000;3(5):462–71. [DOI] [PubMed] [Google Scholar]
- 51.Rose AT, Patel RM. A critical analysis of risk factors for necrotizing enterocolitis. Semin Fetal Neonatal Med. 2018;23(6):374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamoji VM, Dorling JS, Manktelow B, Draper ES, Field DJ. Antenatal umbilical doppler abnormalities: an independent risk factor for early onset neonatal necrotizing enterocolitis in premature infants. Acta Paediatr. 2008;97(3):327–31. [DOI] [PubMed] [Google Scholar]
- 53.Stagnati V, Zanardini C, Fichera A, Pagani G, Quintero RA, Bellocco R, et al. Early prediction of twin-to-twin transfusion syndrome: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;49(5):573–82. [DOI] [PubMed] [Google Scholar]
- 54.Weisz B, Hogen L, Yinon Y, Gindes L, Shrim A, Simchen M, et al. Perinatal outcome of monochorionic twins with selective IUGR compared with uncomplicated monochorionic twins. Twin Res Hum Genet. 2011;14(5):457–62. [DOI] [PubMed] [Google Scholar]
- 55.Yoda H. Fetal and neonatal circulatory disorders in Twin to Twin Transfusion Syndrome (the secondary publication). J Nippon Med Sch. 2019;86(4):192–200. [DOI] [PubMed] [Google Scholar]
- 56.Gagliardi L, Bellu R, Cardilli V, De Curtis M, Network Neonatale L. Necrotising enterocolitis in very low birth weight infants in Italy: incidence and non-nutritional risk factors. J Pediatr Gastroenterol Nutr. 2008;47(2):206–10. [DOI] [PubMed] [Google Scholar]
- 57.Lambert DK, Christensen RD, Henry E, Besner GE, Baer VL, Wiedmeier SE, et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J Perinatol. 2007;27(7):437–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.