Abstract
Background
Larval source management (LSM) is re-emerging as a critical malaria intervention to address challenges associated with core vector control tools, such as insecticide-treated nets (ITNs), and to accelerate progress towards elimination. Presently, LSM is not widely used in rural settings and is instead more commonly applied in urban and arid settings. A systematic entomological assessment was conducted in rural communities of southeastern Tanzania, where insecticide-treated nets (ITNs) are widely used, to explore opportunities for deploying LSM to improve malaria control.
Methods
Aquatic habitat surveys were conducted in 2022 and 2023 to understand habitat usage by different mosquito vectors, covering five villages during the rainy season and seven villages during the dry season. Additionally, samples of adult mosquitoes were collected to assess the role of various Anopheles species in malaria transmission in the area, and to explore opportunities for species sanitation using targeted LSM.
Results
Adult mosquito surveys showed that in this area, the total entomological inoculation rates (EIR) for indoor collections were 20.1 and 6.5 infectious bites per person per year for outdoors. Anopheles funestus and Anopheles arabiensis were the only Anopheles vectors identified. Anopheles funestus was responsible for over 97.6% of the malaria transmission indoors and 95.4% outdoors. The concurrent larval surveys found that habitats with late instar An. arabiensis and An. funestus comprised only a small subset of 11.2%–16.5% of all water bodies in the rainy season, and 9.7%–15.2% in the dry season. In terms of size, these habitats covered 66.4%–68.2% of the total habitat areas in the wet season, reducing to 33.9%–40.6% in the dry season. From the rainy season to the dry season, the surface area of habitats occupied by An. arabiensis and An. funestus decreased by 92.0% to 97.5%, while the number of habitats occupied by An. arabiensis and An. funestus decreased by 38.0% to 57.3%. Anopheles funestus preferred large, permanent habitats with clear water and vegetation year-round, while An. arabiensis showed contrasting seasonal preferences, favouring sunlit still waters in the rainy season and larger, opaque habitats in the dry season.
Conclusion
These findings suggest that An. funestus, which is the dominant malaria vector in the area, mediating over 95% of malaria transmission, preferentially occupies only a small subset of uniquely identifiable aquatic habitats in both wet and dry seasons. This presents an opportunity to expand LSM in rural settings by carefully targeting An. funestus habitats, which might be effective and logistically feasible as a complementary approach alongside existing interventions. Further research should assess the impact of targeted LSM for species sanitation compared to blanket LSM.
Background
Insecticide-treated nets (ITNs) and indoor residual spraying (IRS) are used as primary tools to control malaria transmission in many African countries and have significantly reduced transmission over the past few decades [1, 2]. These interventions are dependent on insecticides and primarily target indoor-biting and resting adult mosquitoes. Unfortunately, extensive use of insecticides for public health and agricultural purposes has led to the adaptation of malaria vectors that allow them to evade the fatal impact of insecticides [3, 4]. These adaptations can manifest through the development and spread of insecticide resistance [5–7] or behavioural changes such as increased early morning and early evening biting, and outdoor biting of mosquitoes [8–10]. Physiological and behavioural resistance to insecticide-based interventions, together with other challenges such as sub-optimal coverage and use of interventions [11], means that many malaria-endemic countries are unlikely to realize the malaria elimination goals, including the ambitious targets set forth by the World Health Organization (WHO)—to eliminate malaria in 35 countries by 2030 and reduce mortality and incidence by 90% compared to 2015 [12].
To address these challenges, larval source management (LSM) is re-emerging as a critical malaria intervention, which could be deployed alongside the primary approaches to accelerate progress towards elimination. LSM consists of four main strategies: (1) habitat modification or source reduction, involving permanent environmental alterations such as land reclamation; (2) habitat manipulation, which includes recurrent activities like stream flushing or clearing of vegetation; (3) larviciding, or regular application of biological or chemical insecticides to water bodies; and (4) biological control, introducing natural predators into water bodies [13, 14].
LSM was historically an important component of malaria control around the world but became less commonly used in the 1950s following the discovery of DDT, which was efficacious and less labour-intensive to apply [15, 16]. The widespread use of ITNs in the past three decades further disincentivized its application in Africa. However, recent successful implementations in several sub-Saharan African countries have revived interest in LSM [17, 18], particularly larviciding, which is now endorsed by the WHO as a supplementary measure to insecticide-treated nets (ITNs) and indoor residual spraying (IRS) in settings with limited and specific types of vector aquatic habitats, typically found in urban and arid areas [14, 19]. LSM is re-emerging as a potential solution to several current challenges facing vector control. The expectation is that by targeting mosquitoes at the source, it would be possible to control their populations effectively regardless of their physiological resistance status or biting behaviours.
Ecological studies suggest that the effectiveness of LSM can vary greatly in Africa, where diverse malaria vector species inhabit different aquatic habitats. For example, Anopheles gambiae sensu lato (s.l.) typically breeds in small, temporary water bodies, while Anopheles funestus s.l. prefers larger, more permanent sites [20, 21]. This variability often makes LSM challenging, particularly in rural areas dominated by An. gambiae, unless ecological or seasonal conditions naturally limit breeding sites to fewer, manageable habitats [13, 14]. Such conditions might include the seasonal drying of water bodies or the concentration of vectors in a small number of distinctive, semi-permanent habitats.
Recent reports indicate that some sites, in part due to environmental factors and wide-scale use of ITNs, have seen a reduction in densities of An. gambiae sensu stricto (s.s.), historically the most efficient vector in Africa [22–24]. As a result, in east and southern Africa, the proportional contribution of An. funestus to overall transmission intensities now exceeds those of other vector species [25]. Interestingly, An. funestus appears to prefer aquatic habitats that are rarer, have specific characteristics, and may fit the WHO criteria of ‘few, fixed, and findable’ [20, 26, 27]. Therefore, it is likely that in areas where An. funestus predominates, malaria transmission might be considerably controlled by preferentially targeting this vector with LSM to complement adult-targeting approaches [26]. The importance of tailoring LSM to suit local vectors, in particular understanding the importance of the vector and the extent and characteristics of their larval habitats in the targeted area, was noted from the works of Watson and Schwellengrebel [28]. In their work, they coined the concept of ‘species sanitation’ i.e. “selective modification of the environment to render a particular anopheline of no importance as a vector” [28]. One example of this is the successful malaria control with sanitation of Anopheles umbrosus in Malaysia, which involved efforts focused on aquatic habitats in shaded areas where this important vector thrived [28]. The concept of targeted LSM has also been proposed using a different approach that involves targeting a small subset of habitats that are productive [29], though there were also strong counter-arguments against this on the basis that it is not always possible to identify such productive habitats [30]—a situation previously evidenced in urban Dar es Salaam, where extensive surveys yielded no recognisable aquatic habitats for malaria vectors [31].
To further explore opportunities for expanding LSM to rural settings, this study was conducted in villages in the Ulanga district, southeastern Tanzania to identify, enumerate, and compare the significance of different aquatic habitats in the area, as well as to assess the contribution of various vector species to malaria transmission. The data was also used to investigate the extent to which the aquatic habitats of the dominant malaria vector might potentially be targeted to improve malaria control.
Methods
Study site
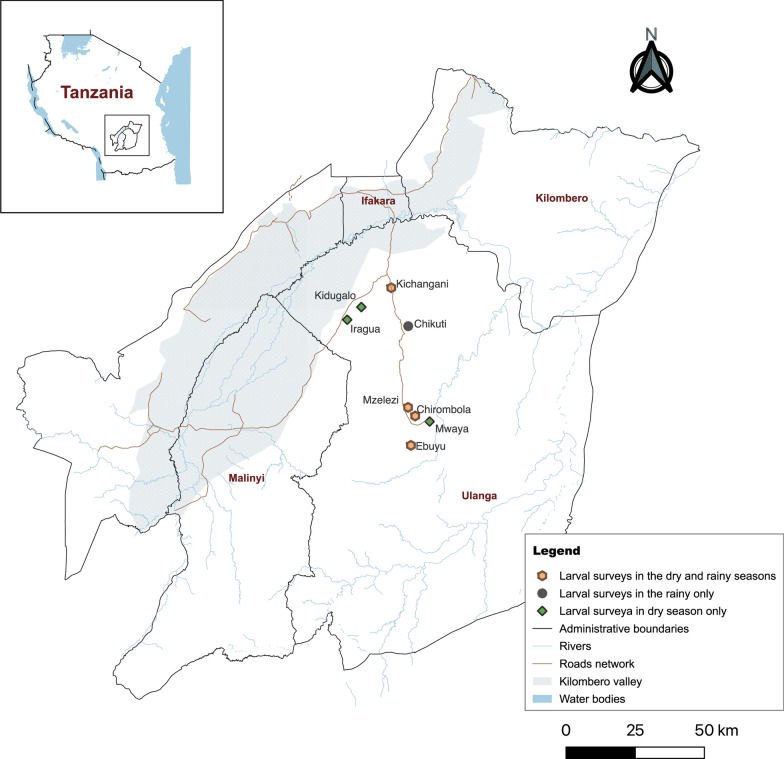
This study was conducted in Ulanga district, south-eastern Tanzania (Fig. 1), which is comprised mostly of rural and semi-urban settlements. Community members here are predominantly crop farmers, with some practising small-scale animal husbandry or fishing, or running small businesses. The rainy season extends from November to May, with peak rains between March to May. Annual rainfall ranges between 800 and 1600 mm, and temperatures between 16 and 32 °C [32, 33]. Malaria transmission is mediated by An. funestus and An. arabiensis, with previous studies suggesting that An. funestus as the predominant vector in several villages [34, 35]. A recent assessment revealed that in the study villages over 90% of An. gambiae s.l. were Anopheles arabiensis, and over 99% of An. funestus s.l. were An. funestus s.s. [34, 36, 37]. Consequently, this manuscript will refer to the two vectors as An. arabiensis and An. funestus. The district is mostly mesoendemic but has significant fine-scale malaria prevalence variability [38].
Fig. 1.
Study villages in Ulanga district where larval surveillance was conducted. Adult mosquitoes were also collected from these villages to assess the role of different Anopheles species in malaria transmission
The study was conducted in eight villages selected with support from malaria focal persons in the Ulanga district. The wards were purposely selected to include wards that had at least 20% malaria test positivity rate among women attending antenatal care clinics between 2019 and 2020 (District Malaria Focal Person, pers. commun.). One village from each of the selected wards was chosen for this study. The final selected villages were Chikuti (− 8.5716, 36.7470), Chirombola (− 8.8975, 36.7681), Ebuyu (− 9.0050, 36.7559), Kichangani (− 8.4311, 36.6866), Kidugalo (− 8.5022, 36.5739), Iragua (− 8.5490, 36.5236), Mzelezi (− 8.8663, 36.7438), and Mwaya (− 8.9183, 36.8253). Each of the selected villages was visited and consent was obtained from the village leaders and household heads to participate in the study.
Survey of adult mosquitoes and their role in malaria transmission
The entomological surveys were conducted between March 2022 and August 2023. Adult mosquito surveillance was conducted in the same villages as aquatic surveys to assess the differential importance of local Anopheles species in malaria transmission. A previous study estimated a mean nightly mosquito catch of 15 (standard deviation of 4.5). Suppose an intervention is to be implemented here with two treatment groups to detect a 45% reduction with 80% power (α = 0.05, coefficient of variation of 0.3 (pers. comm. Moore)), a sample size of 148 houses per arm is required. Therefore, a total of 37 houses were selected in each village. To achieve this, the satellite-generated open building dataset containing the geocoordinates of buildings was downloaded, and a random selection of 40 buildings per village was conducted using QGIS software [39–41]. The geocoordinates of the selected buildings were loaded onto a handheld GPS device to help locate the selected houses for recruitment. However, upon visitation, some of the selected buildings were found to be uninhabitable, and a few of the occupied houses did not consent to participate. To compensate for these exclusions, the remaining houses were randomly selected from a list of households provided by village leaders, bringing the total number of houses in each village to 36. One additional house (i.e. 37th house) was selected purposefully in each village and was also the house of a volunteer who helped distribute the traps each collection day. Mosquito collections in each of the 36 randomly selected houses were done at least once monthly in each of the eight villages (1 trap night × 36 houses × 8 villages, repeated every month). In the purposefully selected houses (i.e. the sentinel houses), mosquitoes were collected three times a week, totalling a minimum of 12 collections per month in each of the eight villages (i.e. 12 trap nights × 1 sentinel house × 8 villages, repeated monthly). In all houses, the mosquito collections were done indoors using a CDC-light trap set near a person sleeping under an ITN. Additionally, in the purposefully selected sentinel houses, we also collected mosquitoes outdoors at least two nights a week using miniaturised double net traps (DN-mini traps [36, 42]), set at least 5 m from the house.
Identification of adult mosquitoes and testing of Plasmodium sporozoites
The collected mosquitoes were first segregated by sex and then further categorised by taxa using morphological features [21, 43]. Female Anopheles mosquitoes were also assessed based on their abdominal status and categorised as unfed, fed, or gravid. Subsequently, female Anopheles of each species were pooled, ensuring each pool contained a maximum of ten individuals with the same physiological state and originating from a single household, collection location (indoor/outdoor), and collected on the same day. These pools were then tested by enzyme-linked immunosorbent assay (ELISA) to detect the presence of circumsporozoite protein, a biomarker for Plasmodium falciparum infection [44]. For definitive identification of P. falciparum, all initially positive samples were subjected to a confirmatory ELISA test after boiling the lysates at 100 °C for 10 min—to eliminate potential false positives caused by heat-labile non-P. falciparum antigens [45].
Survey of aquatic habitats and immature stages of mosquitoes
Surveys to identify and characterize mosquito aquatic habitats in each village were conducted by a team of at least two entomology technicians. The team performed a systematic ground search of all accessible water bodies within the study villages, aided by two to three community members from each village to ensure comprehensive habitat identification. Surveys were conducted in the dry season (in villages, Chirombola, Ebuyu, Iragua, Kichangani, Kidugalo, Mwaya and Mzelezi) and in the wet season (in villages Chikuti, Chirombola, Ebuyu, Kichangani, and Mzelezi).
All identified waterbodies were inspected for the presence of mosquito larvae and characterised based on environmental and physio-chemical characteristics. Habitats were inspected for mosquito presence using either a 350-millilitre dipper or a 10-L bucket, selected based on the depth and surface area of the water body. For water bodies less than 20 cm deep, a 350-ml dipper was employed. Five dips were made for areas up to five square metres, with an additional dip for every extra square metre, up to a maximum of 20 dips. For deeper habitats, a 10-L bucket was used: only one dip was made when the surface area was five-metre square or less, and two dips for habitats with six-metre square, one additional dip was made for every five-metre square increase in the surface area. Extended habitats with flowing water such as rivers, streams, and ditches were divided into segments of 50 m (only a few segments were shorter because they were either toward the end of the ditch or on the periphery of the village) in length and each segment was treated as a separate habitat from which mosquitoes were sampled.
After each dipping effort, the content of the dipper was poured into a white tray for sorting, identification, and counting the larvae of mosquitoes. For early instar larvae and pupae, it was possible to identify the mosquitoes only as either Anopheles or other genera but not to any lower taxonomic levels. However, for late instar larvae of Anopheles mosquitoes, we also distinguished them as either An. funestus s.l., An. gambiae s.l. or other Anopheles mosquitoes, using morphological identification [21].
The aquatic habitats were categorized into distinct types: (i) Rivers and streams, which encompassed various river channel formations such as streams, ditches, drains, and pools; (ii) ground pools, including swamps, marshes, and ponds (iii) Spring/wells including groundwater features like seepages, wells or springs; (iv) rice fields, covering all water collections within cultivated areas; (v) human-made habitats, such as brick and sand pits, construction sites, fishponds, and dug holes; (vi) other small habitats, including puddles, hoofprints, and tyre tracks (Fig. 2).
Fig. 2.
Different aquatic habitat classes and types that identified in the study villages in rural south-eastern Tanzania
For each habitat, the following characteristics were assessed and recorded: (i) water movement (stagnant or flowing); (ii) presence or absence of floating or emergent vegetation; (iii) presence or absence of green algae; (iv) water clarity (clear or opaque); (v) presence or absence of shade; (vi) proximity to the nearest house (more than or less than 100 m); and (vii) size of the habitat (less than 10 m2, 10–100 m2, or more than 100 m2).
Data analysis
Data were collected using paper-based tools and an Open Data Kit (ODK) file was created and used for subsequent data entry. The data was then meticulously verified by a different team by cross-referencing it with the original paper forms to identify any inaccuracies. Lastly, a third party independently examined and verified any data modifications.
To assess the importance of different vectors in malaria transmission, three key metrics were estimated: proportion of sporozoite infection (Sr), human biting rate (HBR), and annual entomological inoculation rate (EIR). The Sr values were determined by dividing the number of mosquito pools that tested positive for P. falciparum by the total number of captured mosquitoes. HBR was calculated by dividing the number of collected mosquitoes by the total number of trapping nights. Finally, EIR was estimated by multiplying Sr, HBR, and 365 (total number of days in a year).
Descriptive statistics i.e. means, totals, and proportions were calculated first, for different attributes of enumerated aquatic habitats. Multivariate logistic regression analyses were then employed to identify environmental predictors associated with the presence of An. funestus and An. arabiensis larvae in the habitats and study villages by season. Initially, all environmental predictors were considered, and to obtain the best-fitting model we used a backward selection approach, systematically removing individual variables and assessing the Akaike Information Criterion (AIC). Ultimately, the model with the lowest AIC value was selected. Odds ratios and their corresponding 95% confidence intervals were reported, and P-values less than 0.05 were considered statistically significant. The analyses were done using R software [46].
Results
Contribution of different Anopheles species to malaria transmission
The comprehensive mosquito survey conducted throughout the study period yielded 20,752 adult female Anopheles. Of all Anopheles collected, An. funestus constituted 80% (n = 16,870), followed by An. gambiae s.l. at 18% (n = 3662). The remainder comprised various species, such as Anopheles coustani (n = 60), Anopheles maculipalpis (n = 27), Anopheles ziemanni (n = 6), and Anopheles pharoensis (n = 3).
Based on the ELISA tests for sporozoite infections, malaria transmission within the study villages was attributed to An. funestus and An. arabiensis only (Table 1). Indoor mosquito collections using CDC-light traps yielded an overall annual EIR of 20.1 infectious bites per person per year (ib/p/y), while outdoor collections using DN-Mini traps resulted in an overall annual EIR of 6.5 ib/p/y. In the comparative analysis of species contribution to EIR, it was observed that An. funestus accounted for 97.6% and 95.4% of indoor and outdoor malaria transmission, respectively. An. arabiensis, on the other hand, contributed 2.4% and 4.6% to indoor and outdoor malaria transmission, respectively (Table 1). No Plasmodium infections were detected in the other Anopheles species mosquitoes collected in the study area (Table 1).
Table 1.
Analysis of malaria vectors, transmission indices, and contribution of different vector species to EIR in the study area
| Parameter | Trap & trap positions | An. funestus | An. arabiensis | Other Anopheles |
|---|---|---|---|---|
| Total Trap nights | CDC light traps (indoors) | 4464 | ||
| DN-mini traps (outdoors) | 1422 | |||
| Number caught | CDC light traps (indoors) | 15,343 | 2771 | 84 |
| DN-mini traps (outdoors) | 1527 | 891 | 12 | |
| No. tested pools | CDC light traps (indoors) | 1134 | 3632 | 70 |
| DN-mini traps (outdoors) | 446 | 716 | 8 | |
| No. pools with P. falciparum sporozoites | CDC light traps (indoors) | 240 | 6 | 0 |
| DN-mini traps (outdoors) | 24 | 1 | 0 | |
| Minimum prevalence of P. falciparum | CDC light traps (indoors) | 1.5% | 0.2% | 0 |
| DN-mini traps (outdoors) | 1.5% | 0.1% | 0 | |
| Annual entomological inoculation rate (EIR) | Indoors (based on CDC-light traps data) | 19.6 | 0.5 | 0 |
| Outdoors (based on DN-mini data) | 6.2 | 0.3 | 0 | |
| Overall EIR | Indoors (based on CDC-light traps data) | 20.1 | ||
| Outdoors (based on DN-mini data) | 6.5 | |||
| Percentage contribution of species to EIR | Indoors (based on CDC-light traps data) | 97.6% | 2.4% | 0% |
| Outdoors (based on DN-mini data) | 95.4% | 4.6% | 0% | |
Potential aquatic habitats in the rainy and dry season
Across the five villages visited during the rainy season (Chikuti, Chirombola, Ebuyu, Kichangani, and Mzelezi), a total of 1923 water bodies (potential habitats) were identified; 48 ground pools (2.5%), %), 225 human-made habitats (11.7%), 103 rice fields (5.4%), 1104 segments of river streams (57.4%), 351 springs/wells (18.3%), and 92 other small habitats (4.8%) (Fig. 2, Table 2). The median number of potential aquatic habitats identified was 342 (range: 204–644) and the median surface area of the habitats was 62,930 m2 (range: 19,913–1,197,974). During the dry season, seven villages were visited (Chirombola, Ebuyu, Iragua, Kichangani, Kidugalo, Mwaya, and Mzelezi), where a total of 1528 potential habitats were identified. These included 74 ground pools (4.8%), 67 human-made habitats (4.4%), 26 rice fields (1.7%), 910 stream segments (59.6%), 401 springs/wells (26.2%), and 50 other small habitats (3.3%). In this season the median number and area of potential aquatic habitats identified were 238 (range: 103–331) and 46,525 m2 (range: 3068–486,362), respectively.
Table 2.
Water bodies (potential mosquito aquatic habitats) found in the study villages
| Season | Habitat category | Total number of habitats, N (%) | Total area of habitats, m2 (%) | Median No. habitats/village, m2 (range) | Median area of habitats/village, m2 (range) |
|---|---|---|---|---|---|
| Rainy season | Ground pools | 48 (2.5%) | 1,160,763 (68.3%) | 9 (0–25) | 12,598 (0–988,309) |
| Human-made habitats | 225 (11.7%) | 28,378 (1.7%) | 28 (19–115) | 3585 (1108–16,903) | |
| Rice fields | 103 (5.4%) | 370,740 (21.8%) | 22 (0–42) | 27,123 (0–175,335) | |
| River/stream segments | 1104 (57.4%) | 125,169 (7.4%) | 192 (150–300) | 21,236 (17,122–35,847) | |
| Springs/wells | 351 (18.3%) | 9128 (0.5%) | 54 (6–150) | 275 (12–8186) | |
| Other small habitats | 92 (4.8%) | 4264 (0.3%) | 5 (4–60) | 62 (58–3705) | |
| Subtotal* | 1923 (100%) | 1,698,441 (100%) | 342 (204–644) | 62,930 (19,913–1,197,974) | |
| Dry season | Ground pools | 74 (4.8%) | 646,362 (72.8%) | 15 (0–22) | 17,828 (0–426,003) |
| Human-made habitats | 67 (4.4%) | 15,682 (1.8%) | 9 (2–25) | 260 (11–7594) | |
| Rice fields | 26 (1.7%) | 87,926 (9.9%) | 5 (0–9) | 2066 (0–61,305) | |
| River/stream segments | 910 (59.6%) | 135,407 (15.3%) | 135 (28–194) | 11,028 (2548–59,918) | |
| Springs/wells | 401 (26.2%) | 1349 (0.2%) | 65 (5–100) | 191 (9–374) | |
| Other small habitats | 50 (3.3%) | 936 (0.1%) | 5 (1–16) | 89 (1–322) | |
| Subtotal* | 1528 (100%) | 887,662 (100%) | 238 (103–331) | 46,525 (3068–486,362) |
*Numbers and areas of habitats were calculated from a different number of villages in rainy (5 villages: Chikuti, Chirombola, Ebuyu, Kichangani, and Mzelezi) and dry (7 villages: Chirombola, Ebuyu, Iragua, Kichangani, Kidugalo, and Mwaya and Mzelezi) seasons
Infestation of aquatic habitats by Anopheles mosquitoes in wet and dry seasons
During the rainy season, across five villages, 37.4% (n = 719) of the 1923 water bodies assessed contained Anopheles mosquito larvae or pupae (Table 3). The median number of habitats in these villages was 66.5 (range: 27–341) and the median surface area of habitats was 22,338 m2 (range: 804–1,099,282). The analysis of the rainy season data also revealed that 317 of the 1923 habitats were infested with late instars of An. funestus, covering an area of 1,127,069 m2 out of all potential habitats (1,698,442 m2) (Table 3). When the prevalence of infestation in different habitat categories was assessed, the ground pools had the highest infestation, reaching 35.4% (17 out of 48 ground pools), which constituted 92.7% of the surface area of total ground pool area (1,160,762 m2); since the infested pools were mostly the large ones. Among all An. funestus-infested habitats rivers and streams were the main contributors in terms of the number of habitats (65.9%, 209 rivers and stream segments out of all 317 An. funestus-infested habitats) and ground pools were the primary contributor in terms of total surface area (95.5%, 1,075,964 m2 out of 1,127,069 m2). For An. arabiensis, 215 of the 1923 habitats were infested with late instars of An. arabiensis, covering an area of 1,158,574 m2 out of the total 1,698,442 m2 of potential aquatic habitats. Springs/wells were the most frequent aquatic habitats for An. arabiensis in terms of the number of locations (45.1%, n = 97), but ground pools still contributed the most surface area (93.8%) for larval development (among a total An. arabiensis-infested surface area of 1,160,762 m2).
Table 3.
Number and area of aquatic habitats infested by different immature stages of different Anopheles in the rainy and dry seasons
| Season | Habitat types | Total number of potential habitats | No. habitats with Anopheles immatures, (% contribution) | Proportion of habitats infested by habitat category (%) | Median No. habitats with Anopheles immatures per village (range) | Total area of potential habitats | Total area (m2) of habitats with Anopheles immatures, (% contribution) | Proportion of area infested by habitat category | Median area (m2) of habitats infested with Anopheles immatures per village (range) |
|---|---|---|---|---|---|---|---|---|---|
| Any Anopheles | |||||||||
| Rainy season | Ground pools | 48 (2.5%) | 27 (3.8%) | 56.2 | 7 (0–10) | 1,160,762 (68.3%) | 1,099,282 (88.3%) | 94.7% | 1113 (0 (− 988,309) |
| Human-made habitats | 225 (11.7%) | 88 (12.2%) | 39.1 | 11 (8–45) | 28,378 (1.7%) | 14,430 (1.2%) | 50.8% | 1182 (313 (− 10,232) | |
| Rice fields | 103 (5.4%) | 45 (6.3%) | 43.7 | 9 (0–23) | 370,741 (21.8%) | 98,407 (7.9%) | 26.5% | 9628 (0 (− 48,699) | |
| River/stream segments | 1104 (57.4%) | 341 (47.4%) | 30.9 | 38 (5–205) | 125,169 (7.4%) | 30,246 (2.4%) | 24.2% | 4903 (450 (− 16,026) | |
| Springs/wells | 351 (18.3%) | 186 (25.9%) | 53.0 | 25 (1–96) | 9128 (0.5%) | 804 (0.1%) | 8.8% | 106 (1 (− 401) | |
| Other small habitats | 92 (4.8%) | 32 (4.5%) | 34.8 | 3 (1–22) | 4264 (0.3%) | 1387 (0.1%) | 32.5% | 53 (4 (− 951) | |
| Subtotal* | 1923 (100%) | 719 (100%) | 37.4 | 66.5 (27–341) | 1,698,441 (100%) | 1,244,555 (100%) | 73.3% | 22,338 (804–1,099,282) | |
| Dry season | Ground pools | 74 (4.8%) | 41 (8.6%) | 55.4 | 7 (0–13) | 646,363 (72.8%) | 576,249 (87.6%) | 89.2% | 17,549 (0 (− 392,015) |
| Human-made habitats | 67 (4.4%) | 32 (6.7%) | 47.8 | 5 (0–9) | 15,680 (1.8%) | 14,884 (2.3%) | 94.9% | 193 (0 (− 7519) | |
| Rice fields | 26 (1.7%) | 7 (1.5%) | 26.9 | 0 (0–3) | 87,926 (9.9%) | 32,530 (4.9%) | 37% | 0 (0 (− 27,273) | |
| River/stream segments | 910 (59.6%) | 334 (70.3%) | 36.7 | 39 (21–91) | 135,408 (15.3%) | 33,468 (5.1%) | 24.7% | 4623 (2234 (− 6733) | |
| Springs/wells | 401 (26.2%) | 43 (9.1%) | 10.7 | 8 (1–10) | 1350 (0.2%) | 214 (0%) | 15.9% | 30 (2 (− 80) | |
| Other small habitats | 50 (3.3%) | 18 (3.8%) | 36.0 | 1 (0–7) | 936 (0.1%) | 495 (0.1%) | 52.9% | 33 (0 (− 191) | |
| Subtotal* | 1528 (100%) | 475 (100%) | 31.1 | 36.5 (7–334) | 887,662 (100%) | 657,841 (100%) | 74.1% | 23,707 (214–576,249) | |
| Late instars of An. funestus | |||||||||
| Rainy season | Ground pools | 48 (2.5%) | 17 (5.4%) | 35.4 | 4 (0–7) | 1,160,762 (68.3%) | 1,075,964 (95.5%) | 92.7% | 1052 (0 (− 965,258) |
| Human-made habitats | 225 (11.7%) | 35 (11.0%) | 15.6 | 5 (4–16) | 28,378 (1.7%) | 6664 (0.6%) | 23.5% | 700 (190 (− 4555) | |
| Rice fields | 103 (5.4%) | 12 (3.8%) | 11.7 | 2 (0–5) | 370,741 (21.8%) | 25,637 (2.3%) | 6.9% | 1648 (0 (− 14,310) | |
| River/stream segments | 1104 (57.4%) | 209 (65.9%) | 18.9 | 16 (5–146) | 125,169 (7.4%) | 17,954 (1.6%) | 14.3% | 1584 (450 (− 12,628) | |
| Springs/wells | 351 (18.3%) | 38 (12.0%) | 10.8 | 7 (1–16) | 9128 (0.5%) | 225 (0%) | 2.5% | 30 (1 (− 89) | |
| Other small habitats | 92 (4.8%) | 6 (1.9%) | 6.5 | 1 (0–2) | 4264 (0.3%) | 625 (0.1%) | 14.7% | 16 (0 (− 558) | |
| Subtotal* | 1923 (100%) | 317 (100%) | 16.5 | 26 (6–209) | 1,698,442 (100%) | 1,127,069 (100%) | 66.4% | 12,309 (225–1,075,964) | |
| Dry season | Ground pools | 74 (4.8%) | 20 (8.6%) | 27.0 | 3 (0–7) | 646,363 (72.8%) | 325,325 (90.2%) | 50.3% | 4618 (0 (− 245,934) |
| Human-made habitats | 67 (4.4%) | 10 (4.3%) | 14.9 | 1 (0–5) | 15,680 (1.8%) | 13,995 (3.9%) | 89.3% | 52 (0 (− 7503) | |
| Rice fields | 26 (1.7%) | 3 (1.3%) | 11.5 | 0 (0–2) | 87,926 (9.9%) | 1370 (0.4%) | 1.6% | 0 (0 (− 1272) | |
| River/stream segments | 910 (59.6%) | 188 (80.7%) | 20.7 | 19 (6–79) | 135,408 (15.3%) | 19,803 (5.5%) | 14.6% | 1924 (1147 (− 6132) | |
| Springs/wells | 401 (26.2%) | 11 (4.7%) | 2.7 | 3 (1–4) | 1350 (0.2%) | 104 (0%) | 7.7% | 22 (1 (− 58) | |
| Other small habitats | 50 (3.3%) | 1 (0.4%) | 2.0 | 0 (0–1) | 936 (0.1%) | 1 (0%) | 0.1% | 0 (0 (− 1) | |
| Subtotal* | 1528 (100%) | 233 (100%) | 15.2 | 10.5 (1–188) | 887,662 (100%) | 360,597 (100%) | 40.6% | 7683 (1–325,325) | |
| Late instars of An. arabiensis | |||||||||
| Rainy season | Ground pools | 48 (2.5%) | 12 (5.6%) | 25.0 | 1 (0–8) | 1,160,762 (68.3%) | 1,086,758 (93.8%) | 93.6% | 100 (0 (− 977,509) |
| Human-made habitats | 225 (11.7%) | 26 (12.1%) | 11.6 | 5 (0–12) | 28,378 (1.7%) | 6537 (0.6%) | 23.0% | 321 (0 (− 5435) | |
| Rice fields | 103 (5.4%) | 23 (10.7%) | 22.3 | 1 (0–17) | 370,741 (21.8%) | 55,814 (4.8%) | 15.1% | 5068 (0 (− 30,745) | |
| River/stream segments | 1104 (57.4%) | 42 (19.5%) | 3.8 | 8 (0–20) | 125,169 (7.4%) | 8062 (0.7%) | 6.4% | 1139 (0 (− 4050) | |
| Springs/wells | 351 (18.3%) | 97 (45.1%) | 27.6 | 24 (1–48) | 9128 (0.5%) | 518 (0%) | 5.7% | 87 (22 (− 322) | |
| Other small habitats | 92 (4.8%) | 15 (7.0%) | 16.3 | 0 (0–13) | 4264 (0.3%) | 886 (0.1%) | 20.8% | 0 (0 (− 527) | |
| Subtotal* | 1923 (100%) | 215 (100%) | 11.2 | 24.5 (12–97) | 1,698,442 (100%) | 1,158,574 (100%) | 68.2% | 7299 (518–1,086,758) | |
| Dry season | Ground pools | 74 (4.8%) | 15 (10.1%) | 20.3 | 0 (0–7) | 646,363 (72.8%) | 268,374 (89.1%) | 41.5% | 0 (0 (− 247,540) |
| Human-made habitats | 67 (4.4%) | 6 (4.1%) | 9.0 | 0 (0–3) | 15,680 (1.8%) | 151 (0.1%) | 1.0% | 0 (0 (− 126) | |
| Rice fields | 26 (1.7%) | 2 (1.4%) | 7.7 | 0 (0–1) | 87,926 (9.9%) | 20,211 (6.7%) | 23.0% | 0 (0 (− 16,324) | |
| River/stream segments | 910 (59.6%) | 100 (67.6%) | 11.0 | 11 (1–40) | 135,407 (15.3%) | 12,048 (4.0%) | 8.9% | 1234 (118 (− 3865) | |
| Springs/wells | 401 (26.2%) | 12 (8.1%) | 3.0 | 1 (1–5) | 1350 (0.2%) | 56 (0%) | 4.2% | 12 (0 (− 30) | |
| Other small habitats | 50 (3.3%) | 13 (8.8%) | 26.0 | 1 (0–4) | 936 (0.1%) | 408 (0.1%) | 43.6% | 33 (0 (− 191) | |
| Subtotal* | 1528 (100%) | 148 (100%) | 9.7 | 12.5 (2–100) | 887,662 (100%) | 301,248 (100%) | 33.9% | 6228 (56–268,374) | |
*Numbers and areas of habitats were calculated from a different number of villages in rainy (5 villages: Chikuti, Chirombola, Ebuyu, Kichangani, and Mzelezi) and dry (7 villages: Chirombola, Ebuyu, Iragua, Kichangani, Kidugalo, and Mwaya and Mzelezi) seasons
In the dry season, among seven villages and 1528 potential habitats, 31.1% (n = 475) contained Anopheles mosquito larvae or pupae (Table 3). The median number of habitats in these villages was 36.5 (range: 7–334), and the median surface area of habitats was 23,707 m2 (range: 214–576,249) m2. Further analysis of this dry season data showed that 233 of the 1528 habitats were infested with late instars of An. funestus, covering an area of 360,597 m2 out of the total 887,662 m2 of potential habitats (Table 3). In the analysis of the prevalence of An. funestus infestation in each habitat category, ground pools again had the highest infestation rate, reaching 27% (20 out of 74 ground pools). Among all An. funestus-infested habitats, rivers and streams were the main contributors in terms of the number of An. funestus-infested aquatic habitats in the dry season (80.7%, 188 out of 233 An. funestus-infested habitats), and ground pools were the primary contributor in terms of total surface area, contributing 90.2% (325,325 m2 out of 360,597 m2 An. funestus-infested habitats). For An. arabiensis, 148 of the 1528 habitats were infested with late instars, covering an area of 301,248 m2 out of the total 887,662 m2 of potential habitats. For this species, ground pools had the highest infestation rate, reaching 20.3% (15 out of 74 ground pools). These infested ground pools represented 41.5% (268,374 m2) of the total ground pool area (646,363 m2). Among all An. arabiensis-infested habitats, rivers and stream segments remained significant, contributing 45.1% (n = 97) to the total number of An. arabiensis-infested habitats. However, in terms of surface area, 93.8% of the An. arabiensis-infested habitats surface area (1,158,574 m2) was attributed to ground pools (Table 3).
Availability of aquatic habitats in the wet season versus the dry season
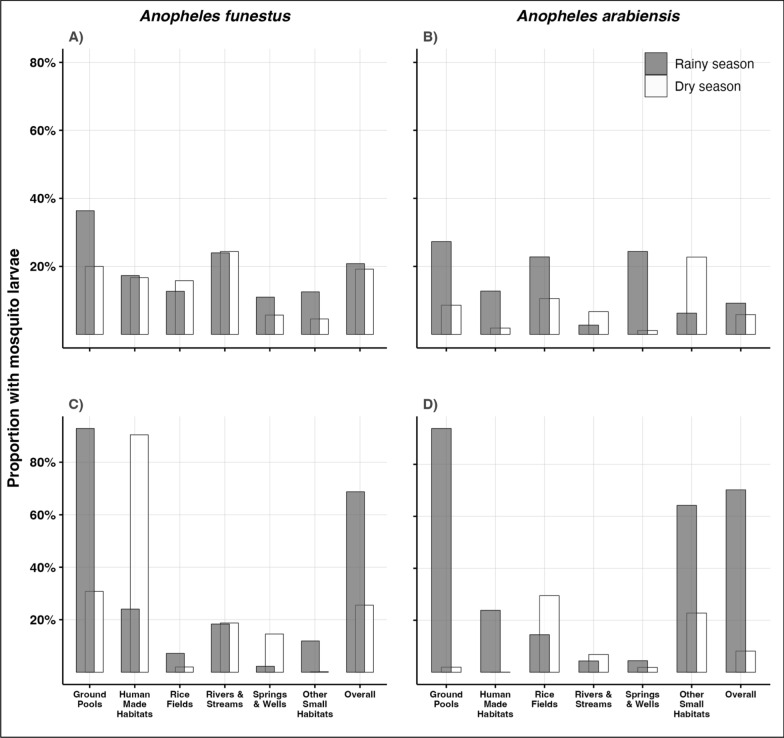
Of all the surveyed villages, four were visited in both wet and dry seasons, namely Chirombola, Ebuyu, Kichangani, and Mzelezi (Fig. 1). In these four villages, habitats with late instar An. arabiensis and An. funestus comprised only a small subset of 9.1%–20.8% of all water bodies in the rainy season, and these figures slightly decreased to 5.8%–19.2% in the dry season. In terms of size, these habitats covered 68.7%–70.2% of the total habitat areas in the rainy season, reducing to 25.6%–8.1% in the dry season (Table 4, Fig. 3). The number of habitats declined by 32.8% between the rainy season and the dry season (1279 vs 860 habitats). This decrease was even more pronounced in terms of surface area, which was reduced by 78.5%. The reduction in both number and surface area varied across habitat types, ranging from 12.4 to 75.9% for the number and from 30.0 to 91.8% for the surface area.
Table 4.
Comparison of the number and surface area of potential habitats and habitats infested by An. funestus and An. arabiensis in the four villages surveyed in both dry and rainy seasons. Percentage reductions in both number and area are included
| Habitats | Habitat category | Rainy season | Dry season | Reduction in number of habitats from rainy season to dry season (%) | Reduction in surface area of habitats from rainy season to dry season (%) | ||
|---|---|---|---|---|---|---|---|
| Total number of habitats, N | Total area of habitats found, m2 | Total number of habitats, N | Total area of habitats found, m2 | ||||
| Late instars of An. funestus | Ground pools | 16 | 1,075,814.3 | 7 | 62,405.9 | 56.2 | 94.2 |
| Human-made habitats | 19 | 5964.3 | 9 | 13,942.8 | 52.6 | +133.8 | |
| Rice fields | 10 | 25,247.6 | 3 | 1369.9 | 70 | 94.6 | |
| River/stream segments | 195 | 16,862 | 135 | 12,044.6 | 30.8 | 28.6 | |
| Springs/wells | 22 | 194.7 | 10 | 103 | 54.5 | 47.1 | |
| Other small habitats | 4 | 66.6 | 1 | 0.7 | 75 | 98.9 | |
| Overall | 266 | 1,124,149.5 | 165 | 89,866.9 | 38 | 92 | |
| Late instars of An. arabiensis | Ground pools | 12 | 1,086,758.3 | 3 | 3916.4 | 75 | 99.6 |
| Human-made habitats | 14 | 5913.9 | 1 | 3.9 | 92.9 | 99.9 | |
| Rice fields | 18 | 50,745 | 2 | 20,211.3 | 88.9 | 60.2 | |
| River/stream segments | 22 | 4011.9 | 37 | 4399.5 | +68.2 | +9.7 | |
| Springs/wells | 49 | 383.6 | 2 | 13.1 | 95.9 | 96.6 | |
| Other small habitats | 2 | 358.7 | 5 | 82.1 | +150 | 77.1 | |
| Overall | 117 | 1,148,171.4 | 50 | 28,626.3 | 57.3 | 97.5 | |
| All potential habitats | Ground pools | 44 | 1,158,902.1 | 35 | 202,464.9 | 20.5 | 82.5 |
| Human-made habitats | 110 | 24,792.9 | 54 | 15,422.3 | 50.9 | 37.8 | |
| Rice fields | 79 | 350,810.5 | 19 | 68,495 | 75.9 | 80.5 | |
| River/stream segments | 813 | 91,851.8 | 554 | 64,257.4 | 31.9 | 30 | |
| Springs/wells | 201 | 8595.8 | 176 | 706.8 | 12.4 | 91.8 | |
| Other small habitats | 32 | 558.6 | 22 | 360.3 | 31.2 | 35.5 | |
| Overall | 1279 | 1,635,511.7 | 860 | 351,706.7 | 32.8 | 78.5 | |
+Indicates an increase in the proportion of the number or surface area in the dry season compared to the rainy season
Fig. 3.
Percentage number and surface area of all potential habitats that were occupied by An. funestus and An. arabiensis in the study area. A Proportion of habitat number infested with An. funestus, B Proportion of habitat number infested with An. arabiensis, C Proportion of surface area infested with An. funestus, D Proportion of surface area infested with An. arabiensis
For habitats infested with the An. funestus larvae, there was a 38.0% decrease in the number of habitats and a substantial 92.0% reduction in the surface area of the infested habitats in the dry season compared to those found infested in the rainy season. Rice fields, puddles, and ground pools experienced the most significant decline, with both the number and surface area of infested habitats dropping by over 50% and 90%, respectively. While the number of human-made habitats infested by An. funestus decreased by 50% during the dry season, the surface area of these infested habitats increased by a factor of 1.3 compared to the rainy season.
An. arabiensis exhibited a similar pattern. The number of infested habitats declined by 57.3% in the dry season, with a corresponding decrease of 97.5% in surface area. Ground pools, human-made habitats, puddles, rice fields, and springs/wells all showed reductions, ranging from 76.9 to 92.5% for habitat numbers. River and stream segments also showed a slight increase in the number of infested habitats (factor of 0.68) during the dry season. However, the reduction in surface area for these habitat types was more pronounced, exceeding 60% for ground pools, human-made habitats, puddles, and rice fields. Notably, the surface area of rivers and stream segments with An. arabiensis increased in the dry season compared to the rainy season.
Cohabitation by An. funestus and An. arabiensis in different aquatic habitats
Among all infested habitats across all seasons, we observed only a limited degree of habitats being co-infested by late instars of both An. funestus and An. arabiensis. Overall, only 8.4% of the habitats were infested with both the malaria vector species. This proportion varied from 6% (n = 43) in the rainy season to 12% (n = 57) in the dry season.
Multivariate logistic regression analysis revealed distinct aquatic habitat characteristics for An. funestus and An. arabiensis across seasons (Table 5). In both rainy and dry seasons, An. funestus exhibited a strong preference for the larger habitats (> 100 m2) characterised by clear water, the presence of floating or emergent vegetations, and the presence of green algae compared to smaller (< 100 m2), opaque habitats lacking these features (p < 0.05). An. arabiensis, on the other hand, displayed contrasting seasonal preferences. During the rainy season, clear water was favoured compared to opaque habitat, while the presence of shade and flowing water significantly reduced the likelihood of An. arabiensis presence (p < 0.05) compared to unshaded stagnant water bodies. In the dry season, An. arabiensis habitats tended to be found in larger habitats with clear water, floating or emergent vegetation, and green algae (p < 0.05) (Table 5).
Table 5.
Characteristics of different aquatic habitat types found with late instars of An. funestus and An. arabiensis in the rainy and dry season
| Season | Habitat characteristics | All water bodies n (%) | No. habitats with late instars of An. funestus (%) | No. habitats with late instars of An. arabiensis (%) | An. funestus | An. arabiensis | ||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95%CI) | p-value | Odds ratio (95%CI) | p-value | |||||
| Rainy season | Water movement | |||||||
| Stagnant | 807 (42%) | 102 (32.4%) | 170 (79.1%) | 1 | 1 | |||
| Flowing | 1113 (58%) | 213 (67.6%) | 45 (20.9%) | 1.1(0.8–1.4) | 0.70 | 0.2 (0.1–0.2) | < 0.01 | |
| Presence of emergent or floating vegetations | ||||||||
| Absent | 1182 (61.6%) | 166 (52.7%) | 110 (51.2%) | 1 | 1 | |||
| Present | 738 (38.4%) | 149 (47.3%) | 105 (48.8%) | 1.4 (1.1–1.8) | 0.01 | 1.3 (1.0–1.8) | 0.06 | |
| Water clarity | ||||||||
| Opaque | 379 (19.7%) | 30 (9.5%) | 49 (22.8%) | 1 | 1 | |||
| Clear | 1541 (80.3%) | 285 (90.5%) | 166 (77.2%) | 2.4 (1.6–3.7) | < 0.01 | 1.8 (1.2–2.6) | < 0.01 | |
| Presence of green algae | ||||||||
| Absent | 1738 (90.5%) | 251 (79.7%) | 188 (87.4%) | 1 | 1 | |||
| Present | 182 (9.5%) | 64 (20.3%) | 27 (12.6%) | 2.8 (2.0–4) | < 0.01 | 1.5 (0.9–2.4) | 0.09 | |
| Habitat shading | ||||||||
| None | 1217 (63.4%) | 202 (64.1%) | 176 (81.9%) | 1 | 1 | |||
| Shaded | 703 (36.6%) | 113 (35.9%) | 39 (18.1%) | 0.9 (0.7–1.2) | 0.69 | 0.4 (0.3–0.6) | < 0.01 | |
| Distance to nearest household | ||||||||
| Greater than 100 m | 874 (45.5%) | 157 (49.8%) | 68 (31.6%) | 1 | 1 | |||
| Less than 100 m | 1046 (54.5%) | 158 (50.2%) | 147 (68.4%) | 0.9 (0.7–1.1) | 0.28 | 1.3 (0.9–1.7) | 0.17 | |
| Habitat size | ||||||||
| Less than 100m2 | 307 (16%) | 24 (7.6%) | 67 (31.2%) | 0.9 (0.7–1.1) | 0.28 | 1 | ||
| Greater than 100m2 | 1613 (84%) | 291 (92.4%) | 148 (68.8%) | 2.1 (1.3–3.4) | < 0.01 | NI | - | |
| Dry season | Water movement | |||||||
| Stagnant | 758 (49.6%) | 85 (36.6%) | 65 (43.9%) | NI | - | 1 | ||
| Flowing | 769 (50.4%) | 147 (63.4%) | 83 (56.1%) | NI | - | 1.0 (0.7–1.5) | 0.97 | |
| Presence of emergent or floating vegetations | ||||||||
| Absent | 1150 (75.3%) | 102 (44%) | 84 (56.8%) | 1 | 1 | |||
| Present | 377 (24.7%) | 130 (56%) | 64 (43.2%) | 3.3 (2.4–4.5) | < 0.01 | 2.0 (1.4–3.0) | < 0.01 | |
| Water clarity | ||||||||
| Opaque | 573 (37.5%) | 51 (22%) | 66 (44.6%) | 1 | 1 | |||
| Clear | 954 (62.5%) | 181 (78%) | 82 (55.4%) | 2.3 (1.6–3.2) | < 0.01 | 0.7 (0.5–1.0) | 0.03 | |
| Presence of green algae | ||||||||
| Absent | 1381 (90.4%) | 187 (80.6%) | 119 (80.4%) | 1 | 1 | |||
| Present | 146 (9.6%) | 45 (19.4%) | 29 (19.6%) | 1.9 (1.2–2.9) | < 0.01 | 2.0 (1.3–3.3) | < 0.01 | |
| Habitat shading | ||||||||
| None | 1014 (66.4%) | 103 (44.4%) | 90 (60.8%) | 1 | 1 | |||
| Shaded | 513 (33.6%) | 129 (55.6%) | 58 (39.2%) | 2.3 (1.7–3.1) | < 0.01 | 1.1 (0.8–1.6) | 0.56 | |
| Distance to nearest household | ||||||||
| Greater than 100 m | 685 (44.9%) | 91 (39.2%) | 64 (43.2%) | 1 | 1 | |||
| Less than 100 m | 842 (55.1%) | 141 (60.8%) | 84 (56.8%) | 1.5 (1.1–2.1) | < 0.01 | 1.2 (0.8–1.7) | 0.32 | |
| Habitat size | ||||||||
| Less than 100m2 | 281 (18.4%) | 5 (2.2%) | 10 (6.8%) | 1 | 1 | |||
| Greater than 100m2 | 1246 (81.6%) | 227 (97.8%) | 138 (93.2%) | 8.8 (3.5–21.9) | < 0.01 | 2.6 (1.3–5.2) | < 0.01 | |
NI Variable not included in the final model
Discussion
Expanding LSM to rural settings in Africa faces a significant obstacle due to perceived logistical challenges arising from the abundance of aquatic habitats. Moreover, the current WHO guidelines have been broadly interpreted in such a way that restricts larviciding to urban and arid settings, where habitats are generally considered “few, fixed and findable”, conditions that are considered rare in rural areas where malaria is more prevalent. In this study, we aimed to understand whether this presumption held in a rural area of south-eastern Tanzania by first identifying the predominant vector species responsible for most malaria transmission and subsequently delineating the habitats of these dominant vectors. This investigation showed that despite the presence of numerous potential aquatic habitats for Anopheles mosquitoes, only a fraction of the total number and the total surface area was infested with the primary malaria vectors. In all surveyed villages, this fraction constituted a small subset of uniquely identifiable habitats. Moreover, the seasonal variability in both the number and size of potential aquatic habitats was pronounced, with more than one-third of the habitats identified during the rainy season disappearing during the dry season and the overall habitat area shrinking significantly, in some cases by up to 90%. These insights contribute to a more nuanced understanding of vector habitat selection and its implications for malaria control strategies. Interestingly, only a very small proportion of habitats were shared between late instars of both An. funestus and An. arabiensis, which suggests limited ecological overlap between the two species in this area.
In rural Africa, where resources are limited, a blanket approach to LSM—though straightforward to implement—can be inefficient in utilising scarce resources and a major drawback for LSM effectiveness [30]. Ideally, LSM should, therefore, take a targeted approach based on key aquatic habitats of dominant mosquito species because not all habitats in these settings are equally important in malaria transmission [28, 29, 47]. Indeed, scientists using mathematical modelling have previously suggested that LSM does not necessarily need to be applied to all habitats but rather to a specific proportion of habitats that are deemed important based on the productivity of adult mosquitoes [29]. While this approach can improve LSM implementation, identifying productive habitats in real-world settings can be challenging [30, 31].
These new field observations accentuate the importance of targeting An. funestus within its aquatic habitats, particularly in regions where An. funestus predominates as the key malaria vector (such as in this study area, where over 95% of malaria transmission is mediated by this one vector species). The findings of this study indicate a reduction of approximately one-third of the habitats of An. funestus during the dry season, suggesting that a significant portion of the habitats in these villages are permanent. It also highlights that the aquatic habitats of this vector in the study area were indeed only a small subset that was uniquely identifiable, manageable, and targetable. This is in line with the earlier assertions by Nambunga et al. [20] that the key habitats of this vector species can indeed be characterised as “fixed, few, and findable”, particularly during the dry season. The advantage of this approach is that it may provide field workers with readily verifiable targets for LSM implementation, which is challenging for other Anopheles species that are plastic in choosing habitats throughout the year, such as An. arabiensis. These targets can be established by implementing habitat management strategies that focus on habitats with characteristics consistently found to harbour An. funestus larvae. Such characteristics include the presence of vegetation, clear water, presence of green algae, shading, and larger sizes. Examples of these habitats are river segments, small streams, spring-fed pools, and other ground pools or ponds, which are critically important as they significantly contribute to the number and surface area of An. funestus habitats. To effectively implement species sanitation for An. funestus, it is crucial to focus on areas where this species is a major malaria vector. In areas where An. funestus plays a minor role, a blanket approach may be more appropriate.
This study also implies that LSM strategies could be more targeted in the dry season, but if deployed in the wet season, then they would need to be more widespread and less focused. However, in areas with seasonal malaria, initiating larviciding during the wet season may be advantageous to control peak transmission periods [48]. Yet, resource limitations or year-round transmission might necessitate a focus on other LSM strategies like habitat modification and larviciding initiated during the dry season. This approach would target the period before heavy rains, potentially limiting the growth of the main malaria vector population. During the dry season, mosquito habitats are scarce, making interventions more impactful and cost-effective due to the critical role these habitats play in sustaining mosquito populations [14, 49]. Seasonal targeting of LSM presents a trade-off between maximising impact and logistical feasibility, particularly in areas with seasonal transmission. While larviciding during the wet season, when the malaria burden is highest, may be most impactful, achieving complete coverage and maintaining larvicide effectiveness can be challenging due to factors like the accessibility of aquatic habitats, dilution, and wash-away of larvicides by the rain. Conversely, the dry season offers easier access to interventions but may coincide with a lower malaria burden. Additionally, long-lasting interventions like habitat modification are ideally suited for the dry season to maximise their impact. This highlights the importance of considering both the impact and feasibility of LSM interventions. Ultimately, the optimal timing for LSM requires careful consideration of both entomological factors, such as mosquito seasonal patterns and malaria transmission patterns, and logistical factors, like intervention accessibility.
Expanded engagement of community members is particularly important as most of the residual water bodies in the dry season are not only mosquito habitats but also serve important domestic purposes, such as being water sources for cattle and domestic uses [50]. This necessitates engaging different groups in the community, i.e. young individuals responsible for livestock grazing, to streamline the process of identifying crucial habitats and ensure targeted interventions [51]. In some settings, communities may express concerns about LSM strategies, which stem from concerns regarding larvicide safety and the potential for environmental damage since some of these habitats serve critical domestic functions [50]. Therefore, the success of LSM in these communities hinges on collaboration with local communities in selecting appropriate LSM strategies for different habitat types, ensuring community buy-in, and maximising programme effectiveness [52].
The substantial variation in habitat types within the study area necessitates a multifaceted approach to LSM beyond just larviciding—i.e. expanding to other forms such as habitat removal or habitat manipulation. In some settings, environmental management strategies that involve the complete removal of habitats, particularly small habitats like puddles and human-made pits, may be prioritised to minimise the need for recurring habitat management.
However, extensive habitats, such as ground pools or streams, that pose logistical challenges for removal can be targeted with alternative methods like larviciding or manipulation. Larviciding, particularly with the growing availability of motorised sprayers or drones, could be important for addressing hard-to-reach habitats or areas within these habitats [53, 54]. Additionally, the use of different larvicide formulations, such as granular larvicide for habitats that are difficult to access, provides flexibility in adapting to diverse environmental conditions [55]. Where available, longer-lasting formulations of larvicides [56] may deliver even greater impact.
To effectively implement species sanitation for An. funestus, it is crucial to address other aquatic habitats that are favourable for this mosquito species, although this may require different approaches for different habitats. For instance, in flowing habitats, such as rivers and streams, these vectors are more prevalent in areas with vegetation and slow-moving water currents. While larviciding might be applicable in these areas, it may require frequent application due to potential dilution or constant wash-away of the larvicides, especially during the rainy season, however, during the dry season, when rivers dry up, stagnant water accumulates in various sections of water channels, making it easier to implement larviciding. In these habitats, the focus should be on employing methods that render these areas unsuitable for oviposition, such as clearing vegetation along the edges of rivers and streams or straightening ditches to ensure proper water flow and prevent stagnation. On the other hand, small and unused human-made pits should be eliminated, such as by filling them with earth to reduce the number of sites requiring ongoing management. Rice fields, despite their minimal contribution to An. funestus habitats can likely also be controlled to aid in malaria control. This exercise can be effectively implemented by engaging with rice farmers and encouraging them to adopt practices that are unfavourable for larval growth, such as weekly flooding and draining routines in their fields [57].
Despite achieving the key objectives, this study also had some limitations. First, field identification of early instar larvae and pupae proved challenging, hindering species identification in habitats containing only these early stages. Consequently, the conclusion regarding aquatic habitat usage is restricted to sites with identifiable late instars of key malaria vectors. Second, resource constraints limited larval surveys to four villages (Chirombola, Ebuyu, Kichangani, and Mzelezi) in both dry and rainy seasons. The remaining villages were surveyed in only one season due to the extensive nature of the surveys and the limitations of a single survey team (not exceeding five individuals). This approach might have underestimated the total surface area surveyed in the rainy season, where only five villages were visited compared to seven in the dry season.
Conclusion
Larval source management (LSM) is gaining renewed attention as a pivotal malaria intervention and could address key limitations of ITNs and IRS, such as insecticide resistance and outdoor biting. However, LSM is currently restricted by the assumption that it would only be feasible where mosquito habitats are “few, fixed and findable”, such as is common in urban and arid areas but not in most rural areas. Our systematic entomological assessment in south-eastern Tanzania, including both adult and aquatic surveys, indicates that the ecology of the main malaria vectors might be readily amenable to LSM beyond its current practice. Adult mosquito surveys identified An. funestus as the primary malaria vector, responsible for over 95% of transmission, with An. arabiensis playing a supplementary role. Concurrent larval surveys revealed a seasonal shift in habitat use by the vector species, with a notable reduction in both the number and size of habitats occupied by vectors during the dry season compared to the wet season. Remarkably, only a fraction of distinctly identifiable habitats was infested by the main vector, An. funestus, which offers significant opportunities to expand LSM as a major intervention in such settings. By strategically targeting these habitats using a species sanitation approach, it may be possible to enhance malaria control efforts and mitigate the burden of malaria transmission in rural areas of south-eastern Tanzania. However, additional research is necessary to assess the effectiveness of these targeted approaches compared to broader strategies.
Acknowledgements
The authors are grateful to the district officials, local leaders, and communities of Ulanga Districts for allowing us to work within their area. We thank local volunteers who helped us in engaging the community and in surveillance.
Author contributions
BJM, FOO, and ALW conceived the study and designed the research framework. MLM guided the selection of study villages. BJM, NSM, DTM, MK, MJ, NFK, and AJL participated in the data collection. BJM analyzed the data and wrote the first and subsequent drafts of the manuscript with inputs from other authors. HSN provided insights during data analysis. PS, ALW, and FOO guided throughout the research process. All authors read and approved the final manuscript.
Funding
Financial support was received from the Bill and Melinda Gates Foundation (BMGF) through the Pan-African Mosquito Control Association (PAMCA) (Grant Number OPP1214408 to FOO, Ifakara Health Institute) to strengthening local capacity for malaria surveillance and elimination in Africa. ALW receives funding from the National Institute for Health Research (NIHR) (Grant Ref: 133144) using UK aid from the UK Government to support global health research and the NIHR (using the UK’s Official Development Assistance (ODA) Funding) and Wellcome [Grant Ref: 220870/Z/20/Z] under the NIHR-Wellcome Partnership for Global Health Research. The funders had no role in the design, data collection, analysis, interpretation of the results, preparation of the manuscript, or decision to publish. The views expressed are those of the authors and not necessarily those of BMGF, PAMCA, Wellcome, the NIHR or the Department of Health and Social Care. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the 783 Author Accepted Manuscript version that might arise from this submission.
Availability of data and materials
All data supporting the main conclusions of this article are included within the article.
Declarations
Ethics approval and consent to participate
Ethics approvals for this study were obtained from the Institutional Review Board of Ifakara Health Institute (Ref no: IHI/IRB/No: 32-2021) and the Medical Research Coordinating Committee (MRCC) at the National Institute for Medical Research (Ref no: NIMR/HQ/R.8a/Vol. IX/3761).
Consent for publication
Permission to publish was granted by The National Institute for Medical Research (NIMR), Tanzania, Ref No. BD.242/437/01C/11.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anne L. Wilson and Fredros O. Okumu contributed equally to this work.
References
- 1.WHO. World malaria report 2022. Geneva: World Health Organization; 2022. [Google Scholar]
- 2.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urio NH, Pinda PG, Ngonzi AJ, Muyaga LL, Msugupakulya BJ, Finda M, et al. Effects of agricultural pesticides on the susceptibility and fitness of malaria vectors in rural south-eastern Tanzania. Parasit Vectors. 2022;15:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranson H, Abdallah H, Badolo A, Guelbeogo WM, Kerah-Hinzoumbé C, Yangalbé-Kalnoné E, et al. Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malar J. 2009;8:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi KS, Christian R, Nardini L, Wood OR, Agubuzo E, Muleba M, et al. Insecticide resistance and role in malaria transmission of Anopheles funestus populations from Zambia and Zimbabwe. Parasit Vectors. 2014;7:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinda PG, Eichenberger C, Ngowo HS, Msaky DS, Abbasi S, Kihonda J, et al. Comparative assessment of insecticide resistance phenotypes in two major malaria vectors, Anopheles funestus and Anopheles arabiensis in south-eastern Tanzania. Malar J. 2020;19:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menze BD, Riveron JM, Ibrahim SS, Irving H, Antonio-Nnkondjio C, Awono-Ambene PH, et al. Multiple insecticide resistance in the malaria vector Anopheles funestus from northern Cameroon is mediated by metabolic resistance alongside potential target site insensitivity mutations. PLoS ONE. 2016;11: e0163261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sangbakembi-Ngounou C, Costantini C, Longo-Pendy NM, Ngoagouni C, Akone-Ella O, Rahola N, et al. Diurnal biting of malaria mosquitoes in the Central African Republic indicates residual transmission may be “out of control.” Proc Natl Acad Sci USA. 2022;119: e2104282119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sougoufara S, Doucouré S, Sembéne PMB, Harry M, Sokhna C. Challenges for malaria vector control in sub-Saharan Africa: resistance and behavioral adaptations in Anopheles populations. J Vector Borne Dis. 2017;54:4–15. [PubMed] [Google Scholar]
- 10.Odero JI, Abong’o B, Moshi V, Ekodir S, Harvey SA, Ochomo E, et al. Early morning anopheline mosquito biting, a potential driver of malaria transmission in Busia County, western Kenya. Malar J. 2024;23:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. World malaria report 2023. Geneva: World Health Organization; 2023. [Google Scholar]
- 12.WHO. Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015. [Google Scholar]
- 13.WHO. Guidelines for malaria vector control. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 14.WHO. Larval source management: a supplementary measure for malaria vector control. Geneva: World Health Organization; 2013. [Google Scholar]
- 15.Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 2020;14: e0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nájera JA, González-Silva M, Alonso PL. Some lessons for the future from the global malaria eradication programme (1955–1969). PLoS Med. 2011;8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database Syst Rev. 2013;2013: CD008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi L, Majambere S, Wilson AL. Larviciding to prevent malaria transmission. Cochrane Database Syst Rev. 2019;2019: CD012736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Guidelines for malaria—2023. Geneva: World Health Organization; 2023. [Google Scholar]
- 20.Nambunga IH, Ngowo HS, Mapua SA, Hape EE, Msugupakulya BJ, Msaky DS, et al. Aquatic habitats of the malaria vector Anopheles funestus in rural south-eastern Tanzania. Malar J. 2020;19:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). Johannesburg: South African Medical Research Institute; 1987. [Google Scholar]
- 22.Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, McCall PJ, et al. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar J. 2014;13:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell TL, Lwetoijera DW, Maliti D, Chipwaza B, Kihonda J, Charlwood JD, et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar J. 2010;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Msugupakulya BJ, Urio NH, Jumanne M, Ngowo HS, Selvaraj P, Okumu FO, et al. Changes in contributions of different Anopheles vector species to malaria transmission in east and southern Africa from 2000 to 2022. Parasit Vectors. 2023;16:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahamba NF, Finda M, Ngowo HS, Msugupakulya BJ, Baldini F, Koekemoer LL, et al. Using ecological observations to improve malaria control in areas where Anopheles funestus is the dominant vector. Malar J. 2022;21:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okumu F, Finda M. Key characteristics of residual malaria transmission in two districts in South-Eastern Tanzania—implications for improved control. J Infect Dis. 2021;223(Suppl 2):S143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley DJ. Watson, Swellengrebel and species sanitation: environmental and ecological aspects. Parassitologia. 1994;36:137–47. [PubMed] [Google Scholar]
- 29.Gu W, Novak RJ. Habitat-based modeling of impacts of mosquito larval interventions on entomological inoculation rates, incidence, and prevalence of malaria. Am J Trop Med Hyg. 2005;73:546–52. [PubMed] [Google Scholar]
- 30.Killeen GF, Tanner M, Mukabana WR, Kalongolela MS, Kannady K, Lindsay SW, et al. Habitat targeting for controlling aquatic stages of malaria vectors in Africa. Am J Trop Med Hyg. 2006;74:517–8. [PubMed] [Google Scholar]
- 31.Sattler MA, Mtasiwa D, Kiama M, Premji Z, Tanner M, Killeen GF, et al. Habitat characterization and spatial distribution of Anopheles sp. mosquito larvae in Dar es Salaam (Tanzania) during an extended dry period. Malar J. 2005;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Weather Online. Morogoro monthly climate averages; 2021. https://www.worldweatheronline.com/morogoro-weather-averages/morogoro/tz.aspx. Accessed 10 Feb 2021.
- 33.Tanzania Meteorological Authority. Monthly climate analysis. Dar es Salaam; 2021. http://maproom.meteo.go.tz/maproom/Climatology/Climate_Analysis/monthly.html?region=bb%3A37.05%3A-8.2125%3A37.0875%3A-8.175%3Abb&YearStart=1995&YearEnd=2020&seasonEnd=Dec. Accessed 10 Feb 2021.
- 34.Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS ONE. 2017;12: e0177807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mapua SA, Hape EE, Kihonda J, Bwanary H, Kifungo K, Kilalangongono M, et al. Persistently high proportions of Plasmodium-infected Anopheles funestus mosquitoes in two villages in the Kilombero valley, South-Eastern Tanzania. Parasite Epidemiol Control. 2022;18: e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limwagu AJ, Msugupakulya BJ, Kilalangongono MM, Mwalugelo YA, Okumu FO, Lyimo IN, et al. Evaluation of the DN-Mini (miniaturized double net) trap for sampling host-seeking Anopheles mosquitoes in malaria-endemic villages of southern Tanzania. PLoS ONE. 2024;19: e0294192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngowo HS, Kaindoa EW, Matthiopoulos J, Ferguson HM, Okumu FO. Variations in household microclimate affect outdoor-biting behaviour of malaria vectors. Wellcome Open Res. 2017;2:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mshani IH, Jackson FM, Minja EG, Abbas S, Lilolime NS, Makala FE, et al. Comparison of fine-scale malaria strata derived from population survey data collected using mRDTs, microscopy and qPCR in South-Eastern Tanzania. medRxiv. 2024. 10.1101/2024.06.24.24309395. [Google Scholar]
- 39.Sirko W, Kashubin S, Ritter M, Annkah A, Bouchareb YSE, Dauphin Y, et al. Continental-scale building detection from high resolution satellite imagery; 2021. http://arxiv.org/abs/2107.12283
- 40.Open Buildings. https://sites.research.google/open-buildings/#download. Accessed 2 Sept 2023.
- 41.QGIS Development Team. QGIS geographic information system. Open Source Geospatial Foundation; 2023. [Google Scholar]
- 42.Limwagu AJ, Kaindoa EW, Ngowo HS, Hape E, Finda M, Mkandawile G, et al. Using a miniaturized double-net trap (DN-Mini) to assess relationships between indoor–outdoor biting preferences and physiological ages of two malaria vectors, Anopheles arabiensis and Anopheles funestus. Malar J. 2019;18:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 2020;19:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 45.Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, et al. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar J. 2011;10:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R Core Team. R: a language and environment for statistical computing. Vienna: R Core Team; 2019. [Google Scholar]
- 47.Gu W, Utzinger J, Novak RJ. Habitat-based larval interventions: a new perspective for malaria control. Am J Trop Med Hyg. 2008;78:2–6. [PubMed] [Google Scholar]
- 48.Runge M, Mapua S, Nambunga I, Smith TA, Chitnis N, Okumu F, et al. Evaluation of different deployment strategies for larviciding to control malaria: a simulation study. Malar J. 2021;20:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charlwood JD, Vij R, Billingsley PF. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. Am J Trop Med Hyg. 2000;62:726–32. [DOI] [PubMed] [Google Scholar]
- 50.Kahamba NF, Tarimo F, Kifungo K, Mponzi W, Kinunda SA, Simfukwe A, et al. Societal uses of the main water bodies inhabited by malaria vectors and implications for larval source management. medRxiv. 2024. 10.1101/2024.05.29.24308146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lupenza ET, Kihonda J, Limwagu AJ, Ngowo HS, Sumaye RD, Lwetoijera DW. Using pastoralist community knowledge to locate and treat dry-season mosquito breeding habitats with pyriproxyfen to control Anophelesgambiae s.l. and Anophelesfunestus s.l. in rural Tanzania. Parasitol Res. 2021;120:1193–202. [DOI] [PubMed] [Google Scholar]
- 52.Mboera LEG, Kramer RA, Miranda ML, Kilima SP, Shayo EH, Lesser A. Community knowledge and acceptance of larviciding for malaria control in a rural district of east-central Tanzania. Int J Environ Res Public Health. 2014;11:5137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Majambere S, Lindsay SW, Green C, Kandeh B, Fillinger U. Microbial larvicides for malaria control in The Gambia. Malar J. 2007;6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrasco-Escobar G, Moreno M, Fornace K, Herrera-Varela M, Manrique E, Conn JE. The use of drones for mosquito surveillance and control. Parasit Vectors. 2022;15:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fillinger U, Kannady K, William G, Vanek MJ, Dongus S, Nyika D, et al. A tool box for operational mosquito larval control: preliminary results and early lessons from the Urban Malaria Control Programme in Dar es Salaam, Tanzania. Malar J. 2008;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.WHO. Prequalified Vector Control Products. 2023. https://extranet.who.int/pqweb/vector-control-products/prequalified-product-list?field_product_type_tid=89&field_pqt_vc_ref_number_value=&title=&field_applicant_tid=&field_active_ingredient_synergis_tid=. Accessed 31 July 2023.
- 57.Chan K, Bottomley C, Saito K, Lines J, Tusting LS. The control of malaria vectors in rice fields: a systematic review and meta-analysis. Sci Rep. 2022;12:19694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the main conclusions of this article are included within the article.