Abstract
Since the discovery of Fe3O4 nanoparticles with enzyme-like activity in 2007, nanozymes have emerged as a promising class of catalysts, offering advantages such as high catalytic efficiency, low cost, mild reaction conditions, and excellent stability. These properties make nanozymes highly suitable for large-scale production. In recent years, the convergence of nanomedicine and nanocatalysis has highlighted the potential of nanozymes in diagnostic and therapeutic applications, particularly in tumor therapy. Despite these advancements, the clinical translation of nanozymes remains hindered by the lack of designs tailored to specific tumor characteristics, limiting their effectiveness in targeted therapy. This review addresses the mechanisms by which nanozymes induce cell death in various tumor types and emphasizes the key design considerations needed to enhance their therapeutic potential. By identifying the challenges and opportunities in the field, this study aims to provide a foundation for future nanozyme development, ultimately contributing to more precise and effective cancer treatments.
Keywords: Nanozyme, Classification, Tumor, Cancer therapy
Background
Recently, cancer has become a growing concern with increasing incidence and mortality rates worldwide [1]. Aberrant cancer metabolism, characterized by enhanced anabolic pathways and aerobic glycolysis, significantly impacts tumorigenesis, drug resistance, and metastasis. Targeting metabolic plasticity has become a key objective in modern cancer therapies, aiming to regulate the expression of metabolic genes and metabolic enzyme activity [2]. The traditional modes of treatment have proven inadequate for addressing the current cancer burden, necessitating the exploration of novel treatment strategies [3].
In the biomedical and health fields, nanobiomaterials and bioprinting techniques have demonstrated broad potential in tissue regeneration, disease treatment, and the development of functional foods [4–6]. Nanomaterials have also been extensively studied as drug delivery vectors in cancer therapy, offering advantages such as enhanced stability, biocompatibility, and the ability to overcome cancer-related drug resistance while improving pharmacokinetics [7, 8]. There is currently a flurry amount of research on how tumors die, such as ferroptosis, pyroptosis, and calcium overload [9–11]. However, the efficiency of certain conventional drugs in achieving the intended mode of death is not high [12]. Natural enzymes can catalyze relevant therapeutic processes; however, they lack stability within the body, are prone to degradation, and present challenges in terms of transportation and storage. Therefore, synthetic nanozymes are more advantageous [13].
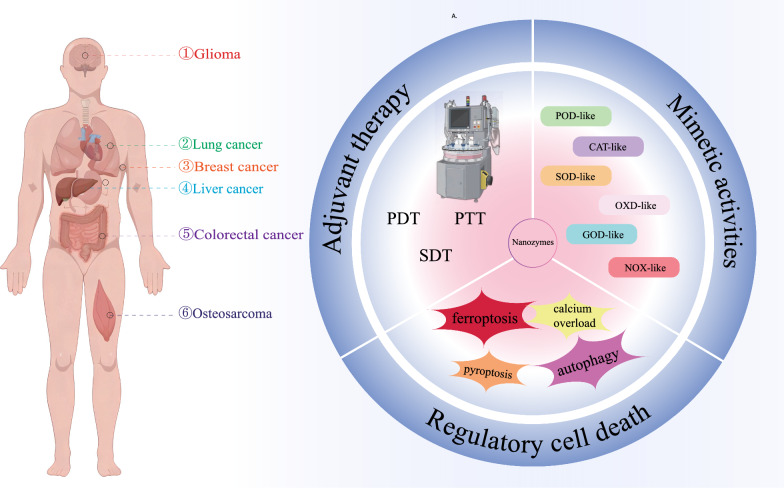
In this regard, nanozymes have emerged as promising therapies for cancer. Recent research on nanozymes has revealed their potential in cancer diagnosis, inducing cancer cell death, and inhibiting tumor growth [14]. A variety of nanozymes are currently being used to treat therapeutic tumors, most of which have a generalized effect on tumors, but the properties of different tumors vary greatly. Considering the specificity of different tumors and therapeutic strategies, summarizing the characteristics of nanozymes required for various types of tumors will play a guiding role in the design and application of nanozymes (Fig. 1), increasing the targeting and clinical translational value of nanozymes.
Fig. 1.
Nanozymes for different types of cancer. Key considerations in designing nanozymes for tumors include: specificity to the tumor; adjuvant therapy; mimetic activities and regulatory cell death (RCD)
This review aims to provide a foundation for the future development of nanozymes by identifying the challenges and opportunities in the field, ultimately contributing to more precise and effective cancer treatments. We conducted a comprehensive literature search using the PubMed and Web of Science databases, covering studies published from 2010 to 2024. We prioritized peer-reviewed articles and excluded non-English publications based on their relevance to the design of nanozymes, mechanisms of cell death, and their biomedical applications in tumor therapy. Key search terms included ‘nanozyme’, ‘tumor therapy’, ‘catalytic activity’, and ‘regulation of cell death’.
Types of mimetic activities by nanozymes
Nanozymes are artificial synthetic enzymes that mimic the activity of natural enzymes. Nanozymes that are widely used in biotherapeutic applications mainly mimic the activity of oxidoreductases, such as peroxidase (POD) [15], catalase (CAT) [16], superoxide dismutase (SOD) [17], and oxidative enzymes (OXDs) [18], which have been widely investigated in recent years. In recent years, scholars have also developed nanozymes with glucose oxidase-like (GOD) [19] and NADH oxidase-like (NOX) [20] properties.
All nanozymes should be designed at the beginning considering the final enzyme-like activity obtained from the material, and nanozymes designed according to different enzyme-like activities have been applied in the treatment of various tumors with remarkable results.
Regulatory cell death
Many current nanozymes have been designed considering that regulatory cell death (RCD) plays an important role in tissue development, internal environment stability and pathogenesis [21]. Many inorganic nanomaterials can induce apoptosis (Table 1), which is a type of regulated cell death caused by massive ROS production leading to intracellular oxidative stress accompanied by caspase-3/7 activation [22]. However, a single apoptosis-inducing effect is susceptible to drug resistance and other characteristics and is not effective in suppressing tumor development [23]. In contrast to apoptosis, newly discovered types of regulatory cell death, such as ferroptosis and pyroptosis, sensitize cancer cells to treatment while releasing large amounts of proinflammatory factors [24]. Additionally, nanozymes with regulatory cell death properties, such as autophagy and necrosis, have been designed, and nanozymes with these properties have been investigated for possible application in the therapeutic field of tumors [25]. This section describes the application of nanomaterials that rely on regulatory cell death to function in tumors (including ferroptosis, calcium overload, pyroptosis and autophagy) Table 1. The Regulatory cell death application of nanozymes in tumor treatment.
Table 1.
The Regulatory cell death application of nanozymes in tumor treatment
| RCD type | Nanozymes | Mimetic activities | Functions | Tumor model | References |
|---|---|---|---|---|---|
| Ferroptosis | mac-DNAFe/PMCS | OXD-,POD-like | Produce ROS; deplete GSH | CT-26 tumor-bearing mouse model | [26] |
| Pyrite nanozymes | POD-,GPx-like | pH-Responsive; produce ROS; deplete GSH | CT-26 tumor-bearing mouse model | [27] | |
| SRF@Hb-Ce6 | CAT-like | Recruitment of CD8 + T, CD4 + T cells, and NK cells into the tumor tissue; produce ROS; deplete GSH | 4T1 tumor-bearing mouse model | [28] | |
| Calcium overload | CaF2 Nanozyme | POD-like | Release Ca2 +; produce ROS;regulating calcium-pumping channels of neoplastic cells | 4T1,H22 tumor-bearing mouse model | [29] |
| CMO NS | POD-,OXD-,CAT-,GPx-,GOx- like | Release Ca2 +; produce ROS;deplete GSH | M109 tumor-bearing mouse model | [30] | |
| CFO-CUR | OXD-、POD-、GPx- like | Release Ca2 +; produce ROS;regulating calcium-pumping channels of neoplastic cells | 4T1 tumor-bearing mouse model | [31] | |
| Pyroptosis | LaFeO3 | POD-,OXD-,CAT-,GPx- like | caspase-1 activation; GSDMD cleavage; IL-1β/LDH release | 4T1 tumor-bearing mouse model | [32] |
| HCS-FeCu | OXD-,POD-like | ROS-Tom20-Bax-Caspase 3-gasdermin E | 4T1 tumor-bearing mouse model | [33] | |
| Autophagy | Fe-CDs@Ang | POD-,OXD-,CAT-,SOD-,Gpx-,TPx- like | Promote autophagy; produce ROS | U87,U251 tumor-bearing mouse model | [17] |
| Cur@MOF-GOx/HA | GOx-, POD-like | Promote autophagy; produce ROS | 4T1 tumor-bearing mouse model | [34] | |
| PN-CeO2 | SOD-, CAT-like | Inhibit autophagy; reduce ROS | SCL-1 tumor-bearing mouse model | [35] | |
| Gd2O3@Ir/MTB-RVG29 | POD-, CAT-like | Inhibit autophagy; reduce ROS | GL261 cells | [36] |
Ferroptosis
Ferroptosis is a regulated cell death (RCD) pathway that was first described by Dr. Brent R Stockwell in 2012 [37]. This pathway is characterized by iron-dependent cell death that occurs due to lipid peroxidation and the accumulation of reactive oxygen radicals. Unlike other RCD pathways, such as apoptosis or necroptosis, ferroptosis is not affected by the regulation of apoptotic effectors or necrosis inducers. Ferroptosis has attracted significant interest from the cancer research community, and many nanozymes have been designed based on the ferroptosis induction mechanism to induce tumor death.
Despite advances in current ferroptosis inducers, their low activity, uncontrolled behavior, and nonselective interactions make efficient systems for triggering ferroptosis still challenging [38]. Qu et al. described the development of a self-adjusting platform for ferroptosis, achieved by attaching a DNA modulator to the surface of single-atom nanozymes (SAzymes). This DNA modulator enhances the ability of SAzymes to specifically produce reactive oxygen species (ROS) and grants them the capacity to consume glutathione (GSH) on demand within tumor cells. This process effectively accelerates ferroptosis in a selective and secure manner [26].
Meng et al. discovered that pyrite nanozymes not only exhibit a high affinity for H₂O₂ but also demonstrate GPx-like activity, oxidizing GSH to produce oxidized glutathione (GSSG) and H₂O₂ [27]. They found that pyrite nanozymes exhibit excellent POD-like activity, effectively catalyzing the conversion of H₂O₂ into hydroxyl radicals (·OH), with a catalytic efficiency that is 4144 times greater than that of Fe₃O₄ nanozymes and 3086 times greater than that of horseradish peroxidase (HRP). Therefore, the dual enzymatic activity of pyrite nanozymes results in the formation of a self-signaling cascade platform that generates a large amount of ·OH and consumes reduced glutathione, thereby inducing apoptosis and ferroptosis in tumor cells and effectively killing apoptosis-resistant tumor cells.
The critical chemical steps for ferroptosis, including ROS production, lipid peroxidation, and GSH depletion, are O2 dependent. However, hypoxic conditions in the tumor microenvironment can significantly inhibit therapeutic efficacy [39]. To solve this problem, Xu et al. linked hemoglobin (Hb) with the photosensitizer chlorin e6 (Ce6) and combined it with sorafenib (SRF, a ferroptosis inducer) to create a dual-function nanoplatform named SRF@Hb-Ce6 [28]. Due to the intrinsic ability of Hb to bind iron and oxygen, it not only supplies oxygen for oxygen-dependent photodynamic therapy (PDT) but also provides iron for iron-dependent ferroptosis. SRF@Hb-Ce6 promoted tumor cell death by enhancing oxygen-augmented PDT combined with an effective ferroptosis trigger.
Cancer cells have higher GSH and ROS levels than normal cells due to their high metabolism, and the enzyme-like properties of nanozymes give them efficient catalytic activity, leading to oxidative stress. In conclusion, the use of ferroptosis as a therapeutic mechanism in nanozymes has been proven to be effective in a variety of cancers, and the research prospects are still very promising; improving the accuracy and catalytic effect of ferroptosis treatment is an important topic that needs to be continuously addressed in the future.
Calcium overload
In cells, mitochondria not only bear the burden of providing energy but are also associated with signaling pathways and closely linked to other organelles [40]. Compared with normal cells, cancer cells exhibit a heightened sensitivity to calcium (Ca2+) regulation, which is attributed to the increased frequency of Ca2+ signaling within these cells [41]. In recent years, nanoparticle development strategies aimed at the calcium overload death mechanism have been increasingly applied in cancer treatment [42–45]. In the field of nanozymes, the application of the calcium overload mechanism in tumors has also received significant attention.
Dong et al. rationally designed and modified a unique valence-stable calcium fluoride (CaF₂) nanozyme with ultrasound (US)-enhanced peroxidase (POD) mimicking activity [29]. This nanozyme efficiently releases exogenous Ca2⁺ ions from Ca2⁺ nanocrystals, while US-amplified POD mimics produce harmful reactive oxygen species (ROS). This combination promotes intracellular Ca2⁺ accumulation and leads to mitochondrial dysfunction by introducing exogenous Ca2⁺ ions and regulating calcium pump channels in tumor cells. Dong et al. demonstrated that valence-stable metal compounds have enzyme-mimicking activities, which is highly important for greatly expanding the understanding and application scope of emerging nanozymes.
Wang et al. designed and modified two-dimensional Ca₂Mn₈O₁₆ nanosheets (CMO NSs) by incorporating glucose oxidase, thereby equipping them with high-performance nanozyme activities that mimic glutathione peroxidase, catalase, oxidase, peroxidase, and glucose oxidase [30]. Through a cascade of catalytic reactions, they disrupt the glucose supply, self-supply of H₂O₂, and subsequent effective ROS generation, thereby resulting in antitumor effects. Additionally, the efficiency of synergistic tumor therapy involving calcium overload, ferroptosis, and apoptosis was further enhanced by the application of exogenous ultrasound stimulation. This work links calcium overload, ferroptosis, and apoptosis through a cascade reaction, emphasizing the crucial role of enzymatic kinetic properties in synergistic tumor therapy through ferroptosis and apoptosis.
Chang et al., aiming to address the issue of rapid restoration of mitochondrial Ca2⁺ concentrations due to natural Ca2⁺ efflux, which could lead to diminished antitumor effects, designed CFO-CUR. This strategy utilizes the characteristic of curcumin (CUR), which can inhibit Ca2⁺ outflow, thereby increasing intracellular Ca2⁺ ion concentrations [31].
CFO utilizes the release of exogenous Ca2⁺ ions to increase intracellular Ca2⁺ accumulation. CUR further prevents Ca2⁺ from being expelled from the cytoplasm through the plasma membrane, thus enhancing mitochondrial-mediated antitumor efficacy. Additionally, Chang et al. amplified mitochondrial dysfunction and antitumor efficacy through a US-induced ionic burst process, providing important insights for the design of nanozymes based on the calcium overload mechanism.
Calcium overload, a novel death mechanism, has garnered significant attention in cancer research. While it has been extensively studied in the field of nanoparticles, its application in nanozymes still requires further exploration. The connections between calcium overload and ferroptosis are particularly notable. Utilizing calcium overload as an anchor point in combination with other modes of cell death represents a promising strategy to enhance the efficacy of cancer treatments.
Pyroptosis
Pyroptosis was first described in 1992 by Zychlinsky et al., [46] and it is a type of lytic cell death induced by pathogen infection or endogenous challenge [47]. As caspase-1-dependent cell death was further investigated and characterized, it became clear that it was distinct from apoptosis. Consequently, in 2001, this form of cell death was officially named pyroptosis [48]. Pyroptosis, a new mechanism of tumor cell death accompanied by the release of large amounts of proinflammatory factors, lactate dehydrogenase, and damage-associated molecular patterns (DAMPs), may be a new strategy for tumor therapy. Recently, nanoplatforms developed using pyroptosis have been widely used in tumor therapy. The development of new methods to induce pyroptosis in cancer cells has been a major focus of research in the field of nanomaterials in recent years. Lin et al. were the pioneers in this area, developing biodegradable Ca2 + nanomodulators (CaNMs) that trigger pyroptosis via mitochondrial Ca2+ overload [49], demonstrating for the first time the relationship between mitochondrial Ca2+ overload and pyroptosis induction. In addition to inhibiting tumor proliferation and lung metastasis, this approach generates a robust immune response, contributing significantly to the development of Ca2+ nanozymes. However, optimizing the design of CaNMs to enhance their stability and specificity is still needed to achieve greater efficacy and reduce potential toxicity.
In recent years, considerable research on pyroptosis has been conducted in the field of nanozymes. This form of programmed cell death, characterized by its inflammatory response, is being increasingly explored for its potential applications in targeted cancer therapies, utilizing nanozymes to selectively induce pyroptosis in tumor cells. The use of ultrasound-enhanced enzyme dynamic (enzyodynamic) therapy by Chen et al. is another promising approach for inducing pyroptosis. By utilizing LaFeO3 (LFO) perovskite nanocrystals to increase the rate of ROS generation, this approach enhances intensive cell pyroptosis. The process of pyroptosis induced by a burst of reactive oxygen species (ROS) is facilitated through the ROS-TXNIP-NLRP3-GSDMD pathway. This pathway is activated by external ultrasound (US) stimulation, resulting in a marked increase in treatments for tumor metastasis and relapse. Despite these advancements, a deeper understanding of the underlying mechanism and the determination of optimal US conditions for the most effective enzymatic effect remain essential for translating this approach into clinical practice.
In conclusion, the study of nanomaterials capable of inducing pyroptosis has received increasing attention, but the underlying mechanisms still require further investigation. GSDMD and GSDME have recently been extensively studied as two important molecules involved in pyroptosis, but other molecules of the gasdermin family (GSDMA, GSDMB, GSDMC, and GSDMF) also play various roles in pyroptosis. Further investigation of the mechanisms of these molecules and their integration with nanomaterial research will be important for the development of nanoresearch in the field of pyroptosis.
Although pyroptosis, as a form of programmed cell death, can release tumor antigens and activate effective tumor immunogenicity, thereby enhancing the efficacy of immune checkpoint blockade (ICB), current treatments leveraging pyroptosis for tumor therapy have limited effectiveness. Tao et al. approached this challenge by designing hollow carbon spheres (HCSs-FeCu) modified with iron and copper atoms, which exhibit multiple enzyme-mimicking activities [33]. These spheres can induce pyroptosis via a pathway activated by light involving reactive oxygen species (ROS), Tom20, Bax, Caspase 3, and gasdermin E (GSDME). Additionally, mild photothermal activation of pyroptosis in combination with anti-PD-1 therapy can enhance antitumor immune treatment. This work presents an effective method for transforming immunologically "cold" tumors into "hot" tumors, which is highly important for clinical immunotherapy.
Currently, while the induction of pyroptosis by bioactive inorganic nanomaterials has garnered increasing attention, the mechanisms underlying this process remain to be clarified. Further elucidation of the mechanisms by which bioactive inorganic nanomaterials induce pyroptosis, as well as their relationship with tumor progression, is crucial for the development of pyroptosis-inducing cancer therapies. This area of research holds significant potential for advancing the effectiveness of treatments that aim to exploit the inflammatory response of pyroptosis to enhance antitumor immunity.
Autophagy
Autophagy occurs in cells as a conserved metabolic process in response to stress. Due to their “self-digesting” nature, cells undergo autophagy to remove nonessential organelles or degrade proteins to maintain homeostasis in the body and are therefore important for cell survival [50]. In the field of cancer research, the activation of autophagy is recognized as a double-edged sword during tumor treatment. Depending on the level of autophagy induced, autophagy can lead to different outcomes in tumor cells [51, 52]. A striking balance between cancer-promoted and nanomaterial-induced autophagy—whether it supports survival or promotes cell death—is crucial in cancer treatment. Manipulating the level of autophagy to induce an overactivated state represents a viable strategy for targeting tumors. This approach not only boosts the effectiveness of tumor therapies but also encourages a greater number of tumor cells to succumb to autophagic death, thereby optimizing antitumor outcomes.
The design of nanomaterials using autophagy mechanisms is a good idea for the treatment of tumors. For example, He et al. developed nanoparticles capable of on-demand amplification of autophagic cascades. These nanoparticles are designed to enhance cancer immunotherapy by inducing overactivated autophagy in cancer cells [53]. It can precisely convert autophagy to an "overactivated" state, which leads to the autophagic death of tumor cells and enhances subsequent tumor antigen processing. Shi et al. subsequently integrated the autophagy mechanism into the realm of nanozymes by creating ultrasmall carbon dots supported by iron single-atom nanozymes (Fe-CDs) [17]. Due to their multiple enzyme-mimetic properties, Fe-CDs, as drug-free enol drugs, can modulate the tumor microenvironment through reactive oxygen species regulation and lysosome-mediated autophagy. Yao et al. constructed a cascade reaction nanozyme, Cur@MOF-GOx/HA, to enhance tumor starvation therapy by inducing excessive autophagy activation [34]. The HA coating allows the Cur@MOF-GOx/HA nanozyme to enter tumor cells through CD44 receptor-mediated endocytosis, subsequently escaping lysosomes and entering the cytoplasm to induce antitumor effects. GOx catalyzes the conversion of glucose to H₂O₂ and gluconic acid, not only leading to tumor starvation but also providing reactants for the MOF-mediated Fenton reaction, which generates ·OH radicals and induces autophagy. As an autophagy agonist, Cur@MOF-GOx/HA leads to overactivation of tumor starvation-induced autophagy, shifting autophagy from a survival-promoting function to a death-promoting process, thereby enhancing the efficacy of cancer therapy.
In addition to activating the autophagy process, in cancer therapy, autophagy inhibitors reduce the protective role of autophagy, which can also inhibit the development of cancer. Wang et al. constructed PN-CeO₂, which acts as an autophagy inhibitor by inducing the catalytic clearance of ROS in tumor therapy to inhibit protective autophagy [35]. Through its unique antioxidant enzyme mimic activity, it effectively catalyzes the degradation of ROS within tumor cells, further inhibiting the activation of the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and p38 mitogen-activated protein kinase (p38MAPK) pathways in human skin squamous cell carcinoma (cSCC). This suppresses protective autophagy and activates apoptosis, thereby effectively killing tumor cells. Yin et al. developed the Gd₂O₃@Ir/MTB-RVG29 nanozyme, which enhances the inhibition of glioma progression through photothermal therapy (PTT) [36]. The iridium-based nanoparticles primarily exhibit catalase (CAT)- and peroxidase (POD)-like activities to scavenge ROS, significantly amplifying the effects of PTT while effectively protecting the functions of normal neuronal cells. Gd₂O₃ also serves as a specific contrast agent for magnetic resonance imaging (MRI), enabling the nanozyme to target exogenous lasers and monitor therapeutic effects. Importantly, the Gd₂O₃@Ir/MTB-RVG29 nanozyme acts as an autophagy flux inhibitor, effectively inhibiting autophagy in gliomas and promoting PTT.
In conclusion, the autophagy mechanism that induces tumor cell death is an effective strategy that can be used as a nanomaterial for cancer treatment, but the relationship between autophagy and tumor immunity is very complex, and much research is still needed to confirm this mechanism; rationally controlling the relationship between nanomaterials and autophagy is one of the most important points.
In recent years, research on nanomaterials for the treatment of cancer by inducing RCD has attracted much attention, and a large number of nanoparticles with varying effects have emerged, providing new therapeutic approaches for previously difficult-to-treat tumors. However, at present, it is uncertain whether nanomaterial-induced RCD treatment is beneficial for treating tumors because normal cells may also die during RCD, and how to design precise and personalized treatment plans is an urgent problem that needs to be solved in the future. The rational design of nanomaterials for different tumors so that the side effects decrease but the therapeutic efficiency improves is important and should receive increased attention in the future in the field of tumor therapy.
Nanozymes in different tumors
Currently, there are three main modes of action of nanozymes used to treat tumors: direct action on tumors, indirect treatment of tumors by augmenting other therapeutic means, and enhancement of drug efficacy by combining drugs to treat tumors.
Since different tumors have different physiological environments and properties, the treatment of tumors with nanozymes cannot be generalized; here, we summarize the design and study of nanozymes in different types of tumors and look for correlations between the design of different types of nanozymes and different tumors (Table 2).
Table 2.
Summary of nanozymes for the treatment of different cancers
| Tumor type | Nanozyme | Activity | References |
|---|---|---|---|
| Glioma | Fe-CDs@Ang | OXD,CAT,SOD,POD,GPX,TPx | [17] |
| G@IT-R | POD | [54] | |
| Surgiflo@PCN | OXD,CAT,POD | [55] | |
| Breast cancer | Fe–N-C SAzyme | POD | [56] |
| OxgeMCC-r | CAT | [57] | |
| PNBCT | CAT,GSHOx,POD | [58] | |
| TLGp | POD,GOx | [59] | |
| CuPP | CAT,GPx,POD | [60] | |
| CMO-R@4T1 | POD,OXD, NOX | [20] | |
| Mn/PSAE | OXD | [61] | |
| HABT-C@HA | CAT,GOD,POD | [19] | |
| LFO@GOx | OXD,POD,GPx,CAT | [32] | |
| SH-CaO2 | CAT | [45] | |
| Ir-N5 SA | OXD,POD,NOX | [62] | |
| Co-SAs@NC | CAT,OXD | [63] | |
| Fe-N5 | CAT | [64] | |
| Lung cancer | AuNCs-NH2 | CAT | [16] |
| PmMn/SAE | CAT,OXDPOD | [65] | |
| D/LArginine@Ru | OXD,NOS | [18] | |
| Liver cancer | FeN3P-SAzyme | POD | [15] |
| Colorectal cancer | CuCP Lipo NPs | POD | [66] |
| BN-GDY | POD | [67] | |
| BTO/MoS2@CA | POD | [68] | |
| MoS2@Pd-Man | POD | [69] | |
| Cutaneous melanoma | CuGQD/PdNPs@PSi | POD,GSHOx | [70] |
| Osteosarcoma | MXene | CAT,POD | [71] |
| RhRu/Ti3C2Tx | CAT,POD | [72] | |
| Hematological neoplasms | Au@Ce | SOD,CAT | [73] |
| PFOB@PLGA@Pt@DOX-CM | POD,SOD,CAT,OXD | [74] | |
| Fe3O4@Pt | CAT,SOD | [75] |
Gliomas
Glioma, a prevalent primary brain tumor, is typically treated through a combination of surgical tumor resection, radiotherapy, and adjuvant chemotherapy. However, the highly invasive nature of glioblastoma multiforme (GBM) makes it nearly impossible to achieve complete surgical resection. Additionally, the effectiveness of traditional chemotherapeutic agents is significantly limited by their inability to penetrate the blood‒brain barrier and reach tumors [76, 77]. Moreover, the remarkable adaptability of glioblastoma multiforme (GBM) cells leads to the development of resistance to both chemotherapy and radiotherapy, further complicating treatment efforts [78].
Since conventional drugs penetrate the blood‒brain barrier very inefficiently, synthetic nanoparticles that can penetrate the blood‒brain barrier have become a new hope in the field of glioma therapy [79]. Most of the current mainstream research has focused on achieving therapeutic goals by wrapping traditional chemotherapeutic drugs for gliomas with nanoplatforms that can penetrate the blood‒brain barrier, while there are few studies on nanozymes applied to gliomas.
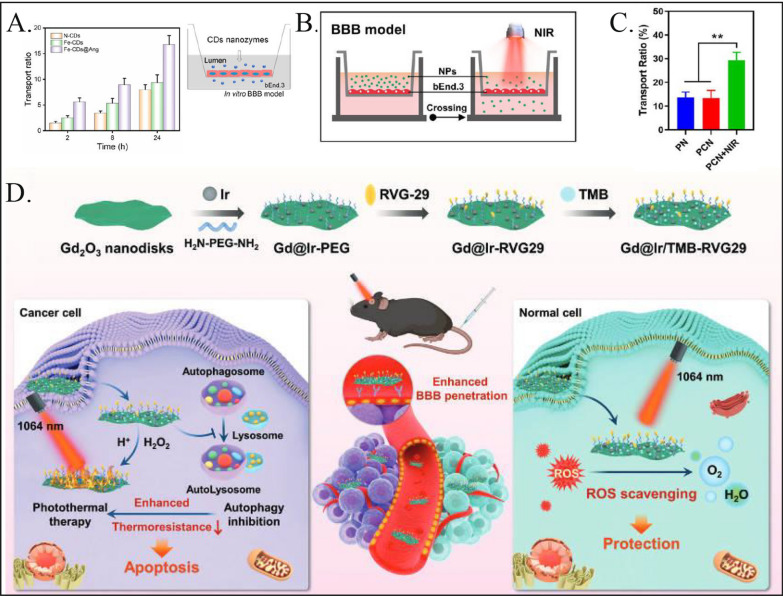
In a recent study, Shi et al. developed ultramicrocarbon dots supporting iron single-atom nanozymes (Fe-CDs) characterized by six enzymatic activities. These Fe-CDs can accurately intervene in the ROS-mediated autophagy signaling pathway, offering a novel strategy to overcome drug resistance in solid glioblastoma multiforme (GBM) tumors [17]. Fe-CDs are designed to accumulate within lysosomes, where they exhibit inherent oxidase/peroxidase (OXD/POD)-like activities that disrupt lysosomal degradation, thereby activating autophagic flux. Additionally, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx)-like enzymes generate reactive oxygen species (ROS), which further enhance both autophagy and lysosomal apoptosis. To ensure precise delivery across the blood‒brain barrier (BBB), the Fe-CDs were functionalized with angiopep-2, resulting in Fe-CDs@Ang, which enhances the efficiency of drug delivery (Fig. 2A). Shi et al. discovered that the autophagy pathway is more effective at targeting drug-resistant glioma cells than the apoptosis pathway, suggesting that influencing the glioma autophagy pathway could provide a significant approach for nanozyme-based glioma therapy.
Fig. 2.
Nanozymes used for treating gliomas. A Fe-CDs@Ang enhances the efficiency of penetrating the blood–brain barrier (BBB) through angiopep-2. Reproduced with permission [17]. Copyright 2022, Elsevier. B, C A Transwell apparatus was used to conduct BBB penetration experiments, and an 808 nm laser at a power of 1.0 W/cm2 was applied to appropriate samples. After 6 h of culture, 13.40% of PCN and 13.63% of PN had penetrated the BBB monolayer. Compared to cells that were not exposed to laser irradiation, the penetration rate for the PCN + near-infrared (NIR) group increased by 2.18 times. This finding suggests that PCN and PN can cross the BBB, and that near-infrared irradiation can significantly enhance this permeability. Reproduced with permission [82].Copyright 2023, ACS NANO. D The G@IT-R nanomachines interact differently with cancer cells and normal cells, enabling the implementation of distinct photothermal therapy (PTT) strategies. Reproduced with permission [54].Copyright 2023, Wiley
In addition to directly targeting gliomas, enhancing current glioma treatments represents a crucial research focus. Recently, photothermal therapy (PTT) has emerged as an effective method for treating gliomas. This approach leverages the conversion of light energy into heat upon exposure to specific wavelengths, selectively damaging or destroying tumor cells while minimizing harm to surrounding healthy tissue [80]. However, challenges such as the nonspecific accumulation of photothermal agents in tissues and the induction of an inflammatory response can adversely affect adjacent brain tissues. These factors may compromise the overall therapeutic efficacy of photothermal therapy (PTT) in the treatment of gliomas, necessitating careful consideration and management to optimize treatment outcomes and minimize collateral damage [81].
Zhang et al. developed a hybrid nanomaterial named Gd2O3@Ir/TMB-RVG29 (G@IT-R) specifically for photothermal therapy (PTT) treatment of gliomas [54]. This nanomaterial is designed to enable tumor-specific PTT while simultaneously mitigating inflammation, thereby safeguarding normal brain tissues. The Ir nanozymes within this system serve a dual purpose: they act as a logical control mechanism that initiates a chromogenic reaction for targeted PTT in tumor cells, and, crucially, in the surrounding normal brain tissue, they function to neutralize the reactive oxygen species (ROS) generated by the treatment. This dual functionality not only enhances the specificity of treatment for tumor cells but also offers protective benefits to adjacent healthy brain tissue, revealing a significant advancement in minimizing collateral damage during glioma treatment. Zhang et al. improved the drug delivery efficiency of nanomaterials by crossing the blood‒brain barrier (BBB) with the rabies virus glycopeptide 29 peptide (RVG29) and targeting gliomas.
In addition, Shi and Huang et al. designed a nanoenzymatic hemostatic matrix system (Surgiflo@PCN) [55], which acts as a photothermal agent while inducing immunogenic cell death after surgical resection of gliomas, which not only assists in enhancing therapeutic treatments but also exerts a killing effect on glioma cells (Fig. 2B, C). While the first role of Surgiflo@PCN is to kill glioma cells via ROS and adjuvant PTT, reversing the immunosuppressive tumor microenvironment and augmenting the antitumor immune response are other critical steps in therapeutic strategies for glioma. It is worth mentioning that the delivery strategy of these nanozymes involves postoperative multifunctional hydrogel implantation, which has good clinical translational value because of its ability to break the blood‒brain barrier. It is important to note that the in vivo validation of the nanozyme combined with photothermal therapy (PTT) for treating glioma used an intracranial mouse model, and since mouse skulls are much thinner than human skulls, potential discrepancies in skull thickness could impact the effectiveness of treatment during clinical translation. PTT is still infrequently used in the clinical treatment of glioma. Therefore, actual clinical features must be carefully considered when designing nanozymes for such therapies.
According to the above-summarized nanozyme treatment modalities, if nanozymes for gliomas are to be administered in vivo, breaking through the blood‒brain barrier is the key issue to be considered, but direct postoperative intracranial administration is also not a poor solution, and it should not be limited to a single design. In addition, it seems that glioma treatment can be more effective by changing the immune microenvironment of glioma than by inducing apoptosis alone. Glioma, as an intracranial tumor, deserves special consideration for therapeutic strategies and approaches more than other tumors, and there is still much to be explored in the design of nanozymes for gliomas, hopefully focusing on the points summarized above.
Breast cancer
Breast cancer is one of the most common malignant tumors, with a 5-year survival rate of only 20% due to drug resistance], high heterogeneity and lack of receptors for hormonal therapy [86, 87]. Generally, low-grade breast cancers are mainly resected, and hormone receptor-positive and HER-2-overexpressing breast cancers can also be treated medically with hormones and targeted agents [88]; however, treatment remains tricky for untargeted and triple-negative breast cancers [89]. Nanozymes can compensate for the shortcomings of breast cancer treatment by catalyzing the production of various factors that can alter the tumor microenvironment and directly or indirectly enhance the death of breast cancer cells [90].
Triple-negative breast cancer has a toxic acidic environment, a high concentration of hydrogen peroxide, hypoxia and other characteristics common to the tumor microenvironment [91]. Owing to the limitations in the catalytic efficiency of typical nanozymes for chemodynamic therapy (CDT), Huang et al. crafted a porous Fe2O3/Au hybrid nanozyme aimed at the Fe2O3/Au hybrid nanozyme operating within the tumor microenvironment, primarily generating hydroxyl radicals due to its exceptional peroxidase-like activity. This activity not only disrupts tumor cells by depleting their energy through glucose consumption but also, crucially, the incorporation of gold nanoparticles significantly boosts the photothermal conversion efficiency of the nanoparticles. Consequently, the Fe2O3/Au hybrid can attack tumors through a multifaceted approach that includes starvation therapy, cascade catalytic reactions, ferroptosis induction, and photothermal treatment modalities. This innovative approach combines the unique properties of both Fe2O3 and Au, enhancing the catalytic activity required for effective CDT and thereby offering a new pathway for the treatment of this challenging cancer type in combination with the synergistic treatment of triple-negative breast cancer [92].
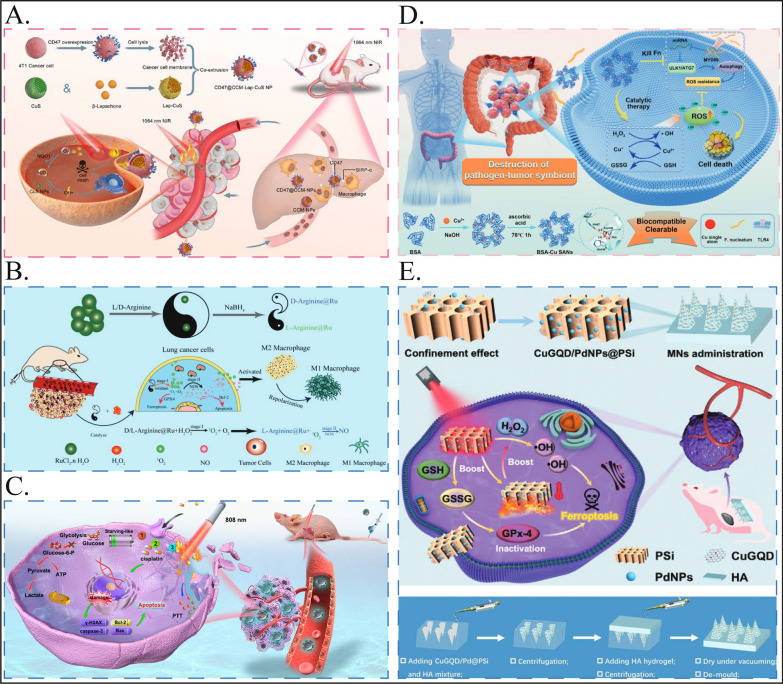
As an emerging noninvasive treatment, photothermal therapy is gradually gaining attention for the treatment of breast cancer [93], but it is limited by the complex pathological barriers of tumors and the uneven dispersion of photosensitizers, which greatly affects the final therapeutic effect. Therefore, the development of nanozymes that can enhance the efficiency of PTT for breast cancer treatment is also a major consideration of current research. Wang et al. reported a novel nanomedicine delivery strategy in which breast cancer cell membranes were extracted and coated with CuS nanoparticles loaded with β-lapachone to inhibit phagocytosis by macrophages via “do not-eat-me” signaling in combination with effective photothermal and chemokinetic precision therapies for the treatment of breast cancer [83]. These nanozymes enable photothermal and chemodynamic precision therapy for breast cancer, creating a new paradigm for safe and limited tumor treatment (Fig. 3A).
Fig. 3.
Nanozymes used for treating other tumors. A The preparation of CD47@CCM-Lap-CuS NPs and the mechanism of CD47@CCM-Lap-CuS NPs mediated precise photothermal and chemodynamic therapy for breast cancer. Reproduced with permission [83]. Copyright 2023, ACS Appl. Mater. Interfaces. B The synthesis process of D/L-Arginine@Ru nanozymes and a schematic diagram of how D/L-Arginine@Ru inhibits lung cancer cell activity through a “cocktail therapy” approach. Reproduced with permission [18]. Copyright 2023, Wiley. C M@TPE-s COF-Au@cisplatin nanoparticles internalize and inactivate the membranes of HepG2 cells, effectively combining photothermal and chemotherapeutic effects. This enables targeted treatment of hepatocellular carcinoma and significantly inhibits tumor growth. Reproduced with permission [84]. Copyright 2023, iScience. D The synthesis of BSA-Cu SAN and its function in disrupting pathogen-tumor symbiosis for antitumor therapy, illustrated schematically. Reproduced with permission [85]. Copyright 2023, Springer Nature. E Schematic illustration of microneedle integrated with PSi loaded with bifunctional nanozymes (including copper-doped graphene quantum dots (CuGQD) and palladium nanoparticles (PdNPs)) to induce ferroptosis of subcutaneous melanoma through nanocatalytic strategy. Reproduced with permission [71]. Copyright 2023, Wiley
In addition to PTT, photodynamic therapy (PDT), which relies on localized oxygen molecules to produce highly cytotoxic single-linear states of oxygen, is one of the therapies worth considering for the treatment of breast cancer [94]. Moreover, nanozymes can improve the therapeutic efficacy of PDT due to hypoxia in solid tumors [95]. OxgeMCC-r single-atom nanozymes with high Ce6 photosensitizer loading capacity can selectively accumulate at breast cancer sites to generate oxygen to improve tumor hypoxia, and their good in vivo tracking imaging capability is a promising anticancer therapeutic agent, as reported by Zhao et al. [57].
Sonic dynamic therapy (SDT) is also an effective way to treat tumors because it can penetrate 7–10 cm deep into tissues, so it is efficient at killing deep tumors and is a good means of treating breast cancer [96, 97]. However, it is similar to PDT and is limited by the lack of an oxygen environment in the tumor, which leads to a greatly reduced therapeutic effect [98]. Therefore, the preparation of sonic sensitizers with multienzyme properties for SDT is also one of the design approaches worth considering. Liu et al. designed a cascade nanozyme-based platform (HABT-C@HA) to modulate hypoxia and immunosuppression in the tumor microenvironment and to enhance the efficiency of SDT, providing a strategy for efficient SDT treatment of breast cancer [19].
In addition to the nanozyme designs summarized above, many new nanozymes are being used for the treatment of breast cancer. Because there are many adjuvant therapies for breast cancer, nanozymes can take full advantage of their efficient catalytic ability to improve the tumor environment and increase the efficiency of other modalities. However, little attention has been given to nanozymes for grading breast cancer with a poor prognosis, such as triple-negative breast cancer, and in the future, more consideration needs to be given to the characteristics of breast cancer itself to design more targeted nanozyme therapeutic agents for breast cancer treatment.
Lung cancer
Lung cancer is one of the most dangerous malignant tumors because it is not easy to diagnose in the early stage and spreads easily in the late stage [99]. Despite significant advances in lung cancer detection and treatment, lung cancer remains the world's deadliest cancer due to the failure to detect lung cancer early and the lack of effective treatments for patients with advanced disease [100]. At present, for early-stage tumors, under the two basic premises of ensuring patient physical function and early-stage disease, surgery is the main treatment; for middle- and late-stage tumors, radiotherapy and chemotherapy are combined with each other [101].
PDT, an advanced minimally invasive ablation technique for localized tumors, has also been used to treat lung cancer because of its minimal invasiveness and easy scalability [102]. Lin et al. designed a catalase-like nanozyme (AuNCs-NH2) to improve the hypoxic environment of lung cancer cells and enhance the therapeutic effect of PDT through the catalytic-like activity of AuNCs-NH2 [16]. Similarly, Liang et al. designed PEG-based mesoporous Mnb single-atom nanozymes (PmMn/SAE) exhibiting catalase-like (CAT), oxidase-like (OXD), and peroxidase-like (POD) activities to ameliorate hypoxia in lung cancer cells, thus synergistically enhancing PTT treatment [65]. However, the above two nanozymes were not designed for lung cancer, and their functions were only verified in lung cancer cells; therefore, more in-depth research is needed to determine their clinical application in lung cancer.
Adjuvant therapy, such as PTT and PDT, is a good adjuvant way to improve early lung cancer and actively cooperate with surgery. For middle- and late-stage lung cancer, from the perspective of lung cancer itself, we should pay more attention to its characteristics, such as immunosuppression and easy metastasis. One of the most prominent problems is immune resistance [103]. In some patients, after the use of immunotherapeutic drugs, tumor cells are still able to evade the attack of the immune system, leading to treatment failure [104]. TAMs are the main cause of immunosuppression in the tumor microenvironment; therefore, inducing macrophage M1 polarization is an effective strategy for remodeling the tumor microenvironment to suppress lung cancer cells [105]. Professor Liu Jie's team proposed a composite ruthenium nanoenzyme (D/L-arginine@Ru), which can mimic the activities of oxidase and nitric oxide synthase (NOS) at the same time, catalyzing the generation of ROS from low concentrations of H2O2 and catalyzing the production of high concentrations of NO, which induces M1 polarization of macrophages. This not only induced apoptosis and ferroptosis in lung cancer cells but also efficiently inhibited their growth and metastasis [18] (Fig. 3B). Liu et al. provided a new strategy for the treatment of lung cancer through the ability of endogenous catalytic therapy to improve the tumor microenvironment and activate autoimmunotherapy.
The use of nanozymes in the treatment of lung cancer has not been well characterized, especially for the early diagnosis of lung cancer, for which much research is still lacking. To further improve the diagnostic and therapeutic effects of nanozymes, nanozymes can be combined with multidisciplinary techniques such as pharmacology and pharmacology. Different dosage forms of nanozymes (solution, inhaler or gel, etc.) can be designed according to the site of disease (mouth, lung, airway, etc.).
Liver cancer
Liver cancer is a common malignant tumor [106]. Summarizing the characteristics of liver cancer, we summarize them as the “five most”: the most difficult to detect, the most difficult to diagnose, the most difficult to treat, the fastest development, and the worst prognosis [107–111]. Hepatocellular carcinoma has the second highest mortality rate of all malignant tumors after lung cancer [112]. Its early symptoms are not obvious, while there is almost no effective treatment available to control it in the late stage [113]. Therefore, early detection, early diagnosis and early treatment are the only means to improve the prognosis of liver cancer patients. We summarize the research on nanozymes in the field of hepatocellular carcinoma, which is generally categorized into early prevention and improvement of adjuvant therapeutic strategies for hepatocellular carcinoma.
The combination of targeted nanoprobe-based photothermal therapy, which has the advantages of precision and efficiency, provides an effective method for the diagnosis and treatment of liver cancer and enables the simultaneous diagnosis and treatment of early-stage liver cancer using a single highly efficient material. Zhang and Wang et al. reported a biomimetic multifunctional COF nanoenzyme (M@TPE-s COF-Au@cisplatin) [84]. The nanoenzyme was endocytosed on the membrane of inactivated HepG2 cells, and under laser irradiation, the COF nanoenzyme underwent high-temperature cleavage, resulting in the release of COF nanozymes and a high concentration of drugs, which efficiently exerted combined photothermal and drug therapeutic effects and were able to target hepatocellular carcinoma and significantly inhibit tumor growth. Covalidated in three animal models, TPE-s COF-Au nanozymes can be specifically fused with autologous hepatocellular carcinoma cells for dual imaging and combination therapy (Fig. 3C).
Liver fibrosis is considered a major risk factor for hepatocellular carcinoma. Associate Professor Nan Li’s team proposed a therapeutic strategy of “remodeling the hepatic fibrosis microenvironment” and developed nilotinib (NIL)-loaded hyaluronic acid (HA)-coated Ag@Pt nanodelta nanohydrolase (APNH NT) to inhibit hepatic stellate cell activation and remodel the hepatic fibrosis microenvironment [114]. Compared with conventional drugs, this nanodelivery system can simultaneously scavenge reactive oxygen species, improve the oxygen-depleted microenvironment, and degrade extracellular collagen at the liver site, thus hindering the progression of liver fibrosis and preventing the progression of hepatocellular carcinoma from an early stage.
Currently, there are few studies on nanozymes in the early diagnosis and treatment of liver cancer, and researchers need to focus on how to improve the efficiency of early diagnosis, which is more clinically relevant for the treatment of liver cancer.
Colorectal cancer
Colorectal cancer is the third most common cancer and the fourth leading cause of cancer deaths worldwide and is a major public health problem [115]. Despite improvements in surgical and oncologic treatments, it continues to have high morbidity and mortality rates. Surgical resection is the cornerstone of colorectal cancer treatment and is traditionally the only curative treatment; however, it often causes considerable inconvenience and pain to patients after surgery [116]. Neoadjuvant radiotherapy is used for locally progressive rectal cancer to increase the complete resection rate and reduce the risk of recurrence, and this method is also suitable for patients who need strong organ preservation [117]. Improving therapeutic strategies for colorectal cancer through nanozymes is an important goal of current research. We summarize the research on nanozymes in the field of colon cancer, providing a variety of new ideas on nanozymes for therapeutic management of colon cancer from early disease progression to development.
Chronic inflammation is a well-recognized carcinogen in colitis-associated colorectal cancer, and concomitant anti-inflammatory and antitumor therapies are required clinically [118, 119]. Zha and Huang et al. reported the use of polyethylene glycol (PEG)-coated ultrasmall rhodium nanodots (Rh-PEG NDs) as metalloenzymes. It possesses reactive oxygen and nitrogen species (RONS) scavenging properties as well as photothermal activity for anti-inflammatory and antioxidant purposes [120]. Rh-PEG NDs have good anti-inflammatory effects while being able to completely ablate CT-26 colon tumors by virtue of their high photothermal conversion efficiency, providing a paradigm for the potential management of colon disease using metal nanozymes and preventing the progression of colon cancer at an early stage.
SDT is an emerging modality for colorectal cancer treatment because of its high tissue penetration and noninvasive advantages [121]. However, the outcome of SDT is usually hampered by inefficient generation of reactive oxygen species (ROS) and activation of protective autophagy. To address the limitations faced by sonodynamic therapy (SDT) in treating colorectal cancer, such as the inefficient generation of reactive oxygen species (ROS) and the activation of protective autophagy, Zhang et al. innovatively created a cascade nanoreactor. This design cleverly incorporates the acoustic sensitizer Ce6 and the autophagy inhibitor chloroquine into hollow polydopamine nanocarriers. These carriers are uniquely modified with membranes from homologous tumor cells and predoped with platinum nanoribonuclease, culminating in the creation of CCP@HP@M. This strategic integration is aimed at enhancing the generation of ROS and inhibiting autophagy, thereby improving the antitumor efficacy of SDT for colorectal cancer [122]. When subjected to ultrasound irradiation, CCP@HP@M demonstrated the ability to effectively alleviate hypoxia and reduce the resistance of colon cancer to sonodynamic therapy (SDT). This synergistic approach not only enhances the production of reactive oxygen species (ROS) but also prevents the initiation of ROS-induced protective autophagy. By addressing these two major hurdles, the effectiveness of SDT is fundamentally assured, making this strategy a promising option for tumor treatment. This innovative method has the potential to significantly improve therapeutic outcomes in colon cancer by optimizing the conditions for ROS generation and minimizing cellular defense mechanisms against therapy-induced stress.
The microbiota is considered a major oncogenic factor in CRC and influences treatment outcomes, and there is growing evidence that intratumoral bacteria enhance the survival of circulating tumor cells and promote metastasis [123]. Qin et al. designed a copper single-atom nanoenzyme (BSA-Cu SAN) that kills the intratumoral pathogen Clostridium nucleatum to disrupt symbiosis and synergistically kill colorectal cancer (CRC) cells [85]. Nanozymes are based on the structure of proteins, are natural enzymes with metal elements as active centers, and act as catalytic therapeutics by generating reactive oxygen species (ROS) and consuming GSH. This study provides a promising pathogen-centric approach to cancer therapy by disrupting a pathogen-tumor symbiosis that is not commonly used as a therapeutic target, providing new ideas for the treatment of CRC (Fig. 3D).
The treatment of colorectal cancer is evolving toward a more individualized approach, emphasizing the assessment of individual risk and fostering patient-centered shared decision-making. This shift is complemented by advances in nanozyme therapeutic methods, facilitating organ preservation strategies via local excision or combined radiotherapy and immunotherapy. Moreover, the advent of precision tumor therapy, grounded in comprehensive whole-tumor genome sequencing and monitoring of therapeutic efficacy by circulating tumor DNA, represents significant strides in tailoring treatment to the specific genetic and molecular profile of the tumor. This evolution toward personalized medicine aims to optimize treatment efficacy, minimize unnecessary exposure to systemic therapies, and improve overall patient outcomes in colorectal cancer care.
Cutaneous melanoma
Cutaneous melanoma (CM) is a fatal malignant cancer of the skin [124]. CM differs from other malignant tumors in that it occurs predominantly in the epidermal and dermal layers of the skin rather than in deeper tissues, and an important reason for the high mortality rate of CM is the lack of reliable and effective treatments.
Exploring the response of malignant cells to intracellular metabolic stress is critical for understanding pathological processes and developing anticancer therapies. Recently, Wu et al. prepared a dual-nanozyme-loaded porous silicon nanocatalytic composite system (CuGQD/PdNPs@PSi) using porous silicon obtained by electrochemical etching as a novel nanoenzyme carrier [70]. The enzyme possesses peroxidase-like (POD) and glutathione oxidase (GSHOx) activities, and the enzyme-like activity of the nanozyme complex can be further enhanced by the photothermal effect induced by near-infrared light. The integration of CuGQD/PdNPs@PSi into MNs can enable the nanozyme complex to penetrate the epidermal barrier and form reversible microchannels to enter CM lesion sites, which can achieve efficient nanocatalytic induction of ferroptosis and thus efficient treatment of melanoma (Fig. 3E). MNs encapsulating CuGQD/PdNPs@PSi could not only provide a potential nanocatalytically induced ferroptosis strategy for melanoma treatment but also meet the medical need for eradicating superficial tumors.
Currently, there are few studies on the application of nanozymes in treating melanoma, and drug delivery for superficial tumors such as melanoma is a key point to consider. Wu et al. provided a good idea to make nanozymes penetrate the skin barrier by using MNs as the medium, and the method of drug delivery works in superficial tumors. Combining nanozymes with penetrating ointment for treating dermatologic diseases may be a new idea for nanozyme design.
Osteosarcoma
Osteosarcoma is a common primary malignant bone tumor that occurs mostly in children and adolescents [125]. The current standard of care is an integrated neoadjuvant chemotherapy-surgery-adjuvant chemotherapy model consisting of doxorubicin, cisplatin, and high-dose methotrexate (MAP) [126]. Although perioperative chemotherapy has dramatically improved 5-year survival and limb preservation in patients with osteosarcoma [127], no further breakthroughs in survival benefit from chemotherapy have been achieved in the last 40 years, and the current state of treatment is in dire need of improvement.
Liang et al. designed a two-dimensional titanium carbide (Ti3C2Tx) carrier composed of RhRu alloy nanoclusters (RhRu/Ti3C2Tx), which possessed good catalase (CAT) and peroxidase (POD)-like activities [72]. Liang et al. verified the synergistic CDT/PDT/PTT effect of RhRu/Ti3C2Tx on osteosarcoma by in vitro and in vivo experiments, which is expected to provide a new research direction for the treatment of osteosarcoma and other tumors.
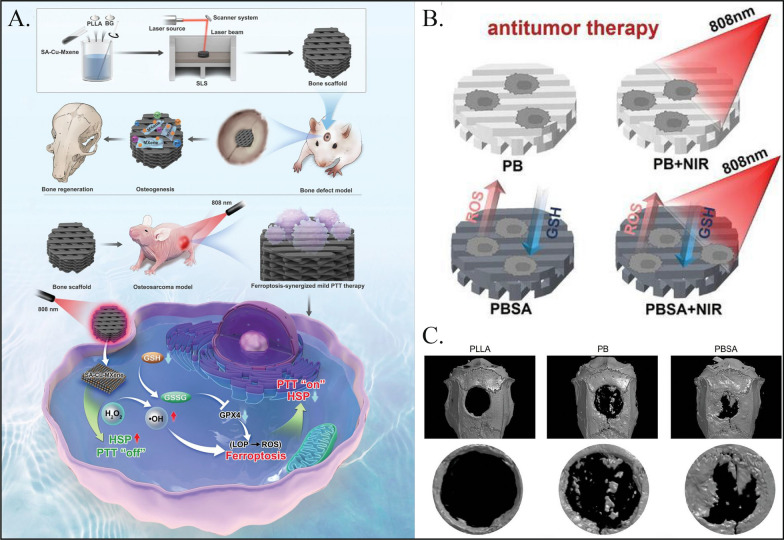
The rapid proliferation of residual tumor cells and poor quality of new bone reconstruction are considered the main challenges in the postoperative treatment of osteosarcoma [128]. Photothermal therapy (PTT) combined with bone regeneration induction in a composite bone scaffold holds promise for the local treatment of osteosarcoma [129]. However, the high heat generated by PTT inevitably damages the surrounding normal tissues of the tumor, and mild hyperthermia has limited effectiveness in tumor therapy because the damage is easily repaired by stress-induced heat shock proteins (HSPs). Yan et al. designed a novel single-atom copper nanozyme-loaded bone scaffold that exhibits excellent photothermal conversion properties and simulates the activities of peroxidase and glutathione oxidase in in vitro experiments (Fig. 4A) [130]. This leads to the upregulation of lipid peroxidation (LPO) and reactive oxygen species (ROS), ultimately triggering ferroptosis (Fig. 4B). The accumulation of LPO and ROS also leads to HSP70 inactivation, thereby maximizing the efficiency of photothermal therapy (PTT) against tumors at the appropriate treatment temperature and minimizing damage to surrounding normal tissues. Additionally, the bone scaffold promoted bone regeneration by continuously releasing biologically active ions (Ca2+, P5+, Si4+, and Cu2+) (Fig. 4C). In vivo experimental results demonstrated that the bone scaffold can inhibit tumor growth and promote bone repair.
Fig. 4.
Nanozymes used for treating osteosarcoma. A Synthetic procedure of PLLA/BG/SA-Cu-MXene composite bone scaffolds and the mechanism of composite bone scaffolds for ferroptosis-synergized mild photothermal therapy in osteosarcoma treatment. Reproduced with permission [130]. Copyright 2024, Weliy. B Schematic diagram of bone scaffolds for in vitro antitumor. Viability of Saos-2 cells treated of PBSA with different SA-Cu-MXene contents (0.5%, 1.0%, 2.0%, and 3.0%). Reproduced with permission [130]. Copyright 2024, Weliy. C 3D reconstructed micro-CT images of bone defect region after implanted for 8 weeks. Reproduced with permission [130]. Copyright 2024, Wiley
The design of nanozymes for the treatment of osteosarcoma plays a key role in the treatment of osteosarcoma. Yan et al. not only considered the ability of nanomaterials to kill OS but also promoted the postsurgical recovery of OS by taking advantage of the osteogenic properties of the nanomaterials, which gave full play to the value of the nanozymes in clinical translation, and it is worthwhile to learn from them in the future in the area of nanozyme design.
Hematological neoplasms
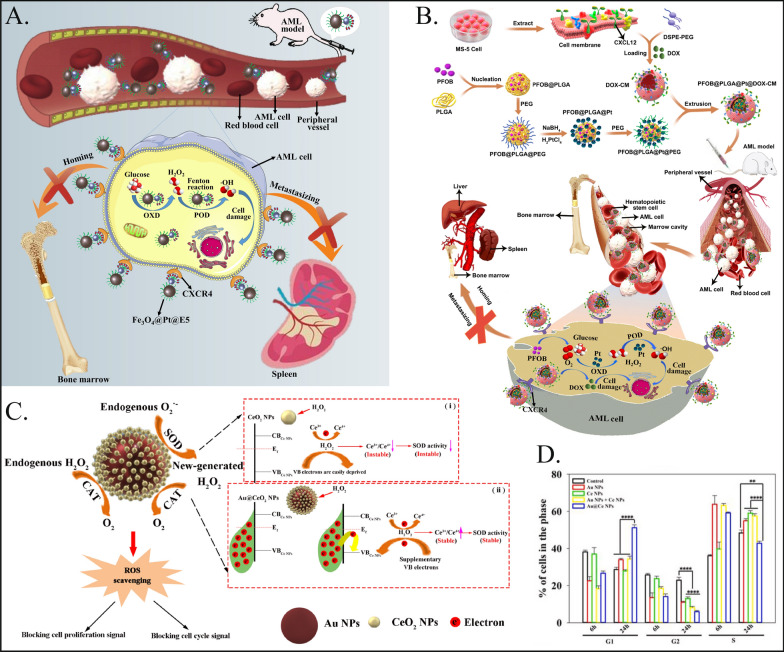
Acute myeloid leukemia (AML) is an aggressive hematopoietic malignancy characterized by proliferation of the patient's primitive cells and destruction of hematopoietic function [131]. Chemotherapy, radiation therapy, and stem cell transplantation are routine treatments for AML [132]. In recent years, most patients have struggled to achieve long-term disease-free survival due to the vulnerability of residual AML cells to relapse [133]. Once relapse occurs, drug resistance increases significantly, and mortality rates are much greater. Therefore, there is an urgent need for new therapeutic strategies for AML patients. Recent hematological studies have shown that an imbalance in intracellular redox (redox) homeostasis leading to abnormally elevated intracellular ROS levels in AML cells can cause DNA damage, promote apoptosis, and overcome drug resistance [134, 135]. The catalytic properties of nanozymes can bidirectionally regulate ROS, which is highly important for the treatment of AML.
To address the critical issue of residual acute myeloid leukemia (AML) cells persisting in patients following conventional chemotherapy due to the interaction of chemokine receptor 4 and chemokine ligand 12 (CXCR4/CXCL12), Kong et al. developed a multifunctional nanoplatform [75]. This innovative approach involves the use of Fe3O4@Pt composite nanozymes that are equipped with CXCR4 antagonists (Fig. 5A). This study aimed to target and eliminate these trace AML cell residues by disrupting the CXCR4/CXCL12 interaction, representing a targeted strategy to enhance the efficacy of leukemia treatment and reduce the likelihood of relapse. The nanoplatform effectively generates ROS and blocks the CXCR4/CXCL12 axis, resulting in a significant therapeutic effect by blocking AML cell migration and adhesion. The nanoplatform effectively generates reactive oxygen species (ROS) and blocks the CXCR4/CXCL12 axis, resulting in significant therapeutic efficacy by blocking the migration and adhesion of AML cells and increasing the blood circulation time, providing a new weapon for improving the prognosis of AML patients. Subsequently, Kong et al. further improved the design idea and designed the biomimetic nanocomposite PFOB@PLGA@Pt@DOX-CM coated with bone marrow stromal cell membranes. Pt nanozymes generate excessive ROS and induce apoptosis in leukemia cells [74]. The bone marrow stromal cell membrane coating enabled the nanoplatform to home to the bone marrow and act as a CXCR4 antagonist to block leukemia cell-stromal adhesion interactions, increasing the susceptibility of AML cells to conventional therapy (Fig. 5B). The nanocomplexes proposed by Kong et al. ultimately exert synergistic anti-AML effects by integrating chemotherapy, nanoenzyme-induced cascade catalytic activity and CXCR4 antagonism.
Fig. 5.
Nanozymes used for treating hematological neoplasms. A Schematic diagram of Fe3O4@Pt@E5 nanoplatform for the treatment of AML. Fe3O4@Pt@E5 entered the body through blood circulation, targeted and enriched around AML cells through E5, and was absorbed by AML cells. Fe3O4@Pt@E5 generated mass ROS through the sequential catalytic reactions, which induced cells apoptosis and prevented AML cells metastasis to bone marrow and spleen. Reproduced with permission [75]. Copyright 2021, Elsevier.B Schematic illustration of biomimetic nanocomposites designed for enhancing anti-leukemia efficacy. Through the CXCR4-CXCL12 axis, the PFOB@PLGA@Pt@DOX-CM binds to the leukemia cells in the blood, where released PFOB, DOX, and Pt in AML cells. The PFOB@PLGA@Pt@DOX-CM generated chemotoxicity and excessive ROS resulted from Pt nanozyme to induce leukemia cell apoptosis. Moreover, bone marrow stromal cell membrane coating enabled the PFOB@PLGA@Pt@DOX-CM to home to the bone marrow, where acted as CXCR4 antagonists to block the leukemia cell-stroma adhesive interactions, thus making AML cells better accessible to conventional therapies, inhibiting the growth of residual AML cells in the bone marrow, and preventing the infiltration of AML cells to other normal organs, like the liver and spleen. Reproduced with permission [74]. Copyright 2022, Elsevier. C Shows the mechanism that Au@Ce NPs interfere with ROS homeostasis and arrest cell cycle and proliferation through its high-performance SOD activity as well as the relay conversion of O2•- → H2O2 → O2. (i) Schematic of CeO2 NPs SOD activity that was suppressed by the oxidation of H2O2. (ii) Schematic of Au@Ce NPs SOD activity that can maintain the stability against the oxidation of H2O2 according to the electron compensation effect from Au substrate. Reproduced with permission [73]. Copyright 2022, Elsevier. D After treating HL-60 cells with nanomaterials for 24 h, the corresponding statistical results for the G1 phase, S phase, and G2 phase [73]. Copyright 2022, Elsevier
In addition, Sun et al. proposed the design of Au@Ce nanoparticles [73]. This particle has SOD mimetic enzyme activity while retaining peroxidase function and catalase function (Fig. 5C). It can effectively clear ROS produced by AML cells, significantly block the G1 phase cell cycle through endogenous ROS signaling, and inhibit the proliferation of AML cells (Fig. 5D). This approach has achieved satisfactory results in reducing liver and spleen leukocyte infiltration, providing an alternative method for the treatment of AML.
In summary, nanozymes represent a novel concept in the treatment of hematologic malignancies. However, clinical research on their application in hematologic oncology is still relatively limited. Given the nonsubstantial and widespread nature of hematologic malignancies, the targeting effect of nanozymes must be precise. The design strategy for enhancing receptor inhibitors through enzyme-like activities warrants extensive exploration but requires substantial experimental validation for clinical translation. Furthermore, targeting the cell cycle of leukemia cells may be a key approach for nanozymes in addressing hematologic malignancies. Combining the advantages of both approaches could yield significant benefits.
The potential of nanoenzymes as innovative alternative therapies
The integration of nanozymes in biomedical applications presents significant advantages over traditional therapies. Nanozymes offer enhanced catalytic efficiency, stability, and versatility in mimicking enzymatic activities, leading to more precise tumor targeting and improved treatment outcomes. Their ability to induce regulatory cell death mechanisms, such as ferroptosis and pyroptosis, offers novel therapeutic avenues for overcoming drug resistance in tumors. These properties make nanozymes a promising alternative for future cancer therapies, especially in cases where conventional treatments have limited efficacy.
In the reviewed literature, various experimental conditions were applied to evaluate the performance of different nanozymes in tumor treatment. Common parameters include nanozyme type, concentration, and environmental conditions mimicking the tumor microenvironment, such as hypoxia, oxidative stress, and acidic pH. For instance, in studies using manganese oxide (MnO₂) nanozymes, concentrations ranged from 100 to 300 μg/mL in in vivo breast cancer models. These nanozymes were tested for their ability to generate reactive oxygen species (ROS) and modulate the tumor immune microenvironment, showing a significant reduction in tumor size by over 60% when combined with photodynamic therapy.
In another example, cerium oxide (CeO₂) nanozymes were utilized in colorectal cancer models, where they demonstrated catalase-like activity under oxidative stress conditions. The experimental setup involved maintaining a low pH environment (pH 6.5) to simulate the tumor microenvironment. These nanozymes were capable of selectively scavenging excessive ROS in normal cells while enhancing ROS levels in tumor cells, leading to a significant decrease in tumor cell viability by up to 70%.The key data across these studies consistently show that nanozymes can induce various forms of regulatory cell death, including ferroptosis and autophagy, while enhancing the immune response. These outcomes were typically achieved under conditions that mimic the tumor's natural environment, making the results more relevant for clinical translation.
These findings underscore the potential of nanozymes as innovative therapeutic alternatives. Their versatility in catalyzing specific reactions tailored to tumor environments—such as enhancing ROS production, disrupting calcium signaling, or modulating immune responses—provides a more targeted approach compared to traditional therapies. By leveraging their stability, catalytic efficiency, and tumor selectivity, nanozymes offer promising new strategies to address challenges such as drug resistance and off-target toxicity.
Conclusion
The application of nanozymes in tumors has led to new ideas for tumor therapy and has a very bright future, but at present, limited by the differences in disciplines, most of the design of nanozymes is still based on the perspective of material science to achieve functional value, which makes it difficult for most nanozymes to achieve clinical translation. Although tumors have many common characteristics at the microenvironmental level, such as hypoxia and acidic environmental pH [136], the characteristics of different tumors still vary significantly, and the action environment of nanozymes cannot be easily generalized. Moreover, the treatment guidelines for tumors of different stages and grades are different, and nanozymes should be more targeted to better realize the value of clinical conversion. For example, the impact of the blood‒brain barrier should be taken into account in gliomas, skin or superficial tumors can focus more on photothermal therapy or photodynamic therapy, and the characteristics of metastasis and early diagnosis of advanced cancers should be taken into account in the development of nanozymes for lung or liver cancers.
The use of nanozymes as a therapeutic approach presents several significant advantages over existing cancer treatments, positioning them as a solid alternative. Traditional cancer therapies, such as chemotherapy and radiotherapy, are often limited by drug resistance, non-specificity, and significant side effects due to damage to healthy tissues. Nanozymes, on the other hand, offer enhanced selectivity and specificity towards tumor cells by exploiting the unique characteristics of the tumor microenvironment, such as hypoxia, acidity, and high reactive oxygen species (ROS) levels. For instance, the ability of nanozymes to induce ferroptosis—a regulated cell death pathway less prone to resistance—offers a promising strategy to overcome the limitations of apoptosis-based therapies commonly used in conventional treatments. Furthermore, nanozymes demonstrate robust catalytic activities that can mimic or surpass natural enzymes, enabling precise control over therapeutic processes like ROS generation and glutathione (GSH) depletion in tumor cells. Their stability under physiological conditions also ensures a longer therapeutic window and reduced degradation compared to natural enzymes, making them a durable option for prolonged treatments. For example, studies have shown that nanozymes, such as Fe-CDs and CaF₂ nanozymes, can significantly enhance tumor cell death rates while minimizing damage to surrounding healthy tissues, thereby improving the therapeutic index.
Many recent studies on nanozymes have begun to develop more targeted nanozymes to treat or assist in the treatment of specific tumors based on the characteristics of tumors, and nanozymes in the field of oncology will be more precise in the future. In addition, there are still many issues that need to be considered, such as the metabolism and side effects of nanozymes in vivo, which should not only address their effects but also pay more comprehensive attention to relevant research on the design of nanozymes in the future.
In summary, while challenges such as large-scale production and biocompatibility need to be addressed, nanozymes represent a highly promising and innovative alternative to current therapeutics. Their ability to target tumor-specific pathways, coupled with their versatility in combination therapies (e.g., with immunotherapy or phototherapy), solidifies their potential to reshape the future landscape of cancer treatment.
Acknowledgements
Not applicable.
Author contributions
ZTJ and ZZJ conceived and designed the study; XQL and JPH wrote the manuscript; QZ and YFW analyzed the data. HL and XQL interpreted results and wrote the manuscript. XQL and JPH contributed equally to this work. All authors read and approved the final version of the manuscript.
Funding
The research was supported by the National Natural Science Foundation of China (No. 82072794).
Availability of data and materials
The datasets obtained and analyzed during the current study were made available from the corresponding authors through request. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Consent to publish has been obtained from all authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinqiao Li and Jinpeng Hu have contributed equally to this work.
Contributor Information
Qi Zhao, Email: qi.zhao1@northwestern.edu.
Weifeng Yao, Email: yaoweifeng@shiep.edu.cn.
Zhitao Jing, Email: jingzhitao@hotmail.com.
Zhizhong Jin, Email: zhizhongjin0311@outlook.com.
References
- 1.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. [DOI] [PubMed] [Google Scholar]
- 2.Rosic G, Selakovic D, Omarova S. Cancer signaling, cell/gene therapy, diagnosis and role of nanobiomaterials. Adv Biol Earth Sci. 2024;9:11–34. [Google Scholar]
- 3.Pei Z, Chen S, Ding L, et al. Current perspectives and trend of nanomedicine in cancer: a review and bibliometric analysis. J Control Release. 2022;352:211–41. [DOI] [PubMed] [Google Scholar]
- 4.Amirova M. Specific biochemical indicators and inflammatory markers in rheumatoid arthritis (RA). Adv Biol Earth Sci. 2024;9(1):175–83. [Google Scholar]
- 5.Miryusifova K, et al. The saffron effects on the dynamics of experimental epilepsy. Adv Biol Earth Sci. 2024;9(1):196–202. [Google Scholar]
- 6.Karadağ M, et al. Use of Prunusarmeniaca L. seed oil and pulp in health and cosmetic products. Adv Biol Earth Sci. 2024;9(1):105–10. [Google Scholar]
- 7.Eftekhari A, Kryschi C, Pamies D, et al. Natural and synthetic nanovectors for cancer therapy. Nanotheranostics. 2023;7(3):236–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salahshour P, et al. Nanobiomaterials/bioinks based scaffolds in 3D bioprinting for tissue engineering and artificial human organs. Adv Biol Earth Sci. 2024;9:97–104. [Google Scholar]
- 9.Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22(7):381–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong X, Tang R, Xiao M, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao J, Peng H, Qiu Y, et al. Nanoplatform-mediated calcium overload for cancer therapy. J Mater Chem B. 2022;10(10):1508–19. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Yi X, Liu L, et al. Advances in tumor nanotechnology: theragnostic implications in tumors via targeting regulated cell death. Apoptosis. 2023;28(7–8):1198–215. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Chen X, Zhao Y. Nanozymes: versatile platforms for cancer diagnosis and therapy. Nano Micro Lett. 2022;14(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomaa EZ. Nanozymes: a promising horizon for medical and environmental applications. J Cluster Sci. 2022;33(4):1275–97. [Google Scholar]
- 15.Ji S, Jiang B, Hao H, et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat Catal. 2021;4(5):407–17. [Google Scholar]
- 16.Liu C-P, Wu T-H, Liu C-Y, et al. Self-supplying O2 through the catalase-like activity of gold nanoclusters for photodynamic therapy against hypoxic cancer cells. Small. 2017;13(26):1700278. [DOI] [PubMed] [Google Scholar]
- 17.Muhammad P, Hanif S, Li J, et al. Carbon dots supported single Fe atom nanozyme for drug-resistant glioblastoma therapy by activating autophagy-lysosome pathway. Nano Today. 2022;45: 101530. [Google Scholar]
- 18.Chen X, Yang Y, Ye G, et al. Chiral ruthenium nanozymes with self-cascade reaction driven the no generation induced macrophage M1 polarization realizing the lung cancer “cocktail therapy.” Small. 2023;19:2207823. [DOI] [PubMed] [Google Scholar]
- 19.Tao N, Li H, Deng L, et al. A cascade nanozyme with amplified sonodynamic therapeutic effects through comodulation of hypoxia and immunosuppression against cancer. ACS Nano. 2022;16(1):485–501. [DOI] [PubMed] [Google Scholar]
- 20.Jana D, He B, Chen Y, et al. A defect-engineered nanozyme for targeted NIR-II photothermal immunotherapy of cancer. Adv Mater. 2022;36:2206401. [DOI] [PubMed] [Google Scholar]
- 21.Peng F, Liao M, Qin R, et al. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther. 2022;7(1):1–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ketelut-Carneiro N, Fitzgerald KA. Apoptosis, pyroptosis, and necroptosis-oh my! the many ways a cell can die. J Mol Biol. 2022;434(4): 167378. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 24.Tang R, Xu J, Zhang B, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Bai Y, Yion C, et al. Single-atom nanocatalytic therapy for suppression of neuroinflammation by inducing autophagy of abnormal mitochondria. ACS Nano. 2023;17(8):7511–29. [DOI] [PubMed] [Google Scholar]
- 26.Cao F, Sang Y, Liu C, et al. Self-adaptive single-atom catalyst boosting selective ferroptosis in tumor cells. ACS Nano. 2022;16(1):855–68. [DOI] [PubMed] [Google Scholar]
- 27.Meng X, Li D, Chen L, et al. High-performance self-cascade pyrite nanozymes for apoptosis-ferroptosis synergistic tumor therapy. ACS Nano. 2021;15(3):5735–51. [DOI] [PubMed] [Google Scholar]
- 28.Xu T, Ma Y, Yuan Q, et al. Enhanced ferroptosis by oxygen-boosted phototherapy based on a 2-in-1 nanoplatform of ferrous hemoglobin for tumor synergistic therapy. ACS Nano. 2020;14(3):3414–25. [DOI] [PubMed] [Google Scholar]
- 29.Dong C, Dai X, Wang X, et al. A calcium fluoride nanozyme for ultrasound-amplified and Ca2+ -overload-enhanced catalytic tumor nanotherapy. Adv Mater (Deerfield Beach, Fla). 2022;34(43): e2205680. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Wang X, Dai X, et al. 2D catalytic nanozyme enables cascade enzyodynamic effect-boosted and Ca2+ overload-induced synergistic ferroptosis/apoptosis in tumor. Adv Mater (Deerfield Beach, Fla). 2024;36: e2312316. [DOI] [PubMed] [Google Scholar]
- 31.Chang M, Zhang L, Zhang T, et al. Ultrasound-augmented enzyodynamic-Ca2+ overload synergetic tumor nanotherapy. Biomaterials. 2024;307: 122513. [DOI] [PubMed] [Google Scholar]
- 32.Chang M, Wang Z, Dong C, et al. Ultrasound-amplified enzyodynamic tumor therapy by perovskite nanoenzyme-enabled cell pyroptosis and cascade catalysis. Adv Mater. 2022;35:2208817. [DOI] [PubMed] [Google Scholar]
- 33.Tao N, Jiao L, Li H, et al. A mild hyperthermia hollow carbon nanozyme as pyroptosis inducer for boosted antitumor immunity. ACS Nano. 2023;17(22):22844–58. [DOI] [PubMed] [Google Scholar]
- 34.Yao H, Gong X, Geng M, et al. Cascade nanozymes based on the “butterfly effect” for enhanced starvation therapy through the regulation of autophagy. Biomater Sci. 2022;10(14):4008–22. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Huang Y, Fu Y, et al. Reductive damage induced autophagy inhibition for tumor therapy. Nano Res. 2023;16(4):5226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin N, Wang Y, Huang Y, et al. Modulating nanozyme-based nanomachines via microenvironmental feedback for differential photothermal therapy of orthotopic gliomas. Adv Sci. 2023;10(3): e2204937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of non-apoptotic cell death. Cell. 2012;149(5):1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Z, Song J, Yung BC, et al. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater. 2018;30(12):1704007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou H, Guo M, Li J, et al. Hypoxia-triggered self-assembly of ultrasmall iron oxide nanoparticles to amplify the imaging signal of a tumor. J Am Chem Soc. 2021;143(4):1846–53. [DOI] [PubMed] [Google Scholar]
- 40.Zheng P, Ding B, Shi R, et al. A multichannel Ca2+ nanomodulator for multilevel mitochondrial destruction-mediated cancer therapy. Adv Mater. 2021;33(15):2007426. [DOI] [PubMed] [Google Scholar]
- 41.Pesakhov S, Nachliely M, Barvish Z, et al. Cancer-selective cytotoxic Ca 2+ overload in acute myeloid leukemia cells and attenuation of disease progression in mice by synergistically acting polyphenols curcumin and carnosic acid. Oncotarget. 2016;7(22):31847–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen J, Yu H, Shu Y, et al. A robust ROS generation strategy for enhanced chemodynamic/photodynamic therapy via H2O2/O2 self-supply and Ca2+ overloading. Adv Func Mater. 2021;31(50):2106106. [Google Scholar]
- 43.Chu X, Jiang X, Liu Y, et al. Nitric oxide modulating calcium store for ca2+-initiated cancer therapy. Adv Func Mater. 2021;31(13):2008507. [Google Scholar]
- 44.Gong F, Xu J, Liu B, et al. Nanoscale CaH2 materials for synergistic hydrogen-immune cancer therapy. Chem. 2022;8(1):268–86. [Google Scholar]
- 45.Zhang M, Song R, Liu Y, et al. Calcium-overload-mediated tumor therapy by calcium peroxide nanoparticles. Chem. 2019;5(8):2171–82. [Google Scholar]
- 46.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigellaflexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–9. [DOI] [PubMed] [Google Scholar]
- 47.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–4. [DOI] [PubMed] [Google Scholar]
- 49.Zheng P, Ding B, Zhu G, et al. Biodegradable Ca 2+ nanomodulators activate pyroptosis through mitochondrial Ca 2+ overload for cancer immunotherapy. Angew Chem Int Ed. 2022;61(36): e202204904. [DOI] [PubMed] [Google Scholar]
- 50.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11(9):709–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Lin Y-X, Wang J, et al. In situ manipulation of dendritic cells by an autophagy-regulative nanoactivator enables effective cancer immunotherapy. ACS Nano. 2019;13(7):7568–77. [DOI] [PubMed] [Google Scholar]
- 52.Duo Y, Yang M, Du Z, et al. CX-5461-loaded nucleolus-targeting nanoplatform for cancer therapy through induction of pro-death autophagy. Acta Biomater. 2018;79:317–30. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Li M, Ren K, et al. On-demand autophagy cascade amplification nanoparticles precisely enhanced oxaliplatin-induced cancer immunotherapy. Adv Mater. 2020;32(32):2002160. [DOI] [PubMed] [Google Scholar]
- 54.Yin N, Wang Y, Huang Y, et al. Modulating nanozyme-based nanomachines via microenvironmental feedback for differential photothermal therapy of orthotopic gliomas. Adv Sci. 2023;10(3):2204937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nie D, Ling Y, Lv W, et al. In situ attached photothermal immunomodulation-enhanced nanozyme for the inhibition of postoperative malignant glioma recurrence. ACS Nano. 2023;17(14):13885–902. [DOI] [PubMed] [Google Scholar]
- 56.Qian X, Shi R, Chen J, et al. The single-atom iron nanozyme mimicking peroxidase remodels energy metabolism and tumor immune landscape for synergistic chemodynamic therapy and photothermal therapy of triple-negative breast cancer. Front Bioeng Biotechnol. 2022;10:1026761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang D, Wu H, Phua SZF, et al. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat Commun. 2020;11(1):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie Y, Wang M, Qian Y, et al. Novel PdPtCu nanozymes for reprogramming tumor microenvironment to boost immunotherapy through endoplasmic reticulum stress and blocking IDO-mediated immune escape. Small. 2023;19:2303596. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Wang A, Liu S, et al. A titanium nitride nanozyme for pH-responsive and irradiation-enhanced cascade-catalytic tumor therapy. Angew Chem Int Ed. 2021;60(48):25328–38. [DOI] [PubMed] [Google Scholar]
- 60.Zeng W, Yu M, Chen T, et al. Polypyrrole nanoenzymes as tumor microenvironment modulators to reprogram macrophage and potentiate immunotherapy. Adv Sci. 2022;9(23):2201703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Y, Wang W, Cheng J, et al. Stimuli-responsive manganese single-atom nanozyme for tumor therapy via integrated cascade reactions. Angew Chem. 2021;133(17):9566–74. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Wang B, Zhu J, et al. Single-atom nanozyme with asymmetric electron distribution for tumor catalytic therapy by disrupting tumor redox and energy metabolism homeostasis. Adv Mater. 2023;35:2208512. [DOI] [PubMed] [Google Scholar]
- 63.Cai S, Liu J, Ding J, et al. Tumor-microenvironment-responsive cascade reactions by a cobalt-single-atom nanozyme for synergistic nanocatalytic chemotherapy. Angew Chem Int Ed. 2022;61(48): e202204502. [DOI] [PubMed] [Google Scholar]
- 64.Xu B, Li S, Zheng L, et al. A bioinspired five-coordinated single-atom iron nanozyme for tumor catalytic therapy. Adv Mater. 2022;34(15):2107088. [DOI] [PubMed] [Google Scholar]
- 65.Ye J, Lv W, Li C, et al. Tumor response and NIR-II photonic thermal co-enhanced catalytic therapy based on single-atom manganese nanozyme. Adv Func Mater. 2022;32(47):2206157. [Google Scholar]
- 66.Yuan H, Xia P, Sun X, et al. Photothermal nanozymatic nanoparticles induce ferroptosis and apoptosis through tumor microenvironment manipulation for cancer therapy. Small. 2022;18(41):2202161. [DOI] [PubMed] [Google Scholar]
- 67.Zhang C, Chen L, Bai Q, et al. Nonmetal graphdiyne nanozyme-based ferroptosis-apoptosis strategy for colon cancer therapy. ACS Appl Mater Interfaces. 2022;14(24):27720–32. [DOI] [PubMed] [Google Scholar]
- 68.Wang L, Zhang X, You Z, et al. Charges-enhanced molybdenum disulfide nanozyme activity for ultrasound-mediated cascade-catalytic tumor ferroptosis. Angew Chem Int Ed. 2022;135:202217448. [DOI] [PubMed] [Google Scholar]
- 69.Wei Y, Wu S, Liu Z, et al. Tumor associated macrophages reprogrammed by targeted bifunctional bioorthogonal nanozymes for enhanced tumor immunotherapy. Mater Today. 2022;56:16–28. [Google Scholar]
- 70.Zhao J, Duan W, Liu X, et al. Microneedle patch integrated with porous silicon confined dual nanozymes for synergistic and hyperthermia-enhanced nanocatalytic ferroptosis treatment of melanoma. Adv Func Mater. 2023;33(47):2308183. [Google Scholar]
- 71.Tang M, Shi Y, Lu L, et al. Dual active nanozyme-loaded MXene enables hyperthermia-enhanced tumor nanocatalytic therapy. Chem Eng J. 2022;449: 137847. [Google Scholar]
- 72.Liang Y, Liao C, Guo X, et al. RhRu alloy-anchored mxene nanozyme for synergistic osteosarcoma therapy. Small. 2023;19(22):2205511. [DOI] [PubMed] [Google Scholar]
- 73.Sun Y, Liu X, Wang L, et al. High-performance SOD mimetic enzyme Au@Ce for arresting cell cycle and proliferation of acute myeloid leukemia. Bioact Mater. 2022;10:117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kong F, He H, Bai H, et al. A biomimetic nanocomposite with enzyme-like activities and CXCR4 antagonism efficiently enhances the therapeutic efficacy of acute myeloid leukemia. Bioact Mater. 2022;18:526–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kong F, Bai H, Ma M, et al. Fe3O4@Pt nanozymes combining with CXCR4 antagonists to synergistically treat acute myeloid leukemia. Nano Today. 2021;37: 101106. [Google Scholar]
- 76.Sminia P, Westerman BA. Blood-brain barrier crossing and breakthroughs in glioblastoma therapy. Br J Clin Pharmacol. 2016;81(6):1018–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Y, Peng Z, Seven ES, et al. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018;270:290–303. [DOI] [PubMed] [Google Scholar]
- 80.Li X, Geng X, Chen Z, et al. Recent advances in glioma microenvironment-response nanoplatforms for phototherapy and sonotherapy. Pharmacol Res. 2022;179: 106218. [DOI] [PubMed] [Google Scholar]
- 81.Lee C, Hwang HS, Lee S, et al. Rabies virus-inspired silica-coated gold nanorods as a photothermal therapeutic platform for treating brain tumors. Adv Mater (Deerfield Beach, Fla). 2017;29(13): e28134459. [DOI] [PubMed] [Google Scholar]
- 82.Ou M, Cho H-Y, Fu J, et al. Inhibition of autophagy and induction of glioblastoma cell death by NEO214, a perillyl alcohol-rolipram conjugate. Autophagy. 2023;19(12):3169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhan Z, Zeng W, Liu J, et al. Engineered biomimetic copper sulfide nanozyme mediates “don’t eat me” signaling for photothermal and chemodynamic precision therapies of breast cancer. ACS Appl Mater Interfaces. 2023;15(20):24071–83. [DOI] [PubMed] [Google Scholar]
- 84.Zhou S, Tian T, Meng T, et al. Tumor-derived covalent organic framework nanozymes for targeted chemo-photothermal combination therapy. IScience. 2023;26(8):107348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Chen Q, Zhu Y, et al. Destroying pathogen-tumor symbionts synergizing with catalytic therapy of colorectal cancer by biomimetic protein-supported single-atom nanozyme. Signal Transduct Target Ther. 2023;8(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makki J. Diversity of breast carcinoma: histological subtypes and clinical relevance. Clin Med Insights Pathol. 2015;8:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kabel AM. Tumor markers of breast cancer: new prospectives. J Oncol Sci. 2017;3(1):5–11. [Google Scholar]
- 88.Mitri Z, Constantine T, O’Regan R. The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemother Res Pract. 2012;2012: 743193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pareja F, Geyer FC, Marchiò C, et al. Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. Npj Breast Cancer. 2016;2(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wan G, Chen B, Li L, et al. Nanoscaled red blood cells facilitate breast cancer treatment by combining photothermal/photodynamic therapy and chemotherapy. Biomaterials. 2018;155:25–40. [DOI] [PubMed] [Google Scholar]
- 91.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48. [DOI] [PubMed] [Google Scholar]
- 92.Zeng X, Ruan Y, Chen Q, et al. Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer. Chem Eng J. 2023;452: 138422. [Google Scholar]
- 93.Kadkhoda J, Tarighatnia A, Tohidkia MR, et al. Photothermal therapy-mediated autophagy in breast cancer treatment: progress and trends. Life Sci. 2022;298: 120499. [DOI] [PubMed] [Google Scholar]
- 94.Gustalik J, Aebisher D, Bartusik-Aebisher D. Photodynamic therapy in breast cancer treatment. J Appl Biomed. 2022;20(3):98–105. [DOI] [PubMed] [Google Scholar]
- 95.Liu G, Liu M, Li X, et al. Peroxide-simulating and GSH-depleting nanozyme for enhanced chemodynamic/photodynamic therapy via induction of multisource ROS. ACS Appl Mater Interfaces. 2023;15(41):47955–68. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y, Wang P, Liu Q, et al. Sinoporphyrin sodium triggered sono-photodynamic effects on breast cancer both in vitro and in vivo. Ultrason Sonochem. 2016;31:437–48. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Zhou Q, Deng Z, et al. IR-780 dye as a sonosensitizer for sonodynamic therapy of breast tumor. Sci Rep. 2016;6(1):25968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wan G-Y, Liu Y, Chen B-W, et al. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol Med. 2016;13(3):325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brody H. Lung cancer. Nature. 2020;587(7834):S7. [DOI] [PubMed] [Google Scholar]
- 100.The Lancet Null. Lung cancer: some progress, but still a lot more to do. Lancet (London, England). 2019;394(10212):1880. [DOI] [PubMed] [Google Scholar]
- 101.Chaft JE, Rimner A, Weder W, et al. Evolution of systemic therapy for stages I–III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol. 2021;18(9):547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Allison R, Moghissi K, Downie G, et al. Photodynamic therapy (PDT) for lung cancer. Photodiagn Photodyn Ther. 2011;8(3):231–9. [DOI] [PubMed] [Google Scholar]
- 103.Lahiri A, Maji A, Potdar PD, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou K, Li S, Zhao Y, et al. Mechanisms of drug resistance to immune checkpoint inhibitors in non-small cell lung cancer. Front Immunol. 2023;14:1127071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gravitz L. Liver cancer. Nature. 2014;516(7529):S1. [DOI] [PubMed] [Google Scholar]
- 107.Anwanwan D, Singh SK, Singh S, et al. (2020) Challenges in liver cancer and possible treatment approaches. Biochim Et Biophys Acta. 1873;1:188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li X, Ramadori P, Pfister D, et al. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. 2021;21(9):541–57. [DOI] [PubMed] [Google Scholar]
- 109.Liu C-Y, Chen K-F, Chen P-J. Treatment of liver cancer. Cold Spring Harb Perspect Med. 2015;5(9): a021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in hepatocellular carcinoma: diagnosis, prognosis and treatment response assessment. Cells. 2020;9(6):1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsukuma H, Tanaka H, Ajiki W, et al. Liver cancer and its prevention. Asian Pac J Cancer Prev APJCP. 2005;6(3):244–50. [PubMed] [Google Scholar]
- 112.Tang A, Hallouch O, Chernyak V, et al. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol. 2018;43(1):13–25. [DOI] [PubMed] [Google Scholar]
- 113.Zhu K, Dai Z, Zhou J. Biomarkers for hepatocellular carcinoma: progression in early diagnosis, prognosis, and personalized therapy. Biomark Res. 2013;1(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jing H, Ren Y, Zhou Y, et al. Remodeling of the liver fibrosis microenvironment based on nilotinib-loaded multicatalytic nanozymes with boosted antifibrogenic activity. Acta Pharm Sin B. 2023;13(12):5030–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Favoriti P, Carbone G, Greco M, et al. Worldwide burden of colorectal cancer: a review. Updat Surg. 2016;68(1):7–11. [DOI] [PubMed] [Google Scholar]
- 116.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet (London, England). 2014;383(9927):1490–502. [DOI] [PubMed] [Google Scholar]
- 117.Feeney G, Sehgal R, Sheehan M, et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25(33):4850–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–44. [DOI] [PubMed] [Google Scholar]
- 119.Vong LB, Yoshitomi T, Matsui H, et al. Development of an oral nanotherapeutics using redox nanoparticles for treatment of colitis-associated colon cancer. Biomaterials. 2015;55:54–63. [DOI] [PubMed] [Google Scholar]
- 120.Miao Z, Jiang S, Ding M, et al. Ultrasmall rhodium nanozyme with RONS scavenging and photothermal activities for anti-inflammation and antitumor theranostics of colon diseases. Nano Lett. 2020;20(5):3079–89. [DOI] [PubMed] [Google Scholar]
- 121.Huang S, Ding D, Lan T, et al. Multifunctional nanodrug performs sonodynamic therapy and inhibits TGF-β to boost immune response against colorectal cancer and liver metastasis. Acta Biomater. 2023;164:538–52. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y, Zhao J, Zhang L, et al. A cascade nanoreactor for enhancing sonodynamic therapy on colorectal cancer via synergistic ROS augment and autophagy blockage. Nano Today. 2023;49: 101798. [Google Scholar]
- 123.Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023;20(7):429–52. [DOI] [PubMed] [Google Scholar]
- 124.Strashilov S, Yordanov A. Aetiology and pathogenesis of cutaneous melanoma: current concepts and advances. Int J Mol Sci. 2021;22(12):6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen C, Xie L, Ren T, et al. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021;500:1–10. [DOI] [PubMed] [Google Scholar]
- 126.Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17(10):1396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smeland S, Bielack SS, Whelan J, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019;109:36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meltzer PS, Helman LJ. New horizons in the treatment of osteosarcoma. N Engl J Med. 2021;385(22):2066–76. [DOI] [PubMed] [Google Scholar]
- 129.Chen B, Xiang H, Pan S, et al. Advanced theragenerative biomaterials with therapeutic and regeneration multifunctionality. Adv Func Mater. 2020;30(34):2002621. [Google Scholar]
- 130.Yan Z, Wu X, Tan W, et al. Single-atom Cu nanozyme-loaded bone scaffolds for ferroptosis-synergized mild photothermal therapy in osteosarcoma treatment. Adv Healthc Mater. 2024. 10.1002/adhm.202304595. [DOI] [PubMed] [Google Scholar]
- 131.Aglietta M, De Vincentiis A, Lanata L, Lanza F, Lemoli RM, Menichella G, Tafuri A, Zanon P, Tura S. Peripheral blood stem cells in acute myeloid leukemia: biology and clinical applications. Haematologica. 1996;81:77. [PubMed] [Google Scholar]
- 132.Rautenberg C, Germing U, Haas R, et al. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: prevention, detection, and treatment. Int J Mol Sci. 2019;20(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khan GN, Orchard K, Guinn B. Antigenic targets for the immunotherapy of acute myeloid leukaemia. J Clin Med. 2019;8(2):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou F, Shen Q, Claret FX. Novel roles of reactive oxygen species in the pathogenesis of acute myeloid leukemia. J Leukoc Biol. 2013;94(3):423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang H, Fang H, Wang K. Reactive oxygen species in eradicating acute myeloid leukemic stem cells. Stem Cell Investig. 2014;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets obtained and analyzed during the current study were made available from the corresponding authors through request. The data are not publicly available due to privacy or ethical restrictions.