Abstract
Background
Recent advances in ultrasound technology have led to widespread adoption of ultrasonic energy devices in liver resections. While various studies have assessed the comparative advantages of ultrasonic devices and traditional clamp-crushing, their findings vary. Moreover, a specific systematic review on this topic has not yet been conducted.
Objectives
This study aims to present a comprehensive, up-to-date analysis comparing outcomes between ultrasonic devices and conventional clamp-crushing methods in liver resection, based on currently available literature.
Patients and methods
We conducted a systematic literature search in databases such as PubMed, Embase, Web of Science, and CNKI up to November 2023. Studies that compared the efficacy or safety of ultrasonic devices against traditional clamp-crushing methods in hepatectomy were included. The analysis covered intraoperative outcomes like operating time, blood loss, and transfusion rate, as well as postoperative outcomes such as complication rate, mortality, postoperative bleeding, and bile leakage. Review Manager version 5.3 (Cochrane Collaboration, Oxford, UK) and Stata 17.0 (Stata Corp, College Station, TX, USA) were used for data analysis.
Results
Thirteen studies, involving a total of 1,417 patients (630 using ultrasonic devices and 787 using clamp-crushing methods), were included. The clamp-crush method resulted in a shorter operation time. Contrarily, the ultrasonic device group experienced reduced blood loss and lower transfusion rates. Postoperatively, there was no significant difference in mortality or postoperative bleeding between the groups. However, the ultrasonic group had a lower overall complication rate, particularly a reduced incidence of bile leakage. Overall, the ultrasonic devices were associated with improved perioperative outcomes.
Conclusions
The findings suggest that ultrasonic devices provide better outcomes in hepatectomy compared to traditional clamp-crushing techniques. Nonetheless, large-scale randomized controlled trials are needed to confirm these results due to potential heterogeneity and biases. The choice of using ultrasonic devices should consider the surgeon’s experience and individual patient circumstances.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-024-03575-3.
Keywords: Liver neoplasm, Liver resection, Ultrasonic, Clamp
Introduction
Hepatectomy remains the most effective treatment for patients with benign or malignant liver tumors and those requiring liver transplantation. Over the past two decades, substantial advancements in liver resection techniques have enhanced patient outcomes. Methods for liver parenchyma division have evolved, including clamp crushing, ultrasonic dissection, and other innovative technologies.
Successful hepatectomy demands a thorough understanding of liver anatomy and effective techniques for parenchyma dissection while safeguarding critical vascular and biliary structures [1–6]. To meet diverse clinical needs, various methods have been developed, such as the clamp-crush technique, Cavitron Ultrasonic Surgical Aspirator (CUSA), radiofrequency dissecting sealer, radiofrequency-assisted liver resection, waterjet dissector, and vascular stapler techniques [7]. Each method offers unique benefits: bipolar cautery reduces blood loss by fusing the collagen matrix of vessel walls; CUSA utilizes ultrasonic energy combined with aspiration to minimize damage to liver structures; and clamp-crushing effectively isolates vessels and bile ducts, thereby reducing surgery time and cost [1, 7].
Despite these technological advances, liver resection still poses significant risks. Studies show that the mortality rate has declined to around 5% in high-volume centers, thanks to improved perioperative management and surgical techniques [4, 8–11]. However, morbidity rates remain elevated, ranging from 23 to 56%, depending on the surgical indication [10–13]. Therefore, selecting the appropriate technique is crucial to minimizing morbidity.
Numerous meta-analyses have compared different parenchymal transection techniques to identify the most effective methods. One previous network meta-analysis, which included 12 studies involving nearly 1000 patients, compared the TissueLink and LigaSure dissecting sealers. However, blood loss during parenchymal transection was not reported, even though it is a critical factor influencing both short-term and long-term outcomes [13, 14]. Another meta-analysis, which examined ten parenchymal transection techniques, found bipolar cautery to be the most effective for minimizing blood loss and the harmonic scalpel to be the most effective for reducing overall and major complications [7]. Despite the growing range of hepatectomy techniques, comprehensive evaluations of perioperative outcomes comparing ultrasonic devices to traditional clamp-crush methods are still needed. Our study aims to address this gap by systematically reviewing evidence from RCTs, focusing on which technique offers superior intraoperative and postoperative outcomes, particularly regarding intraoperative blood loss and postoperative bile leakage.
Materials and methods
Literature search
This evidence-based analysis adhered to the PRISMA 2020 guidelines and was registered in PROSPERO (CRD42023482892) (https://www.crd.york.ac.uk/). The PRISMA 2020 checklist is available in Supplementary Material S1. We conducted a comprehensive search of PubMed, Embase, Web of Science, and CNKI databases up to March 2024, focusing on studies comparing the efficacy and safety of ultrasonic devices versus clamps for liver resection. Search terms included “human,” “method*,” “hepatect*,” “ultrasonic,” “liver resection,” “clamp,” and “prognosis,” with the detailed strategy outlined in Supplementary Material S2. References from all eligible studies were also reviewed manually. Two investigators independently conducted the search and study selection, with any disagreements resolved by consensus.
Identification of eligible studies
Studies were included if they met the following criteria: (1) randomized controlled trials, cohort studies, or case–control studies; (2) hepatectomy performed on patients with liver neoplasms; (3) comparison of ultrasonic devices with clamps; (4) evaluation of at least one perioperative outcome, such as operating time, blood loss, hospital stay duration, complication rate, or transfusion rate; and (5) sufficient data to calculate odds ratios (ORs) or weighted mean differences (WMDs). Excluded were reviews, letters, editorials, case reports, conference abstracts, and unpublished studies. Ultrasonication was defined as using ultrasonic devices like the Harmonic scalpel or Cavitron Ultrasonic Surgical Aspirator (CUSA), while clamping referred to the exclusive use of clamps for liver resection. Studies focusing only on clamp hepatectomy or ultrasonic devices were excluded.
Data extraction
Two investigators independently extracted data, with any discrepancies resolved by a third investigator. Extracted data included demographics (sample size, sex, age, BMI), publication details (first author, year, study period, location, design), and operative and postoperative outcomes (e.g., operating time, transfusion rate, blood loss, length of hospital stay, complication rate, bile leakage, and postoperative bleeding). For continuous variables presented as medians with ranges or interquartile ranges, means ± standard deviations were calculated using validated methods [15–18]. Missing or unreported data were requested from corresponding authors where possible.
Quality assessment
The quality of studies was evaluated using the Newcastle–Ottawa Scale (NOS), with scores of seven to nine indicating high quality. We also assessed the level of evidence using the Oxford Centre for Evidence-Based Medicine criteria, employing Review Manager version 5.3 (Cochrane Collaboration, Oxford, UK). Quality assessments were conducted independently by two investigators, with disagreements resolved through discussion. Detailed results of the quality assessments are provided in Supplementary Material S3.
Statistical analysis
Data synthesis was conducted using Review Manager version 5.3. WMDs were used for continuous variables, and ORs for dichotomous variables, both reported with 95% confidence intervals (CIs). Heterogeneity was evaluated using the chi-square (χ2) test (Cochran’s Q) and inconsistency index (I2) [19], with a χ2 p-value < 0.05 or I2 > 50% indicating significant heterogeneity. A random-effects model was applied when significant heterogeneity was present; otherwise, a fixed-effects model was used. Sensitivity analyses were conducted to assess the impact of individual studies on outcomes with significant heterogeneity. Publication bias was assessed visually using funnel plots and through Egger’s regression tests [19, 20] in Stata version 17.0 (Stata Corp, College Station, TX, USA) for outcomes involving five or more studies. A p-value < 0.05 was considered indicative of significant publication bias.
Results
Literature search and study characteristics
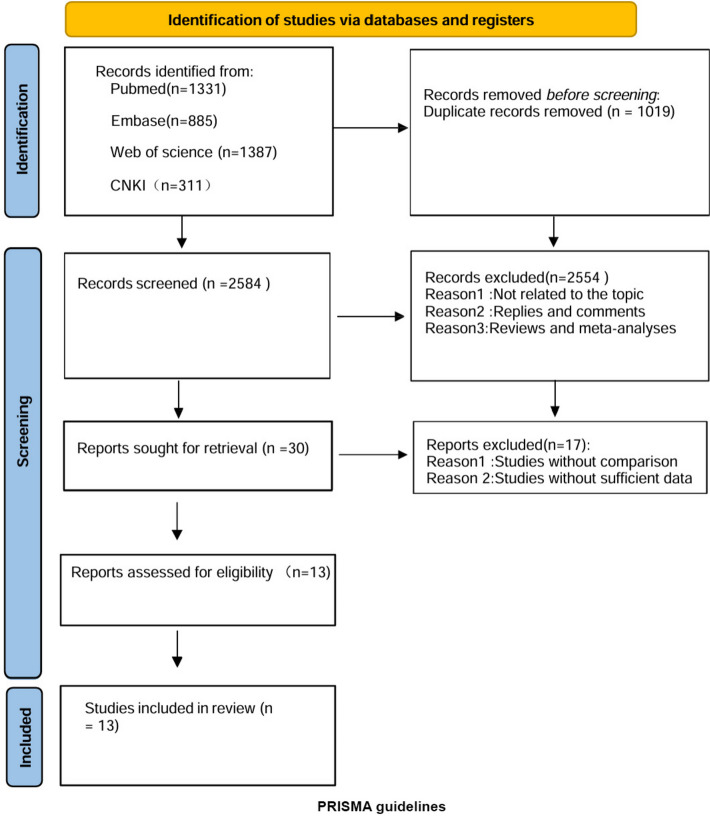
The systematic search process is depicted in Fig. 1. We identified 1,736 relevant articles through searches in PubMed (n = 1331), Embase (n = 885), Web of Science (n = 1387), and CNKI (n = 311). After removing duplicates, 2,584 titles and abstracts were screened, resulting in 13 full-text articles being included in the pooled analysis [21–33], involving 4,493 patients (630 Ultrasonic vs. 787 Clamp). The studies comprised 3 prospective cohort studies [23, 25, 27], 1 retrospective cohort study [33], 1 comparative study [21], and 7 randomized controlled trials [22, 24, 26, 29–32]. Table 1 provides details on study characteristics, evidence levels, and quality scores, with a median quality score of 6 (range 5–7). Eight studies were deemed high quality [22–26, 28, 32, 33]. Quality assessment details are in Supplementary Material S3.
Fig. 1.
Flow diagram of the systematic search and selection process
Table 1.
Baseline characteristics of include studies and methodological assessment
| Authors | Study periods | Country | Study design | Patients(n)ultrasonic/clamp crushing | Level of evidence | Quality score |
|---|---|---|---|---|---|---|
| Ahmad | 2015-2016 | Egypt | Randomized Controlled Trial | 36/36 | A | 6 |
| Bon N. Koo | 2003 | Korea | Randomized Controlled Trial | 25/25 | A | 7 |
| H. G. Rau | 1990-1993 | Germany | Randomized Controlled Trial | 28/61 | A | 5 |
| Doklestić | 2008-2010 | Serbia | Randomized Controlled Triall | 20/20 | A | 7 |
| Luca | 2002-2004 | Italy | Comparative | 100/100 | C | 5 |
| Lesurtel | 2003-2004 | Switzerland | Prospective | 25/25 | C | 6 |
| Nanashima | 2005-2012 | Japan | Retrospective | 24/118 | B | 7 |
| Gotohda | 2011-2012 | Japan | Randomized Controlled Trial | 107/104 | A | 7 |
| Qiao | 2008-2010 | China | Randomized Controlled Trial | 17/20 | A | 6 |
| S.-T. FAN | 1989-1994 | China | Prospective | 69/96 | B | 7 |
| Sun | 2012 | China | Prospective | 80/80 | B | 7 |
| Tadatoshi | 1998-1999 | Japan | Randomized Controlled Trial | 66/66 | A | 7 |
| Zhu | 2013-2018 | China | Retrospective | 33/29 | B | 7 |
Demographic characteristics
No significant differences were found between the ultrasonic and clamp groups in terms of age (WMD: 0.78; 95% CI: -1.07, 2.62; p = 0.41), gender distribution (OR: 0.79; 95% CI: 0.60, 1.04; p = 0.10), BMI (WMD: -0.05; 95% CI: -1.39, 1.29; p = 0.94), cirrhosis prevalence (WMD: 1.05; 95% CI: 0.79, 1.41; p = 0.73), major hepatectomy rates (WMD: 1.08; 95% CI: 0.85, 1.38; p = 0.53), or malignancy rates (WMD: 1.01; 95% CI: 0.71, 1.45; p = 0.95) (Table 2).
Table 2.
Demographics and clinical characteristics of included studies
| Outcomes | Studies | No. of patients | WMD or OR | 95% CI | p-value | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|
| UR/CC | Chi2 | df | p-value | I2 (%) | |||||
| Age(years) | (8) | 304/454 | 0.78 | [-1.07,2.62] | 0.41 | 7.16 | 7 | 0.41 | 2 |
| Gender(male) | (11) | 536/617 | 0.79 | [0.60,1.04] | 0.10 | 4.90 | 9 | 0.84 | 0 |
| BMIa( kg/m2) | (2) | 69/65 | -0.05 | [-1.39,1.29] | 0.94 | 0.89 | 1 | 0.34 | 0 |
| Cirrhosis(yes) | (7) | 463/584 | 1.05 | [0.79,1.41] | 0.73 | 5.02 | 5 | 0.41 | 0 |
| Major Hepatectomyb(yes) | (12) | 613/767 | 1.08 | [0.85,1.38] | 0.53 | 19.97 | 10 | 0.03 | 50 |
| Malignant(yes) | (8) | 606/669 | 1.01 | [0.71,1.45] | 0.95 | 6.30 | 7 | 0.51 | 0 |
aBMI Body mass index
bMajor Hepatectomy(≥3 segments hepatectomy)
Intraoperative outcomes
Operating time
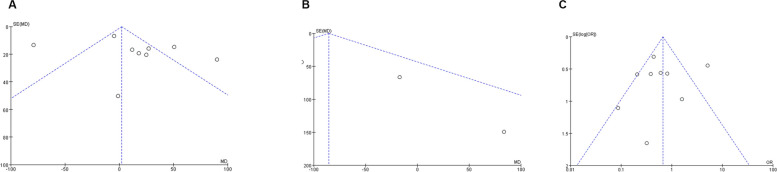
Nine studies including 928 patients (404 ultrasonic vs. 524 clamp) were analyzed for operating time [21–23, 25, 26, 28, 29, 31, 32], showing no significant difference between the groups (WMD: 2.28; 95% CI: -6.95, 11.50; p = 0.63) but with high heterogeneity (I2 = 88%, p < 0.00001)(Fig. 2A). A funnel plot suggested potential publication bias(Fig. 3A), though Egger’s test did not confirm this (p = 0.363). Sensitivity analysis indicated that excluding Amad (2019) [31] and Sun (2015) [25] resulted in shorter operating times in the clamp group (WMD: 33.52; 95% CI: 19.33, 47.72; p < 0.00001) with reduced heterogeneity (I2 = 39%, p = 0.13).
Fig. 2.
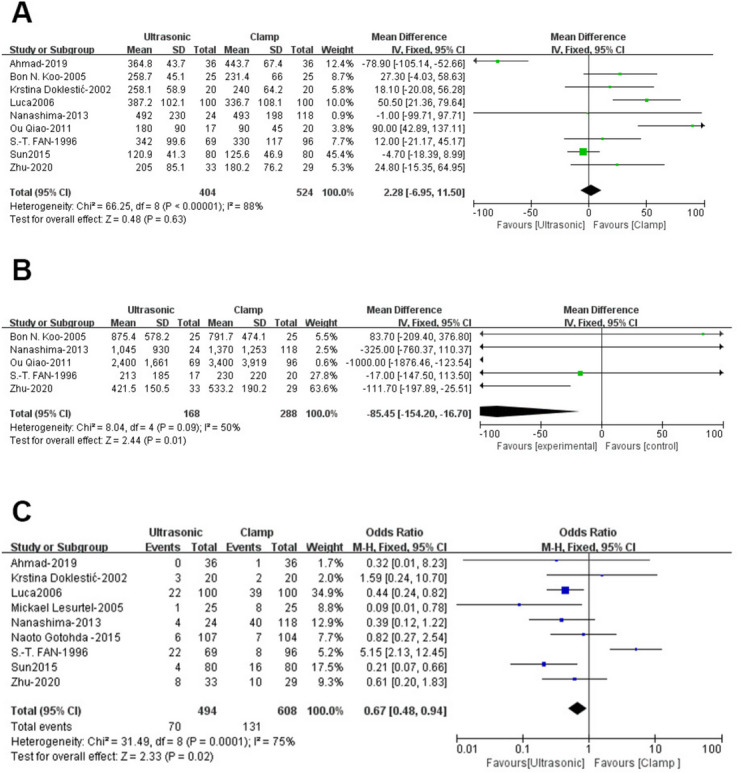
Forest plots of operative outcomes: (A) operating time (B) blood loss (C) transfusion rate
Fig. 3.
Funnel plots of operative outcomes: (A) operating time (B) blood loss (C) transfusion rate
Blood loss
Five studies [23, 26, 28, 29, 33] involving 456 patients (168 ultrasonic vs. 288 clamp) found significantly less blood loss in the ultrasonic group (WMD: -85.45; 95% CI: -154.20, -16.70; p = 0.01) with moderate heterogeneity (I2 = 50%, p = 0.09)(Fig. 2B). Funnel plot analysis indicated slight publication bias(Fig. 3B), but Egger’s test did not (p = 0.489).
Transfusion rate
Data from 9 studies, including 1,102 patients (494 ultrasonic vs. 608 clamp) [21–25, 27, 28, 31, 32], showed a significantly higher transfusion rate in the clamp group (OR: 0.67; 95% CI: 0.48, 0.94; p = 0.02)(Fig. 2C), with substantial heterogeneity (I2 = 75%, p = 0.0001). Neither Egger’s test (p = 0.828) nor visual inspection indicated significant publication bias(Fig. 3C). Sensitivity analysis suggested that excluding studies by Luca (2006) [21] and S-T-FAN (1996) [23] revealed a lower transfusion rate in the ultrasonic group (WMD: 0.42; 95% CI: 0.26, 0.69; p = 0.0006) with reduced heterogeneity (I2 = 15%, p = 0.31).
Sensitivity analysis
We performed one-way sensitivity analyses for operating time and transfusion rate to assess the impact of each study on the combined weighted mean difference (WMD) by sequentially removing individual studies. Sensitivity analysis indicated that removing the studies by Amad (2019) [31] and Sun (2015) [25] affected the WMD for operating time (Fig. 4A), while excluding Luca (2006) [21] and S-T-FAN (1996) [23] impacted the WMD for transfusion rate (Fig. 4B). Excluding Amad (2019) [31] and Sun (2015) [25] reduced the heterogeneity in operating time (I2 = 39%, p = 0.13), suggesting these studies contributed significantly to the heterogeneity. Similarly, removing Luca (2006) [21] and S-T-FAN (1996) [23] resolved the heterogeneity in transfusion rate (I2 = 15%, p = 0.31).
Fig. 4.
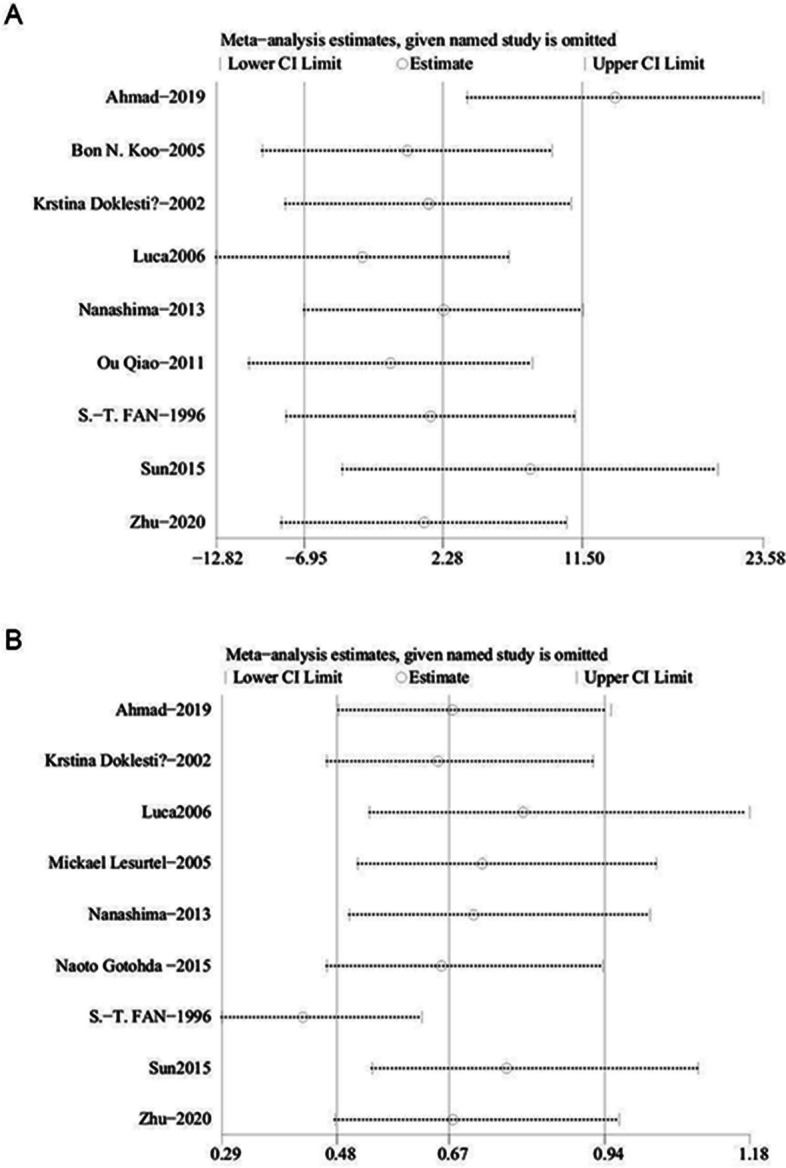
Sensitivity analysis of (A) operating time (B) transfusion rate
Postoperative outcomes
Complication rate
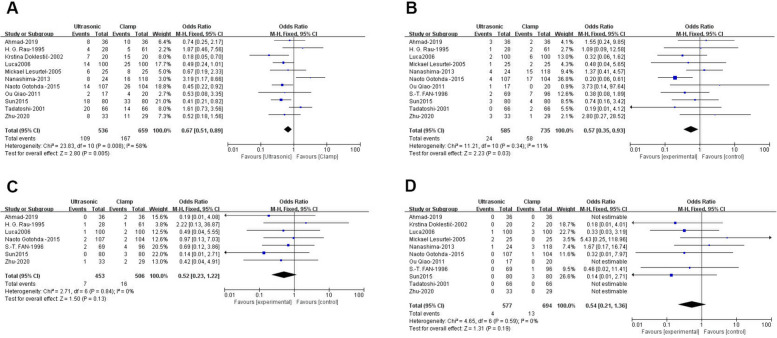
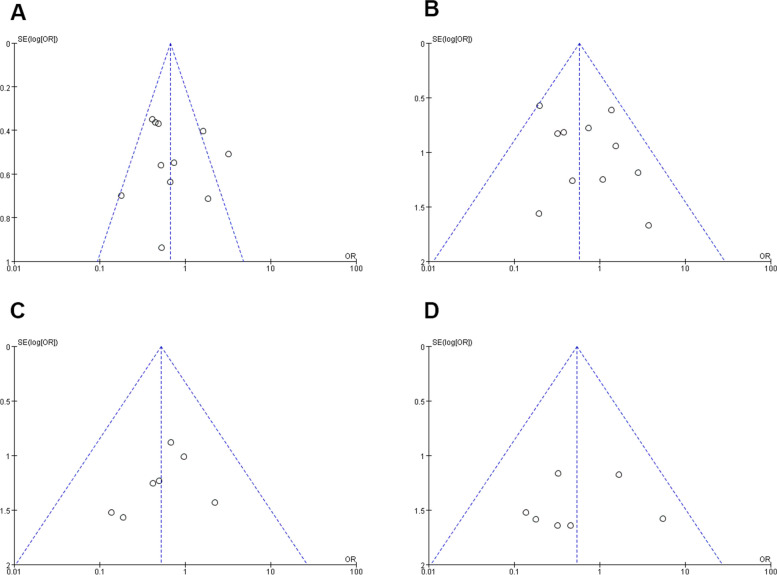
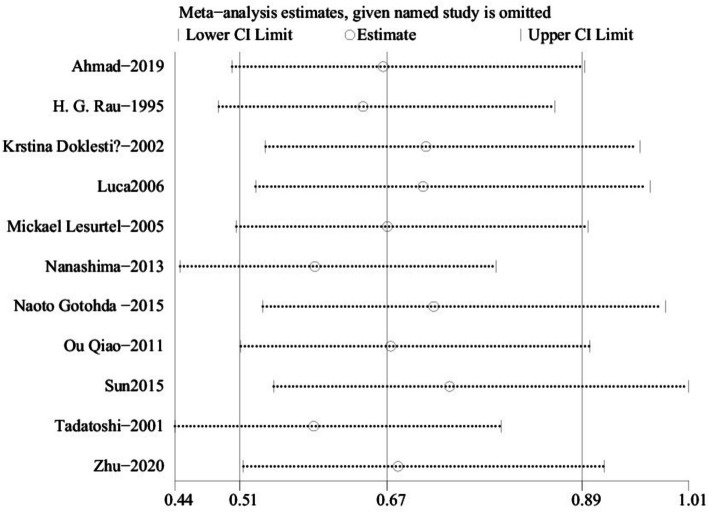
Data from 11 studies involving 1,195 patients (536 ultrasonic vs. 659 clamp) [21, 22, 24, 25, 27–33] showed a significantly lower complication rate in the ultrasonic group (OR: 0.67; 95% CI: 0.51, 0.89; p = 0.005) with moderate heterogeneity (I2 = 58%, p = 0.008)(Fig. 5A). Although visual inspection and Egger’s test did not detect significant publication bias (p = 0.628), high heterogeneity led to a sensitivity analysis(Fig. 6A). Excluding the study by Nanashima [28] revealed a lower complication rate for the ultrasonic group (WMD: 0.59; 95% CI: 0.44, 0.80; p = 0.0005) with reduced heterogeneity (I2 = 35%, p = 0.13).
Fig. 5.
Forest plots of postoperative outcomes: (A) complication rate (B) bile leakage (C) postoperative bleeding (D) mortality
Fig. 6.
Funnel plots of postoperative outcomes: (A) complication rate (B) bile leakage (C) postoperative bleeding (D) mortality
Bile leakage
Eleven studies with 1,320 patients (585 ultrasonic vs. 735 clamp) were analyzed for bile leakage [21, 23–25, 27–33], showing a significantly lower rate in the ultrasonic group (OR: 0.57; 95% CI: 0.35, 0.93; p = 0.03)(Fig. 5B). The heterogeneity was low (I2 = 11%, p = 0.34), and no significant publication bias was found (Egger’s test, p = 0.172)(Fig. 6B).
Subgroup analysis of bile leakage
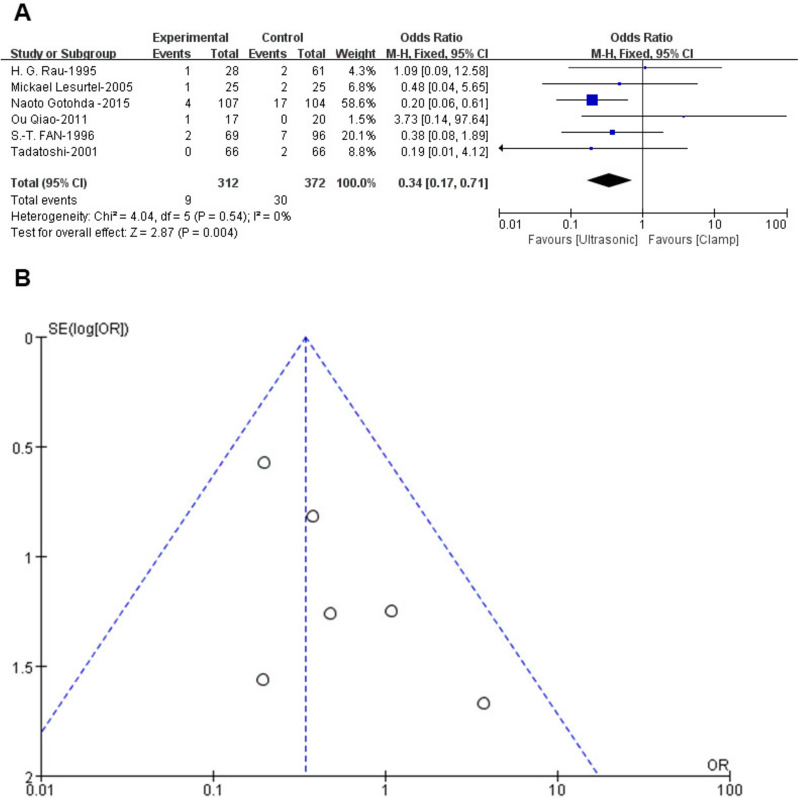
Subgroup analysis revealed differences between ultrasonic devices. Comparison between Cavitron Ultrasonic Surgical Aspirator (CUSA) and Harmonic scalpel showed no significant difference in leakage rates compared to clamps. However, CUSA had a significantly lower bile leakage rate (OR: 0.34; 95% CI: 0.17, 0.71; p = 0.004) with no heterogeneity (I2 = 0%, p = 0.54) (Fig. 7A) or publication bias (Egger’s test, p = 0.167) (Fig. 7B), demonstrating CUSA's superior performance over clamps and Harmonic scalpel.
Fig. 7.
Forest and funnel plot of bile leakage between CUSA and clamp
Postoperative bleeding
Seven studies [21, 23–25, 30, 31, 33] with 959 patients (453 ultrasonic vs. 506 clamp) reported similar postoperative bleeding rates between the groups (OR: 0.52; 95% CI: 0.23, 1.22; p = 0.13). There was no significant heterogeneity (I2 = 0%, p = 0.84) (Fig. 5C) or evidence of publication bias (Egger’s test, p = 0.913) (Fig. 6C).
Mortality
In 11 studies with 1,271 patients (577 ultrasonic vs. 694 clamp) [21–25, 28–33], mortality rates were comparable between the groups (OR: 0.54; 95% CI: 0.21, 1.36; p = 0.19) (Fig. 5D). There was no significant heterogeneity (I2 = 0%, p = 0.59) or publication bias (Egger’s test, p = 0.853) (Fig. 6D).
Sensitivity analysis
One-way sensitivity analyses were conducted to evaluate how the removal of individual studies affected the combined weighted mean difference (WMD) for complication rates. The analysis showed that the WMD remained stable regardless of which study was excluded (Fig. 8A). However, removing the study by Nanashima [28] reduced heterogeneity for complication rates (I2 = 35%, p = 0.13), suggesting that this study contributed significantly to the variability.
Fig. 8.
Sensitivity analysis of complication rate
Discussion
Since the 1970s, various liver transection devices have been developed to minimize intraoperative blood loss and improve patient outcomes [1–6]. Among these, ultrasonic devices are noted for their advanced technology and clinical use. This meta-analysis compared ultrasonic devices with traditional clamp methods, finding that ultrasonic devices were more effective in reducing blood loss and complications, thus improving patient outcomes. However, clamps were associated with shorter operating times, as detailed in our findings.
Blood loss is a key factor in morbidity and mortality after liver resection [13, 14, 34, 35]. Despite advancements in surgical techniques leading to lower mortality rates over the past two decades [8, 9, 11], complication rates remain high, highlighting the need for improved strategies [12, 36]. Our analysis, including 13 studies with 1,417 patients, demonstrated that ultrasonic devices are superior to traditional methods in reducing blood loss, complications, and transfusions.
A recent meta-analysis of blood loss reduction techniques involved 22 studies and nearly 2,360 patients but did not address outcomes of ultrasonic devices versus clamp-crushing techniques [7]. Our study addressed this gap by comparing 13 studies of 1,417 patients—630 with ultrasonic devices and 787 with clamps—ensuring no significant demographic differences.
Intraoperative outcomes showed that clamp-crushing techniques resulted in shorter operating times. While ultrasonic devices reduced blood loss significantly compared to clamps, this raises the possibility of combining these methods to optimize both operating time and blood loss.
Postoperative outcomes indicated that ultrasonic devices were associated with lower rates of complications and bile leakage. A subgroup analysis revealed that the Cavitron Ultrasonic Surgical Aspirator (CUSA) was particularly effective in reducing bile leakage compared to clamps and harmonic scalpels, possibly due to its combined ultrasonic energy and aspiration. No significant differences were observed in mortality or postoperative bleeding between the two groups. This suggests that ultrasonic devices are effective in reducing complications, especially bile leakage, and should guide tool selection.
This study has several strengths. Unlike other analyses that combine various treatments, this meta-analysis evaluated and compared perioperative outcomes of two distinct treatment methods separately. By analyzing both intraoperative and postoperative outcomes, it offers fresh insights into the risk–benefit profiles of ultrasonic devices and clamp-crushing techniques for parenchymal transection. We tackled the issue of inconsistent measurements across studies by synthesizing the available data into a single meta-analysis, reducing the risk of selection bias.
Network meta-analyses allow for the comparison of different treatments by combining direct evidence from individual studies with indirect evidence across various studies. This method enables comparisons that may not have been directly examined before. It also enhances the precision in estimating the relative effects of different treatments, offering greater statistical power compared to traditional pairwise meta-analyses, which depend only on direct evidence. Additionally, network meta-analyses provide a more comprehensive and reliable assessment, helping to interpret a wider range of evidence and facilitating the calculation of treatment rankings with corresponding probabilities [37, 38].
This study has several limitations. First, the limited number of studies included in the analysis may have reduced the statistical power and introduced potential bias. Second, some studies provided incomplete and inconsistent data on intraoperative outcomes like blood loss and operative time [24, 30], and similar inconsistencies were noted in reporting postoperative bile leakage. Third, there was considerable variability in the demographic characteristics of patients undergoing major hepatectomy, which could have affected the results. Additionally, the small number of high-quality randomized controlled trials (RCTs) contributed to the heterogeneity of the findings. We hope to include more RCTs in the future to better evaluate the benefits of both techniques. While these limitations suggest that our meta-analysis should be interpreted cautiously, the findings still offer valuable insights into the effects of different surgical techniques.
We hold that systematic review and meta-analysis offers unquestionable advantages as a research method, combining multiple studies to increase sample size and reduce bias, thereby obtaining more detailed and accurate results. This is the primary reason why it is widely recognized by the academic community. However, it is evident that this method is contingent upon the quality and data integrity of the original studies. In the event that the number of original studies is insufficient or there is a dearth of sufficiently detailed and accurate data, it will lead to difficulties in analyzing part of the systematic review. Consequently, although systematic review represents an efficacious research method for integrating clinical evidence, the inclusion of high-quality randomized controlled trials is still very important, and this is one of the subsequent work we hope to carry out.
Moreover, the studies referenced in this analysis were conducted between 1989 and 2018, with many being over one or two decades old. Hepatectomy peri-operative management has likely improved significantly over this period, complicating the interpretation of outcome comparisons. Although we approached the results with caution, the possibility of misinterpretation remains. Besides this, actually, CUSA and Harmonic work in different way though both technique use the ultrasound which may cause the high heterogeneity of outcomes.
Another limitation is that our meta-analysis relied on summary statistics rather than individual patient data. This meant that certain patient-level covariates, which could impact treatment outcomes, were not included in the analysis, preventing their adjustment [7]. Access to individual patient data would allow for a more detailed understanding of factors influencing outcomes and enhance the statistical strength of the meta-analysis. Lastly, several factors that can increase the risk of bleeding during liver resection—such as cirrhosis, neoadjuvant chemotherapy, central venous pressure management, and hepatic inflow control—were selectively reported in the studies. This selective reporting complicates a thorough assessment of their influence on the outcomes.
These limitations highlight the importance of future studies with larger sample sizes, complete and consistent reporting of surgical outcomes, and access to individual patient data to enable more robust and reliable meta-analyses.
Conclusion
Pooled analyses have shown that ultrasonic devices are more effective and safer than clamp-crushing techniques when considering blood loss, complication rates, bile leakage, and the occurrence of postoperative bleeding during hepatectomy. Among ultrasonic devices, the CUSA appears to be the most effective approach for reducing bile leakage, while clamp crushing is better suited for reducing the operating time. Despite these findings, there was a certain level of heterogeneity and potential bias in the studies analyzed. Thus, surgeons should exercise their clinical judgment when selecting parenchymal transection devices, taking into consideration their own experience and patient-specific factors.
Supplementary Information
Authors’ contributions
(I) Conception and design: YZN and ZM. (II) Administrative support: ZM, ZH. (III) Collection and assembly of the data: YZN, XLL, LL, MYY, WL and JJT. (IV) Data analysis and interpretation: YZN, XLL and LL. (V) Manuscript writing: YZN, ZM, ZH, LL, XLL, MYY, WL and JJT. All the authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82203785, No. 82270648, No. 82473470), Key research and development project of science and technology department of Sichuan Province (2024YFFK0310, 2024YFFK0309), and the Postdoctoral Research Fund, West China Hospital, Sichuan University (2024HXBH073).
Data availability
The raw data supporting the conclusions of this article is available by contacting the correspond author under reasonable request.
Declarations
Ethics approval and consent to participate
Ethical review and approval were not required for the study of human participants in accordance with the local legislation and institutional requirements.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beckingham IJ, Krige JE. ABC of diseases of liver, pancreas, and biliary system. BMJ. 2001;322:477–80. 10.1136/bmj.322.7284.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guro H, et al. Current status of laparoscopic liver resection for hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:212–8. 10.3350/cmh.2016.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moris D, et al. A simple scoring system to estimate perioperative mortality following liver resection for primary liver malignancy-the Hepatectomy Risk Score (HeRS). Hepatobiliary Surg Nutr. 2021;10:315–24. 10.21037/hbsn.2020.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127:S248–260. 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Tateishi R, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419–25. 10.1136/gut.2003.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, et al. 42,573 cases of hepatectomy in China: a multicenter retrospective investigation. Sci China Life Sci. 2018;61:660–70. 10.1007/s11427-017-9259-9. [DOI] [PubMed] [Google Scholar]
- 7.Kamarajah SK, et al. A systematic review and network meta-analysis of parenchymal transection techniques during hepatectomy: an appraisal of current randomised controlled trials. HPB (Oxford). 2020;22:204–14. 10.1016/j.hpb.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Allaire M, et al. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep. 2020;2: 100134. 10.1016/j.jhepr.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung TT, et al. Pure Laparoscopic Hepatectomy Versus Open Hepatectomy for Hepatocellular Carcinoma in 110 Patients With Liver Cirrhosis: A Propensity Analysis at a Single Center. Ann Surg. 2016;264:612–20. 10.1097/SLA.0000000000001848. [DOI] [PubMed] [Google Scholar]
- 10.Sun HC, et al. Risk factors for postoperative complications after liver resection. Hepatobiliary Pancreat Dis Int. 2005;4:370–4. [PubMed] [Google Scholar]
- 11.Virani S, et al. Morbidity and mortality after liver resection: results of the patient safety in surgery study. J Am Coll Surg. 2007;204:1284–92. 10.1016/j.jamcollsurg.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 12.Kingham TP, et al. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg. 2015;220:471–9. 10.1016/j.jamcollsurg.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei AC, Tung-Ping Poon R, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41. 10.1002/bjs.4018. [DOI] [PubMed] [Google Scholar]
- 14.Jarnagin WR, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. 10.1097/01.SLA.0000029003.66466.B3. discussion 406–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805. 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 16.Shi J, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11:641–54. 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, et al. Detecting the skewness of data from the five-number summary and its application in meta-analysis. Stat Methods Med Res. 2023;32:1338–60. 10.1177/09622802231172043. [DOI] [PubMed] [Google Scholar]
- 18.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Aldrighetti L, et al. “Technological” approach versus clamp crushing technique for hepatic parenchymal transection: a comparative study. J Gastrointest Surg. 2006;10:974–9. 10.1016/j.gassur.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Doklestic K, et al. The efficacy of three transection techniques of the liver resection: a randomized clinical trial. Hepatogastroenterology. 2012;59:1501–6. 10.5754/hge11552. [DOI] [PubMed] [Google Scholar]
- 23.Fan ST, et al. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg. 1996;83:117–20. 10.1002/bjs.1800830138. [DOI] [PubMed] [Google Scholar]
- 24.Gotohda N, et al. Impact of energy devices during liver parenchymal transection: a multicenter randomized controlled trial. World J Surg. 2015;39:1543–9. 10.1007/s00268-015-2967-y. [DOI] [PubMed] [Google Scholar]
- 25.Hanyong S, et al. A prospective randomized controlled trial: comparison of two different methods of hepatectomy. Eur J Surg Oncol. 2015;41:243–8. 10.1016/j.ejso.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 26.Koo BN, et al. Hepatic resection by the Cavitron Ultrasonic Surgical Aspirator increases the incidence and severity of venous air embolism. Anesth Analg. 2005;101:966–70. 10.1213/01.ane.0000169295.08054.fa. [DOI] [PubMed] [Google Scholar]
- 27.Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814–22. 10.1097/01.sla.0000189121.35617.d7. discussion 822–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanashima A, et al. Usefulness of vessel-sealing devices combined with crush clamping method for hepatectomy: a retrospective cohort study. Int J Surg. 2013;11:891–7. 10.1016/j.ijsu.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 29.QIAO Ou, H PH, Yan J. Comparing application of cavitron ultrasonic surgical aspirator versus intermittent vascular for liver resection(in Chinese). China J Mod Med. 2011;21:4196–8. [Google Scholar]
- 30.Rau HG, et al. A comparison of different techniques for liver resection: blunt dissection, ultrasonic aspirator and jet-cutter. Eur J Surg Oncol. 1995;21:183–7. 10.1016/s0748-7983(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 31.Sultan AM, et al. Clamp-Crush Technique Versus Harmonic Scalpel for Hepatic Parenchymal Transection in Living Donor Hepatectomy: a Randomized Controlled Trial. J Gastrointest Surg. 2019;23:1568–77. 10.1007/s11605-019-04103-5. [DOI] [PubMed] [Google Scholar]
- 32.Takayama T, et al. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922–8. 10.1001/archsurg.136.8.922. [DOI] [PubMed] [Google Scholar]
- 33.Ji-tao ZY-ZW. Comparison of ultrasonic scalpel and clamp crushing in hepatectomy for giant hepatic hemangioma(in Chinese). J Hepatobiliary Surg. 2020;28:360–3. [Google Scholar]
- 34.Doussot A, et al. Complications after Hepatectomy for Hepatocellular Carcinoma Independently Shorten Survival: A Western. Single-Center Audit Ann Surg Oncol. 2017;24:1569–78. 10.1245/s10434-016-5746-6. [DOI] [PubMed] [Google Scholar]
- 35.Yang T, et al. Impact of postoperative infective complications on long-term survival after liver resection for hepatocellular carcinoma. Br J Surg. 2019;106:1228–36. 10.1002/bjs.11231. [DOI] [PubMed] [Google Scholar]
- 36.Poon RT, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. 10.1097/01.sla.0000141195.66155.0c. discussion 708–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills EJ, et al. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA. 2012;308:1246–53. 10.1001/2012.jama.11228. [DOI] [PubMed] [Google Scholar]
- 38.Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. 2013;11:159. 10.1186/1741-7015-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article is available by contacting the correspond author under reasonable request.