Abstract
Background
The role of Vascular risk factors (VRFs) in the progression of Alzheimer’s Disease (AD) and cognitive decline remains to be elucidated, with previous studies resulting in conflicting findings. The possible impact of age-specific mechanisms of resilience/vulnerability is an under addressed issue. We evaluated the association of VRFs with markers of amyloid deposition, neurodegeneration, and blood-brain-barrier (BBB) permeability (Albumin quotient, Qalb), stratifying patients into early-onset (< 65, EOAD), classic late-onset (65–75, cLOAD) and very late-onset (> 75, vLOAD), to evaluate the moderating effect of age of onset. Moreover, we explored the effects of VRFs on cognitive decline at one year follow-up (ΔMMSE).
Methods
For 368 patients with biologically confirmed AD, we computed eight risk factors in a composite measure of cumulative vascular risk (vascular score, VS). Stratifying patients according to age of onset, we regressed VS and main individual VRFs on p-tau/Aβ42, t-tau and Qalb, and used bootstrapped mediation analysis to test direct and indirect associations of VS with t-tau, using Qalb as mediator. In a subset of 105 patients, we performed multivariate backward regressions to assess the effects of sex, APOE, Qalb, VS, p-tau/Aβ42 and t-tau on ΔMMSE.
Results
VS was positively associated with CSF t-tau in more vulnerable groups burdened by more aggressive disease progression (EOAD: β = 0.256, p = 0.019) or aging (vLOAD: β = 0.007, p < 0.001). Conversely, in patients with classic age of onset VS was associated with higher BBB permeability (cLOAD: β = 0.173, p = 0.015), which simultaneously causes the decrease of CSF t-tau, as a possible resilience response. Cognitive decline was not associated with VS in any of the subgroups. Instead, it was affected by both higher CSF t-tau and increased Qalb values in those with very early or very late onset (EOAD and vLOAD), but by Qalb alone in patients with classic age of onset, where CSF t-tau levels might be buffered by BBB permeability.
Conclusions
Our results show that age of onset weighs on the heterogeneous effects played by VRFs in AD, which do not seem to have direct impact on cognitive decline. These findings stress the importance of a tailored patient-centered approach to the application of vascular prevention strategies in AD.
Keywords: Vascular risk, Cognitive decline, Early-onset, Late-onset, Blood-brain barrier
Introduction
Alzheimer’s Disease (AD) is a neurodegenerative disorder that causes progressive cognitive impairment and loss of autonomy in daily life, holding significant social consequences since it can affect adult patients from midlife to old age [1]. The highly heterogeneous course of AD can be influenced by several factors including demographic features, genetics, and the presence of age-related comorbidities and copathology accompanying amyloid and tau deposition [2–4].
In recent years, cerebrovascular pathology (CVP) has especially gained much attention in the bid toward a more comprehensive characterization of AD (https://aaic.alz.org/diagnostic-criteria.asp#background). However, findings concerning the mutual relationship between CVP and AD pathology are often conflicting. On one hand, the presence of CVP seems to lower the threshold to which AD neuropathological changes cause cognitive decline [5] and, in the presence of amyloid pathology, CVP accelerates tau accumulation rates in early AD [6]. On the other hand, data from in vivo studies show the lack of a robust mechanistic relationship between in-vivo markers of CVP and the progression of either amyloid or tau pathology, so that no temporal association between the two has yet been proven, even when these changes occur in the same regions [7]. Moreover, considering neuropathological evaluations from patients with a clinical diagnosis of AD and dominant amyloid proteinopathy, the presence of CVP has been linked to a lower Braak stage [8]. This suggests that CVP may affect the clinical course of AD, contributing to cognitive decline via additional independent mechanisms other than primary proteinopathies.
The variety of vascular alterations in AD further exacerbates the complexity of this scenario. The wide range of structural and functional vascular changes encompass macroscopic focal alterations in brain circulation as well as microscopic changes, such as the loss of tight junctions at the level of the neurovascular unit and Blood-Brain-Barrier (BBB) breakdown [9, 10]. Notably, BBB dysregulation should be especially taken into account as a contributor to the pathophysiology of AD [11], but most notably since its increased permeability has recently been associated with higher rates of cognitive decline in all dementias [12–14].
Vascular risk factors (VRFs) are not only renowned determinants of both CVP and BBB disruption [8, 15, 16] but are also considered important modifiable determinants of cognitive decline and core components of dementia risk scores, despite findings being again inconclusive. Indeed, an observational study showed no evidence of a synergistic relationship between midlife VRFs and cerebrospinal fluid (CSF) AD biomarkers, with both being independently and additively associated with cognitive decline [17], whereas one study on older adults reported a synergistic association between the two [18].
Overall, the relationship between VRFs, AD biomarker changes, and cognitive decline has yet to be cleared entirely; moreover, whether this relationship engages BBB dysregulation and how it varies with age are under-addressed issues in the literature. For instance, while the most common age of onset for AD is 65 to 75 years old, the prevalence of copathology gradually increases with age [19], and a lack of stratification is likely to hamper the identification of age-range specific disease mechanisms or vulnerability to external factors. Indeed, an increased number of midlife, but not late-life, VRFs has been associated with greater burden at amyloid-PET [20], and a recent neuropathological study highlighted different distribution frequencies of CVP in early-onset (EOAD) versus late-onset AD (LOAD) [21], raising questions on whether differences in age of onset could identify separate cohorts with possibly different response mechanisms to copathology.
Our first aim was to explore the effects of VRFs on CSF measures of AD burden, global neurodegeneration, and BBB permeability, using a multifactorial approach accounting for the age of onset and other known interfering factors (i.e., gender and APOE status) [4, 22] in a real-world clinical cohort of patients with biologically defined AD. Then, we verified whether VRFs act as modifiers of clinical progression in terms of longitudinal cognitive decline, accounting for their potential effect on BBB permeability.
Materials and methods
Patients enrolment and study design
We conducted a prospective observational study including 450 outpatients referred to the UOSD Centro Demenze of the University Hospital “Policlinico Tor Vergata” in Rome upon suspicion of AD between January 2021 and June 2023. As part of the initial assessment, all patients underwent history taking and neurological examination, as well as a complete diagnostic work-up including Mini-Mental State Examination (MMSE), laboratory testing to rule out secondary cognitive decline, 3T brain magnetic resonance imaging, and lumbar puncture (LP).
We considered eligible for the study all patients with an MMSE < 28 and clear-cut decline from previous functioning – thus excluding subjective cognitive decline – willing to undergo a diagnostic LP, regardless of the degree of clinical severity. Exclusion criteria were: [1] refused LP or LP contraindicated; [2] normal biomarker profile or suspected non-AD pathology; [3] presence/history of chemotherapy, inflammatory or autoimmune conditions, decompensated Diabetes Mellitus type 2; [4] manifest acute stroke – Hachinski scale score > 4 – or radiological evidence of focal ischemic lesions at baseline MRI; [5] clinical evidence of other neurological disorders; [6] presence of mutations compatible with genetic AD.
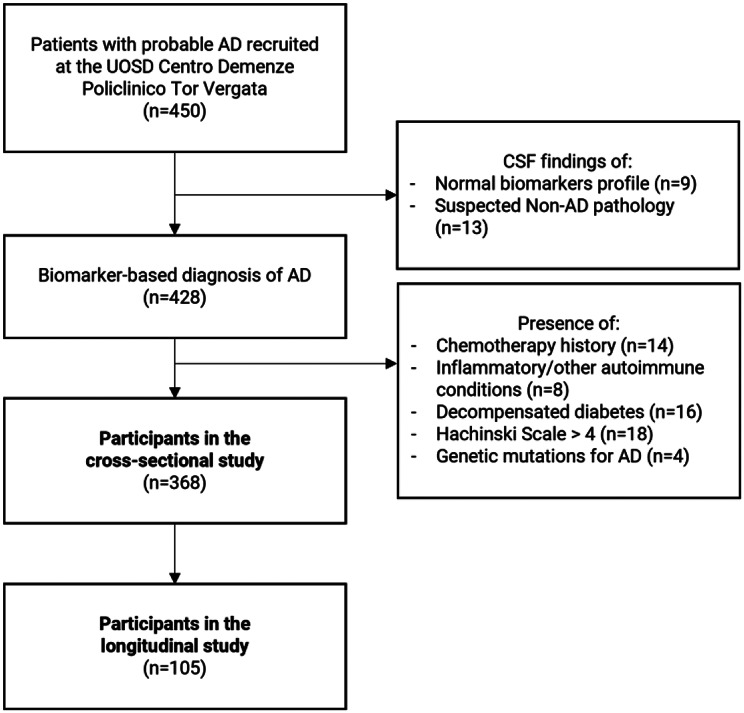
Eventually, 428 patients received a CSF biomarker-based diagnosis of AD, and 368 were included in the study for cross-sectional analysis (see Fig. 1). All patients were further stratified into subgroups according to the reported age of symptoms onset. Patients with clinical onset earlier than 65 years old were classified as early-onset AD (EOAD, age < 65 n = 84). According to the literature, patients with onset later than 65 years old are labeled as late-onset AD; however, to account for the excessive variety in this category and for the potential additive effect of aging, we subdivided this group into classic-onset AD (cLOAD, age range 65–75, n = 178) and late-onset AD (vLOAD, age > 75, n = 106).
Fig. 1.
Legend: Patient selection flowchart summarizing enrolment procedures for the cross-sectional and longitudinal studies
Follow-up visits were performed at 12 ± 3 months from lumbar puncture and included clinical evaluations and administration of MMSE. Cognitive decline was expressed as a negative number obtained as the difference between baseline vs. follow-up MMSE (delta, ΔMMSE). All cases were also reviewed at least once after the conclusion of the follow-up to rule out fluctuations in cognitive symptoms and confirm the progression of cognitive decline.
We excluded from the longitudinal substudy [1] patients who were successfully evaluated and included in phase III double-blind clinical trials available at our center; [2] patients who refused to comply with the longitudinal study procedures; [3] patients without valid follow-up data; [4] patients who developed medical comorbidities with possible consequences on cognitive functions (e.g., sepsis, major surgery); [5] patient passing during the follow-up time. Thus, a final cohort of 105 patients was identified for longitudinal analyses.
We obtained written consent from all participants or legally authorized representatives. All procedures were performed according to the Declaration of Helsinki. The local ethical committee considered the study protocol an observational prospective design.
CSF collection and analysis
A CSF sample of 10 mL was collected for each patient. All lumbar punctures were performed with a sterile technique between 8 and 10 am, and samples were collected in polypropylene tubes and processed according to laboratory standard operating procedures. 2 mL were used for biochemical routine analysis, including cell and protein count. 6 mL were centrifuged at 2000 g at + 4 °C for 10 min, aliquoted in 1 mL portions, and frozen at -80 °C for further analysis.
Commercially available kits were used to carry out biochemical analysis (Flex reagent cartridge, Dimension Vista System, Siemens Healthcare Diagnostics GmbH, Munich, Germany); CSF Aβ42, p-tau, and t-tau concentrations were determined using LUMIPULSE G1200© (Fujirebio, Holdings Inc., Tokyo, Japan), a fully automated chemiluminescent enzyme immunoassay analyzer (CLEIA). The cut-off values were as follows: CSF Aβ42 > 600 pg/ml, CSF p-tau < 65 pg/ml, CSF t-tau < 400 pg/ml. For all patients, the p-tau/Aβ42 ratio was calculated.
Blood samples were also drawn for complimentary analysis including Qalb calculation and APOE genotyping, which was conducted using allelic discrimination technology with real-time PCR (TaqMan; Applied Biosystems).
Vascular score
For each patient, we retrospectively collected information on the presence (score = 1) or absence (score = 0) of 8 cardiovascular risk factors: Hypertension, Atrial Fibrillation, Diabetes Mellitus (DM) type 2, Smoke, Stroke or Transient Ischemic Attack, Acute Myocardial Infarction, Obstructive Sleep Apnea, Dyslipidemia. All information was obtained from the patient’s medical records and information collected during the first visit to our center. The sum of the categorical variables was transformed into a 0 to 1 continuous score representing a percentage (Vascular Score, VS) to account for missing values (up to 2 per patient).
Statistical analysis
Continuous variables were expressed as means ± standard deviation (SD), and categorical variables as frequencies (percentages) [see Table 1]. Patients were classified as APOE4 when carrying one or two ε4 alleles (APOE ε4/ε4 or ε3/ε4). All the remaining patients were identified as APOE3 (ε3/ε3). Differences between age of onset groups were assessed via ANOVA test with post-hoc analyses (continuous variables) or chi-squared test (categorical variables). The association between individual VRFs and VS values were tested using multivariate regression analysis. VRFs showing β values > 0.29 were selected for further analyses.
Table 1.
Demographics, clinical characteristics, and CSF dosages of patients included in the cross-sectional cohort
| EOAD (n = 84) | cLOAD (n = 178) | vLOAD (n = 106) | p | |
|---|---|---|---|---|
| Age (years) | 59.44 ± 3.73 | 70.11 ± 3.10 | 78.96 ± 3.08 | < 0.001*** |
| Sex (%F) | 42.85% | 48.31% | 52.83 | 0.393 |
| APOE (%ε4) | 45.23% | 47.75% | 37.73% | 0.323 |
| MMSE | 19.74 ± 7.01 | 20.70 ± 5.32 | 20.55 ± 5.55 | 0.522 |
| p-tau/Aβ42 | 0.20 ± 0.12 | 0.21 ± 0.14 | 0.21 ± 0.11 | 0.633 |
| t-tau (pg/ml) | 500.74 ± 353.68 | 511.96 ± 317.59 | 517.41 ± 337.00 | 0.941 |
| Qalb | 8.06 ± 4.72 | 7.39 ± 4.31 | 7.36 ± 3.97 | 0.398 |
| Vascular Score | 0.168 ± 0.127 | 0.237 ± 0.197 | 0.252 ± 0.197 | 0.004** |
Legend. EOAD: early-onset AD (< 65 yo); cLOAD: classical onset AD (65–75 yo); vLOAD: late-onset AD (> 75 yo); F: female; APOE: Apolipoprotein E; MMSE: Mini-Mental State Examination; Qalb: albumin quotient; p: p-value. Bold values represent significativity (**= <0.01; ***= <0.001)
To test whether VRFs drive changes in AD neuropathology and BBB permeability biomarker measures, we first assessed cross-sectional associations of VS and the main individual VRFs (independent variables) with CSF p-tau/Aβ42, t-tau, and Qalb (dependent variables). We used multivariate linear regressions, considering the whole sample (All AD) and then patients stratified according to the age of onset (EOAD, cLOAD, vLOAD), controlling for sex and APOE status. Also, we performed cross-sectional bootstrapped mediation analyses with 1000 iterations on each group of patients, using VS as the predictor variable, t-tau as the dependent variable, and Qalb as the mediator. All mediation analyses included sex and APOE genotype as covariates.
The longitudinal analysis focused on a subset of patients with valid follow-up data (n = 104). We performed backward stepwise multivariate linear regressions in the whole group and then in subgroups of patients stratified according to the age of onset (EOAD, n = 25; cLOAD, n = 54; vLOAD, n = 25) using ΔMMSE as the dependent variable and VS, Qalb, p-tau/Aβ42, CSF t-tau, sex, and APOE as covariates.
Statistical analysis and data management were operated via jamovi© (Version 2.4.11 – Computer Software – The jamovi project 2023, https://www.jamovi.org) and GraphPad Prism© version 10.1.0 for Windows (GraphPad Software, San Diego, California USA, www.graphpad.com). The mediation analyses were performed using the R mediation package (https://cran.r-project.org/web/packages/mediation/mediation.pdf) implemented in jamovi. All results were computed with two-tailed significativity tests; values of p < 0.05 were considered statistically significant.
RESULTS
Participants characteristics
The study eventually included 368 patients with a biologically confirmed clinical diagnosis of AD, stratified according to age of onset [see Table 1]. Besides the expected difference in age, the ANOVA test showed the presence of significant differences between groups only in terms of VS [H [2] = 5.564, p = 0.004], with post-hoc comparisons showing that EOAD patients have lower VS than both cLOAD (ptukey = 0.013) and vLOAD (ptukey = 0.006), while no difference was found between cLOAD and vLOAD (ptukey = 0.799). All the remaining variables were comparable among groups.
Looking at the contribution of each VRFs to VS values in each cohort of patients, Hypertension, Smoke, DM type 2, and Dyslipidemia had the highest β coefficients, representing a moderate-to-large strongly significant positive association with VS in both the whole sample and in each subgroup [see Table 2]. The effect sizes of the associations were comparable across EOAD, cLOAD, and vLOAD.
Table 2.
Multivariable regression analysis showing the contribution of each vascular risk factor to cumulative vascular score in each cohort of patients
| All AD | EOAD | cLOAD | vLOAD | |
|---|---|---|---|---|
| β | β | β | β | |
| Hypertension | 0.440*** | 0.493*** | 0.427*** | 0.391*** |
| Atrial Fibrillation | 0.123*** | n.a. | 0.076*** | 0.214*** |
| Smoke | 0.343*** | 0.462*** | 0.301*** | 0.300*** |
| DM type 2 | 0.340*** | 0.346*** | 0.337*** | 0.339*** |
| Stroke or TIA | 0.172*** | n.a.*** | 0.197*** | 0.190*** |
| Myocardial Infarction | 0.215*** | 0.164*** | 0.232*** | 0.216*** |
| OSAS | 0.145*** | 0.164*** | 0.168*** | n.a. |
| Dyslipidemia | 0.440*** | 0.501*** | 0.428*** | 0.414*** |
Legend. EOAD: early-onset AD (< 65 yo); cLOAD: classical onset AD (65–75 yo); vLOAD: late-onset AD (> 75 yo); DM: Diabetes Mellitus; TIA: Transient Ischemic Attack; OSAS: Obstructive Sleep Apnea Syndrome; n.a.: not applicable; ***= pvalue < 0.001; Bold values represent β > 0.29 (moderate effect size)
Cross-sectional results
We performed separate multivariable regression analyses for VS and for each of the main individual VRFs (Hypertension, Smoke, DM2 and Dyslipidemia) to evaluate their association with CSF p-tau/Aβ42, CSF t-tau, and Qalb in the whole cross-sectional sample (All AD), adjusting for sex and APOE as covariates. Then, we repeated the analyses in the three groups of AD patients stratified according to age-of-onset [see Table 3].
Table 3.
Cross-sectional multivariable regressions adjusted for sex and APOE genotype
| All AD | EOAD | cLOAD | vLOAD | |||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | P | β | p | |
| CSF p-tau/Aβ42 | ||||||||
| Vascular Score | 0.010 | 0.846 | 0.077 | 0.491 | −0.038 | 0.607 | 0.040 | 0.687 |
| Hypertension | −0.010 | 0.841 | 0.106 | 0.350 | −0.048 | 0.523 | −0.071 | 0.478 |
| Smoke | −0.065 | 0.248 | −0.077 | 0.493 | −0.137 | 0.090 | 0.169 | 0.152 |
| DM type 2 | 0.0012 | 0.969 | 0.001 | 0.996 | 0.004 | 0.960 | −0.026 | 0.794 |
| Dyslipidemia | 0.018 | 0.758 | 0.164 | 0.180 | −0.050 | 0.535 | 0.025 | 0.835 |
| CSF t-tau | ||||||||
| Vascular Score | 0.152 | 0.003** | 0.256 | 0.019* | 0.007 | 0.925 | 0.339 | < 0.001*** |
| Hypertension | 0.086 | 0.098 | 0.227 | 0.041* | 0.002 | 0.980 | 0.082 | 0.428 |
| Smoke | −0.005 | 0.925 | −0.031 | 0.780 | −0.148 | 0.063 | 0.352 | 0.002** |
| DM type 2 | 0.035 | 0.495 | 0.134 | 0.220 | 0.045 | 0.541 | −0.059 | 0.564 |
| Dyslipidemia | 0.119 | 0.039* | 0.253 | 0.034* | −0.070 | 0.381 | 0.332 | 0.005** |
| Qalb | ||||||||
| Vascular Score | 0.135 | 0.009** | 0.128 | 0.244 | 0.186 | 0.013* | 0.091 | 0.344 |
| Hypertension | 0.176 | < 0.001*** | 0.195 | 0.081 | 0.224 | 0.003** | 0.119 | 0.266 |
| Smoke | −0.071 | 0.207 | −0.148 | 0.178 | −0.050 | 0.544 | −0.041 | 0.714 |
| DM type 2 | 0.107 | 0.038* | 0.028 | 0.799 | 0.180 | 0.017* | 0.057 | 0.588 |
| Dyslipidemia | 0.038 | 0.504 | 0.092 | 0.438 | 0.013 | 0.877 | 0.002 | 0.987 |
Legend. EOAD: early-onset AD (< 65 yo); cLOAD: classical onset AD (65–75 yo); vLOAD: late-onset AD (> 75 yo); CSF: Cerebrospinal fluid; Qalb: Cerebrospinal fluid/serum albumin quotient; DM: Diabetes Mellitus; p: p-value; *=p < 0.05; **=p < 0.01; ***=p < 0.011. Bold values represent statistical significativity. All values are adjusted for Sex and APOE genotype
First, we found that values of CSF p-tau/Aβ42 are not influenced by VS or any of the individual VRFs in any of the subgroups.
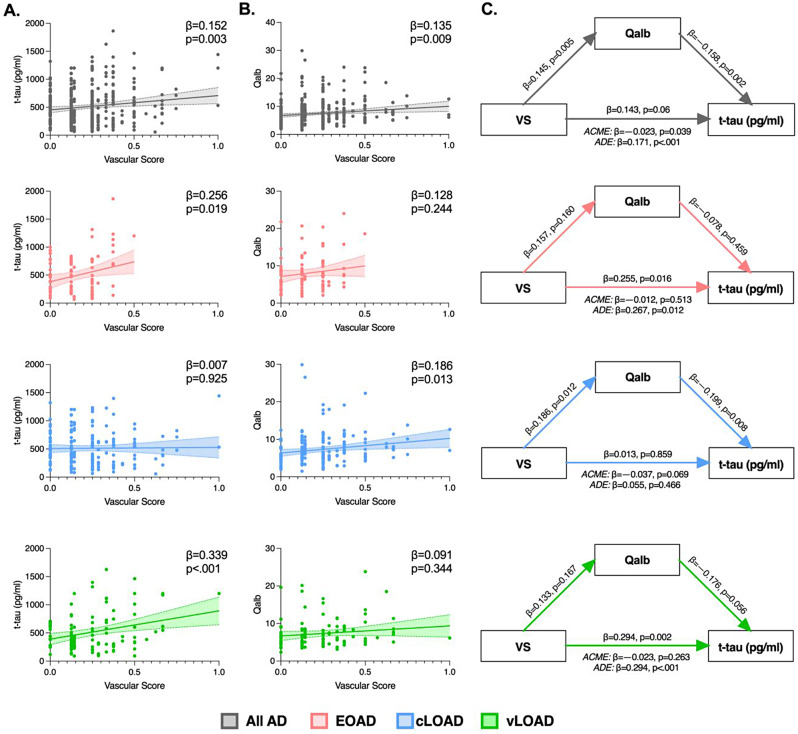
On the other hand, VS values were positively associated with levels of CSF t-tau in All AD (β = 0.152, p = 0.003) as well as both in EOAD (β = 0.256, p = 0.019) and vLOAD (β = 0.339, p < 0.001), but not in cLOAD (β = 0.007, p = 0.925) [see Fig. 2A]. Similarly, Dyslipidemia was positively associated with CSF t-tau in All AD (β = 0.119, p = 0.039), EOAD (β = 0.253, p = 0.034) and vLOAD (β = 0.256, p = 0.019). Moreover, a significant association with CSF t-tau was found for Hypertension in EOAD (β = 0.227, p = 0.041) and Smoke in vLOAD (β = 0.352, p = 0.002). None of the VRFs showed significant associations with CSF t-tau in cLOAD.
Fig. 2.
legend: Associations between Vascular Score (VS), CSF t-tau levels and Qalb in patients with AD. Results on All AD are reported in gray, EOAD are displayed in pink, cLOAD in blue and vLOAD in green. All models are controlled for sex and APOE status. (A) Cross-sectional linear regressions between VS and CSF t-tau, (B) and between VS and Qalb, in All AD (gray) EOAD (pink), cLOAD (blue) and vLOAD (green). Colored areas represent 95% confidence interval. Standardized beta-estimates (β) and p-values were derived from linear regressions. (C) Cross-sectional mediation analyses with VS as predictor, Qalb as mediator, and CSF t-tau as the dependent variable in All AD, EOAD, cLOAD and vLOAD. Standardized Beta-estimates (β) and p-values for each path are displayed on the respective arrow. The average causal mediation effect (ACME) and the average direct effect (ADE) are reported under each mediation triangle
Looking at BBB permeability, we retrieved a significant positive association between Qalb values and VS in All AD (β = 0.135, p = 0.009), that was selectively confirmed in the cLOAD subgroup (β = 0.173, p = 0.021), but not in EOAD or vLOAD [see Fig. 2B]. Hypertension (All AD: β = 0.176, p < 0.001; cLOAD: β = 0.224, p = 0.003) and DM type 2 (All AD: β = 0.107, p = 0.038; cLOAD: β = 0.180, p = 0.017) were also positively associated with Qalb values in the same subgroups of patients. None of the VRFs showed significant associations with Qalb in either EOAD or vLOAD.
Using bootstrapped mediation analysis on the whole cross-sectional cohort (All AD), we identified a direct positive association between VS and CSF t-tau as well as a partial negative indirect effect mediated by Qalb values (ACME: 95%CI: −79.29 to − 1.98, β = −0.023, p = 0.039; ADE: 95%CI: 123.58 to 484.32, β = 0.171, p < 0.001; Total: 95%CI: 74.29 to 435.18, β = 0.143, p = 0.006) [see Fig. 2]. Stratifying patients according to age of onset we additionally found that the association between higher VS and higher CSF t-tau levels is direct and not mediated by Qalb in neither EOAD (ACME: 95%CI: −132.99 to 66.38, β = −0.012, p = 0.513; ADE: 95%CI: 164.55 to 1327.98, β = 0.267, p = 0.012; Total: 95%CI: 132.64 to 1293.29, β = 0.255, p = 0.016) nor vLOAD (ACME: 95%CI: −109.81 to 29.99, β = −0.023, p = 0.263; ADE: 95%CI: 233.68 to 849.71, β = 0.317, p < 0.001; Total: 95%CI: 189.81 to 813.75, β = 0.294, p = 0.002). Instead, we confirmed the absence of a direct effect of VS on CSF t-tau in cLOAD, but in this group the mediating effect of Qalb reached a trend of significativity (ACME: 95%CI: −124.33 to 4.59, β = −0.037, p = 0.069; ADE: 95%CI: −149.25 to 325.98, β = 0.055, p = 0.466; Total: 95%CI: −216.309 to 259.41, β = 0.013, p = 0.859) [see Fig. 2C].
Longitudinal results
A subset of 104 patients were enrolled in the longitudinal study and were stratified according to age of onset. There were no differences between groups for all the variables considered; specifically, the ANOVA test showed comparable values of both ΔMMSE and VS between groups [see Table 4].
Table 4.
Demographics, clinical characteristics, and CSF dosages of patients included in the longitudinal cohort
| EOAD (n = 25) | cLOAD (n = 54) | vLOAD (n = 25) | p | |
|---|---|---|---|---|
| Age (years) | 59.16 ± 3.72 | 70.63 ± 3.08 | 77.88 ± 1.54 | < 0.001*** |
| Sex (%F) | 40.00% | 53.70% | 68.00% | 0.139 |
| APOE (%ε4) | 56.00% | 57.41% | 44.00% | 0.479 |
| ΔMMSE | −2.99 ± 3.73 | −2.30 ± 2.59 | −2.56 ± 1.84 | 0.589 |
| p-tau/Aβ42 | 0.23 ± 0.14 | 0.24 ± 0.14 | 0.24 ± 0.12 | 0.890 |
| t-tau (pg/ml) | 635.09 ± 382.77 | 565.74 ± 310.64 | 602.46 ± 368.57 | 0.694 |
| Qalb | 8.20 ± 5.41 | 7.55 ± 4.48 | 6.47 ± 2.06 | 0.359 |
| Vascular Score | 0.185 ± 0.136 | 0.227 ± 0.188 | 0.260 ± 0.236 | 0.378 |
Legend. EOAD: early-onset AD (< 65 yo); cLOAD: classical onset AD (65–75 yo); vLOAD: late-onset AD (> 75 yo); F: female; APOE: Apolipoprotein E; ΔMMSE: one-year differences in Mini-Mental State Examination score; Qalb: albumin quotient; p: p-value. Bold values represent significativity ***= <0.001)
The backward stepwise linear regression explored the potential influence of sex, APOE genotype, Qalb, CSF p-tau/Aβ42, CSF t-tau levels, and VS on the rate of cognitive decline (ΔMMSE). Collinearity among variables was excluded through the evaluation of the Variance Inflation Factors (all values < 1.5) [23]. At each step, variables with the least contribution to the model (highest p-value) were progressively excluded.
All results in the subgroups are reported in Table 5. The analysis on the whole sample showed that higher values of Qalb (β = −0.435, p < 0.001) and of CSF-tau (β = −0.320, p < 0.001) were associated with more severe cognitive decline (ΔMMSE). When stratifying patients according to age of onset, Qalb remained negatively associated with cognitive decline in all subgroups. Instead, CSF t-tau levels showed a significant association with ΔMMSE selectively in EOAD (β = −0.413; p = 0.009) alongside sex (β = −0.362; p = 0.020), and in vLOAD (β = −0.368; p = 0.037) alongside APOE (β = −0.421; p = 0.032). Interestingly, our regression model showed a higher goodness-of-fit in EOAD [R2 = 0.567] and vLOAD [R2 = 0.444] than in cLOAD [R2 = 0.108].
Table 5.
Backward-entry stepwise regressions considering ΔMMSE as the dependent variable
| All AD (n = 104) | EOAD (n = 25) | cLOAD (n = 54) | vLOAD (n = 25) | |||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Model 1 | ||||||||
| Sex | −0.080 | 0.399 | −0.374 | 0.027* | 0.103 | 0.555 | 0.079 | 0.690 |
| APOE (ε3 = 0; ε4 = 1) | −0.098 | 0.298 | −0.077 | 0.630 | −0.103 | 0.522 | −0.453 | 0.033* |
| Qalb | −0.462 | < 0.001*** | −0.430 | 0.021* | −0.393 | 0.011* | −0.517 | 0.023* |
| Vascular Score | −0.027 | 0.759 | −0.201 | 0.272 | 0.020 | 0.886 | −0.171 | 0.390 |
| CSF p-tau/Aβ42 | 0.014 | 0.897 | 0.124 | 0.526 | −0.135 | 0.430 | −0.144 | 0.565 |
| CSF t-tau | −0.297 | 0.005** | −0.445 | 0.020* | −0.107 | 0.511 | −0.299 | 0.433 |
| R2 = 0.278 | R2 = 0.602 | R2 = 0.154 | R2 = 0.477 | |||||
| Best Model | ||||||||
| Sex | . | . | −0.362 | 0.020* | . | . | . | . |
| APOE (ε3 = 0; ε4 = 1) | . | . | . | . | . | . | −0.421 | 0.032* |
| Qalb | −0.435 | < 0.001*** | −0.531 | 0.001** | −0.328 | 0.016* | −0.512 | 0.011* |
| Vascular Score | . | . | . | . | . | . | . | . |
| CSF p-tau/Aβ42 | . | . | . | . | . | . | . | . |
| CSF t-tau | −0.320 | < 0.001*** | −0.413 | 0.009** | . | . | −0.368 | 0.037* |
| R2 = 0.259 | R2 = 0.567 | R2 = 0.108 | R2 = 0.444 | |||||
| R 2 change | −0.019 | −0.035 | −0.046 | −0.033 | ||||
Legend. EOAD: early-onset AD (< 65 yo); cLOAD: classical late-onset AD (65–75 yo); vLOAD: very late-onset AD (> 75 yo); CSF: cerebrospinal fluid; F: female; Qalb: Cerebrospinal fluid/serum albumin quotient; MMSE: Mini-Mental State Examination; R2: coefficient of determination; p = p-value; *=p < 0.05; **=p < 0.01; ***=p < 0.011. Bold values represent statistical significativity
Of note, VS was not associated with values of ΔMMSE in any of the subgroups.
Discussion
Copathology is an important factor in the multifaceted and heterogeneous course of AD [2]. However, coexistence does not imply causality, nor does it necessarily reflect the existence of a mechanistic link between the two processes. In the present work we addressed the role of VRFs to disentangle their effects on AD-specific neurobiological changes, BBB permeability and cognitive decline according to age of onset.
Age of onset moderates the effects of vascular risk factors on AD biomarkers and blood-brain-barrier permeability
As a composite score, VS reflects a holistic view of how cumulative VRFs affect vascular health and has recently been applied to investigate the relationship between vascular risk and AD biomarkers [24]. In our cohort, some of the VRFs (Hypertension, Smoke, DM type 2 and Dyslipidemia) contributed more to the total VS than others, but overall the entity of their impact was similar across all groups regardless of age of onset.
Considering cumulative vascular risk, we first found that in our cohort VS was not associated with AD neuropathological burden as measured by CSF p-tau/Aβ42 [25], regardless of age of onset. On the other hand, higher VS was associated with higher CSF levels of t-tau, reflecting greater neuronal damage, in both the extreme ages of onset (EOAD and vLOAD) but not in cLOAD.
Other previous studies reported that vascular health has a significant impact on neurodegeneration but not on amyloid deposition [26]. Our results add new and interesting insights on how this impact could be modified by the age of onset, hinting at the presence of different age-dependent susceptibility to neurodegeneration, likely sustained by distinguished drivers of vulnerability or better resilience to vascular injuries in each group. At raw data observation, EOAD and vLOAD seem to share a positive association between VRFs and CSF t-tau levels. In EOAD, this could reflect the failure to bring into play defensive strategies against disease progression, due to accentuated vulnerability. Notably, individuals with EOAD often have shorter relative survival time and steeper cognitive decline [27], supported by a greater pathological burden [28]. Moreover, since AD neurobiological changes start developing up to 20 years before symptoms onset [29], it is interesting to speculate that the earlier clinical debut in EOAD could be at least partly driven by this less resilience to damage accumulation. Indeed, a more damaged brain could be more vulnerable to the presence of aggravating external factors, such as VRFs, or, from an opposite point of view, the advent of VRFs themselves could alter homeostasis and precipitate the AD cascade toward the development of clinical symptoms. Conversely, aging is a well-known risk factor for both AD and VRFs, and findings of a different impact of midlife vs. late-life VRFs on AD biological changes has been reported in older cognitively unimpaired subjects [20, 30]. In this study, we highlight that increasing age-related frailty could have additive damaging effects, boosting vulnerability to VRFs in LOAD, which is reflected in the presence of an association between VS and CSF t-tau only in vLOAD but not in cLOAD.
Moreover, we analyzed the role of those VRFs that had a higher weight within the VS. In both the groups in which VS was positively associated with higher CSF t-tau, EOAD and vLOAD, a concomitant association with Dyslipidemia was always confirmed. On the other hand, Hypertension was additionally linked with higher CSF t-tau only in EOAD, while Smoke had a detrimental role only in vLOAD. Of note, even though the effects of single VRFs seems to play out differently in different clinical contexts, their potential relationship with CSF t-tau is always captured by a background association with VS. Thus, while a more granular analysis could help to verify and to dissect the role of each individual VRF, VS could be trusted as a reliable tool to seize the cumulative effect of VRFs.
Our study also addressed the influence of age of onset on the relationship between VRFs and BBB integrity [31]. Specifically, VS does not seem to influence Qalb in either EOAD or vLOAD, but higher VS was linked with higher Qalb values in both All AD and in cLOAD, with this association being mainly supported by Hypertension and DM type 2 among the individual VRFs. Moreover, our mediation analysis in all AD shows that BBB permeability plays an indirect mediating effect in the relationship between VRFs and CSF t-tau, that is also retrievable as a trend selectively in the cLOAD subgroups while being absent in EOAD and vLOAD. This may indicate that the lack of association between vascular risk and t-tau in cLOAD could partially be due to a potential uncoupling mediated by the modulation of BBB integrity. Indeed, solid data demonstrate that VRFs and vascular injury can alter BBB integrity and Qalb values during the course of AD [15]. At the same time, previous literature shows that BBB permeability affects CSF t-tau levels via an inverse relationship [32, 33], so that higher Qalb values correspond to lower CSF t-tau and this finding is also confirmed in our own results [see Fig. 2]. Thus, while VRFs are able to directly support the progression of neurodegeneration in EOAD and vLOAD, it is possible to speculate the existence of some degree of resilient response to vascular stress in cLOAD, involving the modulation of BBB permeability.
Cognitive decline is influenced by blood-brain-barrier and total tau but not by vascular risk factors
The longitudinal analysis showed the absence of a statistical association between individual vascular risk and worsening cognitive functions in our cohort of AD patients. While this finding is supported by other observational studies in the literature [34], to our knowledge, this is the first study to consider patients with a biologically based diagnosis of AD. Previous research reported differential effects of CVP and of some risk factors on the cognitive trajectories of EOAD and LOAD [30, 35, 36] supporting the use of a stratification based on the age of onset to identify patients with different vulnerability to aggravating factors. Our results highlight that cumulative VRFs do not impact the rate of cognitive decline regardless of age of onset. However, it is not possible to exclude that some specific factors, e.g., hypertension or diabetes, could have specific repercussions in some categories of patients, and further studies are needed to corroborate this hypothesis.
On the other hand, our results highlight that both higher CSF t-tau and increased BBB permeability are associated with higher rates of cognitive decline when considering the whole AD sample [14, 37]. Nevertheless, by stratifying patients into subgroups according to age of onset we observed differences in the extent to which cognitive decline is affected by the variables in our model, as well as in the type of associated variables. First, our regression model showed a higher goodness-of-fit in EOAD and vLOAD than in cLOAD, accounting respectively for 57% and 44% of ΔMMSE variability vs. 10% in the latter. Second, we retrieved different apparent vulnerability to risk factors, since the association with CSF t-tau is observed, again, selectively in EOAD and vLOAD, while higher BBB permeability seems to be the only determinant of cognitive worsening in cLOAD.
The relevance of BBB permeability in determining cognitive decline is thoroughly reported, despite the mechanisms lying behind this relationship are still not entirely cleared yet [13]. Indeed, the regulation of BBB permeability seems to be engaged by complex mechanisms that can favor cognitive worsening, including the development of brain capillary damage in the hippocampus, irrespective of amyloid and tau pathology [12]. At the same time, under certain circumstances, BBB modulation has been associated with regulatory or even homeostatic effects in response to disease progression [32, 38].
Our findings shed light on a new element of complexity related to the age of onset, as the importance of BBB permeability appears to be particularly significant in cLOAD. In this subgroup higher Qalb values, which increase in response to VRFs, are associated with steeper cognitive decline but also with lower levels of CSF t-tau. However, this ambivalent and eluding role of BBB integrity seems to be downsized by the limited effects of Qalb on ΔMMSE in this cohort, as shown by low goodness-of-fit in our regression analysis. Thus, it is possible that a harmful increase in Qalb could be an acceptable cost to be paid to mitigate neurodegeneration. In contrast, this buffer-like effect seems less effective in categories such as EOAD and vLOAD, in which compensatory events, including BBB modulation, could be hindered by faster degeneration and aging. Notably, in these subgroups, VRFs directly impact CSF t-tau levels, which in turn weigh on clinical course.
In summary, these results show that cumulative vascular risk does not seem to directly impact cognitive decline in AD, but rather, it could support the progression of neurodegeneration in both younger and very old patients with AD. Instead, in patients debuting in the classic age range – 65 to 75 - the effects of VRFs seem to induce increased BBB permeability, which is a determinant of cognitive worsening while also partially acting as a buffer against neurodegeneration.
Limitations
This study has limitations, such as the lack of a more detailed neuropsychological follow-up. Indeed, previous literature reported summative effects of amyloid plaques and CVP on longitudinal executive functions [39], and expanding our analysis to explore this matter further will be important. Also, verifying the presence of these associations on more selected groups of patients, such as carriers of APOE ε3 over ε4 genotype, and exploring the differential effects of strictly vascular (i.e., hypertension, hypercholesterolemia) and more metabolic (i.e., diabetes, BMI) risk factors could be relevant [40]. Moreover, the paucity of our sample size– especially for some of the subgroups in the longitudinal study (i.e. EOAD and vLOAD) – did not allow us to fully address the impact of each individual VRFs on cognitive decline.
In addition, the effects of other vascular changes, such as white matter hyperintensities, lacunes, and microhemorrhages, have not been discussed in this study. Widening the analysis to a more extended cohort including multimodal measurements of neurodegenerative processes, such as neurofilament light chains or MRI measurements, will be crucial both to reinforce and expand our findings.
Conclusions
Overall, this study stresses the importance of a global characterization of AD patients, which needs to consider the age of onset as an important player in both the disease’s development and progression. Specifically, a further stratification of late-onset patients seemed to unravel differences likely tied to the additional effect of aging happening along or intertwined with the course of AD.
Moreover, our results could lay foundation for interesting considerations aimed at improving patient care, especially concerning the management of vascular comorbidities. Indeed, VRFs are considered important and modifiable elements in AD, and many studies focused on optimizing vascular prevention as an alternative approach to improve cognitive outcomes [34]. However, clinical trials such as the FINGER study report low effect sizes for these interventions in terms of their ability to prevent the onset or to slow the progression of cognitive decline [41]. Such ineffectiveness should be reassessed considering the limits brought by an overly broad horizontal application of vascular prevention. It is possible that the absent or even detrimental role played by some of these strategies in some subgroups of patients could mask their effectiveness in other specific categories. A vigorous intervention on VRFs could help reduce the burden of neurodegeneration in some patients while possibly altering complex equilibriums in others, and clustering patients according to the anticipated effects of vascular prevention strategies could be helpful in light of a tailored patient-centered approach. More interventional studies accounting for these differences are needed to explore the full potential of VRFs management in the treatment of AD.
Acknowledgements
None.
Abbreviations
- AD
Alzheimer’s Disease
- CSF
Cerebrospinal fluid
- VRFs
Vascular risk factors
- BBB
Blood-brain-barrier
- Qalb
Albumin quotient
- EOAD
Early-onset Alzheimer’s Disease
- cLOAD
Classic late-onset Alzheimer’s Disease
- vLOAD
Very late-onset Alzheimer’s Disease
- VS
Vascular score
- Aβ
Amyloidβ
- p-tau
Phosphorylated tau
- t-tau
Total tau
- CVP
Cerebrovascular pathology
- APOE
Apolipoprotein E
- MMSE
Mini-Mental State Examination
- LP
Lumbar puncture
- ACME
Average causal mediation effect
- ADE
Average direct effect
Author contributions
CGB, CM and AM conceptualized the study and revised the manuscript. CGB and CM analyzed and interpreted the data, drafted, and revised the manuscript, did the statistical analysis, and prepared the figures. MDD, MP and MN collected all laboratory and anamnestic data from the patients. NBM, SB and GK participated in the interpretation of the data and revision of the manuscript. All authors read and approved the final manuscript.
Funding
This study was not supported by any grant.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All participants or legal representatives signed a written informed consent for the anonymization, storage, and analysis of all clinical and biological data. The Ethics committee of the Policlinico Tor Vergata in Rome reviewed and approved this study, which was conducted according to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s Disease International. World Alzheimer Report 2023: Reducing Dementia Risk: Never too early, never too late. 2023.
- 2.Hampel H, Au R, Mattke S, van der Flier WM, Aisen P, Apostolova L, et al. Designing the next-generation clinical care pathway for Alzheimer’s disease. Nat Aging 2022. 2022;2(8):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coulthard EJ, Love S. A broader view of dementia: multiple co-pathologies are the norm. Brain. 2018;141(7):1894–7. [DOI] [PubMed] [Google Scholar]
- 4.Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141(7):2181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapasi A, Schneider JA. Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochim et Biophys Acta - Mol Basis Disease. 2016;1862(5):878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coomans EM, van Westen D, Pichet Binette A, Strandberg O, Spotorno N, Serrano GE et al. Interactions between vascular burden and amyloid-β pathology on trajectories of tau accumulation. Brain. 2023. [DOI] [PMC free article] [PubMed]
- 7.Cogswell PM, Lundt ES, Therneau TM, Mester CT, Wiste HJ, Graff-Radford J et al. Evidence against a temporal association between cerebrovascular disease and Alzheimer’s disease imaging biomarkers. Nature Communications 2023 14:1. 2023;14(1):1–12. [DOI] [PMC free article] [PubMed]
- 8.Bangen KJ, Nation DA, Delano-Wood L, Weissberger GH, Hansen LA, Galasko DR, et al. Aggregate effects of vascular risk factors on cerebrovascular changes in autopsy-confirmed Alzheimer’s disease. Alzheimer’s Dement. 2015;11(4):394–e4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman J, Sun HS, Feng ZP. Microvascular alterations in Alzheimer’s Disease. Front Cell Neurosci. 2021;14:618986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction—the disregarded partner of Alzheimer’s disease. Alzheimer’s Dement. 2019;15(1):158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC, Weiner MW, et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 2016. 2016;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019. 2019;25(2):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barisano G, Montagne A, Kisler K, Schneider JA, Wardlaw JM, Zlokovic BV. Blood-brain barrier link to human cognitive impairment and Alzheimer’s Disease. Nat Cardiovasc Res. 2022;1(2):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puig-Pijoan A, Jimenez-Balado J, Fernández-Lebrero A, García-Escobar G, Navalpotro-Gómez I, Contador J, et al. Risk of cognitive decline progression is associated to increased blood-brain-barrier permeability: a longitudinal study in a memory unit clinical cohort. Alzheimer’s & Dementia; 2023. [DOI] [PMC free article] [PubMed]
- 15.He JT, Zhao X, Xu L, Mao CY. Vascular risk factors and Alzheimer’s Disease: blood-brain barrier disruption, metabolic syndromes, and Molecular Links. J Alzheimer’s Disease. 2020;73(1):39–58. [DOI] [PubMed] [Google Scholar]
- 16.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimer’s Dementia: J Alzheimer’s Association. 2015;11(6):710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettigrew C, Soldan A, Wang J, Wang MC, Arthur K, Moghekar A, et al. Association of midlife vascular risk and AD biomarkers with subsequent cognitive decline. Neurology. 2020;95(23):e3093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabin JS, Schultz AP, Hedden T, Viswanathan A, Marshall GA, Kilpatrick E, et al. Interactive associations of vascular risk and β-Amyloid Burden with Cognitive decline in clinically normal Elderly individuals: findings from the Harvard Aging Brain Study. JAMA Neurol. 2018;75(9):1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302–9. [DOI] [PubMed] [Google Scholar]
- 20.Gottesman RF, Schneider ALC, Zhou Y, Coresh J, Green E, Gupta N, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spina S, La Joie R, Petersen C, Nolan AL, Cuevas D, Cosme C, et al. Comorbid neuropathological diagnoses in early versus late-onset Alzheimer’s disease. Brain. 2021;144(7):2186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YT, Therriault J, Servaes S, Tissot C, Rahmouni N, Macedo AC et al. Sex-specific modulation of amyloid-β on tau phosphorylation underlies faster tangle accumulation in females. Brain. 2023. [DOI] [PMC free article] [PubMed]
- 23.Johnston R, Jones K, Manley D. Confounding and collinearity in regression analysis: a cautionary tale and an alternative procedure, illustrated by studies of British voting behaviour. Qual Quantity. 2018;52(4):1957–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapkota S, Erickson K, Fletcher E, Tomaszewski Farias SE, Jin LW, DeCarli C. Vascular Risk Predicts Plasma Amyloid β 42/40 Through Cerebral Amyloid Burden in Apolipoprotein E ε4 Carriers. Stroke [Internet]. 2023 May 1 [cited 2023 Jun 9];54(5):1227–35. https://pubmed.ncbi.nlm.nih.gov/37021572/ [DOI] [PMC free article] [PubMed]
- 25.Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66(3):382–9. [DOI] [PubMed] [Google Scholar]
- 26.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Lowe VJ, Graff-Radford J, et al. Age, vascular health, and Alzheimer disease biomarkers in an elderly sample. Ann Neurol. 2017;82(5):706–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Early-onset Alzheimer’s disease is associated with greater pathologic burden. J Geriatr Psychiatr Neurol. 2007;20(1):29–33. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie K, Ritchie CW, Jaffe K, Skoog I, Scarmeas N. Is late-onset Alzheimer’s disease really a disease of midlife? Alzheimer’s & dementia: Translational Research &. Clin Interventions. 2015;1(2):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boivin-Proulx LA, Brouillette J, Dorais M, Perreault S. Association between cardiovascular diseases and dementia among various age groups: a population-based cohort study in older adults. Sci Rep. 2023;13(1). [DOI] [PMC free article] [PubMed]
- 31.Andjelkovic AV, Situ M, Citalan-Madrid AF, Stamatovic SM, Xiang J, Keep RF. Blood-brain barrier dysfunction in normal aging and neurodegeneration: mechanisms, impact, and treatments. Stroke. 2023;54(3):661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tijms BM, Gobom J, Reus L, Jansen I, Hong S, Dobricic V, et al. Pathophysiological subtypes of Alzheimer’s disease based on cerebrospinal fluid proteomics. Brain. 2020;143(12):3776–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricci F, Martorana A, Bonomi CG, Serafini C, Mercuri NB, Koch G, et al. Effect of vascular risk factors on blood-brain barrier and cerebrospinal fluid biomarkers along the Alzheimer’s Disease Continuum: a retrospective observational study. J Alzheimer’s Disease. 2023;Preprint(Preprint):1–9. [DOI] [PubMed] [Google Scholar]
- 34.Cheng YW, Chiu MJ, Chen YF, Cheng TW, Lai YM, Chen TF. The contribution of vascular risk factors in neurodegenerative disorders: from mild cognitive impairment to Alzheimer’s disease. Alzheimer’s Res Therapy. 2020;12(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Woo SY, Kim S, Jang H, Kim J, Kim J, et al. Differential effects of risk factors on the cognitive trajectory of early- and late-onset Alzheimer’s disease. Alzheimer’s Res Therapy. 2021;13(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo X, Hong H, Li K, Zeng Q, Wang S, Li Z, et al. Distinct cerebral small vessel disease impairment in early- and late-onset Alzheimer’s disease. Ann Clin Transl Neurol. 2023;10(8):1326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hessen E, Nordlund A, Stalhammar J, Eckerström M, Bjerke M, Eckerström C, et al. T-Tau is Associated with Objective memory decline over two years in persons seeking help for subjective cognitive decline: a report from the Gothenburg-Oslo MCI Study. J Alzheimer’s Disease. 2015;47(3):619–28. [DOI] [PubMed] [Google Scholar]
- 38.Bruno M, Bonomi CG, Ricci F, Di Donna MG, Mercuri NB, Koch G, et al. Blood–brain barrier permeability is associated with different neuroinflammatory profiles in Alzheimer’s disease. Eur J Neurol. 2024;31(1):e16095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali DG, Abner EL, Bahrani AA, El Khouli R, Gold BT, Jiang Y, et al. Amyloid-PET and White Matter Hyperintensities have Independent effects on Baseline cognitive function and synergistic effects on Longitudinal executive function. Brain Sci. 2023;13(2):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruthirakuhan M, Cogo-Moreira H, Swardfager W, Herrmann N, Lanctot KL, Black SE. Cardiovascular Risk factors and risk of Alzheimer Disease and Mortality: a latent class Approach. J Am Heart Association. 2023;12(1):25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.