Abstract
Background
Growing epidemiological evidence suggests an association between exposure to air pollutants and breast cancer. Yet, the underlying mechanisms remain poorly understood. This study explored the mediating role of thirteen metabolic health biomarkers in the relationship between exposure to three air pollutants, i.e. nitrogen dioxide (NO2), polychlorinated biphenyls 153 (PCB153), and benzo[a]pyrene (BaP), and breast cancer risk.
Methods
We used data from a nested case–control study within the French national prospective E3N-Generations cohort, involving 523 breast cancer cases and 523 matched controls. The four-way decomposition mediation of total effects for thirteen biomarkers was applied to estimate interaction and mediation effects (controlled direct, reference interaction, mediated interaction, and pure indirect effects).
Results
The analyses indicated a significant increase in breast cancer risk associated with BaP exposure (odds ratio (OR)Q4 vs Q1 = 2.32, 95% confidence intervals (CI): 1.00–5.37). PCB153 exposure showed a positive association only in the third quartile (ORQ3 vs Q1 = 2.25, CI 1.13–4.57), but it appeared to be non-significant in the highest quartile (ORQ4 vs Q1 = 2.07, CI 0.93–4.61). No association was observed between NO2 exposure and breast cancer risk. Estradiol was associated with an increased risk of breast cancer (OR per one standard deviation (SD) increment = 1.22, CI 1.05–1.42), while thyroid-stimulating hormone was inversely related to breast cancer risk (OR per 1SD increase = 0.87, CI 0.75–1.00). We observed a suggestive mediated effect of the association between the three pollutants and breast cancer risk, through albumin, high-density lipoproteins cholesterol, low-density lipoprotein cholesterol, parathormone, and estradiol.
Conclusion
Although limited by a lack of statistical power, this study provides relevant insights into the potential mediating role of certain biomarkers in the association between air pollutant exposure and breast cancer risk, highlighting the need for further in-depth studies in large populations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01913-7.
Keywords: Breast cancer, Air pollutants, Biomarkers, Mediation, Interaction
Background
Outdoor air pollution, a complex mixture of atmospheric pollutants including gases, particles, metals, and organic compounds, is a major contributor to global mortality [1].The International Agency for Research on Cancer (IARC) classified outdoor air pollution as a whole as carcinogenic in humans [2]. Among these, nitrogen dioxide (NO2) is a common pollutant that has been linked to various adverse health effects, including breast cancer development [3, 4]. NO2 is primarily emitted from the combustion of fossil fuels (heating, power generation) and motor vehicles [5]. Polychlorinated biphenyls (PCB) and benzo[a]pyrene (BaP) are two endocrine-disrupting pollutants (EDP) that are associated with an increased incidence of numerous diseases, notably breast cancer risk [6–8]. These compounds are mainly emitted from industrial activities and biomass combustion [7, 9].
Recent epidemiological studies increasingly link air pollution to breast cancer, suggesting statistically significant relationships between certain air pollutants and an increased risk of breast cancer, though the findings are not entirely inconsistent [10–13]. NO2 has been associated with a higher risk of breast cancer in case-control studies [14, 15]. A recent meta-analysis further supported this association, indicating NO2 as a common marker of traffic-related air pollutants (TRAP) linked to breast cancer [16]. Similarly, elevated levels of BaP and polycyclic aromatic hydrocarbons (PAHs) have been associated with increased breast cancer risk [17, 18], and PCBs (including PCB153) have shown a positive association with breast cancer across several epidemiological studies, including meta-analyses [7, 19, 20].
Although not fully elucidated, several biological mechanisms that might explain how these pollutants are involved in the development of breast cancer have been proposed. EDP can bind to estrogen receptors [21] and the aryl hydrocarbon receptor [22, 23], activating pathways involved in the carcinogenesis. These EDP increase levels of endogenous hormone levels [24], particularly estrogen and progesterone, which are directly linked to breast cancer [25]. Sex Hormone-Binding Globulin (SHBG) also plays a crucial role in the pathophysiology of breast cancer, primarily by regulating circulating estradiol [26]. Consequently, a decrease in SHBG levels is associated with a higher risk of breast cancer development. Additionally, androgens, such as testosterone, significantly influence on breast cancer [27]. Moreover, exposure to the three air pollutants (NO2, BaP, and PCB153) can lead to a number of changes and perturbation as hallmarks in cancer development [28], including chronic inflammations through increase in blood levels of pro-inflammatory factors [29] and C-reactive protein (CRP) [30], and disturbances in lipid metabolism, such as elevated cholesterol levels [31, 32].
Yet, the precise roles of these biomarkers, considering their interacting and mediating effects in the associations between the air pollutants of interest and breast cancer, remain unclear. A mediation approach, which considers both mediation and interaction, is a valuable tool for better understanding the underlying mechanisms and unravelling the different pathways of the association of air pollutants with breast cancer. Mediation analysis is generally applied to evaluate to what extent the effect of an exposure is explained or not, by a set of hypothetical mediators. In recent years, integrating causal inference approaches has significantly advanced mediation analysis, resulting in more robust and generalizable methods for understanding direct and indirect effects [33].
The objective of the present study was, therefore, to explore the mediating role of various biomarkers of metabolic health in the relationship between three air pollutants (NO2, BaP and PCB153) and risk of breast cancer using a four-way decomposition mediation analysis [34].
Methods
Study population
The present study was conducted using a sub-sample of 523 breast cancer cases and 523 matched controls from the XENAIR study [35], for whom measurements of biomarkers were available. This nested case-control study within the national E3N-Generations cohort, included 5,222 cases of invasive breast cancer and 5,222 matched controls followed from 1990 (at baseline) to 2011 [17, 20]. As described in our previous studies, controls were randomly selected from women who were free of breast cancer, based on incidence density sampling and matched to controls according to age, date, menopausal status, residential area, and blood sample [35]. The flowchart of study participants selection is provided in the Supplementary Fig. 1.
The E3N-Generations prospective study, a continuing French cohort study, was established as an extension of the E3N cohort of women (Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale), which includes the E3N women’s children, their fathers and, in the future, their grandchildren.
The E3N-cohort Generations 1 was started in 1990 to investigate the key risk factors for cancer and chronic diseases among women [36]. At recruitment (1990-1991), a total of 98,995 French women aged 40 to 65 years old, and insured with MGEN (a national health insurance scheme covering primarily teachers) were recruited. Participants completed self-administered questionnaires that collect data on socio-demographic characteristics, lifestyle, reproductive factors, anthropometry, past medical history, and familial history of cancer. The addresses of the cohort participants were collected at baseline and at each of the thirteen follow-ups questionnaires. Self-reported cases were validated through the retrieval of medical records from treating physicians, with pathological confirmation received for 93% of cases. The study was approved by the French National Commission for Data Protection and Privacy (CNIL), and informed consent was obtained from each participant.
Pollutant exposure assessment
As previously described [14], long-term exposure levels of the three pollutants (NO2, BaP and PCB153) were estimated at the subjects’ residential addresses using two models in accordance of the existence of measurement and emission data of the pollutants of interest for the study period (1990-2011).
BaP and PCB153 were estimated using is a chemistry-transport model “CHIMERE”. This model, with a spatial resolution of 0.125° × 0.0625° (around 7 × 7 km) simulates pollutant transport from local to continental scales, by utilizing data (e.g. emission, meteorological fields, and boundary conditions) as inputs and runs a set of equations reflecting the physicochemical steps associated with the evolution of concentrations [37]. CHIMERE takes into account main particles that are directly emitted and whether they are anthropogenic or natural, and models the concentrations levels of each particle with aerodynamic diameters varying from a few nanometers to 10 μm [37]. NO2 levels were evaluated using a land use regression (LUR, 50 × 50 m) model, a widely used approach to model and to predict spatial variations in air pollution concentrations [38, 39]. The model employs proximity measures like circular buffers of different sizes, to capture geographical features that explain variability in monitored concentrations at specific locations (i.e. monitoring sites or addresses) [40, 41]. In the present study, a LUR model (50 × 50 m) was developed using the average annual NO2 data for the period of 2010 to 2012 [14]. This “baseline” model further incorporated inputs from COPERNIC (a chemical transport model providing NO2 background concentrations across France) and localised variables related to road traffic and land use, available throughout the country[41, 42]. The model underwent validation through comparisons with measurements across France using a hold-out validation approach with independent monitoring sites. The LUR model was retrospectively extrapolated to 1990 using annual local trends derived from the CHIMERE model [43].
For each woman, annual mean concentration of NO2, BaP and PCB153 were evaluated at their geocoded residential addresses for each year from 1990 to 2011. The average of these annual mean concentration for each pollutant were then calculated for each woman from the year they entered into the cohort until their index date (which corresponds to the date of breast cancer diagnosis for cases and date of selection for controls).
Metabolic health biomarker assays
Biomarker levels were measured from the cohort blood samples collected between 1995 and 1998 [36]. The biomarkers investigated in this study were chosen based on their previously established individual associations with breast cancer risk and air pollutants [21, 22, 24, 27, 29, 30, 44]. These included pre-diagnostic circulating levels of albumin (g/L), c-reactive protein (CRP) (mg/L), triglycerides (mmol/L), cholesterol (mmol/L), high-density lipoproteins cholesterol (HDL) (mmol/L), low-density lipoproteins cholesterol (LDL) (mmol/L), parathormone (PTH) (pg/mL), thyroid-stimulating hormone (mlU/L), prolactin (mIU/L), estradiol (pmol/L), testosterone (nmol/L), SHBG (nmol/L) and progesterone (nmol/L).
Albumin and CRP were quantified by bromocresol green (BCG) analysis and immunoturbidimetric-high sensitivity analysis, respectively, using a Hitachi 911 analyzer (Roche Diagnostics, US) [45]. Using a modular analyzer (Roche Diagnostics, US), triglycerides, cholesterol, HDL, and LDL were quantified employing enzyme immune-inhibition analysis [45]. PTH, thyroid-stimulating hormone, prolactin, estradiol, testosterone, SHBG and progesterone were quantified by electrochemiluminescence immunoassay (ECLIA) method using the Elecsys analyzer (Roche Diagnostics, US) [45].
Statistical analysis
The main characteristics of the population and biomarker levels were described distinctly for cases and controls, using means, standard deviations (SDs), percentiles, minimum and maximum values for continuous variables, and counts and percentages for qualitative variables. Pearson correlation analyses were performed to check correlations between biomarkers. The linearity of the pollutant-cancer and mediator-cancer associations was verified using restricted cubic splines with four degrees of freedom [46]. Conditional logistic regressions were employed to calculate odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) for the associations between exposure to each pollutant and the risk of breast cancer. We modelled the pollutants as continuous variables (one SD increase) and as categorical variables (quartiles). Linear regression analyses were used to estimate the associations between each pollutant level and each biomarker of metabolic health with adjustments for confounders. The effect of each biomarker on breast cancer (per one SD increase) was estimated using conditional logistic regression analyses.
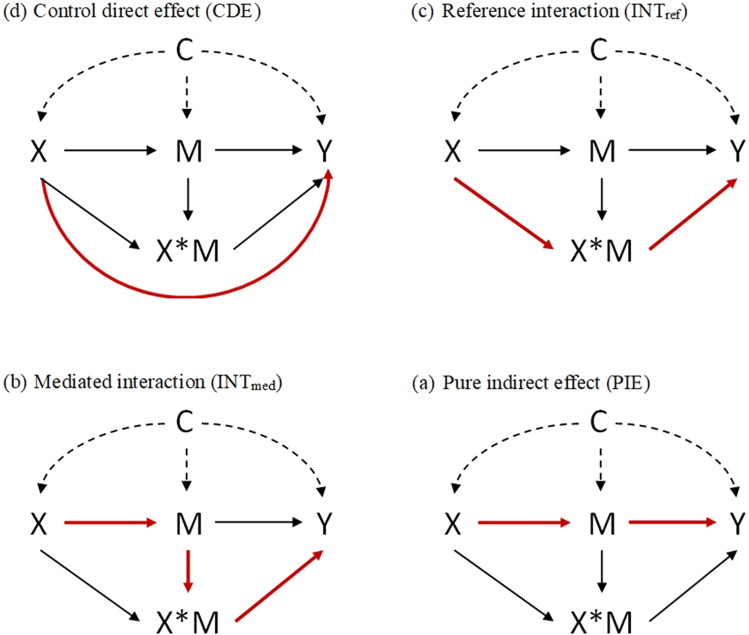
A four-way decomposition mediation analysis was fitted to assess whether the associations between atmospheric pollutants and breast cancer risk were mediated by selected biomarkers [34]. Data on n individuals were observed as independent and identically distributed (C, X, M, Y), with Y being the binary outcome of interest, X the exposure, M a continuous mediator variable measured after X but before Y, and C representing pre-exposure confounders of the effects of (X, M) on Y. (Figure 1). The four-way decomposition analysis assumes that after adjusting for the potential confounders, there is no unobserved confounding that affects the relationship between exposure and outcome, and between exposure and mediator, and there are no confounders of the mediator-outcome relationship that may be affected by the exposure (post-exposure confounders) [47]. This approach allows us to determine the controlled direct effect (CDE), the reference interaction effect (INTref), the mediated interaction effect (INTmed) and the Pure Indirect Effect (PIE) (Fig. 1), assuming the following regression models:
| 1 |
Fig. 1.
Causal diagram with the interaction representing a 4-way decomposition X: the exposure, M: the mediator, X × M: the interaction between the exposure and the mediator, Y: the outcome, C: a set of confounders. Red line shows each effect
And
| 2 |
VanderWeele and Vansteelandt derived expressions for the CDE and the PIE all on the risk ratio scale. The total effect (TE), CDE, and PIE were given by:
| 3 |
The control direct effect is given by:
| 4 |
The reference interaction is given by:
| 5 |
The mediated interaction is given by:
| 6 |
The pure indirect effect is given by:
| 7 |
In this study, the CDE corresponds to the effect of the pollutant on breast cancer risk without mediation by the biomarker and without interaction between the pollutant and the biomarker. The INTmed corresponds to the effect of the pollutant on the breast cancer risk due to both the mediation of the biomarker and the interaction between the pollutant and the biomarker. The INTref corresponds to the effect of the pollutant on the breast cancer risk due solely to the interaction between the pollutant and the biomarker. The PIE corresponds to the effect of the pollutant on the breast cancer risk due solely to the mediation by the biomarker.
The sum of these four effects (i.e. CDE, INTref, INTmed, PIE) equals the total effect (TE) of the pollutant on breast cancer risk. The proportion of each of the four effects is calculated relative to the TE, thus, their sum equals 1. Of note, in some situations, negative proportions and proportions exceeding 100% may be observed. A negative proportion indicates that the indirect effect is opposite to the TE. In this case, the proportions of other effects may exceed 100%. This scenario typically arises when the associations between exposure and biomarker, and between biomarker and outcome are in opposite directions. Mediation analyses were conducted for biomarkers that have previously been demonstrated to have significant associations with breast cancer. Mediation analysis considered causal effects for changes in pollutant levels from the 25th to the 75th percentile and each mediator fixed at its median level. To test the robustness of our results, we further performed sensitivity mediation analyses, using average exposure from the inclusion to the date of blood collection. All multivariable models were adjusted for confounding factors identified by a direct acyclic graph (Supplementary Fig. 2), including body mass index, menopausal hormone replacement therapy use, urban/rural status at birth, urban/rural status at inclusion, alcohol drinking, breastfeeding, mammography before inclusion, oral contraceptive use, age at full-term pregnancy and parity, smoking status, total physical activity.
Analyses were conducted using R software version 4.2.3. Mediation analyses were conducted using STATA 14.
Results
Study population
Descriptive characteristics of the study population are shown in Supplementary Table 1, comprising 523 breast cancer cases and 523 matched controls. The mean age (± SD) at inclusion was 49.9 (± 6.3) years. Alcohol consumption was slightly lower in cases as compared to controls, with 52.0% of cases and 56.2% of controls reporting drinking more than 6.7 g/day. Education levels were generally high, with over 85% of participants having at least a 1- to 2-year university degree, with no difference between cases and controls. With the exception of breastfeeding, slightly more common in controls than in cases (62.5% vs. 59.1%), all other reproductive factors (age at first menstruation, use of oral contraceptives, and the number of children and age at first pregnancy) were overall similar between cases and controls. The distribution of body mass index, physical activity levels, and smoking status were also comparable between cases and controls.
Biomarker levels and annual mean concentration levels of pollutant exposure (NO2, BaP and PCB153) between cases and controls are shown in Supplementary Table 2 and Supplementary Table 3. There was no strong difference in the mean levels of all biomarkers between cases and controls. The average (±SD) of annual mean concentrations was 37.08 (±16.94) μg/m3, 0.21 (±0.12) ng/m3 and 11.06 (±4.04) ng/m3, for NO2, BaP and PCB153, respectively. The averages of annual mean concentrations of the three air pollutants were similar between cases and controls.
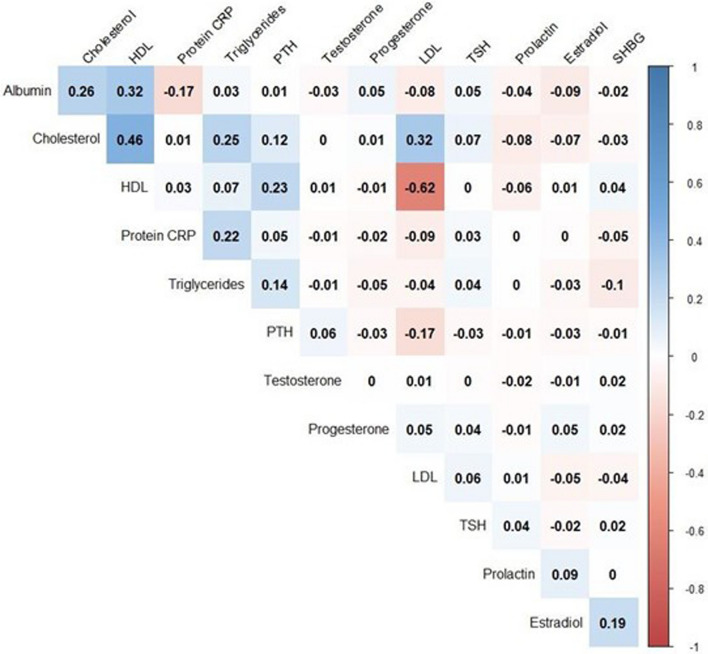
Figure 2 presents Pearson correlation coefficients between biomarkers. Overall, with the exception of between HDL and LDL cholesterol (coef. = − 0.62), there were no strong correlations between biomarkers.
Fig. 2.
Pearson correlations between biomarkers. HDL: High-density lipoprotein cholesterol, LDL: Light-density lipoprotein cholesterol, TSH: Thyroid-stimulating hormone, SHGB: Sex Hormone-Binding Globulin, PTH: Parathormone, Protein CRP: C-reactive protein
Pollutant exposure and breast cancer risk
Table 1 presents the results of multivariable-adjusted associations between the three pollutants of interest and breast cancer risk. Overall, in continuous analyses, each SD increment in exposure to BaP (0.126 ng/m3) and NO2 (17.0 μg/m3) was associated with ORs of 1.04 (CI 0.81–1.34) and 1.04 (CI 0.81–1.34) of breast cancer risk, respectively. In contrast, exposure to PCB153 showed a borderline positive association, with an OR of 1.30 (CI 0.98–1.73) for each 1 SD increment in PCB153 levels (3.92 ng/m3).
Table 1.
Associations between each pollutant and breast cancer risk
| NO2 | BaP | PCB153 | ||
|---|---|---|---|---|
| Cases/Controls | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Continuous (For each 1SD increase) | 523/523 | 1.04 (0.81, 1.34) | 1.04 (0.81, 1.34) | 1.30 (0.98, 1.73) |
| Quartiles | ||||
| I | 121/131 | 1 (ref) | 1 (ref) | 1 (ref) |
| II | 134/131 | 1.11 (0.74, 1.66) | 1.58 (0.96, 2.61) | 1.19 (0.68, 2.06) |
| III | 132/130 | 1.14 (0.71, 1.83) | 2.03 (1.05, 3.93) | 2.25 (1.13, 4.57) |
| IV | 136/131 | 1.18 (0.66, 2.11) | 2.32 (1.00, 5.37) | 2.07 (0.93, 4.61) |
Conditional logistic regression models were used for estimating ORs and 95%CI, adjusted for body mass index, menopausal hormone replacement therapy uses, urban/rural status at birth, urban/rural status at inclusion, alcohol drinking, breastfeeding, mammography before inclusion, oral contraceptive use, age at full-term pregnancy and parity, smoking status, total physical activity.
The ORs (95% CI) correspond to an increment of 1 SD level in controls, NO2: 17.0 μg/m3, PCB153: 3.92 ng/m3, BaP: 0.126 ng/m3
Quartiles’ cut-offs for NO2 based on the distribution among controls: ≤ 24.2, ≤ 32.4, ≤ 46.5 µg/m3
Quartiles’ cut-offs for PCB153 based on the distribution among controls: ≤ 8.13, ≤ 10.12, ≤ 12.91 ng/m3
Quartiles’ cut-offs for BaP based on the distribution among controls: ≤ 0.133, ≤ 0.179, ≤ 0.240 ng/m3
SD Standard deviation, OR odds ratio, 95% CI 95% confidence intervals, NO2 nitrogen dioxide, BaP: benzo[a]pyrene, PCB153: polychlorinated biphenyls
In the analysis by quartiles, an increase in breast cancer risk was shown with increasing quartiles of BaP exposure (ORQ3 vs Q1 = 2.03, CI 1.05–3.93; and ORQ4 vs Q1 = 2.32, CI 1.00–5.37). Similarly, an increased risk of breast cancer associated with PCB153 exposure was observed for the third quartile (ORQ3 vs Q1 = 2.25, CI 1.13–4.57). However, the association became statistically non-significant in the highest exposed quartile (ORQ4 vs Q1 = 2.07, CI 0.93–4.61).
Biomarkers and breast cancer risk
Table 2 shows the multivariable-adjusted ORs of the relationship between biomarkers of interest and breast cancer risk. Thyroid-stimulating hormone was inversely associated with breast cancer risk (OR = 0.87, CI 0.75–1.00, for each 1 SD increment), while estradiol was related to an increased risk of breast cancer (OR = 1.22, CI 1.05–1.42, for each 1 SD increment).
Table 2.
Associations between each biomarker and breast cancer risk
| Biomarkers | Cases/Controls | OR (CI 95%) | P value |
|---|---|---|---|
| Albumin | 478 / 478 | 1.05 (0.90, 1.23) | 0.53 |
| Protein C-reactive | 481 / 481 | 1.05 (0.90, 1.23) | 0.52 |
| Triglycerides | 430 / 430 | 0.96 (0.83, 1.11) | 0.56 |
| Cholesterol | 450 / 450 | 0.94 (0.82, 1.09) | 0.41 |
| HDL cholesterol | 420 / 420 | 0.95 (0.76, 1.18) | 0.62 |
| LDL cholesterol | 423 / 423 | 0.89 (0.72, 1.10) | 0.29 |
| Parathormone | 470 / 470 | 0.92 (0.80, 1.06) | 0.23 |
| Thyroid-stimulating hormone | 480 / 480 | 0.87 (0.75, 1.00) | 0.04 |
| Prolactin | 484 / 484 | 1.01 (0.87, 1.16) | 0.93 |
| Estradiol | 479 / 479 | 1.22 (1.05, 1.42) | 0.01 |
| Testosterone | 472 / 472 | 1.03 (0.89, 1.20) | 0.68 |
| SHBG | 440 / 440 | 0.97 (0.84, 1.11) | 0.63 |
| Progesterone | 481 / 481 | 1.07 (0.93, 1.22) | 0.35 |
Conditional logistic regression models were used for estimating ORs and 95%CI, for each 1SD biomarker increment, adjusted for body mass index, menopausal hormone replacement therapy uses, urban/rural status at birth, urban/rural status at inclusion, alcohol drinking, breastfeeding, mammography before inclusion, oral contraceptive use, age at full-term pregnancy and parity, smoking status, total physical activity.
OR: odds ratio; 95% CI: 95% confidence intervals, HDL cholesterol: High-density lipoprotein cholesterol, LDL cholesterol: Light-density lipoprotein cholesterol, SD: standard deviation, SHGB: Sex Hormone-binding globulin
P value was obtained based on Wald test.
Pollutants and biomarkers associations
Results for the associations between pollutants (NO2, BaP and PCB153) and biomarkers are presented in Table 3. There was evidence of positive associations between albumin and each of the three pollutants. HDL cholesterol and LDL cholesterol were respectively, positively and inversely related to BaP. PTH was inversely associated with PCB153 and NO2. CRP and estradiol showed, respectively, inverse and positive associations with BaP.
Table 3.
Beta coefficients and P values for associations between pollutants and biomarkers, a nested case–control study within the E3N-Generations cohort, 1990–2011
| BaP | PCB153 | NO2 | |||||
|---|---|---|---|---|---|---|---|
| Biomarkers | n | Beta | P value | Beta | P value | Beta | P value |
| Albumin | 1001 | 0.088 | 0.007 | 0.094 | 0.003 | 0.071 | 0.048 |
| Protein C-reactive | 1003 | − 0.061 | 0.057 | − 0.034 | 0.264 | − 0.045 | 0.181 |
| Triglycerides | 945 | − 0.022 | 0.545 | − 0.038 | 0.250 | − 0.045 | 0.227 |
| Cholesterol | 972 | 0.009 | 0.806 | − 0.011 | 0.751 | − 0.017 | 0.649 |
| HDL cholesterol | 936 | 0.104 | 0.004 | 0.043 | 0.194 | − 0.048 | 0.198 |
| LDL cholesterol | 937 | − 0.092 | 0.012 | − 0.060 | 0.079 | 0.039 | 0.305 |
| Parathormone | 992 | − 0.053 | 0.131 | − 0.116 | 0.001 | − 0.155 | 0.000 |
| TSH | 1003 | − 0.018 | 0.582 | 0.019 | 0.551 | 0.020 | 0.577 |
| Prolactin | 1007 | 0.035 | 0.282 | 0.045 | 0.154 | 0.043 | 0.223 |
| Estradiol | 1002 | 0.063 | 0.054 | 0.052 | 0.102 | 0.050 | 0.161 |
| Testosterone | 993 | − 0.048 | 0.148 | − 0.010 | 0.751 | − 0.026 | 0.475 |
| SHBG | 960 | 0.036 | 0.291 | − 0.006 | 0.848 | − 0.004 | 0.920 |
| Progesterone | 1004 | 0.031 | 0.374 | 0.029 | 0.391 | 0.013 | 0.736 |
Linear regression models were used for estimating beta value, per 1 SD biomarker and pollutant increment,adjusted for body mass index, menopausal hormone replacement therapy uses, urban/rural status at birth, urban/rural status at inclusion, alcohol drinking, breastfeeding, and mammography before inclusion, oral contraceptive use, age at full-term pregnancy and parity, smoking status, total physical activity.
P value was obtained based on student test.
SD: standard deviation, HDL cholesterol: High-density lipoprotein cholesterol, LDL cholesterol: Light-density lipoprotein cholesterol, TSH: Thyroid-stimulating hormone, SHGB: Sex Hormone-Binding Globulin, NO2: nitrogen dioxide, BaP: benzo[a]pyrene, PCB153: polychlorinated biphenyls
Four-way decomposition mediation analysis
Table 4 presents the results of the causal mediation analysis with the four-way decomposition (i.e. control direct effect (CDE), reference interaction (INTref), mediated interaction (INTmed), pure indirect effect (PIE)) of the effect of NO2 on breast cancer risk mediated individually by different biomarkers. This mediation analysis considered the causal effects of changes in pollutant levels from the 25th to the 75th percentile, with each mediator set at its median value. The CDEs (the effect in the absence of mediation or interaction) of NO2 on breast cancer risk were very high, ranging from 80.6 to 121.1%, when holding estradiol and testosterone at their median levels, respectively. The overall mediated effects (sum of PIE and mediation interaction) through estradiol and PTH were suggestively positive, at 18.8 and 13.6% respectively (Table 4).
Table 4.
Four-way decomposition of each mediator of the associations between NO2 and breast cancer risk
| Mediation | Effect | Estimate (CI 95%) | P value | Proportion | P value |
|---|---|---|---|---|---|
| Albumin | TE | 0.1178 (− 0.2666, 0.5022) | 0.548 | ||
| CDE | 0.1126 (− 0.2724, 0.4976) | 0.566 | 95.6% | < 0.001 | |
| INTref | 0.0031 (− 0.0260, 0.0323) | 0.833 | 2.7% | 0.842 | |
| INTmed | − 0.0014 (− 0.0136, 0.0109) | 0.829 | − 1.1% | 0.838 | |
| PIE | 0.0034 (− 0.0098, 0.0167) | 0.612 | 2.9% | 0.695 | |
| O_M | 1.8% | 0.766 | |||
| CRP | TE | 0.1206 (− 0.2608, 0.5020) | 0.536 | ||
| CDE | 0.1335 (− 0.2459, 0.5130) | 0.490 | 110.7% | < 0.001 | |
| INTref | − 0.0127 (− 0.0708, 0.0455) | 0.670 | − 10.5% | 0.738 | |
| INTmed | 0.0004 (− 0.0052, 0.0060) | 0.882 | 0.4% | 0.887 | |
| PIE | − 0.0007 (− 0.0099, 0.0084) | 0.877 | − 0.6% | 0.881 | |
| O_M | − 0.2% | 0.893 | |||
| Triglycerides | TE | 0.1260 (− 0.2776, 0.5297) | 0.541 | ||
| CDE | 0.1254 (− 0.2815, 0.5324) | 0.546 | 99.5% | < 0.001 | |
| INTref | − 0.0026 (− 0.0339, 0.0287) | 0.873 | − 2.0% | 0.873 | |
| INTmed | 0.0009 (− 0.0104, 0.0122) | 0.879 | 0.7% | 0.879 | |
| PIE | 0.0023 (− 0.0099, 0.0145) | 0.713 | 1.8% | 0.749 | |
| O_M | 2.5% | 0.681 | |||
| Cholesterol | TE | 0.0836 (− 0.2955, 0.4628) | 0.665 | ||
| CDE | 0.0828 (− 0.2969, 0.4624) | 0.669 | 99.0% | < 0.001 | |
| INTref | − 0.0011 (− 0.0104, 0.0083) | 0.824 | − 1.3% | 0.835 | |
| INTmed | 0.0012 (− 0.0066, 0.0090) | 0.768 | 1.4% | 0.796 | |
| PIE | 0.0008 (− 0.0051, 0.0066) | 0.802 | 0.9% | 0.829 | |
| O_M | 2.3% | 0.787 | |||
| HDL cholesterol | TE | 0.1667 (− 0.2575, 0.5908) | 0.441 | ||
| CDE | 0.1692 (− 0.2587, 0.5971) | 0.438 | 101.5% | < 0.001 | |
| INTref | − 0.0073 (− 0.0437, 0.0291) | 0.695 | − 4.4% | 0.715 | |
| INTmed | 0.0030 (− 0.0134, 0.0195) | 0.720 | 1.8% | 0.736 | |
| PIE | 0.0017 (− 0.0145, 0.0179) | 0.834 | 1.0% | 0.840 | |
| O_M | 2.8% | 0.680 | |||
| LDL cholesterol | TE | 0.2178 (− 0.2414, 0.6770) | 0.353 | ||
| CDE | 0.2164 (− 0.2313, 0.6641) | 0.343 | 99.4% | < 0.001 | |
| INTref | 0.0150 (− 0.0381, 0.0681) | 0.579 | 6.9% | 0.552 | |
| INTmed | − 0.0073 (− 0.0325, 0.0180) | 0.573 | − 3.3% | 0.554 | |
| PIE | − 0.0064 (− 0.0278, 0.0151) | 0.561 | − 2.9% | 0.629 | |
| O_M | − 6.2% | 0.495 | |||
| Parathormone | TE | 0.1378 (− 0.2679, 0.5436) | 0.506 | ||
| CDE | 0.1247 (− 0.2761, 0.5254) | 0.542 | 90.5% | < 0.001 | |
| INTref | − 0.0055 (− 0.0234, 0.0123) | 0.544 | − 4.0% | 0.626 | |
| INTmed | 0.0103 (− 0.0195, 0.0402) | 0.497 | 7.5% | 0.600 | |
| PIE | 0.0083 (− 0.0179, 0.0346) | 0.533 | 6.1% | 0.622 | |
| O_M | 13.6% | 0.510 | |||
| TSH | TE | 0.0289 (− 0.3257, 0.3835) | 0.873 | ||
| CDE | 0.0310 (− 0.3270, 0.3889) | 0.865 | 107.3% | 0.168 | |
| INTref | − 0.0004 (− 0.0295, 0.0286) | 0.978 | − 1.4% | 0.978 | |
| INTmed | 0.0033 (− 0.0176, 0.0241) | 0.760 | 11.3% | 0.893 | |
| PIE | − 0.0050 (− 0.0363, 0.0264) | 0.757 | − 17.1% | 0.890 | |
| O_M | − 5.9% | 0.888 | |||
| Prolactin | TE | 0.1544 (− 0.2442, 0.5531) | 0.448 | ||
| CDE | 0.1568 (− 0.2435, 0.5570) | 0.443 | 101.5% | < 0.001 | |
| INTref | 0.0025 (− 0.0148, 0.0198) | 0.777 | 1.6% | 0.786 | |
| INTmed | − 0.0115 (− 0.0381, 0.0150) | 0.394 | − 7.5% | 0.537 | |
| PIE | 0.0067 (− 0.0153, 0.0288) | 0.549 | 4.4% | 0.624 | |
| O_M | − 3.1% | 0.712 | |||
| Estradiol | TE | 0.0935 (− 0.2865, 0.4734) | 0.630 | ||
| CDE | 0.0776 (− 0.3007, 0.4559) | 0.688 | 83.0% | 0.027 | |
| INTref | − 0.0017 (− 0.0124, 0.0089) | 0.751 | − 1.8% | 0.700 | |
| INTmed | − 0.0035 (− 0.0232, 0.0162) | 0.730 | − 3.7% | 0.807 | |
| PIE | 0.0210 (− 0.0133, 0.0554) | 0.230 | 22.5% | 0.647 | |
| O_M | 18.8% | 0.619 | |||
| Testosterone | TE | 0.1167 (− 0.2850, 0.5184) | 0.569 | ||
| CDE | 0.1414 (− 0.2610, 0.5437) | 0.491 | 121.1% | 0.004 | |
| INTref | − 0.0195 (− 0.0708, 0.0317) | 0.455 | − 16.7% | 0.666 | |
| INTmed | − 0.0119 (− 0.0495, 0.0258) | 0.536 | − 10.2% | 0.659 | |
| PIE | 0.0068 (− 0.0153, 0.0289) | 0.548 | 5.8% | 0.680 | |
| O_M | − 4.4% | 0.659 | |||
| SHBG | TE | 0.1452 (− 0.2664, 0.5569) | 0.489 | ||
| CDE | 0.1446 (− 0.2669, 0.5561) | 0.491 | 99.6% | < 0.001 | |
| INTref | 0.0005 (− 0.0082, 0.0092) | 0.912 | 0.3% | 0.912 | |
| INTmed | − 0.0005 (− 0.0069, 0.0058) | 0.866 | − 0.4% | 0.869 | |
| PIE | 0.0007 (− 0.0069, 0.0082) | 0.860 | 0.5% | 0.864 | |
| O_M | 0.1% | 0.926 | |||
| Progesterone | TE | 0.1063 (− 0.2766, 0.4891) | 0.586 | ||
| CDE | 0.0856 (− 0.2986, 0.4699) | 0.662 | 80.6% | 0.103 | |
| INTref | 0.0226 (− 0.0569, 0.1021) | 0.578 | 21.3% | 0.684 | |
| INTmed | − 0.0011 (− 0.0094, 0.0072) | 0.788 | − 1.1% | 0.808 | |
| PIE | − 0.0008 (− 0.0071, 0.0055) | 0.801 | − 0.8% | 0.818 | |
| O_M | − 1.8% | 0.794 |
TE: total effect (total excess relative risk), CDE: excess relative risk due to controlled direct effect, INTref: excess relative risk due to reference interaction, INTmed: excess relative risk due to mediated interaction, PIE: excess relative risk due to pure indirect effect, O_M: overall mediated
CRP: C-reactive protein, HDL: High-density lipoprotein cholesterol, LDL: Light-density lipoprotein cholesterol, SHBG: Sex Hormone-Binding Globulin, TSH: Thyroid-stimulating hormone, SHGB: Sex Hormone-binding globulin
Output of mediation analysis with causal effects estimated for a change in pollutant levels from the 25th to the 75th percentile
Adjusted for body mass index, menopausal hormone replacement therapy uses, urban/rural status at birth, urban/rural status at inclusion, alcohol drinking, breastfeeding, mammography before inclusion, oral contraceptive use, age at full-term pregnancy and parity, smoking status, total physical activity
Controlled direct effects are computed fixing the mediators at their median levels
For PCB153, the proportions of CDE were elevated, ranging from 95.2 to 106.0%, when holding estradiol and CRP at their median levels, respectively (Table 5). Although not statistically significant, small proportions of the association between PCB153 and breast cancer were mediated by estradiol and PTH, with the overall mediated effect being 6.4 and 4.1%, respectively (Table 5).
Table 5.
Four-way decomposition of each mediator of the associations between PCB153 and breast cancer risk
| Mediation | Effect | Estimate (CI 95%) | P value | Proportion | P value |
|---|---|---|---|---|---|
| Albumin | TE | 0.4563 (− 0.0831, 0.9957) | 0.097 | ||
| CDE | 0.4369 (− 0.1007, 0.9746) | 0.111 | 95.7% | < 0.001 | |
| INTref | 0.0204 (− 0.0320, 0.0728) | 0.445 | 4.5% | 0.475 | |
| INTmed | − 0.0139 (− 0.0443, 0.0164) | 0.368 | − 3.1% | 0.413 | |
| PIE | 0.0129 (− 0.0119, 0.0377) | 0.306 | 2.8% | 0.375 | |
| O_M | − 0.2% | 0.946 | |||
| CRP | TE | 0.4211 (− 0.1049, 0.9471) | 0.117 | ||
| CDE | 0.4462 (− 0.0882, 0.9806) | 0.102 | 106.0% | < 0.001 | |
| INTref | − 0.0251 (− 0.0821, 0.0319) | 0.388 | − 6.0% | 0.435 | |
| INTmed | − 0.0021 (− 0.0131, 0.0090) | 0.711 | − 0.5% | 0.715 | |
| PIE | 0.0021 (− 0.0088, 0.0130) | 0.705 | 0.5% | 0.714 | |
| O_M | 0.0% | 0.994 | |||
| Triglycerides | TE | 0.4763 (− 0.0883, 1.0410) | 0.098 | ||
| CDE | 0.4948 (− 0.0772, 1.0667) | 0.090 | 103.9% | < 0.001 | |
| INTref | − 0.0184 (− 0.0507, 0.0139) | 0.265 | − 3.9% | 0.305 | |
| INTmed | − 0.0001 (− 0.0151, 0.0150) | 0.997 | 0.0% | 0.997 | |
| PIE | 0.0001 (− 0.0018, 0.0018) | 0.997 | 0.0% | 0.997 | |
| O_M | 0.0% | 0.997 | |||
| Cholesterol | TE | 0.5117 (− 0.0634, 1.0868) | 0.081 | ||
| CDE | 0.5158 (− 0.0611, 1.0928) | 0.080 | 100.8% | < 0.001 | |
| INTref | − 0.0017 (− 0.0232, 0.0197) | 0.875 | − 0.3% | 0.875 | |
| INTmed | − 0.0027 (− 0.0184, 0.0129) | 0.730 | − 0.5% | 0.732 | |
| PIE | 0.0003 (− 0.0039, 0.0046) | 0.873 | 0.1% | 0.873 | |
| O_M | − 0.5% | 0.739 | |||
| HDL cholesterol | TE | 0.5204 (− 0.0624, 1.1032) | 0.080 | ||
| CDE | 0.5379 (− 0.0546, 1.1303) | 0.075 | 103.4% | < 0.001 | |
| INTref | − 0.0118 (− 0.0427, 0.0191) | 0.455 | − 2.3% | 0.455 | |
| INTmed | − 0.0066 (− 0.0268, 0.0136) | 0.523 | − 1.3% | 0.526 | |
| PIE | 0.0009 (− 0.0128, 0.0147) | 0.894 | 0.2% | 0.894 | |
| O_M | − 1.1% | 0.624 | |||
| LDL cholesterol | TE | 0.5723 (− 0.0436, 1.1882) | 0.069 | ||
| CDE | 0.5481 (− 0.0487, 1.1449) | 0.072 | 95.8% | < 0.001 | |
| INTref | 0.0121 (− 0.0355, 0.0597) | 0.618 | 2.1% | 0.604 | |
| INTmed | 0.0079 (− 0.0177, 0.0335) | 0.545 | 1.4% | 0.525 | |
| PIE | 0.0042 (− 0.0117, 0.0200) | 0.605 | 0.7% | 0.611 | |
| O_M | 2.1% | 0.497 | |||
| Parathormone | TE | 0.3785 (− 0.1522, 0.9092) | 0.162 | ||
| CDE | 0.3702 (− 0.1577, 0.8982) | 0.169 | 97.8% | < 0.001 | |
| INTref | − 0.0073 (− 0.0255, 0.0109) | 0.430 | − 1.9% | 0.456 | |
| INTmed | 0.0109 (− 0.0123, 0.0341) | 0.357 | 2.9% | 0.393 | |
| PIE | 0.0047 (− 0.0144, 0.0237) | 0.631 | 1.2% | 0.638 | |
| O_M | 4.1% | 0.332 | |||
| TSH | TE | 0.3490 (− 0.1430, 0.8410) | 0.164 | ||
| CDE | 0.3608 (− 0.1374, 0.8591) | 0.156 | 103.4% | < 0.001 | |
| INTref | − 0.0071 (− 0.0309, 0.0167) | 0.559 | − 2.0% | 0.565 | |
| INTmed | 0.0027 (− 0.0091, 0.0145) | 0.654 | 0.8% | 0.689 | |
| PIE | − 0.0075 (− 0.0354, 0.0205) | 0.600 | − 2.1% | 0.631 | |
| O_M | − 1.4% | 0.631 | |||
| Prolactin | TE | 0.4295 (− 0.0944, 0.9534) | 0.108 | ||
| CDE | 0.4299 (− 0.0946, 0.9543) | 0.108 | 100.1% | < 0.001 | |
| INTref | 0.0003 (− 0.0051, 0.0058) | 0.905 | 0.1% | 0.905 | |
| INTmed | − 0.0022 (− 0.0181, 0.0136) | 0.782 | − 0.5% | 0.784 | |
| PIE | 0.0015 (− 0.0133, 0.0163) | 0.841 | 0.4% | 0.842 | |
| O_M | − 0.2% | 0.940 | |||
| Estradiol | TE | 0.3752 (− 0.1330, 0.8835) | 0.148 | ||
| CDE | 0.3570 (− 0.1500, 0.8641) | 0.168 | 95.2% | < 0.001 | |
| INTref | − 0.0060 (− 0.0195, 0.0074) | 0.381 | − 1.6% | 0.248 | |
| INTmed | 0.0079 (− 0.0160, 0.0318) | 0.517 | 2.1% | 0.474 | |
| PIE | 0.0163 (− 0.0102, 0.0427) | 0.227 | 4.3% | 0.345 | |
| O_M | 6.4% | 0.266 | |||
| Testosterone | TE | 0.3552 (− 0.1657, 0.8762) | 0.181 | ||
| CDE | 0.3759 (− 0.1457, 0.8975) | 0.158 | 105.8% | < 0.001 | |
| INTref | − 0.0175 (− 0.0597, 0.0247) | 0.416 | − 4.9% | 0.502 | |
| INTmed | − 0.0075 (− 0.0344, 0.0194) | 0.584 | − 2.1% | 0.601 | |
| PIE | 0.0044 (− 0.0118, 0.0206) | 0.595 | 1.2% | 0.624 | |
| O_M | − 0.9% | 0.649 | |||
| SHBG | TE | 0.5035 (− 0.0961, 1.1030) | 0.100 | ||
| CDE | 0.5021 (− 0.0975, 1.1016) | 0.101 | 99.7% | < 0.001 | |
| INTref | 0.0015 (− 0.0180, 0.0211) | 0.878 | 0.3% | 0.879 | |
| INTmed | − 0.0034 (− 0.0168, 0.0101) | 0.624 | − 0.7% | 0.636 | |
| PIE | 0.0032 (− 0.0089, 0.0154) | 0.600 | 0.6% | 0.614 | |
| O_M | 0.0% | 0.979 | |||
| Progesterone | TE | 0.4212 (− 0.0995, 0.9420) | 0.113 | ||
| CDE | 0.4321 (− 0.0897, 0.9539) | 0.105 | 102.6% | < 0.001 | |
| INTref | − 0.0138 (− 0.0936, 0.0661) | 0.735 | − 3.3% | 0.744 | |
| INTmed | − 0.0015 (− 0.0115, 0.0086) | 0.772 | − 0.4% | 0.778 | |
| PIE | 0.0044 (− 0.0095, 0.0183) | 0.537 | 1.0% | 0.561 | |
| O_M | 0.7% | 0.646 |
TE: total effect (total excess relative risk), CDE: excess relative risk due to controlled direct effect, INTref: excess relative risk due to reference interaction, INTmed: excess relative risk due to mediated interaction, PIE: excess relative risk due to pure indirect effect, O_M: overall mediated
CRP: C-reactive protein, HDL: High-density lipoprotein cholesterol, LDL: Light-density lipoprotein cholesterol, SHBG: Sex Hormone-Binding Globulin, TSH: Thyroid-stimulating hormone, SHGB: Sex Hormone-binding globulin
Output of mediation analysis with causal effects estimated for a change in pollutant levels from the 25th to the 75th percentile
Adjusted for body mass index, menopausal hormone replacement therapy uses, urban/rural status at birth, urban/rural status at inclusion, alcohol drinking, breastfeeding, mammography before inclusion, oral contraceptive use, age at full-term pregnancy and parity, smoking status, total physical activity
Controlled direct effects are computed fixing the mediators at their median levels
Table 6 displays the results of the causal mediation analysis with four-way decompositions of the effect of BaP on breast cancer mediated individually by different biomarkers. The CDEs ranged from 66.4 to 176.5% while holding albumin and progesterone at their median levels, respectively. The overall mediated effects through albumin (24.3%), LDL cholesterol (22.8%), and estradiol (27.0%) were suggestively positive. In contrast, there was a non-significant negative mediated effect through HDL cholesterol (− 18.7%).
Table 6.
Four-way decomposition of each mediator of the associations between BaP and breast cancer risk
| Mediation | Effect | Estimate (CI 95%) | P value | Proportion | P value |
|---|---|---|---|---|---|
| Albumin | TE | 0.0143 (− 0.2134, 0.2421) | 0.902 | ||
| CDE | 0.0095 (− 0.2189, 0.2380) | 0.935 | 66.4% | 0.813 | |
| INTref | 0.0013 (− 0.0175, 0.0201) | 0.890 | 9.3% | 0.928 | |
| INTmed | − 0.0007 (− 0.0101, 0.0088) | 0.889 | − 4.7% | 0.927 | |
| PIE | 0.0042 (− 0.0096, 0.0179) | 0.553 | 29.0% | 0.903 | |
| O_M | 24.3% | 0.903 | |||
| CRP | TE | 0.0530 (− 0.2032, 0.3091) | 0.685 | ||
| CDE | 0.0705 (− 0.1792, 0.3201) | 0.580 | 133.0% | 0.216 | |
| INTref | − 0.0160 (− 0.0737, 0.0418) | 0.587 | − 30.2% | 0.769 | |
| INTmed | 0.0019 (− 0.0057, 0.0094) | 0.629 | 3.5% | 0.779 | |
| PIE | − 0.0034 (− 0.0132, 0.0064) | 0.501 | − 6.3% | 0.736 | |
| O_M | − 2.8% | 0.762 | |||
| Triglycerides | TE | 0.0287 (− 0.2204, 0.2778) | 0.821 | ||
| CDE | 0.0255 (− 0.2236, 0.2746) | 0.841 | 88.9% | 0.127 | |
| INTref | 0.0027 (− 0.0161, 0.0215) | 0.778 | 9.4% | 0.858 | |
| INTmed | − 0.0002 (− 0.0027, 0.0022) | 0.839 | − 0.9% | 0.877 | |
| PIE | 0.0007 (− 0.0046, 0.0061) | 0.789 | 2.5% | 0.861 | |
| O_M | 1.7% | 0.865 | |||
| Cholesterol | TE | 0.0935 (− 0.1848, 0.3717) | 0.510 | ||
| CDE | 0.0973 (− 0.1822, 0.3768) | 0.495 | 104.0% | < 0.001 | |
| INTref | 0.0006 (− 0.0191, 0.0204) | 0.949 | 0.7% | 0.949 | |
| INTmed | − 0.0056 (− 0.0172, 0.0059) | 0.339 | − 6.0% | 0.547 | |
| PIE | 0.0012 (− 0.0074, 0.0099) | 0.783 | 1.3% | 0.793 | |
| O_M | − 4.7% | 0.580 | |||
| HDL cholesterol | TE | 0.0977 (− 0.2007, 0.3962) | 0.521 | ||
| CDE | 0.1207 (− 0.1885, 0.4299) | 0.444 | 123.5% | < 0.001 | |
| INTref | − 0.0047 (− 0.0451, 0.0357) | 0.821 | − 4.8% | 0.828 | |
| INTmed | − 0.0262 (− 0.0599, 0.0076) | 0.129 | − 26.8% | 0.487 | |
| PIE | 0.0078 (− 0.0287, 0.0443) | 0.674 | 8.0% | 0.705 | |
| O_M | − 18.7% | 0.562 | |||
| LDL cholesterol | TE | 0.0560 (− 0.2016, 0.3135) | 0.670 | ||
| CDE | 0.0436 (− 0.2102, 0.2973) | 0.736 | 77.9% | 0.147 | |
| INTref | − 0.0004 (− 0.0079, 0.0071) | 0.920 | − 0.7% | 0.920 | |
| INTmed | − 0.0003 (− 0.0200, 0.0195) | 0.979 | − 0.5% | 0.979 | |
| PIE | 0.0130 (− 0.0149, 0.0410) | 0.361 | 23.3% | 0.683 | |
| O_M | 22.8% | 0.670 | |||
| Parathormone | TE | 0.0337 (− 0.2019, 0.2693) | 0.779 | ||
| CDE | 0.0324 (− 0.2020, 0.2668) | 0.786 | 96.1% | 0.002 | |
| INTref | − 0.0020 (− 0.0186, 0.0147) | 0.815 | − 5.9% | 0.860 | |
| INTmed | 0.0026 (− 0.0055, 0.0107) | 0.532 | 7.7% | 0.790 | |
| PIE | 0.0007 (− 0.0043, 0.0056) | 0.783 | 2.1% | 0.837 | |
| O_M | 9.7% | 0.787 | |||
| TSH | TE | 0.0376 (− 0.1991, 0.2742) | 0.756 | ||
| CDE | 0.0342 (− 0.2051, 0.2735) | 0.779 | 91.1% | 0.012 | |
| INTref | 0.0004 (− 0.0066, 0.0075) | 0.904 | 1.2% | 0.922 | |
| INTmed | − 0.0006 (− 0.0041, 0.0030) | 0.761 | − 1.5% | 0.839 | |
| PIE | 0.0035 (− 0.0097, 0.0166) | 0.604 | 9.3% | 0.785 | |
| O_M | 7.8% | 0.779 | |||
| Prolactin | TE | 0.0369 (− 0.1988, 0.2727) | 0.759 | ||
| CDE | 0.0348 (− 0.1997, 0.2693) | 0.771 | 94.2% | < 0.001 | |
| INTref | 0.0012 (− 0.0114, 0.0138) | 0.854 | 3.2% | 0.869 | |
| INTmed | 0.0020 (− 0.0046, 0.0086) | 0.548 | 5.5% | 0.780 | |
| PIE | − 0.0011 (− 0.0062, 0.0041) | 0.685 | − 2.9% | 0.810 | |
| O_M | 2.6% | 0.799 | |||
| Estradiol | TE | 0.0434 (− 0.1963, 0.2831) | 0.722 | ||
| CDE | 0.0321 (− 0.2067, 0.2709) | 0.792 | 74.0% | 0.320 | |
| INTref | − 0.0004 (− 0.0043, 0.0035) | 0.837 | − 0.9% | 0.765 | |
| INTmed | 0.0005 (− 0.0077, 0.0086) | 0.910 | 1.1% | 0.899 | |
| PIE | 0.0112 (− 0.0062, 0.0287) | 0.208 | 25.9% | 0.731 | |
| O_M | 27.0% | 0.714 | |||
| Testosterone | TE | 0.0249 (− 0.2116, 0.2613) | 0.837 | ||
| CDE | 0.0251 (− 0.2073, 0.2576) | 0.832 | 101.1% | 0.008 | |
| INTref | 0.0006 (− 0.0187, 0.0200) | 0.948 | 2.6% | 0.947 | |
| INTmed | − 0.0001 (− 0.0043, 0.0040) | 0.948 | − 0.6% | 0.946 | |
| PIE | − 0.0008 (− 0.0058, 0.0043) | 0.765 | − 3.1% | 0.872 | |
| O_M | − 3.6% | 0.853 | |||
| SHBG | TE | 0.0810 (− 0.1913, 0.3533) | 0.560 | ||
| CDE | 0.0807 (− 0.1918, 0.3533) | 0.561 | 99.7% | < 0.001 | |
| INTref | 0.0001 (− 0.0097, 0.0099) | 0.986 | 0.1% | 0.986 | |
| INTmed | − 0.0006 (− 0.0053, 0.0040) | 0.784 | − 0.8% | 0.802 | |
| PIE | 0.0008 (− 0.0050, 0.0066) | 0.783 | 1.0% | 0.801 | |
| O_M | 0.2% | 0.879 | |||
| Progesterone | TE | 0.0326 (− 0.2022, 0.2674) | 0.785 | ||
| CDE | 0.0576 (− 0.1825, 0.2976) | 0.638 | 176.5% | 0.550 | |
| INTref | − 0.0271 (− 0.0789, 0.0247) | 0.305 | − 83.1% | 0.794 | |
| INTmed | − 0.0038 (− 0.0125, 0.0050) | 0.399 | − 11.5% | 0.795 | |
| PIE | 0.0059 (− 0.0061, 0.0179) | 0.335 | 18.1% | 0.794 | |
| O_M | 6.5% | 0.813 |
TE: total effect (total excess relative risk), CDE: excess relative risk due to controlled direct effect, INTref: excess relative risk due to reference interaction, INTmed: excess relative risk due to mediated interaction, PIE: excess relative risk due to pure indirect effect, O_M: overall mediated
CRP: C-reactive protein, HDL: High-density lipoprotein cholesterol, LDL: Light-density lipoprotein cholesterol, SHBG: Sex Hormone-Binding globulin, TSH: Thyroid-stimulating hormone, SHGB: Sex Hormone-binding globulin
Output of mediation analysis with causal effects estimated for a change in pollutant levels from the 25th to the 75th percentile
Adjusted for body mass index, menopausal hormone replacement therapy uses, urban/rural status at birth, urban/rural status at inclusion, alcohol drinking, breastfeeding, mammography before inclusion, oral contraceptive use, age at full-term pregnancy and parity, smoking status, total physical activity
Controlled direct effects are computed fixing the mediators at their median levels
The sensitivity mediation analyses, which restricted pollutants exposure to the period from inclusion to the date of biomarker collection, yielded comparable mediating effects to those observed when exposure was measured until the index date (Supplementary Tables 4, 5 and 6).
Discussion
This study is, to date, the first to assess whether specific biomarkers act as potential mediators of the association between exposure to three major air pollutants (NO2, BaP and PCB153) and risk of breast cancer. Our analyses revealed a significantly increased risk of breast cancer with increasing quartile levels of BaP and PCB153 exposures. A positive but not statistically significant association was observed between exposure to NO2 and the risk of breast cancer. There was evidence of an inverse association between thyroid-stimulating hormone and breast cancer risk, whereas estradiol showed an increased risk of breast cancer. The four-way decomposition mediation analysis showed a suggestive mediation through estradiol and PTH in the association of NO2 and PCB153 exposures with breast cancer risk, whereas albumin, estradiol, LDL and HDL cholesterol may play a role in the association between BaP and breast cancer risk.
BaP and PCB153 are recognized as EDP [7]. Steroid hormones, especially estradiol, have been strongly linked to the risk of breast cancer [48, 49]. Certain EDP may promote tumor growth through pathways mediated by estrogen, progesterone, or other hormonal responses, particularly by modifying the levels of these steroid hormones [50, 51]. Moreover, PAHs, such as BaP, exhibit estrogenic properties and could, therefore, stimulate the proliferation of breast cells [23]. Certain BaP metabolites can bind to estrogen receptors and activate estrogen-dependent signalling pathways, potentially promoting the growth of breast cells [52]. Although a direct link between NO2 and estradiol has not been established, NO2 can contribute to both endocrine disruption (ED) and carcinogenic effects through indirect mechanisms [53–55]. Notably, its potential carcinogenicity can arise from pathways such as oxidative stress and chronic inflammation, influencing various cancer-related processes (such as angiogenesis, apoptosis, cell cycle regulation, invasion, and metastasis) or enhancing the effects of other environmental carcinogens. Overall, these findings are in agreement with our mediation results observed for estradiol, suggesting a potential role in the association between these pollutants and breast cancer development.
Furthermore, we noted an elevated but statistically non-significant mediated proportion for PTH in the association between both NO2 and PCB153 exposures and breast cancer risk. PTH is a peptide hormone secreted by the parathyroid glands, playing a crucial role in the metabolism of calcium and phosphorus [56]. A few studies have suggested that PTH might be involved in the development of breast cancer [57, 58]. Although the association between PTH and PCB153 or NO2 has not yet been well studied, some studies have found associations between PTH and other air pollutants [59, 60]. Specifically, an inverse association was observed between particulate matter <2.5 μm diameter (PM2.5) exposure and PTH levels [59]. Another study provides insights into the impact of ozone (O3) on PTH levels [60].
While we observed a higher positive indirect effect through LDL cholesterol (21%), there was a suggestive negative through HDL cholesterol (− 17%) in the association between BaP exposure and breast cancer. This negative effect is mainly due to the opposite associations between exposure-biomarker and biomarker-outcome, indicating antagonistic associations between the effect of BaP on HDL cholesterol (positive association) and the association of HDL cholesterol with breast cancer risk (inverse association), resulting in an overall negative mediated proportion. Several studies have identified associations between high levels of LDL cholesterol or low levels of HDL cholesterol and an increased risk of breast cancer [61–63]. Furthermore, studies have demonstrated a link between exposure to certain EDP, such as bisphenol A or perfluorinated compounds and cholesterol [64, 65]. However, a direct link between BaP and cholesterol has not been investigated in previous studies.
In contrast, we estimated an important mediation through albumin (i.e., proportion of pure indirect effect = 28%) for the association between BaP and risk of breast cancer. Regarding the role of albumin, its levels have been reported to be associated with breast cancer risk [66]. The mediating effect of albumin in the association between BaP and breast cancer development has not been investigated in other studies yet.
Although we did not identify potential mediating effects of metabolic/inflammatory markers, previous studies reported that chronic inflammatory and metabolism conditions play a role in the underlying mechanisms linking air pollution and breast cancer risk [54, 66]. Both BaP and PCB exposures can result in perturbation of inflammation mediators, leading to an inflammation microenvironment (via TNF-α and NFκB leading to IL-6 upregulation) that facilitates and contributes to the migration and invasion of breast cancer cells [67, 68]. Taken together, all these conditions can stimulate the growth of breast cancer cells and contribute to the development and progression of breast cancer.
Our study has several strengths. One main strength is the use of four-way decomposition mediation analyses to explore potential mediating pathways linking air pollutants to the risk of breast cancer. The method used in this study has several advantages compared to other mediation analysis approaches, including the ability to estimate the reference interaction and mediated interaction, greater flexibility and better control of confounding variables. In addition, this study has investigated several biomarkers of metabolic health, adjusted all the models for a comprehensive list of confounding variables. While this present study is the first to explore the potential mediation role of several biomarkers of metabolic health, the findings offer insights into the potential biological pathways through which these pollutants could influence the risk of breast cancer development, and suggest promising research perspectives. In the present study, biases due to exposure occurring after biomarker assessment are unlikely, as our additional sensitivity mediation analyses using the average exposure from the time of inclusion to the date of biomarker assessment, revealed no substantial differences as compared to the exposure calculated from inclusion to the index date. These findings confirm the robustness of the estimates and suggest that the timing of exposure relative to biomarker collection did non influence the results observed in our mediation analyses. However, further studies with larger sample sizes are needed to confirm and extend these findings. A better understanding of underlying mechanisms could lead to more effective preventive strategies for breast cancer.
A notable limitation of the present study is the limited statistical power due to small sample size, which may reduce our ability to detect significant associations, especially in mediation analyses. Additionally, the small sample size precluded us from performing stratified analyses. Despite the extensive efforts to adjust for a potential confounder, residual confounding cannot be entirely excluded. We noted some negative proportions and proportions exceeding 100%. As mentioned earlier, negative effects can occur when the associations between exposure-biomarker and biomarker-outcome are in the opposite direction, leading to proportions of the overall effect exceeding 100%. However, in some cases, these negative effects may be attributable to confounding or interaction with other variables, or measurement biases. It should be noted that due to sample size limitations, we were not able to perform multiple-mediator models; future studies, with larger sample sizes should consider the simultaneous analysis of multiple mediators, which could provide insights into how each biomarker contributes to the overall mediated effect. Additionally, limitations associated with multiple-mediator models, such as collinearity, should be carefully managed to ensure robust findings. It is also important to note the lack of representativeness in the study sample, since the analysis was based on a subsample of the E3N cohort participants, who were predominantly teachers. Thus, caution is warranted in interpreting these results or extrapolating them to the general population. Finally, the results should also be interpreted with caution due to the wide confidence intervals, which may indicate a degree of uncertainty and precision in the estimates.
Conclusion
Overall, this pioneering study provides additional insights into the potential role of several metabolic health biomarkers in mediating the association between air pollutants and breast cancer risk. Although not statistically significant, there was a suggestive mediation through estradiol and PTH in the association of NO2 and PCB153 exposures with breast cancer risk. Similarly, albumin, estradiol, and both LDL and HDL cholesterol may play a role in linking BaP exposure to breast cancer risk. These findings emphasize the need and importance of further investigation into the role of biomarkers linking air pollutant exposure to the occurrence of breast cancer, a major public health issue. This study also highlights the value of mediation analysis in unravelling the complex mechanisms through which environmental exposures may impact global human health.
Supplementary Information
Acknowledgements
We acknowledge the ongoing support of the MGEN, the Gustave Roussy Institute and the French League Against Cancer (Ligue contre le cancer) and the Institut National de la Santé et de la Recherche (Inserm) that runs the cohort. We are grateful to all participants for providing data and physicians for supplying pathology reports. Special thank Céline Kernaleguen, Maxime Valdenaire, Laureen Dartois, Roselyn Gomes-Rima, and Amandine Gelot for the data management and the assistance for the cohort data collection. We also appreciate the valuable advice from the project’s scientific committee of on the exposure assessment.
Abbreviations
- BaP
Benzo[a]pyrene
- BC
Breast cancer
- BCG
Bromocresol green
- CI
Confidence intervals
- CDE
Controlled direct effect
- CNIL
National Commission for Data Protection and Privacy
- CRP
C-reactive protein
- E3N
Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale
- ECLIA
Electrochemiluminescence immunoassay
- ED
Endocrine-disrupting
- HDL
High-density lipoproteins cholesterol
- IARC
International Agency for Research on Cancer
- INTmed
Mediated interaction effect
- INTref
Reference interaction effect
- LDL
Low-density lipoproteins cholesterol
- LUR
Land use regression
- NO2
Nitrogen dioxide
- ORs
Odds ratios
- O3
Ozone
- PAHs
Polycyclic aromatic hydrocarbons
- PCBs
Polychlorinated biphenyls
- PCB153
Group III of the Wolff’s classification of polychlorinated biphenyls
- PTH
Parathormone
- PIE
Pure Indirect Effect
- SHBG
Sex hormone-binding globulin
- SD
Standard deviation
- TE
Total effect
- TRAP
Traffic-related air pollutants
Author contributions
Benoît Mercoeur: Formal analysis, Methodology, Conceptualization, Writing—Review & Editing, Writing—Original Draft. Béatrice Fervers: Project administration, Conceptualization, Resources, Writing—Review & Editing. Delphine Praud Data Curation, Writing—Review & Editing. Hwayoung Noh: Data Curation, Writing—Review & Editing, Validation. Thomas Coudon: Resources Writing—Review & Editing, Supervision, Conceptualization. Camille Giampiccolo: Data Curation, Writing—Review & Editing, Validation. Lény Grassot: Data Curation, Writing—Review & Editing. Elodie Faure: Resources, Data Curation, Writing—Review & Editing. Florian Couvidat: Resources, Data Curation, Writing—Review & Editing. Gianluca Severi: Resources, Data Curation, Writing—Review & Editing. Francesca Romana Mancini: Resources, Data Curation, Writing—Review & Editing. Pascal Roy: Project administration, Conceptualization, Resources, Writing—Review & Editing. Amina Amadou: Project administration, Conceptualization, Resources, Writing—Review & Editing. All authors have approved the final manuscript and agreed to the submission.
Funding
This study received support from ADEME (21ESD0020), the Regional Committee of the French League against Cancer of the Savoie Region (18–383-C). CG is supported by a doctoral fellowship of the Regional Committee of the French League Against Cancer of Rhône. The research was carried out using data from the Inserm (French National Institute for Health and Medical Research) E3N-Generation cohort, which was established and maintained with the support of the Mutuelle Générale de l’Education Nationale (MGEN), Gustave Roussy, and the French League against Cancer (LNCC). The E3N-Generation cohort is also supported by the French National Research Agency (ANR) under the Investment for the future Program (PIA; ANR-10-COHO-0006) and by the French Ministry of Higher Education, Research and Innovation (subsidy for public service charges No. 2102 918823, 2103236497, and 2103586016).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Our research utilized data from the existing French prospective cohort E3N. All participants provided informed consent and the study received approval from the French National Commission for Data Protection and Privacy (CNIL). Due to ethical considerations and the specific consent signed by the participants, the datasets generated and/or analysed during the current study are not publicly available, however, data can be obtained from the E3N-Generations team through the corresponding author upon reasonable request.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Turner MC, Andersen ZJ, Baccarelli A, Diver WR, Gapstur SM, Pope CA III, et al. Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA Cancer J Clin. 2020;70(6):460–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loomis D, Grosse Y, Lauby-Secretan B, Ghissassi FE, Bouvard V, Benbrahim-Tallaa L, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14(13):1262–3. [DOI] [PubMed] [Google Scholar]
- 3.Gabet S, Lemarchand C, Guénel P, Slama R. Breast cancer risk in association with atmospheric pollution exposure: a meta-analysis of effect estimates followed by a health impact assessment. Environ Health Perspect. 2021;129(5):057012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei W, Wu BJ, Wu Y, Tong ZT, Zhong F, Hu CY. Association between long-term ambient air pollution exposure and the risk of breast cancer: a systematic review and meta-analysis. Environ Sci Pollut Res. 2021;28(44):63278–96. [DOI] [PubMed] [Google Scholar]
- 5.Krzyzanowski M, Cohen A. Update of WHO air quality guidelines. Air Qual Atmos Health. 2008;1(1):7–13. [Google Scholar]
- 6.Liu H, Sun Y, Ran L, Li J, Shi Y, Mu C, et al. Endocrine-disrupting chemicals and breast cancer: a meta-analysis. Front Oncol. 2023;9(13):1282651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leng L, Li J, Luo XM, Kim JY, Li YM, Guo XM, Chen X, Yang QY, Li G, Tang NJ. Polychlorinated biphenyls and breast cancer: a congener-specific meta-analysis. Environ Int. 2016;1(88):133–41. [DOI] [PubMed] [Google Scholar]
- 8.Arif I, Adams MD, Johnson MTJ. A meta-analysis of the carcinogenic effects of particulate matter and polycyclic aromatic hydrocarbons. Environ Pollut. 2024;11:123941. [DOI] [PubMed] [Google Scholar]
- 9.Premnath N, Mohanrasu K, Rao RG, Dinesh GH, Prakash GS, Ananthi V, et al. A crucial review on polycyclic aromatic hydrocarbons-environmental occurrence and strategies for microbial degradation. Chemosphere. 2021;280:130608. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg MS, Labrèche F, Weichenthal S, Lavigne E, Valois MF, Hatzopoulou M, et al. The association between the incidence of postmenopausal breast cancer and concentrations at street-level of nitrogen dioxide and ultrafine particles. Environ Res. 2017;1(158):7–15. [DOI] [PubMed] [Google Scholar]
- 11.Mordukhovich I, Beyea J, Herring AH, Hatch M, Stellman SD, Teitelbaum SL, et al. Vehicular traffic-related polycyclic aromatic hydrocarbon exposure and breast cancer incidence: the long island breast cancer study project (LIBCSP). Environ Health Perspect. 2016;124(1):30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen ZJ, Stafoggia M, Weinmayr G, Pedersen M, Galassi C, Jørgensen JT, Oudin A, Forsberg B, Olsson D, Oftedal B, Marit AG. Long-Term exposure to ambient air pollution and incidence of postmenopausal breast cancer in 15 European cohorts within the ESCAPE project. Environ Health Perspect. 2017;125(10):107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shmuel S, White AJ, Sandler DP. Residential exposure to vehicular traffic-related air pollution during childhood and breast cancer Risk. Environ Res. 2017;159:257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amadou A, Praud D, Coudon T, Deygas F, Grassot L, Dubuis M, et al. Long-term exposure to nitrogen dioxide air pollution and breast cancer risk: a nested case-control within the French E3N cohort study. Environ Pollut. 2023;15(317):120719. [DOI] [PubMed] [Google Scholar]
- 15.Crouse DL, Goldberg MS, Ross NA, Chen H, Labrèche F. Postmenopausal breast cancer is associated with exposure to traffic-related air pollution in montreal, canada: a case-control study. Environ Health Perspect. 2010;118(11):1578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Praud D, Deygas F, Amadou A, Bouilly M, Turati F, Bravi F, et al. Traffic-related air pollution and breast cancer risk: a systematic review and meta-analysis of observational studies. Cancers. 2023;15(3):927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amadou A, Praud D, Coudon T, Deygas F, Grassot L, Faure E, et al. Risk of breast cancer associated with long-term exposure to benzo[a]pyrene (BaP) air pollution: evidence from the French E3N cohort study. Environ Int. 2021;1(149):106399. [DOI] [PubMed] [Google Scholar]
- 18.Shen J, Liao Y, Hopper JL, Goldberg M, Santella RM, Terry MB. Dependence of cancer risk from environmental exposures on underlying genetic susceptibility: an illustration with polycyclic aromatic hydrocarbons and breast cancer. Br J Cancer. 2017;116(9):1229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan M, Deoraj A, Felty Q, Roy D. Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Mol Cell Endocrinol. 2017;5(457):89–102. [DOI] [PubMed] [Google Scholar]
- 20.Deygas F, Amadou A, Coudon T, Grassot L, Couvidat F, Bessagnet B, et al. Long-term atmospheric exposure to PCB153 and breast cancer risk in a case-control study nested in the French E3N cohort from 1990 to 2011. Environ Res. 2021;1(195):110743. [DOI] [PubMed] [Google Scholar]
- 21.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Pub Health. 2020;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donini CF, El Helou M, Wierinckx A, Győrffy B, Aires S, Escande A, et al. Long-term exposure of early-transformed human mammary cells to low doses of Benzo[a]pyrene and/or Bisphenol a enhances their cancerous phenotype via an AhR/GPR30 interplay. Front Oncol. 2020;10:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White AJ, Bradshaw PT, Herring AH, Teitelbaum SL, Beyea J, Stellman SD, et al. Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ Int. 2016;89–90:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calaf GM, Ponce-Cusi R, Aguayo F, Muñoz JP, Bleak TC. Endocrine disruptors from the environment affecting breast cancer. Oncol Lett. 2020;20(1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satpathi S, Gaurkar SS, Potdukhe A, Wanjari MB. Unveiling the role of hormonal imbalance in breast cancer development: a comprehensive review. Cureus. 2023;15(7):e41737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortunati N, Catalano MG, Boccuzzi G, Frairia R. Sex hormone-binding globulin (SHBG), estradiol and breast cancer. Mol Cell Endocrinol. 2010;316(1):86–92. [DOI] [PubMed] [Google Scholar]
- 27.Hormones E, Breast Cancer Collaborative Group. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. The lancet oncology. 2013; 14(10): 1009–19. [DOI] [PMC free article] [PubMed]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 29.Reaves DK, Ginsburg E, Bang JJ, Fleming JM. Persistent organic pollutants & obesity: potential mechanisms for breast cancer promotion? Endocr Relat Cancer. 2015;22(2):R69-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48(4):155–70. [DOI] [PubMed] [Google Scholar]
- 31.Kitahara CM, de González AB, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29(12):1592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borgquist S, Giobbie-Hurder A, Ahern TP, Garber JE, Colleoni M, Láng I, et al. Cholesterol, cholesterol-lowering medication use, and breast cancer outcome in the BIG 1–98 study. J Clin Oncol. 2017;35(11):1179–88. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TQ, Schmid I, Stuart EA. Clarifying causal mediation analysis for the applied researcher: defining effects based on what we want to learn. Psychol Methods. 2020. 10.1037/met0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiol Camb Mass. 2014;25(5):749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amadou A, Coudon T, Praud D, Salizzoni P, Leffondre K, Lévêque E, et al. Chronic low-dose exposure to xenoestrogen ambient air pollutants and breast cancer Risk: XENAIR protocol for a case-control study nested within the french E3N cohort. JMIR Res Protoc. 2020;9(9):e15167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clavel-Chapelon F. E3N study group. Cohort profile: the French E3N cohort study. Int J Epidemiol. 2015;44(3):801–9. [DOI] [PubMed] [Google Scholar]
- 37.Guerreiro CBB, Horálek J, de Leeuw F, Couvidat F. Benzo(a)pyrene in Europe: ambient air concentrations, population exposure and health effects. Environ Pollut. 2016;1(214):657–67. [DOI] [PubMed] [Google Scholar]
- 38.Lee M, Brauer M, Wong P, Tang R, Tsui TH, Choi C, et al. Land use regression modelling of air pollution in high density high rise cities: a case study in Hong Kong. Sci Total Environ. 2017;15(592):306–15. [DOI] [PubMed] [Google Scholar]
- 39.Ryan PH, LeMasters GK. A Review of Land-use regression models for characterizing Intraurban air pollution exposure. Inhal Toxicol. 2007;19(sup1):127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Hoogh K, Gulliver J, van Donkelaar A, Martin RV, Marshall JD, Bechle MJ, Cesaroni G, Pradas MC, Dedele A, Eeftens M, Forsberg B. Development of West-European PM2.5 and NO2 land use regression models incorporating satellite-derived and chemical transport modelling data. Environ Res. 2016;1(151):1. [DOI] [PubMed] [Google Scholar]
- 41.Gulliver J, de Hoogh K, Hansell A, Vienneau D. Development and back-extrapolation of NO2 land use regression models for historic exposure assessment in Great Britain. Environ Sci Technol. 2013;47(14):7804–11. [DOI] [PubMed] [Google Scholar]
- 42.Levy I, Levin N, Yuval, Schwartz JD, Kark JD. Back-Extrapolating a land use regression model for estimating past exposures to traffic-related air pollution. Environ Sci Technol. 2015;49(6):3603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coudon T, Grassot L, Couvidat F, Salizzoni P, Fervers B, Gulliver J. Spatiotemporal modeling of outdoor PM10, PM2.5, NO2 and O3 concentrations in France from 2010 to 1990. submitted in Environ Int.;
- 44.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–82. [DOI] [PubMed] [Google Scholar]
- 45.Berger E, Dudouet R, Dossus L, Baglietto L, Gelot A, Boutron-Ruault MC, et al. Biological embodiment of educational attainment and future risk of breast cancer: findings from a French prospective cohort. submitted in BMJ Open.;
- 46.Harrell , FE. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis [Internet]. Cham: Springer International Publishing; 2015 [cited 2023 May 23]. (Springer Series in Statistics). Available from: 10.1007/978-3-319-19425-7
- 47.VanderWeele TJ. Bias formulas for sensitivity analysis for direct and indirect effects. Epidemiol Camb Mass. 2010;21(4):540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johansson Å, Schmitz D, Höglund J, Hadizadeh F, Karlsson T, Ek WE. Investigating the effect of estradiol levels on the risk of breast, endometrial, and ovarian cancer. J Endocr Soc. 2022;6(8):bvac100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brisken C, Hess K, Jeitziner R. Progesterone and overlooked endocrine pathways in breast cancer pathogenesis. Endocrinology. 2015;156(10):3442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudel RA, Ackerman JM, Attfield KR, Brody JG. New exposure biomarkers as tools for breast cancer epidemiology, biomonitoring, and prevention: a systematic approach based on animal evidence. Environ Health Perspect. 2014;122(9):881–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HR, Hwang KA, Nam KH, Kim HC, Choi KC. Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem Res Toxicol. 2014;27(5):834–42. [DOI] [PubMed] [Google Scholar]
- 52.Hýžd′alová M, Pivnička J, Zapletal O, Vázquez-Gómez G, Matthews J, Neča J, et al. Aryl hydrocarbon receptor-dependent metabolism plays a significant role in estrogen-like effects of polycyclic aromatic hydrocarbons on cell proliferation. Toxicol Sci. 2018;165(2):447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Callahan CL, Bonner MR, Nie J, Han D, Wang Y, Tao MH, et al. Lifetime exposure to ambient air pollution and methylation of tumor suppressor genes in breast tumors. Environ Res. 2018;1(161):418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodgers KM, Udesky JO, Rudel RA, Brody JG. Environmental chemicals and breast cancer: an updated review of epidemiological literature informed by biological mechanisms. Environ Res. 2018;1(160):152–82. [DOI] [PubMed] [Google Scholar]
- 55.Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, et al. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect. 2016;124(6):713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dawale K, Agrawal A. Parathyroid hormone secretion and related syndromes. Cureus. 2022;14(10):e30251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCarty MF. Parathyroid hormone may be a cancer promoter—an explanation for the decrease in cancer risk associated with ultraviolet light, calcium, and vitamin D. Med Hypotheses. 2000;54(3):475–82. [DOI] [PubMed] [Google Scholar]
- 58.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control. 2005;16(2):83–95. [DOI] [PubMed] [Google Scholar]
- 59.Prada D, Zhong J, Colicino E, Zanobetti A, Schwartz J, Dagincourt N, et al. Association of air particulate pollution with bone loss over time and bone fracture risk: analysis of data from two independent studies. Lancet Planet Health. 2017;1(8):e337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kheirouri S, Department of Nutrition, Tabriz University of Medical Sciences Faculty of Nutrition and Food Sciences, Tabriz, Iran, Shanehbandi D, Immunology research center, Tabriz University of Medical Sciences Faculty of Medicine, Tabriz, Iran, Khordadmehr M, Department of Pathobiology, University of Tabriz Faculty of Veterinary Medicine, Tabriz, Iran, et al. Effects of Air Pollution, Ozone, and Sulfur Dioxide on Kidney Oxidative-Stress Enzymes, Histopathology, and Apoptosis Gene Expressions in Rats. Turk J Nephrol. 2020; 33(2): 153–60.
- 61.Wu J, Lei X, Pan X, Zeng X, Li W. Association between serum lipids and breast cancer risk in premenopausal women: systematic review and meta-analysis. J Int Med Res. 2021;49(11):03000605211061033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Llanos AA, Makambi KH, Tucker CA, Wallington SF, Shields PG, Adams-Campbell LL. Cholesterol, lipoproteins, and breast cancer risk in African-American women. Ethn Dis. 2012;22(3):281–7. [PMC free article] [PubMed] [Google Scholar]
- 63.Cedó L, Reddy ST, Mato E, Blanco-Vaca F, Escolà-Gil JC. HDL and LDL: potential new players in breast cancer development. J Clin Med. 2019;8(6):853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu WS, Lai YT, Chan HL, Li SY, Lin CC, Liu CK, et al. Associations between perfluorinated chemicals and serum biochemical markers and performance status in uremic patients under hemodialysis. PLoS ONE. 2018;13(7):e0200271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li R, Yang S, Gao R, Deng Y, Liu J, Yuan C, et al. Relationship between the environmental endocrine disruptor bisphenol a and Dyslipidemia: a five-year prospective study. Endocr Pract. 2020;26(4):399–406. [DOI] [PubMed] [Google Scholar]
- 66.Kühn T, Sookthai D, Graf ME, Schübel R, Freisling H, Johnson T, et al. Albumin, bilirubin, uric acid and cancer risk: results from a prospective population-based study. Br J Cancer. 2017;117(10):1572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malik DE, David RM, Gooderham NJ. Mechanistic evidence that benzo [a] pyrene promotes an inflammatory microenvironment that drives the metastatic potential of human mammary cells. Arch Toxicol. 2018;92:3223–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prunicki M, Cauwenberghs N, Ataam JA, Movassagh H, Kim JB, Kuznetsova T, et al. Immune biomarkers link air pollution exposure to blood pressure in adolescents. Environ Health. 2020;19(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.