Abstract
Background
The spontaneous echo contrast (SEC) in patients with atrial fibrillation (AF) indicates a prethrombic state that ultimately progresses into thrombus formation. A comprehensive understanding of specific plasma metabolomics characteristics may protect AF patients from thrombus, particularly in the early stage.
Objectives
Through the investigation of metabolic pathways, we endeavor to uncover the metabolomic characteristics associated with SEC states, and to examine the differential metabolites by which may exert their influence on thrombotic states.
Methods
Patients with AF were enrolled, and the participants were divided into three groups based on the results of the echocardiogram: non-SEC, low-SEC and high-SEC group. Samples were collected and subjected to non-targeted metabolomics analysis. The analytical process included data quality control, metabolite difference analysis, component analysis, Kegg cluster analysis, etc.
Results
Our metabolic phenotype revealed a clear differential metabolic pattern between the SEC and non-SEC. Specifically, we identified 35 and 142 significantly differential metabolites in venous and atrial plasma, respectively, suggesting that SEC may be involved in pervasive metabolic dysregulation and that the degree of metabolic dysregulation in atrial plasma is more severe than that in venous blood.
Conclusion
Patients with SEC have a significantly different metabolic pattern compared to those without SEC. Our work promoted the understanding of mechanism of the occurrence and development of SEC, facilitated the screening of the target metabolites for its therapeutic intervention, and provided evidence for the prevention and treatment of SEC or thrombosis in AF. Our work also provided new directions for subsequent research in related fields. In conclusion, our study not only provides a theoretical basis for understanding the occurrence and development of SEC in AF, but also provides recommendations for the daily diet of AF patients with SEC, such as a balanced intake of essential amino acids, avoiding excessive intake of benzoic acid, and intake of appropriate inositol.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04306-y.
Keywords: Atrial fibrillation, Spontaneous echo contrast, Differential metabolites, Plasma metabolomics
Introduction
Atrial fibrillation (AF), one of the most prevalent cardiac arrhythmias, affects approximately 2–4% of adults and steadily increases with age. AF is an independent risk factor for stroke, leading to significant economic and healthcare burdens in terms of thrombus-related deaths [1]. It has been recognized that spontaneous echo contrast (SEC) within the heart chambers indicates stagnant blood flow and is a precursor to thrombosis [2, 3]. However, the pathogenesis of SEC is complex, with multiple interrelated contributing factors. Therefore, in-depth exploration of the mechanism of SEC formation and attenuation of these causative factors may reduce SEC and thus prevent thrombosis to some extent. In light of these considerations, the prevention and treatment of AF centers around reducing thrombotic events [4]. Consequently, this study utilized non-targeted metabolomic profiling to examine metabolic pattern and biomarkers in the plasma of SEC in AF patients, providing insights for evaluating the risk of SEC and thrombus formation.
Materials and methods
Study populations
The study population consisted of patients with non-valvular AF who underwent radiofrequency ablation therapy at the Atrial Fibrillation Center of the Second Hospital of Tianjin Medical University between March and September 2023. The inclusion criteria involved all patients during the study period who sought medical care at the Atrial Fibrillation Center and were diagnosed with non-valvular AF by experienced experts in cardiac arrhythmias. Exclusion criteria were: valvular AF, poorly controlled diabetes, chronic obstructive pulmonary disease, severe liver dysfunction (alanine aminotransferase level > 135 U/l), heart failure, severe renal dysfunction, thyroid disease and malignant tumors. In addition, we also excluded patients who were unwilling to participate in the study, those who were unable to complete necessary examinations (such as transesophageal echocardiography), and those with incomplete clinical data. This study has been approved by the Ethics Committee of the Second Hospital of Tianjin Medical University (No: KY2023K058) and adheres to the principles of the Helsinki Declaration. All participants are included voluntarily and have signed informed consent forms.
Trans Esophageal echocardiography examination
Experienced experts in cardiac arrhythmias assessed and confirmed the suitability of trans esophageal echocardiography examination (TEE) for each patient. For patients in good condition, after fasting for at least 12 h, the examination was conducted by experienced sonographers in the Ultrasound Department using GE HealthCare Vivid E95 echocardiography equipment (GE Healthcare; Vingmed Ultrasound, Horten, Norway). GE HealthCare EchoPAC was used to analyze TEE test results. SEC refers to a smoky echo of swirling blood flow in the left atrial appendage and/or left atrial. Its presence is associated with slow blood flow velocity, abnormal blood cell aggregation, locally hypercoagulable state and can be considered a pre-thromboembolic condition [5]. The severity assessment methodology of SEC was proposed by Fatkin et al. [5], According to the evaluation criteria, three experienced sonographers categorize the observed SEC into three classes: none-SEC group, low-SEC group and high-SEC group [6]. Three experienced cardiac sonographers each independently evaluated the same TEE images, and patients were included in the study only if the reports from the three experts were the same.
Sample collection
For AF patients who require ablation surgery, we asked them to temporarily stop taking anticoagulants (NOACs or low molecular weight heparin) once on the day of surgery [7, 8]. In the atrial fibrillation ablation procedure at our Atrial Fibrillation Research Center, heparin is not used after femoral venous puncture, but only after successful transseptal puncture. For this study, in order to avoid the influence of heparin on the selection of SEC-related differential metabolites, we did not administer a standard dose of heparin until appropriate amounts of blood were collected immediately after transseptal puncture. During transseptal puncture, we used a conventional septal puncture needle, not a radiofrequency needle. After successful femoral vein puncture, an appropriate amount of peripheral venous blood was collected, and left atrial blood was collected immediately after successful transseptal puncture.
All blood samples obtained were placed in an EDTA-anticoagulated vacuum blood collection tube and temporarily stored in a 4℃ freezer for no more than 4 h. The blood samples were then centrifuged at 3000 rpm for 15 min, and the super-clarified plasma was aliquoted in cryovials and stored at -80℃.
Metabolomics testing
Plasma non-targeted metabolomic analysis was conducted by Novogene Co., Ltd. We prepared samples in strict accordance with the testing requirements before testing. Firstly, 400 µL of 80% methanol-water solution (V/V) was added to 100 µL of plasma, followed by vortex mixing. The mixture was then placed in an ice bath for 5 min and finally centrifuged at 15,000 g for 20 min at 4℃. An appropriate amount of supernatant was added to mass spectrometry grade water and diluted to a methanol content of 53% (V/V), then centrifuged at 15,000 g for 20 min at 4℃ and an appropriate amount of supernatant was taken for LC-MS analysis. Mass spectrometer: Q Exactive™ HF/Q Exactive™ HF-X(Thermo Fisher), Chromatograph: Vanquish UHPLC (Thermo Fisher), Columns: Hypesil Gold column (100 × 2.1 mm, 1.9 μm) (Thermo Fisher).
Statistical analysis
The KEGG database (https://www.genome.jp/kegg/pathway.html), HMDB database (httpshmdb.ca/metabolites), and LIPMaps database (http://wwwipidmaps.org/) were utilized for annotating the identified metabolites. For the multivariate statistical analysis, the metabolomics data processing software metaX was used to convert the data, and then the principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were performed to obtain the Variable important in projection (VIP) value of each metabolite. In the univariate analysis, the student’s t test was used to calculate the statistical significance (P value) of each metabolite between the two groups, and the fold change (FC value) of the metabolite between the two groups was calculated to quantify the differences in metabolite levels between the two groups.
The default criteria for initial screening for differential metabolites are VIP-value > 1, P-value < 0.05 and FC ≥ 1.2 or FC ≤ 0.8. The differential metabolites were assigned different weight points according to different rules for further screening, and the metabolites that met the two or more rules were preferentially selected. Rule 1: Metabolites presented in 2 or 3 comparison groups were assigned a score of 0.5 and 1, respectively. Rule 2: The metabolites of VIP > 2 was given weight points of 1 point; Rule 3: Metabolites present in the KEGG-enrichment pathway were assigned a weight score of 1 point.
The volcano map was plotted using the R package ggplot2, which can be combined with the VIP value of metabolites, log2 (Fold Change) and -log10 (P-value) to screen metabolites. Clustering heatmap, plotted with the R package Pheatmap, normalized metabolite data using z-score. Correlation analysis between differential metabolites (Pearson correlation) was performed using R package cor(), statistical significance was calculated with R package cor.mtest(), P-value < 0.05 was considered statistically different, and correlation plots were plotted with R package corrplot. The bubble plot was plotted with the R package ggplot2. The KEGG database was used to study the function and metabolic pathways of metabolites, and when x/n > y/n, the metabolic pathways were considered to be enriched; When the P value of the metabolic pathway was less than 0.05, it was considered to be significantly enriched. GraphPad Prism 8 was used for histogram plotting and statistical analysis, the baseline data was analyzed by R package compare Groups, the one-way ANOVA was used for comparison between multiple groups, and the student’s t test was used for comparison between two groups, and P < 0.05 was statistically significant.
Results
Study population characteristics
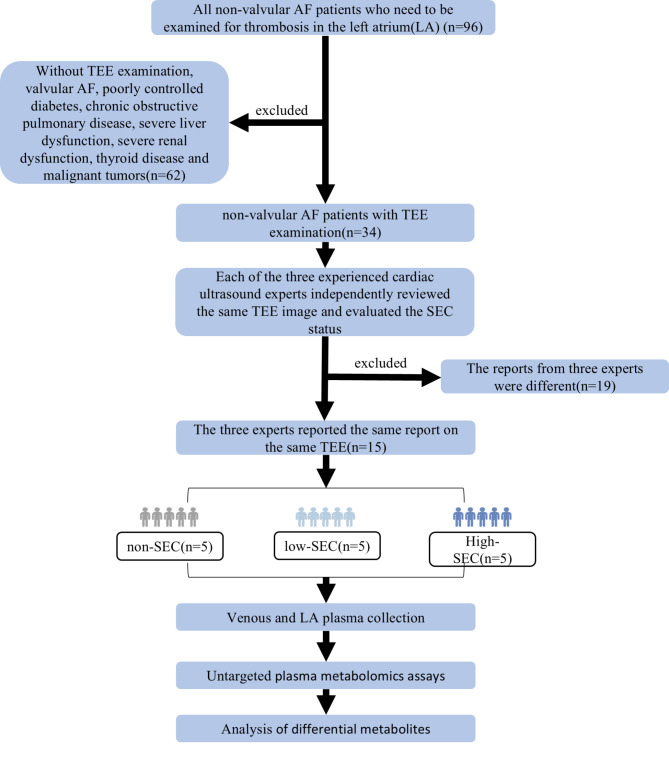
A total of 15 patients were included in this study (Fig. 1). Based the different TEE presentations, they were divided into three groups: non-SEC group (5 patients), low-SEC group (5 patients) and high-SEC group (5 patients). The clinical baseline data of these groups are shown in Table 1. We found that as the severity of SEC increased, the amount of LDLc gradually decreased and the LAD gradually increased. There were no significant differences in the remaining items.
Fig. 1.
Research flowcharts. TEE, Trans Esophageal echocardiography; SEC, spontaneous echo contrast
Table 1.
Baseline characteristics of participants
| Variable | All (N = 15) | Non-SEC (N = 5) | low-SEC (N = 5) | high-SEC (N = 5) | P (low-SEC VS. Non-SEC) | P (high-SEC VS. Non-SEC) |
|---|---|---|---|---|---|---|
| Female (n, %) | 8 (53.3%) | 3 (60.0%) | 3 (60.0%) | 2 (40.0%) | 1.000 | 0.549 |
| Age(years) | 69.0 [63.0;77.0] | 66.0 [60.0;69.0] | 74.0 [64.0;78.0] | 70.0 [60.0;79.0] | 0.12 | 0.349 |
| Smoke (n, %) | 4 (26.7%) | 1 (20.0%) | 1 (20.0%) | 2 (40.0%) | 1.000 | 0.513 |
| Drink (n, %) | 3 (20.0%) | 0 (0.00%) | 1 (20.0%) | 2 (40.0%) | 0.317 | 0.134 |
| SBP (mmHg) | 125[112.0;134.0] | 127 [119.0;145.5] | 115 [107.5;149.5] | 121 [108.0;125.5] | 0.686 | 0.118 |
| DBP (mmHg) | 78.0 [72.5;88.0] | 93.0 [77.0;96.5] | 75.0 [60.0;86.5] | 77.0 [72.5;78.5] | 0.118 | 0.066 |
| HR (bpm) | 79.0 [65.0;98.0] | 70.0 [65.5;102.0] | 67.0 [61.5;85.0] | 85.0 [72.0;120] | 0.408 | 0.393 |
| Hypertension (n, %) | 8 (53.3%) | 2 (40.0%) | 3 (60.0%) | 3 (60.0%) | 0.549 | 0.549 |
| Diabetes (n, %) | 2 (13.3%) | 1 (20.0%) | 1 (20.0%) | 0 (0.00%) | 1.000 | 0.317 |
| Coronary heart disease (n, %) | 7 (46.7%) | 1 (20.0%) | 3 (60.0%) | 3 (60.0%) | 0.221 | 0.221 |
| HDLc (mmol/L) | 1.22 [1.05;1.34] | 1.26 [1.12;1.39] | 1.26[1.17;1.35] | 1.03 [0.84;1.22] | 1.000 | 0.064 |
| LDLc(mmol/L) | 2.78 [2.04;3.34] | 3.32 [2.89;3.84] | 2.53 [2.00;3.06] | 2.30 [1.76;2.97] | 0.039 | 0.039 |
| Prothrombin time (s) | 13.7 [13.0;22.8] | 12.9 [12.5;13.4] | 13.9 [13.6;23.6] | 14.0 [13.5;23.4] | 0.09 | 0.09 |
| Prothrombin percentage activity (%) | 93.0[37;100] | 100 [96.5;101.5] | 88.0 [35.5;94.5] | 90.0 [36.0;99.0] | 0.069 | 0.109 |
| INR | 1.04 [1.00;2.03] | 1.00 [1.00;1.02] | 1.08 [1.03;2.08] | 1.06 [1.01;2.07] | 0.112 | 0.126 |
| Partial thromboplastin time (s) | 42.4 [33.5;44.2] | 39.7 [30.5;43.7] | 43.0 [35.3;64.2] | 42.4 [34.6;43.5] | 0.112 | 0.126 |
| Fibrinogen(g/L) | 3.06 [2.95;3.53] | 3.01 [2.72;3.17] | 4.16 [3.27;5.64] | 2.95 [2.72;3.34] | 0.059 | 0.792 |
| Thrombin time (s) | 18.1 [17.7;18.6] | 18.1 [17.85;19.1] | 18.6 [18.05;19.9] | 18.0 [16.7;18.25] | 0.473 | 0.188 |
| BNP (pg/ml) |
1029.8 [276.6;3795.35] |
290.6 [71.4;2086.25] |
375.9 [233.15;6456.1] |
3795.35 [935;6655.7] | 0.467 | 0.104 |
| Thyrotropin (mIU/ml) | 2.04 [0.98;2.8] | 1.12 [0.49;3.345] | 2.04 [0.775;3.265] | 2.76 [2.1;3.425] | 0.812 | 0.309 |
| CHA2DS2−VASc | 4 [2.0;4.0] | 2 [1.0;3.5] | 4 [2.5;4.0] | 4 [3.5;4.5] | 0.189 | 0.055 |
| IVS (mm) | 8.8[8;9.8] | 8[6.95;9.25] | 8.8[8.2;10.35] | 9.8[8.7;15.3] | 0.258 | 0.161 |
| LVDd (mm) | 49.1[43.3;50.3] | 46.3[44.2;48.65] | 42[40.8;49.55] | 53.5[49.8;65.5] | 0.457 | 0.075 |
| LVDs(mm) | 26.8[25.1;28.7] | 26.5[25;29.35] | 26[22.95;28.5] | 27.5[26.4;55.1] | 0.477 | 0.218 |
| RA (mm) | 49[45.8;56] | 45.9[43.6;52.9] | 46.9[46.1;62.55] | 50.5[47.1;56.15] | 0.343 | 0.281 |
| LAD (mm) | 42.1[39.2;48.2] | 39.8[34.6;41.8] | 40.1[38.7;49.65] | 48.2[47.25;52.8] | 0.229 | 0.001 |
| RVAW (mm) | 3.4[2.9;3.7] | 2.9[2.5;3.45] | 3.4[3.15;3.95] | 3.6[2.95;3.95] | 0.086 | 0.149 |
| RVDd (mm) | 21[20;23.6] | 21.9[20.6;23.2] | 20[19.45;26.25] | 20.6[19.85;24.85] | 0.868 | 0.944 |
| AoD (mm) | 22.1[21.8;25] | 22.1[22.05;23.25] | 22[20.85;23.75] | 26.1[19.7;26.9] | 0.753 | 0.487 |
| PAD (mm) | 21.2[19.5;24.2] | 20.6[19.1;21.85] | 21.6[19.4;34.55] | 21.2[18.95;34] | 0.301 | 0.281 |
| LVEF (%) | 59[55;68] | 62[57.5;68.5] | 59[57.5;71.5] | 52[38;62.5] | 0.899 | 0.12 |
| IVC (mm) | 15.3[14.1;18.3] | 15.3[9.85;20.15] | 17.3[15.65;19.2] | 14.6[13.75;15.4] | 0.373 | 0.845 |
| Na+(mmol/l) | 141.5[138.6;142.5] | 142.3[140.05;145] | 141.5[139.5;142.45] | 139.8[138.1;142.6] | 0.429 | 0.273 |
| K+(mmol/l) | 4[3.95;4.2] | 4[3.725;4.15] | 4.1[3.95;4.55] | 4[3.9;4.2] | 0.213 | 0.553 |
| Cl−(mmol/l) | 107.4[104.7;109.5] | 107.4[105.4;109.4] | 109.1[104.3;114.4] | 105.1[104.7;109.9] | 0.544 | 0.745 |
| CO2CP (mmol/l) | 24.1[23.3;25.6] | 23.3[21.75;24.85] | 25.1[16.4;26.1] | 24.3[24.05;25.55] | 0.705 | 0.143 |
| glucose(mmol/l) | 5.75[4.63;7.06] | 4.9[4.235;8.035] | 5.3[4.455;5.82] | 6.71[5.685;8.855] | 0.475 | 0.353 |
| TP(g/l) | 66.2[62.6;68.4] | 68.9[61.35;69.9] | 66.4[61.15;68.05] | 64.3[62.35;66.15] | 0.697 | 0.496 |
| Albumin(g/l) | 39.8[38.2;42] | 41.7[38.3;44.15] | 39.5[37.5;43.3] | 39.2[37.75;40.65] | 0.623 | 0.253 |
| Globulin(g/l) | 24.9[22.3;27.2] | 26.9[21.05;27.9] | 24.4[21.25;28.4] | 25.1[23.5;26.6] | 0.937 | 0.959 |
| ALT(U/l) | 16[10.4;22.7] | 18.1[11.15;29.1] | 16[8.7;27.9] | 15.8[12.05;19.5] | 0.788 | 0.488 |
| IBIL (ummol/l) | 12.8[11.7;14] | 13.1[10.65;13.55] | 12.3[7.65;13.4] | 14.1[9.65;22.425] | 0.521 | 0.401 |
| CB (ummol/l) | 2.3[2;3] | 2.3[1.85;2.75] | 2.3[1.95;6.35] | 2.55[2.1;3.0] | 0.383 | 0.457 |
| AST(U/l) | 16.8[14.1;24.8] | 15.1[14;21.35] | 24.8[14.7;27.5] | 16.8[14.75;18.85] | 0.273 | 0.902 |
| TBIL (ummol/l) | 15[14.1;16.1] | 15[13.3;15.7] | 14.4[13.85;15.6] | 16.1[12.25;25.2] | 0.947 | 0.376 |
| ALP(U/l) | 63.85[59.56;72.1] | 79.5[59.3;118.9] | 69.7[56.58;71.35] | 63.3[60;67.975] | 0.168 | 0.141 |
| γ-GGT(U/l) | 25.1[17.8;34.7] | 20.7[19.05;31.95] | 17.8[14.65;31.735] | 30.675[19.75;41.6] | 0.665 | 0.371 |
| TG (mmol/l) | 1.53[0.97;2.01] | 1.53[0.64;2.71] | 1.02[0.86;1.175] | 2.01[1.74;2.38] | 0.303 | 0.515 |
| BUN (mmol/l) | 6.6[5.7;8.5] | 6.7[5.55;9.55] | 6.3[5.5;16.65] | 6.6[5.55;8.2] | 0.533 | 0.655 |
| Cr(mmol/l) | 60.6[53.1;79.1] | 55.4[48.4;73.45] | 56.7[52.5;178.65] | 75.2[66.05;85] | 0.395 | 0.072 |
| Ua (mmol/l) | 355.9[245.2;422.4] | 256.3[221.4;524.4] | 292.4[210.35;459.9] | 391.1[347.25;422.9] | 0.832 | 0.672 |
| WBC(*109/L) | 6.58[5.4;7.55] | 7.39[6.42;8.385] | 5.96[4.91;9.015] | 5.52[4.965;7.225] | 0.591 | 0.095 |
| NEUT(*109/L) | 4.12[3.39;5.39] | 4.41[3.945;5.68] | 4.12[3.41;5.5] | 3.39[2.605;4.81] | 0.64 | 0.182 |
| LY(*109/L) | 1.93[1.54;2.12] | 2.12[1.715;2.245] | 1.67[0.915;2.8] | 1.69[1.585;2.02] | 0.729 | 0.23 |
| Mono(*109/L) | 0.42[0.38;0.51] | 0.51[0.445;0.595] | 0.41[0.33;0.48] | 0.38[0.36;0.48] | 0.085 | 0.053 |
| PLT(*109/L) | 209[174;240] | 209[179;252.5] | 225[166;271] | 201[127;227.5] | 0.866 | 0.301 |
| FT3(pmol/l) | 4.32[3.99;4.82] | 4.34[4.175;5.7425] | 3.99[3.515;4.245] | 4.72[3.60;5.2] | 0.110 | 0.591 |
| FT4(pmol/l) | 15.7[13.94;16.3] | 15.9[12.7;19.66] | 15.8[15.3;17.6] | 14.3[11.45;16.25] | 0.926 | 0.383 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; HDLc, high density lipoprotein cholesterol; LDLc, low density lipoprotein cholesterol; INR, international normalized ratio; BNP, Brain Natriuretic Peptide. IVS, Interventricular septum thickness; LVDd, Left ventricular end diastolic diameter; LVDs, Left ventricular end systolic diameter; RA, right atrium diameter; LAD, left atrial diameter; RVAW, right ventricular anterior wall thickness; RVDd, right ventricular end diastolic diameter; AoD, aortic diameter; PAD, pulmonary artery diameter; LVEF, Left Ventricular Ejection Fraction; IVC, Inferior caval vein diameter; CO2CP, carbon dioxide combining power; TP, total protein; ALT, alanine aminotransferase; IBIL, indirect bilirubin; CB, conjugated bilirubin; AST, aspartate aminotransferase; TBIL, total bilirubin; ALP, Alkaline phosphatase; γ-GGT, γ-glutamyl transpeptidase; TG, triglyceride; BUN, urea nitrogen; Cr, creatinine; Ua, uric acid; WBC, White blood cell count; NEUT, Neutrophil count; LY, Lymphocyte count; Mono, Monocyte count; PLT, platelet count; FT3, Free T3; FT4, Free T4
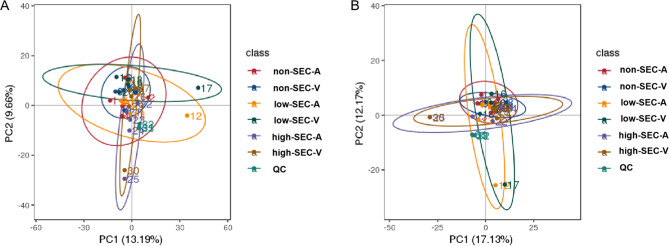
Metabolomics data quality assessment
Constructing PCA models for quality control (QC) samples and plasma samples in positive and negative modes in LC-MS. In the study, the QC samples exhibited good clustering, and all plasma samples fell within the 95% confidence interval in the PCA model, indicating minimal systematic errors during sample handling and detection processes (Fig. 2A and B). The metabolic profile of the plasma sample aligns with a typical chromatogram (Supplementary Fig. 1A-D). A total of 913 distinct metabolites were identified under the positive mode in LC/MS analysis(Supplementary Fig. 2A), while the negative mode, 470 different metabolites were detected(Supplementary Fig. 2B).The metabolites encompass various classes, including lipids and like-lipid molecules, organic acids and their derivatives, aromatic compounds, nucleosides, nucleotides and analogs, heterocyclic organic compounds, organic oxides, phenylpropanoids and polyketides, organic halogen compounds, organic nitrogen compounds, alkaloids and their derivatives, organic sulfur compounds, and more(Supplementary Fig. 2).
Fig. 2.
PCA plots of all plasma samples and QC samples in positive mode (A)and negative mode (B). The QC samples exhibited good clustering, and all plasma samples fell within the 95% confidence interval in the PCA model. non-SEC-A, atrial plasma of non-SEC; non-SEC-V, venous plasma of non-SEC; low-SEC-A, atrial plasma of low-SEC; low-SEC-V, venous plasma of low-SEC; high-SEC-A, atrial plasma of high-SEC; high-SEC-A, atrial plasma of high-SEC; high-SEC-V, venous plasma of high-SEC
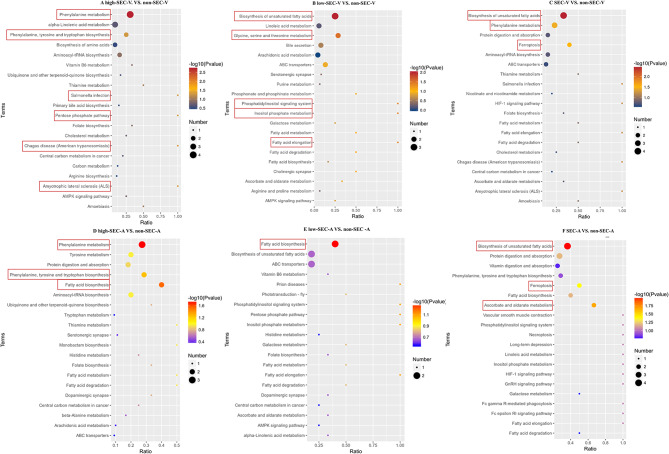
Pathway investigation of differential metabolites
First, the heat map showed that the venous and atrial plasma metabolism patterns of patients in the low-SEC group and high-SEC group were changed compared with the non-SEC group, and with the aggravation of the degree of SEC, the metabolomics changes were more and more, and the changes in atrial plasma were more obvious than those in venous(Fig. 3).This suggested that changes in metabolomics may play an important role in the occurrence and development of SEC, so we further explored the pathway analysis of differential metabolites. KEGG analysis revealed that the metabolic pathway was most altered in the high-SEC group compared to the non-SEC group, regardless of venous or atrial plasma(P<0.05)(Fig. 4). In the comparison between the high-SEC group and the non-SEC group, both venous and atrial plasma metabolic pathways were enriched in Phenylalanine metabolism and Phenylalanine, Tyrosine, and Tryptophan biosynthesis(Fig. 4A and D).This suggested that the metabolism of Phenylalanine, Tyrosine, and Tryptophan is markedly influential in the pathogenesis and development of SEC. Additionally, in the venous plasma metabolism, significant changes were also observed in the Pentose phosphate pathway, Amyotrophic Lateral Sclerosis (ALS), Salmonella infection, and Chagas disease (American trypanosomiasis) pathways, while in atrial plasma, notable alterations were detected in Fatty acid biosynthesis(Fig. 4A and D). In the low-SEC group vs. the non-SEC group, the metabolic pathways related to fatty acid metabolism were the most significantly changed in the venous and atrial plasma(Fig. 4B and E) .For the SEC group (low-SEC group plus high-SEC group) VS. non-SEC group, both venous and atrial plasma metabolic pathways were enriched in the Biosynthesis of unsaturated fatty acids and Ferroptosis, with the atrial plasma exhibiting the most substantial changes (Fig. 4C and F).The findings above indicated that distinct metabolic dysregulation patterns were presented at different stages of SEC.
Fig. 3.
Heatmap of metabolic patterns in venous and atrial plasma in positive(left) and negative(right) modes. The metabolism patterns of patients in the low-SEC group and high-SEC group were changed compared with the non-SEC group, and with the aggravation of the degree of SEC, the metabolomics changes were more and more, but the changes in atrial plasma were more obvious than those in venous. non-SEC-A, atrial plasma of non-SEC; non-SEC-V, venous plasma of non-SEC; low-SEC-A, atrial plasma of low-SEC; low-SEC-V, venous plasma of low-SEC; high-SEC-A, atrial plasma of high-SEC; high-SEC-A, atrial plasma of high-SEC; high-SEC-V, venous plasma of high-SEC
Fig. 4.
KEGG analysis of differential metabolites. KEGG pathways that are significantly different are selected in the box. non-SEC-A, atrial plasma of non-SEC; non-SEC-V, venous plasma of non-SEC; low-SEC-A, atrial plasma of low-SEC; low-SEC-V, venous plasma of low-SEC; high-SEC-A, atrial plasma of high-SEC; high-SEC-A, atrial plasma of high-SEC; high-SEC-V, venous plasma of high-SEC
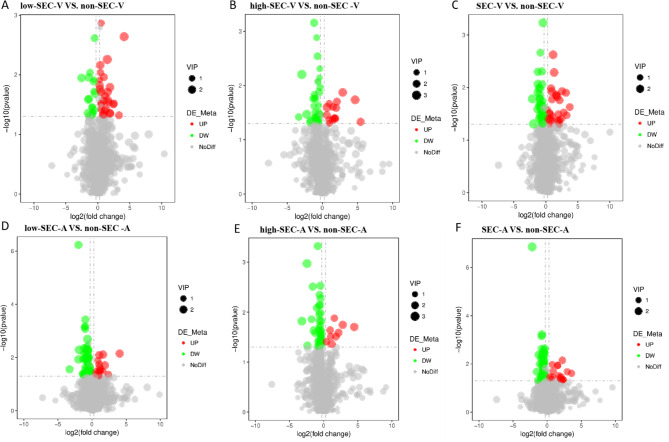
Inter-group comparison and screening of differential metabolites
We created a Venn diagram to represent the changes in differential metabolites between the different comparison groups (Fig. 5). Based on the results of statistical analysis and the criteria for the initial screening of metabolites that have been specified, in venous plasma samples, a total of 46 differential metabolites were identified from the low-SEC group vs. non-SEC group(Fig. 6A, Supplementary Table 1), including 29 elevated metabolites(Fig. 5A) and 17 decreased metabolites(Fig. 5B), 48 differential metabolites were identified from the high-SEC group vs. non-SEC group(Fig. 6B, Supplementary Table 2), including 15 elevated metabolites(Fig. 5A) and 33 decreased metabolites(Fig. 5B), and 74 differential metabolites were identified from the SEC group vs. non-SEC group(Fig. 6C, Supplementary Table 3), including 28 elevated metabolites(Fig. 5A) and 46 decreased metabolites(Fig. 5B).In the atrial plasma samples, 69 differential metabolites were identified from the low-SEC group vs. non-SEC group(Fig. 6D, Supplementary Table 4), including 16 elevated metabolites(Fig. 5C) and 53 decreased metabolites(Fig. 5D), 51 differential metabolites were identified from the high-SEC group vs. non-SEC group(Fig. 6E, Supplementary Table 5), including 9 elevated metabolites(Fig. 5C) and 42 decreased metabolites(Fig. 5D), and 84 differential metabolites were identified from the SEC group vs. non-SEC group(Fig. 6F, Supplementary Table 6), including 17 elevated metabolites(Fig. 5C) and 67 decreased metabolites(Fig. 5D).
Fig. 5.
Volcano diagram of differential metabolites. The abscissa indicates the expression times of metabolites in different groups number change (log2FC), the ordinate represents the difference significance level (-log10(p-value)), and each point in the plot represents one metabolite, the size of the dots represent VIP values, metabolites that are significantly upregulated are indicated by red dots, and metabolites that are significantly downregulated objects are indicated by green dots. In venous plasma samples, a total of 46 differential metabolites were identified from the low-SEC group vs. non-SEC group including 29 elevated metabolites and 17 decreased metabolites (A), 48 differential metabolites were identified from the high-SEC group vs. non-SEC group, including 15 elevated metabolites and 33 decreased metabolites (B), and 74 differential metabolites were identified from the SEC group vs. non-SEC group, including 28 elevated metabolites and 46 decreased metabolites (C).In the atrial plasma samples, 69 differential metabolites were identified from the low-SEC group vs. non-SEC group including 16 elevated metabolites and 53 decreased metabolites (D), 51 differential metabolites were identified from the high-SEC group vs. non-SEC group, including 9 elevated metabolites and 42 decreased metabolites (E), and 84 differential metabolites were identified from the SEC group vs. non-SEC group, including 17 elevated metabolites and 67 decreased metabolites (F). non-SEC-A, atrial plasma of non-SEC; non-SEC-V, venous plasma of non-SEC; low-SEC-A, atrial plasma of low-SEC; low-SEC-V, venous plasma of low-SEC; high-SEC-A, atrial plasma of high-SEC; high-SEC-A, atrial plasma of high-SEC; high-SEC-V, venous plasma of high-SEC
Fig. 6.
The Venn plots to reflect the changes in differential metabolites between different comparison groups. non-SEC-A, atrial plasma of non-SEC; non-SEC-V, venous plasma of non-SEC; low-SEC-A, atrial plasma of low-SEC; low-SEC-V, venous plasma of low-SEC; high-SEC-A, atrial plasma of high-SEC; high-SEC-A, atrial plasma of high-SEC; high-SEC-V, venous plasma of high-SEC
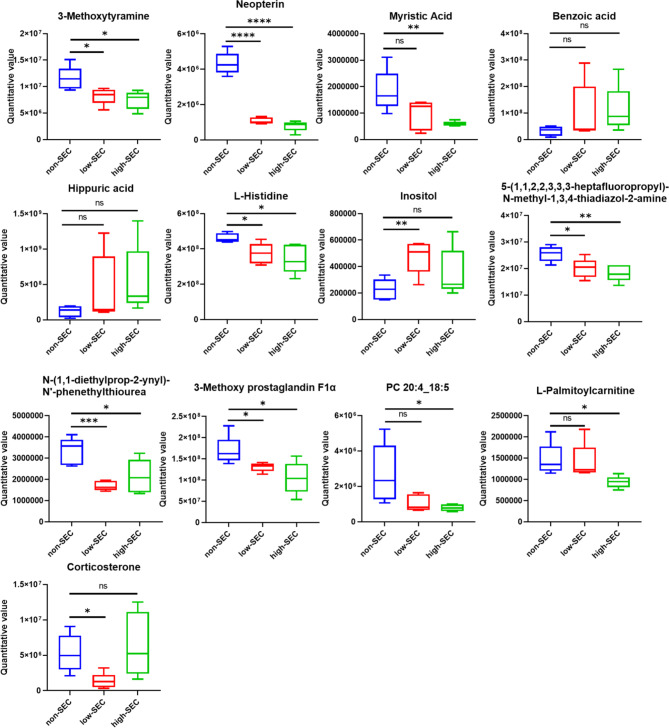
To identify the pivotal metabolites may involve in the onset and progression of SEC, we assigned different weight points to the differential metabolites based on diverse criteria, ultimately selecting those with a cumulative weight score of two or higher. Rule 1: Metabolites present in 2 or 3 comparison groups were assigned a score of 0.5 and 1, respectively. In venous plasma, five metabolites were concurrently present across the three groups (high-SEC vs. non-SEC, low-SEC vs. non-SEC, SEC vs. non-SEC), while 35 metabolites were found in two of the groups. In atrial, 6 metabolites were concurrently identified in the three of groups (high-SEC vs. non-SEC, low-SEC vs. non-SEC, SEC vs. non-SEC), with 50 metabolites present in two groups concurrently. Rule 2: The metabolites of VIP > 2 was given weight points of 1; In both venous and atrial plasma, 55 and 49 metabolites, respectively, were filtered out in accordance with the established criteria. Rule 3: Metabolites present in the KEGG-enrichment pathway were assigned a weight score of 1. Within the venous and atrial plasma, respectively, 12 and 27 metabolites conformed to the specified criteria. Based on a cumulative total of weight scores of ≥ 2, 13 key metabolites were respectively selected from both the venous and atrial plasma (Tables 2 and 3). The expression levels were depicted in the figures (Figs. 7 and 8). As indicated by the Venn diagram(Fig. 9), three metabolites: Benzoic acid、Inositol and 5-(1,1,2,2,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine are all present in both the venous and atrial plasma.
Table 2.
Venous plasma differential metabolite screening
| Metabolites_ID | Name | Venn | VIP > 2 | KEGG | weight point | Up_Down# |
|---|---|---|---|---|---|---|
| Com_6047_neg | Lignoceric Acid | ○ | ◆ | ■ | 2.5 | down |
| Com_192_neg | 8Z,11Z,14Z-Eicosatrienoic acid | ○ | ◆ | ■ | 2.5 | down |
| Com_1152_neg | 2-Hydroxyhippuric acid | ○ | ◆ | ■ | 2.5 | up |
| Com_11014_neg | 5-(1,1,2,2,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine | ● | ◆ | 2 | down | |
| Com_24259_pos | N-Acetyl-Dl-glutamic acid | ● | ◆ | 2 | up | |
| Com_10599_pos | 3’-Adenosine monophosphate (3’-AMP) | ● | ◆ | 2 | up | |
| Com_889_neg | Lauric acid ethyl ester | ● | ◆ | 2 | down | |
| Com_20378_pos | PE 19:2_19:2 | ● | ◆ | 2 | down | |
| Com_12898_pos | D-Erythrose 4-phosphate | ◆ | ■ | 2 | up | |
| Com_360_pos | Benzoic acid | ◆ | ■ | 2 | up | |
| Com_779_pos | Choline | ◆ | ■ | 2 | up | |
| Com_100_pos | Creatine | ◆ | ■ | 2 | down | |
| Com_20691_neg | Inositol | ◆ | ■ | 2 | up | |
| Com_11053_pos | PC 18:1_20:5 | ○ | ◆ | 1.5 | down | |
| Com_22568_pos | PC O-38:8 | ○ | ◆ | 1.5 | down | |
| Com_801_neg | FAHFA 16:0/18:2 | ○ | ◆ | 1.5 | down | |
| Com_6959_pos | Linolelaidic Acid (C18:2N6T) | ○ | ◆ | 1.5 | up | |
| Com_350_neg | 11(E)-Eicosenoic Acid | ○ | ◆ | 1.5 | down | |
| Com_953_pos | methyl 3,4,5-trihydroxycyclohex-1-ene-1-carboxylate | ○ | ◆ | 1.5 | up | |
| Com_8383_neg | PE O-20:1_20:4 | ○ | ◆ | 1.5 | down | |
| Com_9351_neg | Neopterin | ○ | ◆ | 1.5 | down | |
| Com_4402_neg | 2-[2-(3,4-dichloro-2-cyanophenyl)hydrazono]malononitrile | ○ | ◆ | 1.5 | up | |
| Com_41_neg | Hippuric acid | ○ | ◆ | 1.5 | up | |
| Com_24272_pos | Glycerol 1-hexadecanoate | ○ | ◆ | 1.5 | up | |
| Com_1318_pos | PC 16:0_17:1 | ○ | ◆ | 1.5 | down | |
| Com_5086_pos | Linustatin | ○ | ◆ | 1.5 | up | |
| Com_1356_neg | L-Tyrosine | ○ | ◆ | 1.5 | down | |
| Com_3598_pos | LPC 36:3 | ○ | ◆ | 1.5 | down | |
| Com_1316_neg | (3beta,9xi)-3-(beta-D-Glucopyranosyloxy)-14-hydroxycard-20(22)-enolide | ○ | ◆ | 1.5 | up | |
| Com_13931_pos | Dl-2-Amino-3-phosphonopropionic acid | ○ | ◆ | 1.5 | up | |
| Com_11652_pos | Di(2-ethylhexyl) phthalate | ○ | ◆ | 1.5 | up | |
| Com_292_neg | DL-Malic acid | ○ | ◆ | 1.5 | down | |
| Com_5249_neg | FAHFA 18:3/16:2 | ○ | ◆ | 1.5 | down | |
| Com_25_neg | Palmitic acid | ○ | ■ | 1.5 | down | |
| Com_25968_pos | L-arginine | ○ | ■ | 1.5 | down | |
| Com_24425_pos | ethyl 2-[4,6-dimethyl-3-oxoisothiazolo[5,4-b]pyridin-2(3 H)-yl]acetate | ◆ | 1 | up | ||
| Com_7366_neg | FAHFA 16:0/22:5 | ◆ | 1 | down | ||
| Com_890_pos | 5-(2,4-dichlorobenzyl)-2-mercapto-4,6-dimethylnicotinonitrile | ◆ | 1 | up | ||
| Com_2780_pos | PC 17:0_18:4 | ◆ | 1 | down | ||
| Com_2181_neg | PG O-10:0_18:3 | ◆ | 1 | down | ||
| Com_9698_pos | LPC 19:0 | ◆ | 1 | down | ||
| Com_16106_pos | PC 17:0_18:3 | ◆ | 1 | down | ||
| Com_3742_pos | (2R,3 S,4 S,5R,6R)-2-(hydroxymethyl)-6-(2-phenylethoxy)oxane-3,4,5-triol | ◆ | 1 | up | ||
| Com_11066_pos | PC 19:0_20:4 | ◆ | 1 | down | ||
| Com_27_neg | Glycochenodeoxycholic acid | ◆ | 1 | down | ||
| Com_10664_pos | PC 37:6 | ◆ | 1 | down | ||
| Com_19801_pos | PC O-39:0 | ◆ | 1 | up | ||
| Com_1380_pos | SDMA | ◆ | 1 | up | ||
| Com_6191_neg | LPC O-18:2 | ◆ | 1 | up | ||
| Com_3425_pos | 5,6-dimethyl-4-oxo-4 H-pyran-2-carboxylic acid | ◆ | 1 | up | ||
| Com_4653_pos | MAG (18:2) | ◆ | 1 | up | ||
| Com_20804_pos | Fosfomycin | ◆ | 1 | up | ||
| Com_7640_pos | PC 35:4 | ◆ | 1 | down | ||
| Com_9830_pos | PC 16:0_18:5 | ◆ | 1 | down | ||
| Com_21545_pos | 1-Methylguanosine | ◆ | 1 | up | ||
| Com_2850_pos | Tetrahydrocortisone | ◆ | 1 | up | ||
| Com_7374_pos | 2-Phenylglycine | ◆ | 1 | up | ||
| Com_142_neg | Adrenic acid | ■ | 1 | down | ||
| Com_3270_pos | L-Cystine | ■ | 1 | up | ||
| Com_4999_pos | 15-Deoxy-Δ12,14-prostaglandin J2-2-glycerol ester | ○ | 0.5 | up | ||
| Com_5823_pos | N-(cyclopropylmethyl)-N’-phenylurea | ○ | 0.5 | up | ||
| Com_8882_pos | Ouabain | ○ | 0.5 | up | ||
| Com_45_pos | DL-Stachydrine | ○ | 0.5 | up | ||
| Com_4409_pos | PC 34:1 | ○ | 0.5 | down | ||
| Com_9692_pos | PC O-37:6 | ○ | 0.5 | down | ||
| Com_331_pos | 2-Hydroxyphenylalanine | ○ | 0.5 | down | ||
| Com_9156_pos | LPC 40:6 | ○ | 0.5 | down | ||
| Com_17518_neg | PE O-16:1_22:5 | ○ | 0.5 | down | ||
| Com_260_pos | 2-Hydroxycinnamic acid | ○ | 0.5 | down |
○represented the metabolites that are present between the two comparison groups and was given a weighted score of 0.5. ●represented the metabolites that are present between the three comparison groups and was given a weighted score of 1. ◆represents the metabolite of VIP ≥ 2 and was given a weighted score of 1. ■represents the metabolites enriched in the KEGG pathway and was given a weighted score of 1. #,compared to the non-SEC group
Table 3.
Atrial plasma differential metabolite screening
| Metabolites_ID | Name | Venn | VIP > 2 | KEGG | weight point | Up_Down# |
|---|---|---|---|---|---|---|
| Com_6944_pos | 3-Methoxytyramine | ● | ◆ | ■ | 3 | down |
| Com_9351_neg | Neopterin | ● | ◆ | ■ | 3 | down |
| Com_14927_neg | Myristic Acid | ○ | ◆ | ■ | 2.5 | down |
| Com_360_pos | Benzoic acid | ○ | ◆ | ■ | 2.5 | up |
| Com_41_neg | Hippuric acid | ○ | ◆ | ■ | 2.5 | up |
| Com_534_pos | L-Histidine | ○ | ◆ | ■ | 2.5 | down |
| Com_20691_neg | Inositol | ○ | ◆ | ■ | 2.5 | down |
| Com_11014_neg | 5-(1,1,2,2,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine | ● | ◆ | 2 | down | |
| Com_14291_pos | N-(1,1-diethylprop-2-ynyl)-N’-phenethylthiourea | ● | ◆ | 2 | down | |
| Com_1493_pos | 3-Methoxy prostaglandin F1α | ● | ◆ | 2 | down | |
| Com_16682_pos | PC 20:4_18:5 | ● | ◆ | 2 | down | |
| Com_21879_pos | L-Palmitoylcarnitine | ◆ | ■ | 2 | down | |
| Com_4640_pos | Corticosterone | ◆ | ■ | 2 | up | |
| Com_11492_pos | PC O-40:4 | ○ | ◆ | 1.5 | down | |
| Com_19303_pos | Methandrostenolone | ○ | ◆ | 1.5 | down | |
| Com_2074_pos | Homoarginine | ○ | ◆ | 1.5 | down | |
| Com_209_pos | Indole | ○ | ■ | 1.5 | down | |
| Com_24272_pos | Glycerol 1-hexadecanoate | ○ | ◆ | 1.5 | up | |
| Com_331_pos | 2-Hydroxyphenylalanine | ○ | ◆ | 1.5 | down | |
| Com_4008_pos | Coumarin | ○ | ◆ | 1.5 | down | |
| Com_467_pos | LPC 20:2-SN1 | ○ | ◆ | 1.5 | down | |
| Com_4674_pos | 3-Indoleacrylic acid | ○ | ◆ | 1.5 | down | |
| Com_5086_pos | Linustatin | ○ | ◆ | 1.5 | up | |
| Com_795_pos | N-Benzylformamide | ○ | ◆ | 1.5 | down | |
| Com_1104_pos | N-[2-chloro-6-(trifluoromethoxy)phenyl]-2,2-dimethylpropanamide | ○ | ◆ | 1.5 | down | |
| Com_192_neg | 8Z,11Z,14Z-Eicosatrienoic acid | ○ | ◆ | 1.5 | up | |
| Com_2475_pos | PC O-20:3 | ○ | ◆ | 1.5 | down | |
| Com_25_neg | Palmitic acid | ○ | ■ | 1.5 | down | |
| Com_2782_neg | FAHFA 16:1/18:3 | ○ | ◆ | 1.5 | down | |
| Com_292_neg | DL-Malic acid | ○ | ◆ | 1.5 | down | |
| Com_5249_neg | FAHFA 18:3/16:2 | ○ | ◆ | 1.5 | down | |
| Com_7267_pos | ethyl 3,5-dichloro-4-[(2,2,2-trifluoroacetyl)amino]benzoate | ○ | ◆ | 1.5 | up | |
| Com_733_pos | DLK | ○ | ◆ | 1.5 | down | |
| Com_99_pos | PC O-18:0 | ○ | ◆ | 1.5 | down | |
| Com_10007_pos | 16α-Hydroxydehydroepiandrosterone | ◆ | 1 | down | ||
| Com_10132_pos | PC O-32:1 | ◆ | 1 | up | ||
| Com_11786_neg | Boldione | ◆ | 1 | down | ||
| Com_1356_neg | L-Tyrosine | ■ | 1 | down | ||
| Com_1360_pos | LPC 36:2 | ◆ | 1 | up | ||
| Com_14791_pos | 19-Nortestosterone | ◆ | 1 | down | ||
| Com_20021_pos | Prostaglandin B2 | ■ | 1 | up | ||
| Com_24505_pos | Ethyl paraben | ◆ | 1 | down | ||
| Com_3204_pos | bicyclo[2.2.2]oct-2-en-1-yl 4-methylbenzene-1-sulfonate | ◆ | 1 | down | ||
| Com_370_pos | LPC O-16:0 | ◆ | 1 | down | ||
| Com_3961_pos | HPK | ◆ | 1 | down | ||
| Com_4455_pos | 3-(3-morpholinopropyl)-2-(2-pyridinyl)-2,3-dihydro-4(1 H)-quinazolinone | ◆ | 1 | down | ||
| Com_9328_pos | Palmitoleic Acid | ■ | 1 | up | ||
| Com_12898_pos | D-Erythrose 4-phosphate | ■ | 1 | up | ||
| Com_1316_neg | (3beta,9xi)-3-(beta-D-Glucopyranosyloxy)-14-hydroxycard-20(22)-enolide | ◆ | 1 | down | ||
| Com_13482_pos | Gamithromycin | ◆ | 1 | up | ||
| Com_20378_pos | PE 19:2_19:2 | ◆ | 1 | up | ||
| Com_21545_pos | 1-Methylguanosine | ◆ | 1 | down | ||
| Com_3610_neg | 13(S)-HOTrE | ■ | 1 | down | ||
| Com_3615_neg | Porphobilinogen | ■ | 1 | down | ||
| Com_4424_pos | TKK | ◆ | 1 | down | ||
| Com_761_neg | Prostaglandin A2 | ■ | 1 | up | ||
| Com_7677_pos | Etiocholanolone | ■ | 1 | down | ||
| Com_8878_pos | Berberine | ■ | 1 | up | ||
| Com_90_neg | Stearic acid | ■ | 1 | down | ||
| Com_141_neg | Docosapentaenoic acid | ■ | 1 | down | ||
| Com_142_neg | Adrenic acid | ■ | 1 | down | ||
| Com_20146_pos | 2-hydroxy-3,6-diphenylcyclohexyl acetate | ◆ | 1 | down | ||
| Com_24889_pos | L-Ascorbate | ■ | 1 | down | ||
| Com_2696_pos | Pyridoxamine 5-phosphate | ■ | 1 | down | ||
| Com_46_neg | Arachidonic acid | ■ | 1 | down | ||
| Com_8635_pos | Papaverine | ◆ | 1 | down | ||
| Com_9058_neg | L-Tryptophan | ■ | 1 | down | ||
| Com_10_pos | DL-Tryptophan | ○ | 0.5 | down | ||
| Com_11165_pos | Indole-3-acrylic acid | ○ | 0.5 | down | ||
| Com_1446_neg | (+/-)9(10)-EpOME | ○ | 0.5 | down | ||
| Com_167_pos | 6-Methylquinoline | ○ | 0.5 | down | ||
| Com_17576_pos | LysoPE 18:2 | ○ | 0.5 | down | ||
| Com_260_pos | 2-Hydroxycinnamic acid | ○ | 0.5 | down | ||
| Com_542_pos | 5-acetyl-2,6-dimethyl-1,2,3,4-tetrahydropyridin-4-one | ○ | 0.5 | down | ||
| Com_6300_pos | Gly-Tyr | ○ | 0.5 | down | ||
| Com_7994_pos | N-(2-morpholinophenyl)-2-furamide | ○ | 0.5 | down | ||
| Com_824_pos | Skatole | ○ | 0.5 | down | ||
| Com_890_pos | 5-(2,4-dichlorobenzyl)-2-mercapto-4,6-dimethylnicotinonitrile | ○ | 0.5 | up | ||
| Com_12314_neg | FAHFA 20:1/20:2 | ○ | 0.5 | down | ||
| Com_16790_neg | 2’-Deoxyinosine | ○ | 0.5 | up | ||
| Com_1698_neg | Xanthohumol | ○ | 0.5 | down | ||
| Com_212_neg | 16-Hydroxyhexadecanoic acid | ○ | 0.5 | down | ||
| Com_2181_neg | PG O-10:0_18:3 | ○ | 0.5 | down | ||
| Com_350_neg | 11(E)-Eicosenoic Acid | ○ | 0.5 | down | ||
| Com_3504_pos | LPC 19:1-SN1 | ○ | 0.5 | up | ||
| Com_400_neg | 11(Z),14(Z)-Eicosadienoic Acid | ○ | 0.5 | down | ||
| Com_4402_neg | 2-[2-(3,4-dichloro-2-cyanophenyl)hydrazono]malononitrile | ○ | 0.5 | down | ||
| Com_6872_neg | Docosatrienoic Acid | ○ | 0.5 | up | ||
| Com_889_neg | Lauric acid ethyl ester | ○ | 0.5 | down | ||
| Com_9476_neg | FAHFA 18:0/3:0 | ○ | 0.5 | down | ||
| Com_7366_neg | FAHFA 16:0/22:5 | ○ | 0.5 | up | ||
| Com_15726_pos | 4-Hydroxyretinoic Acid | 0 | down | |||
| Com_16455_neg | LPA 18:0 | 0 | down | |||
| Com_2179_neg | CerP 15:0;2O/10:0 | 0 | down | |||
| Com_2593_pos | CAR 20:3 | 0 | down | |||
| Com_298_pos | DL-Lysine | 0 | down | |||
| Com_10125_pos | cyclohexyl{4-[4-nitro-2-(1 H-pyrrol-1-yl)phenyl]piperazino}methanone | 0 | down | |||
| Com_1027_pos | 13-HPODE | 0 | down | |||
| Com_1063_neg | trans-10-Heptadecenoic Acid | 0 | down | |||
| Com_11354_pos | LPC 42:12-SN1 | 0 | up | |||
| Com_1265_pos | LPC 20:1 | 0 | up | |||
| Com_13598_pos | PC O-38:9 | 0 | down | |||
| Com_157_pos | MAG (18:3) | 0 | down | |||
| Com_19370_pos | Lysopa 18:0 | 0 | up | |||
| Com_1951_neg | FAHFA 18:0/20:2 | 0 | down | |||
| Com_20671_neg | IDP | 0 | down | |||
| Com_21937_neg | O1-(4-chlorobenzoyl)-4-nitrobenzene-1-carbohydroximamide | 0 | down | |||
| Com_225_neg | 4-[2-(2-oxo-1-imidazolidinyl)ethyl]-1lambda ~ 6~,4-thiazinane-1,1-dione | 0 | down | |||
| Com_232_pos | LPC 20:1-SN1 | 0 | up | |||
| Com_2674_pos | 2-(Methylthio)benzothiazole | 0 | up | |||
| Com_2768_neg | (+/-)8(9)-DiHET | 0 | down | |||
| Com_309_neg | (±)9-HpODE | 0 | down | |||
| Com_4634_neg | Chenodeoxycholic acid-3-beta-D-glucuronide | 0 | up | |||
| Com_5197_neg | 1-(2,4-diphenyl-2,3-dihydro-1 H-1,5-benzodiazepin-1-yl)propan-1-one | 0 | up | |||
| Com_5207_pos | Ingenol-3-angelate | 0 | up | |||
| Com_5619_neg | MGDG O-24:4_16:0 | 0 | up | |||
| Com_577_neg | LPC 22:5 | 0 | down | |||
| Com_597_pos | 2-Arachidonoyl glycerol | 0 | up | |||
| Com_6468_pos | Nervonic ceramide | 0 | down | |||
| Com_683_pos | methyl 2-(acetylamino)-4-amino-4-oxobutanoate | 0 | down | |||
| Com_698_pos | LPC 22:5-SN1 | 0 | up | |||
| Com_869_neg | Ne-(1-Carboxymethyl)-L-lysine | 0 | down | |||
| Com_1078_pos | 2-methyl-2,3,4,5-tetrahydro-1,5-benzoxazepin-4-one | 0 | down | |||
| Com_12312_neg | beta-Nicotinamide mononucleotide | 0 | down | |||
| Com_12460_pos | N1-[1-(2,4-dichlorobenzoyl)-4-piperidyl]cyclohexane-1-carboxamide | 0 | up | |||
| Com_15605_neg | N3-cyclohexyl-3-azabicyclo[3.2.2]nonane-3-carbothioamide | 0 | down | |||
| Com_20327_pos | 4-Pregnen-17alpha,20alpha-Diol-3-One | 0 | up | |||
| Com_2178_pos | CAR 26:0 | 0 | down | |||
| Com_26805_pos | Alloxan | 0 | up | |||
| Com_2773_neg | ST 28:1;O; Hex; FA 18:2 | 0 | down | |||
| Com_3881_pos | CAR 26:2 | 0 | down | |||
| Com_4240_pos | 1,7-bis(4-hydroxyphenyl)heptan-3-one | 0 | down | |||
| Com_437_pos | LPC 20:3-SN1 | 0 | down | |||
| Com_4829_pos | Chlortetracycline | 0 | down | |||
| Com_4888_pos | Maslinic acid | 0 | down | |||
| Com_4999_pos | 15-Deoxy-Δ12,14-prostaglandin J2-2-glycerol ester | 0 | up | |||
| Com_5823_pos | N-(cyclopropylmethyl)-N’-phenylurea | 0 | up | |||
| Com_6671_pos | Asp-Phe | 0 | up | |||
| Com_7400_neg | FAHFA 4:0/17:2 | 0 | up | |||
| Com_8329_pos | N-[1-(4-methoxy-2-oxo-2 H-pyran-6-yl)-2-methylbutyl]acetamide | 0 | down | |||
| Com_8882_pos | Ouabain | 0 | up | |||
| Com_953_pos | methyl 3,4,5-trihydroxycyclohex-1-ene-1-carboxylate | 0 | up |
○ represented the metabolites that are present between the two comparison groups and was given a weighted score of 0.5. ● represented the metabolites that are present between the three comparison groups and was given a weighted score of 1. ◆ represents the metabolite of VIP ≥ 2 and was given a weighted score of 1. ■ represents the metabolites enriched in the KEGG pathway and was given a weighted score of 1. #,compared to the non-SEC group
Fig. 7.
Relative expression levels of differential metabolites among groups. Based on a cumulative total of weight scores of ≥ 2, 13 key metabolites were selected from the venous plasma. The expression levels are depicted in the figure
Fig. 8.
Relative expression levels of differential metabolites among groups. Based on a cumulative total of weight scores of ≥ 2, 13 key metabolites were selected from the atrial plasma. The expression levels are depicted in the figure
Fig. 9.

Metabolites selected from the venous and atrial plasma. Three metabolites: Benzoic acid、Inositol and 5-(1,1,2,2,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine are all present in both the venous and atrial plasma
Discussion
Spontaneous echo contrast (SEC) is considered to be a pre-thrombotic state in the atrial of patients with AF, and it is likely to progress to thrombosis eventually, which is very harmful [9]. The occurrence and development of SEC may require the participation of many metabolites, so exploring the metabolomic changes of SEC can protect the AF patients from thrombosis and provide ideas for the development of new antithrombotic drugs. But so far, few studies have systematically reported SEC-related metabolomic changes in patients with AF, and our study explored not only venous plasma metabolomic changes in patients with SEC atrial fibrillation, but also atrial plasma. This paper attempts to explore the characteristics of SEC-related metabolic changes from different perspectives, so as to provide a basis for the prevention and treatment of SEC.
In this study, patients with AF who completed TEE testing were divided into three groups: non-SEC group, low-SEC group, and high-SEC group according to the different degrees of SEC, and a comprehensive analysis of venous and atrial plasma metabolomics was performed. Our metabolic phenotype revealed a clear differential metabolic pattern among the three groups (Fig. 3). Specifically, we identified 35 and 142 significantly differential metabolites in venous and atrial plasma, respectively (Fig. 5, Supplementary Tables 1–6), suggesting that SEC may be involved in pervasive metabolic dysregulation and that the degree of metabolic dysregulation in atrial plasma is more severe than that in venous blood. Then, according to the rule of VIP ≥ 2, we screened 13 significant differential metabolites from atrial and venous plasma, respectively, and it is worth noting that Benzoic acid, Inositol and 5-(1,1,2,2,3,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine displayed significant alterations in both venous and atrial plasma, potentially indicating their pivotal role in the onset and progression of SEC.
In view of the large number of differential metabolites identified, we will focus on some substances that are most likely to play an important role in the development of the SEC and AF, so as to provide theoretical basis for subsequent research and related drug development. Benzoic acid (C7H6O2, BA), an organic acidulant, is an colorless crystalline solid and constitutes the simplest aromatic carboxylic acid, frequently utilized as a food and pharmaceutical additive [10]. Its absorption and transport predominantly occur in the small intestine [11]. Following oral administration, BA is entirely metabolized to hippuric acid in humans and an additional twenty species of animals, and is subsequently excreted via urine [12]. It has been reported that excessive intake of BA can lead to dysfunction and damage to various organs [13]. In consonance with previous research findings, our study demonstrates that BA and hippuric acid levels are elevated in the SEC group (low-SEC group plus high-SEC group) compared to the non-SEC group, with the most pronounced increase observed in the high-SEC group, suggesting that BA levels may influence the onset and progression of SEC. On the other hand, as an acidulant, BA can lower the pH of body fluids [14–16]. Studies have proved that a reduced pH can affect coagulation system function, potentially leading to a hypercoagulable state [17]. Consequently, it is plausible to infer from our findings that the increased content of BA in the SEC group may lead to a decrease in blood pH, thereby promoting the occurrence and development of SEC. However, it is regrettable that routine clinical examinations for patients do not include pH level assessments. In addition, it has been shown that excess BA can impair normal cell function, which may be related to impaired redox status regulated by the Nrf2 pathway [18], which also plays an important role in the occurrence and development of AF [19, 20]. Furthermore, it has been reported that BA and its derivatives can inhibit the activity of acetylcholinesterase to a certain extent, thereby reducing the degradation of acetylcholine [21–23], and the elevated content of acetylcholine can induce or aggravate atrial fibrillation [24, 25], resulting in a series of pathophysiological effects. From the above evidence, we can speculate that BA may have an impact on the occurrence and development of SEC and AF through multiple pathways.
In particular, it is important to note that dietary intake is one of the primary sources of BA within the human body [26]. Therefore, our results showed that the increase of BA in the SEC group was most likely due to excessive dietary intake, indicating that attention should be paid to the BA content in food, particularly providing crucial dietary recommendations for patients with AF. Our discovery affords evidence for the prevention of SEC and atrial thrombosis in patients with AF through dietary means.
Inositol, apart from regulating intracellular osmolarity in the brain [27], is also a structural component in the formation of phosphatidylinositol and phosphatidylinositides, which act as second messengers. Phosphatidylinositides participate in cellular signal transduction through pathways involving phosphatidylinositol kinases [28, 29], protein kinase C, and nuclear factor κB (NFκB) [30, 31]. NFκB signaling plays a role in the regulation of adhesion molecules, cytokines, chemokines, growth factors and matrix metalloproteinases (MMPs) [32, 33]. Inflammatory cytokines are considered to be associated with AF-related Sect. [34]. Our results similarly support this inference, with elevated levels of inositol observed in the SEC group compared to the control group, which may lead to increased inflammation and subsequently affect the occurrence of SEC. Interestingly, our results showed that while the SEC group had higher levels of inositol than the control group, the increase was significant in the low-SEC group and less in the high-SEC group. This phenomenon reflected that the degree of inositol metabolic disorder was different between the low-SEC group and high-SEC group. Studies have shown that inositol can exert antioxidant properties by scavenging free radicals and increasing the activity of antioxidant enzymes [35, 36]. Oxidative stress is closely related to coagulation, and it plays a role at different aspects of the coagulation process [37]. Besides, oxidative stress contributes to the development of AF [38, 39]. In general, atrial fibrillation, oxidative stress, and coagulation interact with each other, and once the balance is broken, it will lead to the development of pathological states. Therefore, based on our results, it can be inferred that the metabolic disorders of the antioxidant system in the low-SEC group were still in the compensated stage, and can still produce enough inositol to exert antioxidant effects and avoid thrombosis. The antioxidant system of patients in the high group was in the decompensated stage and cannot produce enough inositol, resulting in excessive accumulation of oxidized substances affecting the coagulation system and even thrombus.
Furthermore, the increased inositol can exacerbate the imbalance of MMPs activity and tissue inhibitor of matrix metalloproteinase (TIMP) by upregulating growth factors and inflammatory cytokines, thereby intensifying the structural remodeling of atrial fibrillation [40]. This structural remodeling, in turn, can promote the onset and progression of SEC, creating a vicious cycle [41]. Hence, the metabolic dysregulation of inositol is of significant concern, as its disturbance can simultaneously impact SEC, thrombosis, and atrial structural remodeling.
Inositol can be ingested from food or synthesized in the body [42]. Based on our findings, dietary inositol should be considered due to the growing problems posed by unhealthy diets and living conditions, as they have antioxidant effects, especially in AF patients with SEC.
5-(1,1,2,2,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine, an amine derivative, exhibits an undefined mechanism in the pathogenesis and progression of SEC in AF, although literature suggests it possesses antioxidant properties [43]. The role of 5-(1,1,2,2,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine in medicine is very little, but we can speculate its function based on its chemical structure. Compounds containing 1,3,4-thiadiazol usually have a wide range of biological activities, including anti-inflammatory, anti-tumor, etc [44, 45]. , and 5-(1,1,2,2,3,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine contains the same chemical structure. Therefore, we speculate that it may also have anti-inflammatory effects in addition to antioxidant effects. Importantly, we found that the content of 5-(1,1,2,2,3,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine in the SEC group was significantly reduced, suggesting that the occurrence and development of SEC may be affected by it. Based on the above conjectures about the possible effects of 5-(1,1,2,2,3,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine, we believed that low concentration of it may lead to decreased antioxidant capacity, increased levels of oxidative stress, and possibly increased levels of inflammation, which in turn affected the pathophysiology of SEC and AF. Reports have associated increased oxidative stress in AF patients with SEC, contributing to the development of SEC and thrombosis [46], which supports our hypothesis. There are very few studies about this substance in the field of AF-related treatments, and our research provided a theoretical basis for its feasibility.
Moreover, within the venous plasma metabolites, we identified a group previously implicated as potential metabolites associated with thrombosis in AF, including Lignoceric acid [47], 8Z,11Z,14Z-Eicosatrienoic acid [48, 49], Lauric acid ethyl ester [50, 51], PE 19:2_19:2 [52], Choline [53, 54], Creatine [55], N-Acetyl-DL-glutamic acid [56, 57], D-Erythrose 4-phosphate [58], and 2-Hydroxyhippuric acid, which is a metabolite of salicylic acid and may be related to the administration of drugs such as aspirin.
Additionally, 3’-Adenosine monophosphate (3’-AMP) is derived from the hydrolysis of 2’,3’-cAMP, a compound recently discovered to regulate immune function [59]. Normally, there is a dynamic equilibrium between the metabolism of 2’,3’-cAMP and 3’-AMP. Studies have shown that 3’-AMP has a pro-inflammatory and vasoconstrictive effect [60]. Our study revealed an increase in its concentration within the SEC group, suggesting a possible dysregulation of 3’-AMP metabolism. Because it has a pro-inflammatory effect, an abnormally high level of 3’-AMP in the SEC group may lead to an inflammatory state in the body, which promotes the onset and progression of SEC. This provides a new perspective for the prevention and treatment of SEC in AF patients.
It is worth noting that we also studied the metabolic changes in the atrial plasma that is spatially closer to the left atrium. Their changes may have a more direct impact on the development of SEC. In addition to the previously discussed Benzoic acid, Inositol, and 5-(1,1,2,2,3,3,3-heptafluoropropyl)-N-methyl-1,3,4-thiadiazol-2-amine, the level of 3-Methoxytyramine (3-MT) significantly decreased in the SEC group (as depicted in the Fig. 8). 3-MT is not only a metabolite of dopamine, but also a neuromodulator [61]. Neuroelectrophysiological experiments showed that 3-MT could affect the activity of the dopaminergic nervous system through the trace amine associated receptor 1 (TAAR1), which showed that 3-MT could activate TAAR1 to reduce the spontaneous electrical rate of dopaminergic neurons, otherwise, it could increase the activity of the dopaminergic nervous system [62]. Studies have shown that excessive activity of the dopaminergic nervous system can promote the occurrence of SEC by activating platelets [63]. Combined with the above results, we can surmise that the reduction of 3-MT in the SEC group may increase the activity of the dopaminergic nervous system through TAAR1, which may affect the progression of SEC and AF. Thus, our findings implied that AF patients with SEC may have dopaminergic nervous system dysfunction mediated by 3-MT, and it also provided a basis for the cross-study of nervous system and heart diseases. Patients with AF should pay attention to the regulation of nervous system function.
Neopterin is a pterin analogue produced by the oxidation of 7,8-dihydroneopterin, which is a potent free radical scavenger and antioxidant [64–66]. Therefore, the content of neopterin is negatively correlated with the level of oxidative stress in the body, that is, the level of oxidative stress in the body is high when the neopterin content is low. Our results showed a significant decrease in neopterin content in the SEC group compared to the control group, and combined with previous discussions, we believed that the decrease in neopterin content in the SEC group may be involved in the process of increased oxidative stress levels, which led to the progression of SEC and AF. Over all, our study showed that the reduced neopterin content of AF patients with SEC may means that the body’s antioxidant capacity is reduced, which affected the pathophysiology of SEC and AF.
L-Histidine, an essential amino acid, possesses unique physicochemical properties that make it with acid-base buffering capabilities, thus preventing organ damage from drastic fluctuations in pH [67]. Moreover, it can exert antioxidant effects through chelation of iron ions and singlet oxygen, and it also reduces the viscosity of solutions, enhancing fluidity [68]. The metabolic disorder of histidine in the SEC group, characterized by a significant decrease in its content, may consequently lower the patient’s antioxidant capacity and increase blood viscosity, contributing to the onset and progression of SEC and AF. This suggested a balanced intake of essential amino acids, as they are not only nutrients but also affect the pathophysiological processes of the body.
L-Myristic Acid, with its anti-inflammatory effects [69], was found to be reduced in the SEC group in this study, potentially leading to a decrease in anti-inflammatory action and thus contributing to the onset and progression of SEC. L-Palmitoylcarnitine (L-PC), an important long-chain acylcarnitine, has been shown in previous studies to exert antithrombotic effects by enhancing plasmin and tissue-type plasminogen activator (tPA) activity. Moreover, reduced levels of plasma long-chain acylcarnitines have been associated with venous thrombosis [70]. Our results support these findings, with the change in L-PC levels indicating that carnitine metabolism dysregulation may aid in the occurrence and progression of SEC. The reduction in L-PC content in the SEC group may, like previously reported cases, promote SEC by affecting plasmin and tPA activity. Additionally, we identified significant metabolic abnormalities in atrial plasma for compounds such as N-(1,1-diethylprop-2-ynyl)-N’-phenethylthiourea, 3-Methoxy prostaglandin F1α, PC 20:4_18:5, and Corticosterone, although their specific impact on SEC is yet to be further investigated. These findings suggest that there are multiple metabolic pathways and mediators that may be involved in the pathophysiology of SEC, and that a comprehensive understanding of these mechanisms could lead to better diagnostic and therapeutic strategies for AF patients with SEC.
Conclusion
We reported a comprehensive metabolomics assessment to characterize the metabolic derangement of SEC associated with AF. This study showed a significant change in metabolic patterns in SEC compared to non-SEC. Our work promoted the understanding of mechanism of the occurrence and development of SEC, facilitated the screening of the target metabolites for its therapeutic intervention, and provided evidence support for the prevention and treatment of SEC and thrombosis associated with AF. At the same time, our work also provided a direction for subsequent research in related fields. In conclusion, our study not only provides a theoretical basis for understanding the occurrence and development of SEC in AF, but also provides recommendations for the daily diet of AF patients with SEC, such as a balanced intake of essential amino acids, avoiding excessive intake of benzoic acid, and intake of appropriate inositol.
Limitations
First, our study sample size was relatively small. TEE is the traditional test to determine whether there is atrial thrombosis before AF ablation, but it also has obvious drawbacks, such as esophageal damage and unbearable nausea, etc. Therefore, according to the patient’s preference and for the patient’s safety, most of them chose the more acceptable and less invasive examination—Left Atrium Computed Tomography Angiography test, which led to a sharp decrease in the number of patients eligible for our study. In addition, in order to minimise the impact of diagnostic bias on the results of the study, we rigorously reviewed the results of TEE. Three experienced cardiac sonographers each independently evaluated the same TEE images, and patients were included in the research only if the reports from the three experts were the same, which further limited the number of people included in the research. However, the rigorous selection process made our findings more credible and representative.
The small sample size may restrict the generalizability of the findings, suggesting future studies with larger cohorts. To assess whether the results of our research were representative, we made cross-sectional comparisons with other similar studies. Since there is no research on the characteristics of plasma metabolomics in AF patients with SEC, which is also the innovation of our project, we compared it with other metabolomics studies related to coagulation and found that our sample size is similar to that of most studies [71–79], which indicates that although the small sample size limits the generalizability of the results, it can also reflect the scientific problems to a certain extent and provide a theoretical basis and direction for subsequent research.
Furthermore, we did not employ alternative metabolomic platforms to detect a broader spectrum of metabolites, nor did we quantitate the potential biomarkers absolutely. Lastly, due to the absence of in-depth exploration through molecular biology, cellular biology, and pharmacological methodologies, the metabolic mechanisms underlying disease onset and progression remain undetermined.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- SEC
Spontaneous echo contrast
- AF
Atrial fibrillation
- TEE
Trans esophageal echocardiography examination
- PCA
Principal component analysis
- PLS-DA
Partial least squares discriminant analysis
- VIP
Variable important in projection
- FC
Fold change
- QC
Quality control
- ALS
Amyotrophic Lateral Sclerosis
- BA
Benzoic acid
- NFκB
Nuclear factor κB
- MMPs
Matrix metalloproteinases
- TIMP
Tissue inhibitor of matrix metalloproteinase
- 3’-AMP
3’-Adenosine monophosphate
- 3-MT
3-Methoxytyramine
- L-PC
L-Palmitoylcarnitine
- tPA
Tissue-type plasminogen activator
Author contributions
B.s. , R. S. and X. L. designed the study; B.s. ,X.w. , D.q. , X.y.,R.n.,H.p. and W.h. collected and analyzed the data; B.s. writed the manuscript; G.p., X. L., T. L. and X. L. corrected the initial manuscript; G.p., X. L. and T. L. reviewed and edited the final manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China [grant number 82370320, 81900314], The Science and Technology Development Fund of Tianjin Education Commission for Higher Education (2021KJ227, 2023KJ029), and Tianjin Key Medical Discipline (Specialty) Construction Project [grant number TJYXZDXK-029 A].
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study has been approved by the Ethics Committee of the Second Hospital of Tianjin Medical University (No: KY2023K058) and adheres to the principles of the Helsinki Declaration. All participants are included voluntarily and have signed informed consent forms.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bingshuo Shi and Rong Suo contributed equally to this work.
Contributor Information
Tong Liu, Email: liutongdoc@126.com.
Xing Liu, Email: liuxing0626@163.com.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 update: a Report from the American Heart Association. Circulation. 2019;139(10):e56–528. 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Gramiak R, Shah PM. Detection of intracardiac blood flow by pulsed echo-ranging ultrasound. Radiology. 1971;100(2):415–8. 10.1148/100.2.415. [DOI] [PubMed] [Google Scholar]
- 3.Beppu S, Nimura Y, Sakakibara H, Nagata S, Park YD, Izumi S. Smoke-like echo in the left atrial cavity in mitral valve disease: its features and significance. J Am Coll Cardiol. 1985;6(4):744–9. 10.1016/s0735-1097(85)80476-9. [DOI] [PubMed] [Google Scholar]
- 4.Mohanty S, Torlapati PG, La Fazia VM, Kurt M, Gianni C, MacDonald B, Mayedo A, Allison J, Bassiouny M, Gallinghouse GJ, Burkhardt JD, Horton R, Di Biase L, Al-Ahmad A, Natale A. Best anticoagulation strategy with and without appendage occlusion for stroke-prophylaxis in postablation atrial fibrillation patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. 2024;35(7):1422–8. 10.1111/jce.16308. [DOI] [PubMed] [Google Scholar]
- 5.Fatkin D, Loupas T, Jacobs N, Feneley MP. Quantification of blood echogenicity: evaluation of a semiquantitative method of grading spontaneous echo contrast. Ultrasound Med Biol. 1995;21(9):1191–8. 10.1016/0301-5629(95)02006-3. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Suwa M, Kobashi A, Yagi H, Nakamura T, Miyazaki S, Kitaura Y. Integrated backscatter assessment of left atrial spontaneous echo contrast in chronic nonvalvular atrial fibrillation: relation with clinical and echocardiographic parameters. J Am Soc Echocardiogr. 2000;13(7):666–73. 10.1067/mje.2000.104739. [DOI] [PubMed] [Google Scholar]
- 7.Douketis JD, Spyropoulos AC, Duncan J, Carrier M, Le Gal G, Tafur AJ, Vanassche T, Verhamme P, Shivakumar S, Gross PL, Lee AYY, Yeo E, Solymoss S, Kassis J, Le Templier G, Kowalski S, Blostein M, Shah V, MacKay E, Wu C, Clark NP, Bates SM, Spencer FA, Arnaoutoglou E, Coppens M, Arnold DM, Caprini JA, Li N, Moffat KA, Syed S, Schulman S. Perioperative Management of patients with Atrial Fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179(11):1469–78. 10.1001/jamainternmed.2019.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulman S, Carrier M, Lee AY, Shivakumar S, Blostein M, Spencer FA, Solymoss S, Barty R, Wang G, Heddle N, Douketis JD, Periop Dabigatran Study Group. Perioperative Management of Dabigatran: a prospective cohort study. Circulation. 2015;132(3):167–73. 10.1161/CIRCULATIONAHA.115.015688. [DOI] [PubMed] [Google Scholar]
- 9.Zeng D, Zhang X, Chang S, Zhong Y, Cai Y, Huang T, Wu J. A nomogram for predicting left atrial thrombus or spontaneous echo contrast in non-valvular atrial fibrillation patients using hemodynamic parameters from transthoracic echocardiography. Front Cardiovasc Med. 2024;11:1337853. 10.3389/fcvm.2024.1337853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim GA, Robertson JM, Goodwin TH. The crystal and molecular structure of benzoic acid. Acta Cryst. 1955;8:157–64.
- 11.Cong D, Fong AK, Lee R, Pang KS. Absorption of benzoic acid in segmental regions of the vascularly perfused rat small intestine preparation. Drug Metab Dispos. 2001;29(12):1539–47. [PubMed] [Google Scholar]
- 12.Bridges JW, French MR, Smith RL, Williams RT. The fate of benzoic acid in various species. Biochem J. 1970;118(1):47–51. 10.1042/bj1180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu Y, Yu B, He J, et al. Excess of dietary benzoic acid supplementation leads to growth retardation, hematological abnormality and organ injury of piglets. Livest Sci. 2016;190:94–103. 10.1016/j.livsci.2016.06.010. [Google Scholar]
- 14.Chen J, Yu CD. Effects of benzoic acid on growth performance, organ indexes and gastrointestinal content pH of weaned piglets. Chin J Anim Nutr. 2015;27:238–46. [Google Scholar]
- 15.Gao Z, Zheng YB. Effects of Benzoic acid on intestinal microflora and metabolites of piglets. Chin J Anim Nutr. 2014;26:1044–54. [Google Scholar]
- 16.Diao H, Gao Z, Yu B, Zheng P, He J, Yu J, Huang Z, Chen D, Mao X. Effects of benzoic acid (VevoVitall®) on the performance and jejunal digestive physiology in young pigs. J Anim Sci Biotechnol. 2016;7:32. 10.1186/s40104-016-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorter KA, Stehouwer MC, Van Putte BP, Vlot EA, Urbanus RT. Acidosis induced by carbon dioxide insufflation decreases heparin potency: a risk factor for thrombus formation. Perfusion. 2017;32(3):214–9. 10.1177/0267659116677307. [DOI] [PubMed] [Google Scholar]
- 18.Z G. Regulatory effects of Benzoic Acid on Digestive Physiology and Nutritional Metabolism of Young pigs. Sichuan Agricultural University; 2013.
- 19.Wu L, Li Z, Xu L, Fan Y, Mao D, Sun H, Zhuang W. Nrf2 ameliorates Atrial Fibrosis during Antithrombotic Therapy for Atrial Fibrillation by modulating CYP2C9 activity. J Cardiovasc Pharmacol. 2024;84(4):440–50. 10.1097/FJC.0000000000001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badreldin H, El-Karef A, Ibrahim T, Elshal M. Targeting Nrf2/HO-1 and NF-κB/TNF-α signaling pathways with empagliflozin protects against atrial fibrillation-induced acute kidney injury in rats. Toxicology. 2024;506:153879. 10.1016/j.tox.2024.153879. [DOI] [PubMed] [Google Scholar]
- 21.Zhao ZX, Fu J, Ma SR, Peng R, Yu JB, Cong L, Pan LB, Zhang ZG, Tian H, Che CT, Wang Y, Jiang JD. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics. 2018;8(21):5945–59. 10.7150/thno.28068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalaycı M, Türkeş C, Arslan M, Demir Y, Beydemir Ş. Novel benzoic acid derivatives: synthesis and biological evaluation as multitarget acetylcholinesterase and carbonic anhydrase inhibitors. Arch Pharm (Weinheim). 2021;354(3):e2000282. 10.1002/ardp.202000282. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira C, Bagetta D, Cagide F, Teixeira J, Amorim R, Silva T, Garrido J, Remião F, Uriarte E, Oliveira PJ, Alcaro S, Ortuso F, Borges F. Benzoic acid-derived nitrones: a new class of potential acetylcholinesterase inhibitors and neuroprotective agents. Eur J Med Chem. 2019;174:116–29. 10.1016/j.ejmech.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Mitrokhin V, Hadzi-Petrushev N, Kazanski V, Schileyko S, Kamkina O, Rodina A, Zolotareva A, Zolotarev V, Kamkin A, Mladenov M. The role of KACh channels in Atrial Fibrillation. Cells. 2024;13(12):1014. 10.3390/cells13121014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayer JD, Boukens BJ, Krul SPJ, Roney CH, Driessen AHG, Berger WR, van den Berg NWE, Verkerk AO, Vigmond EJ, Coronel R, de Groot JR. Acetylcholine delays atrial activation to facilitate Atrial Fibrillation. Front Physiol. 2019;10:1105. 10.3389/fphys.2019.01105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Olmo A, Calzada J, Nuñez M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: uses, exposure, and controversy. Crit Rev Food Sci Nutr. 2017;57(14):3084–103. 10.1080/10408398.2015.1087964. [DOI] [PubMed] [Google Scholar]
- 27.Kukuljan M, Vergara L, Stojilkovic SS. Modulation of the kinetics of inositol 1,4,5-trisphosphate-induced [Ca2+]i oscillations by calcium entry in pituitary gonadotrophs. Biophys J. 1997;72(2 Pt 1):698–707. 10.1016/s0006-3495(97)78706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115(5):654–7. 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 29.Badier-Commander C, Couvelard A, Henin D, Verbeuren T, Michel JB, Jacob MP. Smooth muscle cell modulation and cytokine overproduction in varicose veins. An in situ study. J Pathol. 2001;193(3):398–407. 10.1002/path.819. [DOI] [PubMed] [Google Scholar]
- 30.Sansilvestri-Morel P, Rupin A, Jullien ND, Lembrez N, Mestries-Dubois P, Fabiani JN, Verbeuren TJ. Decreased production of collagen type III in cultured smooth muscle cells from varicose vein patients is due to a degradation by MMPs: possible implication of MMP-3. J Vasc Res. 2005;42(5):388–98. 10.1159/000087314. [DOI] [PubMed] [Google Scholar]
- 31.Rapiejko PJ, Northup JK, Evans T, Brown JE, Malbon CC. G-proteins of fat-cells. Role in hormonal regulation of intracellular inositol 1,4,5-trisphosphate. Biochem J. 1986;240(1):35–40. 10.1042/bj2400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78(6):539–52. 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and – 9 production by vascular smooth muscle cells. Cardiovasc Res. 2001;50(3):556–65. 10.1016/s0008-6363(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 34.Kelesoglu S, Elcık D, Zengin I, Ozan R, Inanc MT, Dogan A, Oguzhan A, Kalay N. Association of spontaneous echo contrast with systemic Immune inflammation index in patients with mitral stenosis. Rev Port Cardiol. 2022;41(12):1001–8. 10.1016/j.repc.2021.08.016. English, Portuguese. [DOI] [PubMed] [Google Scholar]
- 35.Rengarajan T, Rajendran P, Nandakumar N, Balasubramanian MP, Nishigaki I. Free radical scavenging and antioxidant activity of D-pinitol against 7, 12 dimethylbenz (a) anthracene induced breast cancer in Sprague Dawley rats. Asian Pac J Trop Dis. 2014;4:384–90. 10.1016/S2222-1808(14)60592-2. [Google Scholar]
- 36.Jiang WD, Wu P, Kuang SY, Liu Y, Jiang J, Hu K, Li SH, Tang L, Feng L, Zhou XQ. Myo-Inositol prevents copper-induced oxidative damage and changes in antioxidant capacity in various organs and the enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian). Aquat Toxicol. 2011;105(3–4):543–51. 10.1016/j.aquatox.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Masselli E, Pozzi G, Vaccarezza M, Mirandola P, Galli D, Vitale M, Carubbi C, Gobbi G. ROS in platelet Biology: functional aspects and methodological insights. Int J Mol Sci. 2020;21(14):4866. 10.3390/ijms21144866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang CX, Liu Y, Xia WF, Tang YH, Huang H. Oxidative stress: a possible pathogenesis of atrial fibrillation. Med Hypotheses. 2009;72(4):466–7. 10.1016/j.mehy.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 39.Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115(2):135–43. 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Peng W, Raffetto JD, Khalil RA. Matrix metalloproteinases in Remodeling of Lower Extremity veins and chronic venous disease. Prog Mol Biol Transl Sci. 2017;147:267–99. 10.1016/bs.pmbts.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.to T, Suwa M. Left atrial spontaneous echo contrast: relationship with clinical and echocardiographic parameters. Echo Res Pract. 2019;6(2):R65–73. 10.1530/ERP-18-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beemster P, Groenen P, Steegers-Theunissen R. Involvement of inositol in reproduction. Nutr Rev. 2002;60(3):80–7. 10.1301/00296640260042748. [DOI] [PubMed] [Google Scholar]
- 43.Kuş C, Ayhan-Kilcigil G, Ozbey S, Kaynak FB, Kaya M, Coban T, Can-Eke B. Synthesis and antioxidant properties of novel N-methyl-1,3,4-thiadiazol-2-amine and 4-methyl-2H-1,2,4-triazole-3(4H)-thione derivatives of benzimidazole class. Bioorg Med Chem. 2008;16(8):4294–303. 10.1016/j.bmc.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 44.Jin X, Qiu T, Xie J, Wei X, Wang X, Yu R, Proud C, Jiang T. Using Imidazo[2,1-b][1,3,4]thiadiazol skeleton to design and synthesize novel MNK inhibitors. ACS Med Chem Lett. 2022;14(1):83–91. 10.1021/acsmedchemlett.2c00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li XY, Wang DP, Li S, Xue WH, Qian XH, Liu KL, Li YH, Lin QQ, Dong G, Meng FH, Jian LY. Discovery of N-(1,3,4-thiadiazol-2-yl)benzamide derivatives containing a 6,7-methoxyquinoline structure as novel EGFR/HER-2 dual-target inhibitors against cancer growth and angiogenesis. Bioorg Chem. 2022;119:105469. 10.1016/j.bioorg.2021.105469. [DOI] [PubMed] [Google Scholar]
- 46.Słaboszewski M, Kolec R, Paszek E, Baran M, Undas A. Prothrombotic plasma fibrin clot phenotype is associated with spontaneous echo contrast in atrial fibrillation: the role of protein carbonylation. Thromb Res. 2024;240:109065. 10.1016/j.thromres.2024.109065. [DOI] [PubMed] [Google Scholar]
- 47.Chung HK, Cho Y, Do HJ, Oh K, Seo WK, Shin MJ. Plasma phospholipid arachidonic acid and lignoceric acid are associated with the risk of cardioembolic stroke. Nutr Res. 2015;35(11):1001–8. 10.1016/j.nutres.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Yeung J, Tourdot BE, Adili R, Green AR, Freedman CJ, Fernandez-Perez P, Yu J, Holman TR, Holinstat M. 12(S)-HETrE, a 12-Lipoxygenase oxylipin of Dihomo-γ-Linolenic acid, inhibits thrombosis via Gαs Signaling in platelets. Arterioscler Thromb Vasc Biol. 2016;36(10):2068–77. 10.1161/ATVBAHA.116.308050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cebo M, Dittrich K, Fu X, Manke MC, Emschermann F, Rheinlaender J, von Eysmondt H, Ferreirós N, Sudman J, Witte A, Pelzl L, Borst O, Geisler T, Rath D, Bakchoul T, Gawaz M, Schäffer TE, Lämmerhofer M, Chatterjee M. Platelet ACKR3/CXCR7 favors antiplatelet lipids over an atherothrombotic lipidome and regulates thromboinflammation. Blood. 2022;139(11):1722–42. 10.1182/blood.2021013097. [DOI] [PubMed] [Google Scholar]
- 50.Choi JY, Jung JM, Kwon DY, Park MH, Kim JH, Oh K, Koh SB, Seo WK. Free fatty acid as an outcome predictor of atrial fibrillation-associated stroke. Ann Neurol. 2016;79(2):317–25. 10.1002/ana.24568. [DOI] [PubMed] [Google Scholar]
- 51.Eun MY, Sung JH, Lee SH, Jung I, Park MH, Kim YH, Jung JM. Predictive value of free fatty acid levels in embolic stroke of undetermined source: a retrospective observational study. Med (Baltim). 2020;99(40):e22465. 10.1097/MD.0000000000022465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staub HL, Bertolaccini ML, Khamashta MA. Anti-phosphatidylethanolamine antibody, thromboembolic events and the antiphospholipid syndrome. Autoimmun Rev. 2012;12(2):230–4. 10.1016/j.autrev.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut Microbial Metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–24. 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witkowski M, Weeks TL, Hazen SL. Gut microbiota and Cardiovascular Disease. Circ Res. 2020;127(4):553–70. 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haris M, Singh A, Cai K, Kogan F, McGarvey J, Debrosse C, Zsido GA, Witschey WR, Koomalsingh K, Pilla JJ, Chirinos JA, Ferrari VA, Gorman JH, Hariharan H, Gorman RC, Reddy R. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat Med. 2014;20(2):209–14. 10.1038/nm.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun H, Swaim A, Herrera JE, Becker D, Becker L, Srivastava K, Thompson LE, Shero MR, Perez-Tamayo A, Suktitipat B, Mathias R, Contractor A, Faraday N, Morrell CN. Platelet kainate receptor signaling promotes thrombosis by stimulating cyclooxygenase activation. Circ Res. 2009;105(6):595–603. 10.1161/CIRCRESAHA.109.198861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrell CN, Sun H, Ikeda M, Beique JC, Swaim AM, Mason E, Martin TV, Thompson LE, Gozen O, Ampagoomian D, Sprengel R, Rothstein J, Faraday N, Huganir R, Lowenstein CJ. Glutamate mediates platelet activation through the AMPA receptor. J Exp Med. 2008;205(3):575–84. 10.1084/jem.20071474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazi TA, Stanhope KL, Elevated Erythritol. A marker of metabolic dysregulation or contributor to the pathogenesis of Cardiometabolic. Disease? Nutrients. 2023;15(18):4011. 10.3390/nu15184011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newell EA, Exo JL, Verrier JD, Jackson TC, Gillespie DG, Janesko-Feldman K, Kochanek PM, Jackson EK. 2’,3’-cAMP, 3’-AMP, 2’-AMP and adenosine inhibit TNF-α and CXCL10 production from activated primary murine microglia via A2A receptors. Brain Res. 2015;1594:27–35. 10.1016/j.brainres.2014.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forman MB, Gillespie DG, Cheng D, Jackson EK. A novel adenosine precursor 2’,3’-cyclic adenosine monophosphate inhibits formation of post-surgical adhesions. Dig Dis Sci. 2014;59(9):2118–25. 10.1007/s10620-014-3139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sotnikova TD, Beaulieu JM, Espinoza S, Masri B, Zhang X, Salahpour A, Barak LS, Caron MG, Gainetdinov RR. The dopamine metabolite 3-methoxytyramine is a neuromodulator. PLoS ONE. 2010;5(10):e13452. 10.1371/journal.pone.0013452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, Hoener MC. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324(3):948–56. 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- 63.Thorner MO. Dopamine is an important neurotransmitter in the autonomic nervous system. Lancet. 1975;1(7908):662–5. 10.1016/s0140-6736(75)91762-6. [DOI] [PubMed] [Google Scholar]
- 64.Baxter-Parker G, Prebble HM, Cross S, Steyn N, Shchepetkina A, Hock BD, Cousins A, Gieseg SP. Neopterin formation through radical scavenging of superoxide by the macrophage synthesised antioxidant 7,8-dihydroneopterin. Free Radic Biol Med. 2020;152:142–51. 10.1016/j.freeradbiomed.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Prebble H, Cross S, Marks E, Healy J, Searle E, Aamir R, Butler A, Roake J, Hock B, Anderson N, Gieseg SP. Induced macrophage activation in live excised atherosclerotic plaque. Immunobiology. 2018;223(8–9):526–35. 10.1016/j.imbio.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Oettl K, Greilberger J, Dikalov S, Reibnegger G. Interference of 7,8-dihydroneopterin with peroxynitrite-mediated reactions. Biochem Biophys Res Commun. 2004;321(2):379–85. 10.1016/j.bbrc.2004.06.157. [DOI] [PubMed] [Google Scholar]
- 67.Kresh JY, Nastala C, Bianchi PC, Goldman SM, Brockman SK. The relative buffering power of cardioplegic solutions. J Thorac Cardiovasc Surg. 1987;93(2):309–11. [PubMed] [Google Scholar]
- 68.Lv JY, Ingle RG, Wu H, Liu C, Fang WJ. Histidine as a versatile excipient in the protein-based biopharmaceutical formulations. Int J Pharm. 2024;662:124472. 10.1016/j.ijpharm.2024.124472. [DOI] [PubMed] [Google Scholar]
- 69.Alonso-Castro AJ, Serrano-Vega R, Pérez Gutiérrez S, Isiordia-Espinoza MA, Solorio-Alvarado CR. Myristic acid reduces skin inflammation and nociception. J Food Biochem. 2022;46(1):e14013. 10.1111/jfbc.14013. [DOI] [PubMed] [Google Scholar]
- 70.Yang J, Cha L, Wang Y, Zhang Q, Tang X, Shao J, Duan Z. L-Palmitoylcarnitine potentiates plasmin and tPA to inhibit thrombosis. Nat Prod Bioprospect. 2023;13(1):48. 10.1007/s13659-023-00413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Z, Mou C, Ji R, Chen H, Ding Y, Jiang X, Meng F, He F, Luo B, Yu J. Alterations in metabolome and lipidome in patients with in-stent restenosis. CNS Neurosci Ther. 2024;30(7):e14832. 10.1111/cns.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martha SR, Levy SH, Federico E, Levitt MR, Walker M. Machine learning analysis of the Cerebrovascular Thrombi Lipidome in Acute ischemic stroke. J Neurosci Nurs. 2023;55(1):10–7. 10.1097/JNN.0000000000000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bykowski EA, Petersson JN, Dukelow S, Ho C, Debert CT, Montina T, Metz GAS. Urinary metabolomic signatures as indicators of injury severity following traumatic brain injury: a pilot study. IBRO Neurosci Rep. 2021;11:200–6. 10.1016/j.ibneur.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung CS, Oldfield EH, Harvey-White J, Espey MG, Zimmermann M, Seifert V, Pluta RM. Association of an endogenous inhibitor of nitric oxide synthase with cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107(5):945–50. 10.3171/JNS-07/11/0945. [DOI] [PubMed] [Google Scholar]
- 75.Engelke UF, Zijlstra FS, Mochel F, Valayannopoulos V, Rabier D, Kluijtmans LA, Perl A, Verhoeven-Duif NM, de Lonlay P, Wamelink MM, Jakobs C, Morava E, Wevers RA. Mitochondrial involvement and erythronic acid as a novel biomarker in transaldolase deficiency. Biochim Biophys Acta. 2010;1802(11):1028–35. 10.1016/j.bbadis.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vorkas PA, Shalhoub J, Lewis MR, Spagou K, Want EJ, Nicholson JK, Davies AH, Holmes E. Metabolic phenotypes of carotid atherosclerotic plaques relate to stroke risk: an exploratory study. Eur J Vasc Endovasc Surg. 2016;52(1):5–10. 10.1016/j.ejvs.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 77.Malý M, Kučerka O, Bechyňská K, Kočí K, Mandys V, Hajšlová J, Kosek V. Plasma lipidome differences in patients with and without significant carotid plaque. Vascul Pharmacol. 2024;155:107377. 10.1016/j.vph.2024.107377. [DOI] [PubMed] [Google Scholar]
- 78.Maekawa K, Sugita C, Yamashita A, Moriguchi-Goto S, Furukoji E, Sakae T, Gi T, Hirai T, Asada Y. Higher lactate and purine metabolite levels in erythrocyte-rich fresh venous thrombus: potential markers for early deep vein thrombosis. Thromb Res. 2019;177:136–44. 10.1016/j.thromres.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 79.Fan Z, Xu S, Deng Y, Wei L, Yang J, Xing X. Disordered gut microbiota and alterations in the serum metabolome are associated with venous thromboembolism. Thromb Res. 2024;235:68–74. 10.1016/j.thromres.2024.01.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.