Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have a reliable hypoglycaemic and weight-loss effect that can intervene in obesity, which is the basis of type 2 diabetes pathology. GLP-1RA therapy has shown potential benefits in reducing the risk of major adverse cardiovascular events and improving kidney outcomes in patients with diabetes at high risk for cardiovascular disease. More recent evidence is expanding their benefits to heart failure with preserved ejection fraction and clinically important renal outcomes in patients with and without diabetes. Some sub-analyses of large clinical trials suggest that GLP-1RA and sodium-glucose cotransporter 2 inhibitor combination therapy may provide more significant reductions in heart failure hospitalization and renal composite events than each alone. Moreover, the addition of finerenone to this combination therapy could potentially provide stronger cardiorenal protective benefits. Further studies are needed to assess the potential cardiovascular and renal benefits of combination therapy and to determine suitable patient population for the therapy.
Graphical abstract
Suspected clinical impacts of GLP-1RAs, SGLT2is, and finerenone oncardiovascular and kidney outcomes. CV, cardiovascular; GLP-1RA, glucagon-like peptide-1 receptor agonist; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MI, myocardial infarction; SGLT2i, sodium-glucose cotransporter 2 inhibitor.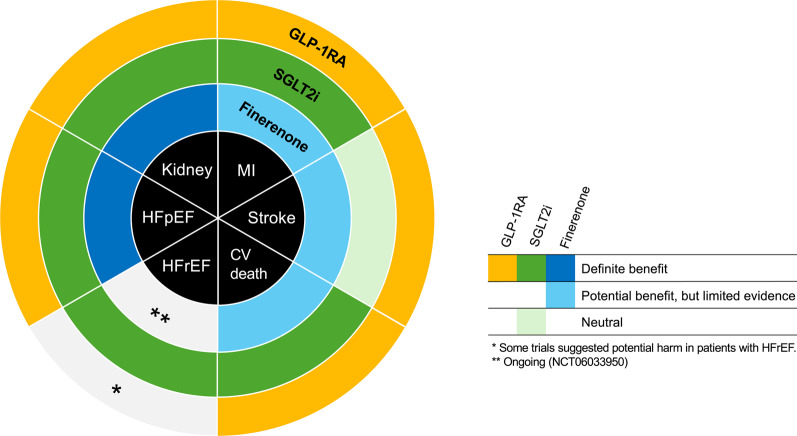
Keywords: Glucagon-like peptide-1 receptor agonist, Sodium-glucose cotransporter 2 inhibitor, Finerenone, Combination therapy, Cardiovascular and kidney outcomes
Diabetes and risk of cardiovascular and kidney complications
Diabetes is one of the most important risk factors for cardiovascular disease (CVD) and chronic kidney disease (CKD). Compared with patients without diabetes, patients with diabetes have a 2–fourfold higher risk of atherosclerotic cardiovascular diseases (ASCVD), including coronary artery disease, stroke, and peripheral artery disease [1]. Moreover, fasting hyperglycaemia was responsible for 2.3 million cardiovascular deaths worldwide in 2021 [2]. Additionally, as the first CVD to develop in patients with diabetes is often heart failure rather than myocardial infarction [1], it is becoming clear that the pathology of diabetes itself causes heart failure through direct effects on cardiomyocytes and other myocardial cell types. CKD is a principal microvascular complication of diabetes and increases the risk of CVD and death [3]. About 40% of patients with diabetes could develop CKD [4], and it could be already present at diagnosis especially in patients with type 2 diabetes [5]. Furthermore, CKD in patients with diabetes is globally the leading cause of end-stage kidney disease and leading to increased health care cost due to renal replacement therapy [4].
Whether hyperglycaemia itself directly affects the pathogenesis of atherosclerosis and heart failure remains inconclusive; this is consistent with the fact that previous large clinical trials have not reached a conclusion on whether cardiovascular disease can be suppressed by strict glycaemic control [6]. Effect of glycaemic control itself on preventing CKD is relatively small, compared with multifactorial therapy in patients with diabetes [7, 8]. Lowering blood glucose levels is the basis of diabetes treatment, not only in terms of preventing microvascular complications, including CKD, but also in preventing cardiovascular events. In contrast, it is difficult to completely prevent the development of cardiovascular events and CKD by hypoglycaemic therapy alone. Thus, in addition to a comprehensive approach encompassing multidisciplinary risk factors (dyslipidaemia, hypertension, obesity, smoking, etc.) [8, 9], considering "what drugs to use to lower blood glucose" by selecting hypoglycaemic agents with evidence-based cardiorenal protective effects, according to patient background and risk status is increasingly important [10].
Glucagon-like peptide-1 receptor agonist (GLP-1RA) and sodium-glucose cotransporter 2 inhibitor (SGLT2i) are currently recommended as first-line hypoglycaemic agents for patients with diabetes with established cardiovascular disease or multiple cardiovascular and renal risks [10]. Particularly, GLP-1RAs have greater hypoglycaemic and weight-loss effects and are attracting attention as a means of intervening in obesity, which is the key basis of type 2 diabetes pathogenesis. The cardiorenal protective effects of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist (MRA), in patients with diabetes and CKD have also been revealed in recent years [11]. The strategies for preventing cardiovascular and renal events in patients with diabetes are becoming increasingly diverse. However, the results of clinical trials suggest that the characteristics of the cardiorenal protective effects achieved by these drugs differ slightly, and consensus for their use and evidence for their optimal use and combined benefits are still lacking. In this article, we primarily summarize the clinical effects of GLP-1RAs on the risk of cardiovascular and renal events and discuss the emerging role of their combined therapy with SGLT2is and finerenone.
Mechanisms of CVD and CKD in patients with diabetes
Vascular damages of ASCVD in patients with diabetes are characterized by endothelial and vascular smooth muscle cell dysfunction [12]. Increased oxidative stress, inflammation and lipotoxicity associated with hyperglycaemia and insulin resistance potentially impair vascular endothelial function and increase endothelial permeability, leading to the penetration of glycated and oxidized low-density lipoprotein (LDL) cholesterol into the endothelium, i.e., initiation of atherosclerosis. Subsequently, macrophages transform into foam cells in the LDL cholesterol-deposited vascular endothelium. Hyperglycaemia has been suggested to cause increased expression of inflammation-related genes in macrophages. In vascular smooth muscle cells, hyperinsulinemia also promotes cell proliferation, contributing to the development of atherosclerosis and vascular remodelling. Independent of ASCVD and other risk factors, such as hypertension, diabetes also increases the risk of heart failure [13]. Myocardial remodelling characterized by fibrosis and hypertrophy, impaired microcirculation, abnormal protein function caused by advanced glycation end products, changes in myocardial metabolism and abnormal mitochondrial function have been noted as associated factors of diabetic cardiomyopathy [14].
The process of CKD progression in diabetes is also complicated [15]. Glomerular hyperfiltration due to hyperglycemia is one of the important features of CKD in diabetes and lead to diabetic glomerulopathy in combination with advanced glycation end products and inflammation. Inflammation and fibrosis can affect tubulo-interstitial lesions, and both glomerular and interstitial injuries will development of CKD. Comorbidities of diabetes (i.e., hypertension, dyslipidemia, obesity) also have crucial roles in pathophysiology of CKD in diabetes.
GLP-1RA therapy on major adverse cardiovascular events
Representative large-scale cardiovascular and kidney outcome trials of GLP-1RAs and their results are shown in Table 1. The first study to demonstrate the effect of GLP-1RAs in reducing cardiovascular events was LEADER, in which 9340 patients with type 2 diabetes at high cardiovascular risk were randomized to liraglutide 1.8 mg per day or placebo [16]. The study showed a 13% reduction in major adverse cardiovascular events (MACE), a composite endpoint for cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke, in patients treated with liraglutide compared with placebo at a mean follow-up of 3.8 years (hazard ratio [HR] 0.87, 95% confidence interval [CI] 0.78–0.97) and a significant reduction in cardiovascular death (HR 0.78, 95% CI 0.66–0.93). In SUSTAIN-6, wherein semaglutide (subcutaneous injection) was administered at 0.5 or 1 mg per week, MACE was also reduced by 26% in the semaglutide group compared with the placebo group (HR 0.74, 95% CI 0.58–0.95) as was stroke (HR 0.61, 95% CI 0.38–0.99) [17]. The risk of MACE was also significantly reduced in consecutive studies (Harmony Outcomes [18], REWIND [19], AMPLITUDE-O [20]). Their effects on the individual components of MACE, myocardial infarction (reduced in LEADER and Harmony Outcomes), and stroke (reduced in SUSTAIN-6 and REWIND), varied in each trial (Table 1). Although the reasons for the variation are still unclear, this might be due to several differences in the individual study design and patient characteristics rather than drug effect [21]. Moreover, in a meta-analysis of eight trials, GLP-1RAs reduced MACE by 14% (HR 0.86, 95% CI 0.79–0.94), cardiovascular death by 13% (HR 0.87, 95% CI 0.78–0.96), and stroke by 16% (HR 0.84, 95% CI 0.76–0.94) [22] (Table 2). Furthermore, in another meta-analysis [23], which excluded ELIXA [24] due to fact that lixisenatide is a short-acting GLP-1RA and all participants had acute coronary syndrome, GLP-1RAs were associated with significant reductions in MACE (HR 0.85, 95% CI 0.80–0.90), cardiovascular death (HR 0.85, 95% CI 0.78–0.93), stroke (HR 0.81, 95% CI 0.74–0.90), and myocardial infarction (HR 0.88, 95% CI 0.81–0.96) (Table 2).
Table 1.
Summary of major large-scale clinical trials with GLP-1RAs
| ELIXA [24] | LEADER [16] | SUSTAIN-6 [17] | EXSCEL [36] | Harmony Outcomes [18] | REWIND [19] | PIONEER-6 [59] | AMPLITUDE-O [20] | SELECT [25] | FLOW [31] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Lixisenatide | Liraglutide | Semaglutide (s.c. injection) | Exenatide | Albiglutide | Dulaglutide | Semaglutide (oral) | Efpeglenatide | Semaglutide (s.c. injection) | Semaglutide (s.c. injection) |
| Dose | 10 or 20 μg per day | 1.8 mg per day | 0.5 or 1.0 mg per week | 2.0 mg per week | 30 or 50 mg per week | 1.5 mg per week | 14 mg per day | 4 or 6 mg per week | 2.4 mg per week | 1.0 mg per week |
| Number | 6068 | 9340 | 3297 | 14,752 | 9463 | 9903 | 3183 | 4076 | 17,604 | 3533 |
| Population | T2DM and history of acute coronary syndrome within 180 days | T2DM with high cardiovascular risk* | T2DM with high cardiovascular risk* | T2DM with or without previous CVD | T2DM with previous CVD | T2DM with previous CVD or risk factors of CVD | T2DM with high cardiovascular risk* | T2DM with previous CVD, or CKD and additional risk of CVD | BMI ≥ 27 kg/m2 and previous CVD without diabetes | T2DM and CKD |
| Observation period, years | 2.1 | 3.8 | 3.1 | 3.2 | 1.6 | 5.4 | 1.3 | 1.8 | 3.3 | 3.4 |
| Age, years | 60 | 64 | 65 | 62 | 64 | 66 | 66 | 65 | 62 | 67 |
| BMI, kg/m2 | 30.1 | 32.5 | 32.8 | 32.7 | 32.3 | 32.3 | 32.3 | 32.7 | 33.3 | 32.0 |
| HbA1c, % | 7.7 | 8.7 | 8.7 | 8 | 8.7 | 7.4 | 8.2 | 8.9 | 5.8 | 7.8 |
| History of CVD, % | 100 | 81 | 60 | 73.1 | 71 | 32 | 85 | 90 | 100 | 22.9 |
| History of CHF, % | 22 | 18 | 24 | 16.2 | 20 | 9 | 12 | 18 | 24 | 19.2 |
| SGLT2i use at baseline, % | – | – | 0.2 |
0.9 (ITT population) |
6.1 | – | 10 | 15.2 | – | 15.6 |
| MACE, HR (95% CI) |
1.02** (0.89–1.17) |
0.87 (0.78–0.97) |
0.74 (0.58–0.95) |
0.91 (0.83–1.00) |
0.78 (0.68–0.90) |
0.88 (0.79–0.99) |
0.79 (0.57–1.11) |
0.73 (0.58–0.92) |
0.80 (0.72–0.90) |
0.82 (0.68–0.98) |
| Cardiovascular death, HR (95% CI) |
0.98 (0.78–1.22) |
0.78 (0.66–0.93) |
0.98 (0.65–1.48) |
0.88 (0.76–1.02) |
0.93 (0.73–1.19) |
0.91 (0.78–1.06) |
0.49 (0.27–0.92) |
0.72 (0.50–1.03) |
0.85 (0.71–1.01) |
0.71 (0.56–0.89) |
| MI, HR (95% CI) |
1.03 (0.87–1.22) |
0.86 (0.73–1.00) |
0.74 (0.51–1.08) |
0.97 (0.85–1.10) |
0.75 (0.61–0.90) |
0.96 (0.79–1.15) |
1.18 (0.73–1.90) |
0.78 (0.55–1.10) |
0.72 (0.61–0.85) |
0.80 (0.55–1.15) |
| Stroke, HR (95% CI) |
1.12 (0.79–1.58) |
0.86 (0.71–1.06) |
0.61 (0.38–0.99) |
0.85 (0.70–1.03) |
0.86 (0.66–1.14) |
0.76 (0.61–0.95) |
0.74 (0.35–1.57) |
0.80 (0.48–1.31) |
0.93 (0.74–1.15) |
1.22 (0.84–1.77) |
| HHF, HR (95% CI) |
0.96 (0.75–1.23) |
0.87 (0.73–1.05) |
1.11 (0.77–1.61) |
0.94 (0.78–1.13) |
0.85*** (0.70–1.04) |
0.93 (0.77–1.12) |
0.86 (0.48–1.55) |
0.61 (0.38–0.98) |
0.79 (0.60–1.13) |
- |
| All cause death, HR (95% CI) |
0.94 (0.78–1.13) |
0.85 (0.74–0.97) |
1.05 (0.74–1.50) |
0.86 (0.77–0.97) |
0.95 (0.79–1.16) |
0.90 (0.80–1.01) |
0.51 (0.31–0.84) |
0.78 (0.58–1.06) |
0.81 (0.71–0.93) |
0.80 (0.67–0.95) |
| Composite kidney events, HR (95% CI) | – |
0.78 (0.67–0.92) |
0.64 (0.46–0.88) |
– | – |
0.85 (0.77–0.93) |
– |
0.68 (0.57–0.79) |
0.78 (0.63–0.96) |
0.76**** (0.66–0.88) |
BMI body mass index, CI confidence interval, CHF congestive heart failure, CVD cardiovascular disease, GLP-1RA glucagon-like peptide-1 receptor agonist, HHF heart failure hospitalization, HR hazard ratio, ITT intention-to-treat, MACE major adverse cardiovascular events, MI myocardial infarction, s.c. subcutaneous, SGLT2i sodium-glucose cotransporter 2 inhibitor, T2DM type 2 diabetes mellitus
*Age of 50 years or more with at least one cardiovascular condition, or age of 60 years or more with at least one cardiovascular risk factor, ** 4-point MACE, *** Composite endpoint of CV death or HHF, **** Excluding macroalbuminuria
Table 2.
Two meta-analyses of large GLP-1RA clinical trials involving patients with diabetes at high cardiovascular risk
| Giugliano et al. [22] 2021 | Sattar et al. [23] 2021 | |
|---|---|---|
| MACE |
0.86 (0.79–0.94) |
0.85 (0.80–0.90) |
| Cardiovascular death |
0.87 (0.78–0.96) |
0.85 (0.78–0.93) |
| MI |
0.91 (0.81–1.01) |
0.88 (0.81–0.96) |
| Stroke |
0.84 (0.76–0.94) |
0.81 (0.74–0.90) |
| HHF |
0.90 (0.83–0.98) |
0.88 (0.79–0.98) |
| All cause death |
0.88 (0.80–0.96) |
0.87 (0.81–0.94) |
| Composite kidney events* |
0.83 (0.73–0.94) |
0.79 (0.73–0.87) |
Data are presented as hazard ratio (95% confidence interval)
GLP-1RA glucagon-like peptide-1 receptor agonist, HHF heart failure hospitalization, MACE major adverse cardiovascular events, MI myocardial infarction
*Including macroalubuminuria
The results of SELECT, which examined the effect of subcutaneous semaglutide injection for the prevention of cardiovascular events in 17,604 patients with obesity (body mass index [BMI] ≥ 27 kg/m2) and established cardiovascular disease but without diabetes, were presented in 2023 [25]. During the mean observation period of 3.3 years, semaglutide at 2.4 mg per week resulted in a mean weight loss of − 9.39% from baseline and a 20% reduction in the risk of MACE (HR 0.80, 95% CI 0.72–0.90), especially a 28% reduction in the risk of myocardial infarction (HR 0.72, 95% CI 0.61–0.85), compared with that in the placebo group. GLP-1RAs not only have glucose-lowering but also greater weight-loss effects as discussed in detail in a previous literature [26], the results of SELECT also suggest the weight-loss effect as an important factor explaining the cardiovascular protective effects of GLP-1RAs regardless of diabetes. However, in Harmony Outcomes, albiglutide reduced the risk of MACE by 22%, with 0.52% and 0.83 kg reductions in HbA1c and weight, respectively, compared with those in the placebo group [18]. Factors independent of glycaemic and weight loss effects have also been suggested to affect the cardiovascular protective effects of GLP-1RAs.
Heart failure therapy by GLP-1RAs
Although a meta-analysis of large-scale clinical trials suggested that GLP-1RAs reduce heart failure hospitalizations (HHF) by approximately 10% in patients with diabetes at high cardiovascular risk [22, 23], the treatment effects on the risk of HHF in individual trials other than AMPLITUDE-O were not significant (Tables 1, 2). Thus, unlike for MACE, the effect of GLP-1RAs in reducing the risk of HHF has not yet been consistently demonstrated in individual trials. FIGHT and LIVE were clinical trials involving the administration of liraglutide in patients with heart failure with reduced ejection fraction (HFrEF). Three hundred patients with HFrEF hospitalized for acute heart failure within 14 days and taking diuretics equivalent to at least 40 mg furosemide were randomized to receive 1.8 mg per day of liraglutide or placebo in FIGHT [27]. Their median left ventricular ejection fraction (LVEF) was 25%, 82% of the patients had ischaemic heart disease, and 59% had type 2 diabetes. At 180 days follow-up, death (HR 1.10, 95% CI 0.57–2.14) and heart failure rehospitalization (HR 1.30, 95% CI 0.89–1.88) did not differ compared with those in the placebo group. In addition, the results were similar for subgroup analysis by diabetes status. In LIVE, 241 clinically stable patients with HFrEF were randomized to receive liraglutide at 1.8 mg per day or placebo [28]. The mean LVEF was 33.7% and 35.4% in the liraglutide and placebo groups, respectively, and 60% of patients had ischaemic heart disease. During the 24-week observation, liraglutide did not improve LVEF but rather caused more death, ventricular arrhythmia, and atrial fibrillation, compared with the placebo. Thus, no evidence currently indicates that patients with HFrEF benefit from the administration of GLP-1RAs, but rather, the increased arrhythmias and death are concerning. One factor that may have contributed to such a result in patients with HFrEF is the increased heart rate caused by GLP-1RAs [21].
On the other hand, it is becoming clear that obesity and diabetes themselves affect the pathogenesis, progression, and prognosis of heart failure with preserved ejection fraction (HFpEF). The effect of semaglutide subcutaneous injection at 2.4 mg per week in patients with HFpEF and obesity (BMI ≥ 30 kg/m2) but without diabetes was tested in STEP-HFpEF [29]. The change in body weight from baseline was − 13.3% and − 2.6% in the semaglutide and placebo groups, respectively. The semaglutide group showed a significantly improved Kansas City Cardiomyopathy Questionnaire clinical summary score and a longer 6-min walking distance from baseline (21.5 m and 1.2 m in the semaglutide and placebo groups, respectively, intergroup difference 20.3 m, 95% CI 8.6–32.1 m), compared with that in the placebo group despite the relatively short observation period of 52 weeks. Similar results were reported in STEP-HFpEF DM, which included patients with diabetes excluded in STEP-HFpEF [30]. Pooled analysis including these two studies in addition to SELECT and FLOW [31] reported that semaglutide reduced the risk of hospitalization or urgent visits due to heart failure by 41% (HR 0.59, 95% CI 0.41–0.82) in HFpEF patients compared with placebo [32]. In contrast, the effect on cardiovascular death did not reach at the statistical significance (HR 0.82, 95% CI 0.57–1.16), possibly due to lower event rate. Nearly all patients were overweight or obesity (BMI ≥ 27 kg/m2) in this analysis, and reduction of worsening heart failure event was more compelling in patients with higher BMI (HR 0.49, 95% CI 0.33–0.70 in BMI ≥ 35 kg/m2, HR 0.96 95% CI 0.67–1.38 in BMI < 35 kg/m2). Weight loss effect of semaglutide could be one of important factors leading to improved quality of life in patients with HFpEF and overweight. However, the effect beyond weight loss was also indicated by decreasing trend of NT-proBNP or C-reactive protein levels independent of weight loss in STEP-HFpEF [33, 34].
Collectively, semaglutide improved the heart failure-related symptoms and reduce the incidence of worsening heart failure events especially in HFpEF patients with higher BMI, suggesting that it could be a promising therapeutic approach for this patient population. However, whether this could be a class effect of GLP-1RA remains uncertain, and the evidence on HFrEF is scarce.
GLP-1RA therapy on kidney outcomes
In individual clinical trials of GLP-1RAs, the therapy improved the kidney outcomes compared with those of placebo treatment, although most of these results emanated from secondary outcomes or exploratory analysis. Zelniker et al. [35] defined kidney outcomes as broad (new onset of macroalbuminuria, worsening estimated glomerular filtration rate [eGFR], end-stage kidney disease, or death attributable to renal causes) and narrow composite (excluding macroalbuminuria from a broad composite) and performed a meta-analysis of ELIXA, LEADER, SUSTAIN-6, and EXSCEL [36]. Broad composite outcomes were significantly reduced compared with those following placebo treatment (HR 0.82, 95% CI 0.75–0.89), but no statistically significant reduction in narrow composite outcomes, except for macroalbuminuria, was observed (HR 0.92, 95% CI 0.80–1.06). In a meta-analysis by Sattar et al., composite kidney outcomes, including macroalbuminuria, were reduced by 21% compared with those in the placebo group (HR 0.79, 95% CI 0.73–0.87). Although the incidence of worsening kidney function outcome based predominantly on eGFR change was nominally reduced after removal of ELIXA (0.82, HR 95% CI 0.69–0.98), composite outcomes excluding macroalbuminuria, mainly declining eGFR and doubling creatinine, were not significant (HR 0.86, 95% CI 0.72–1.02) [23]. Therefore, most of renoprotective effects of GLP-1RAs were likely to be due to the inhibition of progression to macroalbuminuria [35], and the effects on other clinically important renal endpoints, including eGFR decline, initiation of renal replacement therapy and renal death, was unclear [5].
The result of FLOW, a study to assess the effect of subcutaneous semaglutide injection on CKD in patients with type2 diabetes, was reported recently [31]. In this study, 3533 patients with CKD (eGFR of 50–75 ml/min/1.73 m2 and a UACR of > 300 and < 5000 mg/gCr or an eGFR of 25 to < 50 ml/min/1.73 m2 and a UACR of > 100 and < 5000 mg/gCr) and diabetes were enrolled and assigned to 1 mg per week subcutaneous semaglutide injection or a placebo group. The primary outcome defined as time to first kidney failure (persistent eGFR < 15 mL/min/1.73 m2 or initiation of chronic renal replacement therapy), ≥ 50% eGFR decline from baseline, or death from renal or cardiovascular causes was lower in semaglutide group (HR 0.76, 95% CI 0.66–0.88). In the component of primary outcome, persistent ≥ 50% eGFR decline from baseline was significantly reduced in semaglutide group (HR 0.73, 95% CI 0.59–0.89). Initiation of renal replacement therapy tended to decrease, but did not reach to statistical significance (HR 0.84, 95% CI 0.63–1.12). Thus, this study firstly added an insight that semaglutide can favourably affect more clinically important renal endpoints in patients with type 2 diabetes and CKD.
Potential mechanisms underlying clinical benefits of GLP-1RAs
GLP-1 receptors are thought to be expressed on atrial and ventricular cardiomyocytes, endothelial cells in the human heart, and endothelial and smooth muscle cells in blood vessels [21]. However, humans and mice display large differences. Furthermore, information on GLP-1 receptor expression in the heart is still lacking, and whether GLP-1RAs act directly or indirectly on the cardiovascular system remains inconclusive. Nevertheless, in vitro studies on blood vessels have shown that GLP-1RAs inhibit vascular smooth muscle cell proliferation, reduce reactive oxygen species, and increase nitric oxide in vascular endothelial cells [37].
In animal models of heart failure induced by high-frequency pacing, ischaemia, and obesity, GLP-1RAs inhibit left ventricular remodelling and improve left ventricular contractility and diastolic function through anti-inflammatory effects, cardiomyocyte apoptosis inhibition, and glucose metabolism enhancement in cardiomyocytes [38–40]. However, in a LIVE sub-study, myocardial glucose utilization did not improve in the liraglutide group as examined by F-fluorodeoxyglucose positron emission tomography [41], similar to a study using albiglutide [42]. This discrepancy between results in animal models and clinical studies may partly be due to myocardial insulin resistance in severely failing hearts and the use of beta-blockers [21].
Also in kidney, localization of GLP-1 receptor is under investigation, but it is thought to be expressed in vascular smooth muscle cells and immune cells in kidney [43]. Although GLP-1RAs have inhibitory effect on sodium hydrogen exchanger-3 in tubules, it is unclear that renoprotective effect of GLP-1RAs is attributable to hemodynamic effect [44]. Preclinical studies suggested that GLP-1RAs reduced immune cell activity and oxidative stress in kidney and led to decrease in fibrosis [15, 45]. Immunomodulation effects are currently thought to have principal roles in renoprotective effect of GLP-1RAs.
Potential complementary evidence of GLP-1RAs and SGLT2is
In a meta-analysis comparing the effects of GLP-1RAs with SGLT2is, five GLP-1RA (ELIXA, LEADER, SUSTAIN-6, EXSCEL, and Harmony Outcomes) and three SGLT2i trials (EMPA-REG OUTCOME, CANVAS Program, and DECLARE-TIMI 58) with a total of 77,242 patients were include [35]. The risk of MACE was reduced by 12% (HR 0.88, 95% CI 0.84–0.94) with GLP-1RAs and by 11% (HR 0.89, 95% CI 0.83–0.96) with SGLT2is compared with that in placebo groups, suggesting that GLP-1RAs and SGLT2is reduce the risk of MACE to a similar extent. Furthermore, the risks of myocardial infarction (HR 0.91, 95% CI 0.84–0.98 with GLP-1RAs and HR 0.89, 95% CI 0.80–0.98 with SGLT2is) and cardiovascular death (HR 0.88, 95% CI 0.80–0.96 with GLP-1RAs and HR 0.84, 95% CI 0.75–0.94 with SGLT2is) were similarly reduced in both GLP-1RA and SGLT2i groups, while the risk of stroke was reduced only with GLP-1RAs (HR 0.86, 95% CI 0.77–0.97 with GLP-1RAs and HR 0.97, 95% CI 0.86–1.10 with SGLT2is). On the other hand, the risks of HHF (HR 0.93, 95% CI 0.83–1.04 with GLP-1RAs and HR 0.69, 95% CI 0.61–0.79 with SGLT2is) and composite kidney outcomes excluding macroalbuminuria (HR 0.92, 95% CI 0.80–1.06 with GLP-1RAs and HR 0.55, 95% CI 0.48–0.64 with SGLT2is) significantly reduced with SGLT2is only. Particularly for heart failure, several clinical trials and meta-analyses have already confirmed that SGLT2is reduce HHF by approximately 30% in both HFrEF and HFpEF, with or without diabetes [46]. Thus, the clinical benefits for the risk of heart failure and kidney outcomes, except albuminuria, are likely to be a strength of SGLT2is, whereas those for the risk of stroke are a strength of GLP-1RAs (Graphical Abstract).
It may be reasonable to focus on whether the combination of GLP-1RAs and SGLT2is, which are expected to have complementary effects based on large-scale clinical trial results, can further reduce cardiovascular and renal events in patients with diabetes. However, currently, no trial has directly examined the effects of this combination therapy. In previous large-scale clinical trials of GLP-1RAs, 15.2% (n = 618) of AMPLITUDE-O and 6.1% (n = 575) of Harmony Outcomes patients used SGLT2is at baseline [18, 20] (Table 1). In the AMPLITUDE-O post-hoc analysis, the HR for MACE was 0.70 (95% CI 0.37–1.30) with baseline SGLT2is and 0.74 (95% CI 0.58–0.94) without SGLT2is (P-value for interaction 0.70), and the HR for HHF was 0.23 (95% CI 0.05–0.97) with SGLT2is and 0.70 (95% CI 0.42–1.17) without SGLT2is (P-value for interaction 0.35) [47]. For composite kidney outcomes, the HR was 0.52 (95% CI 0.33–0.83) with SGLT2is and 0.70 (95% CI 0.59–0.83) without SGLT2is (P-value for interaction 0.38) (Table 3). Therefore, considering cardiorenal protective effects, no interaction was observed between baseline SGLT2i use and efpeglenatide. The Harmony Outcomes post-hoc analysis also showed a similar trend with respect to MACE and HHF, although kidney outcomes were not examined (Table 3) [48]. No difference in adverse events was observed with the SGLT2i combination in the respective Harmony Outcomes and AMPLITUDE-O post-hoc analyses. In addition, a meta-analysis of 1193 patients with baseline SGLT2i use in AMPLITUDE-O and Harmony Outcomes revealed an HR for MACE of 0.78 (95% CI 0.49–1.24) with SGLT2is and 0.77 (95% CI 0.68–0.87) without SGLT2is (P-value for interaction 0.95), and an HR for HHF of 0.34 (95% CI 0.12–0.96) with SGLT2is and 0.72 (95% CI 0.55–0.92) without SGLT2is (P-value for interaction 0.18) [48]. Moreover, a similar trend has been observed in the post-hoc analysis of clinical trials for SGLT2is. In DECLARE-TIMI58 with dapagliflozin, 4.4% of the patients were using GLP-1RAs at baseline, and dapagliflozin reduced the risks of MACE, HHF, and composite kidney outcomes regardless of baseline GLP-1RA use [49]. Specifically, the HR for HHF was 0.20 (95% CI 0.07–0.60) with GLP-1RAs and 0.77 (95% CI 0.64–0.92) without GLP-1RAs (P-value for interaction 0.014). Although the CIs were wide because of the small number of events in subgroups, point estimates showed that the combination of GLP-1RAs and SGLT2is tended to reduce the incidence of HHF more than either alone. Additive effect on kidney outcomes including macroalbuminuria was also expected from AMPLITUDE-O substudy, however, additive effect of semaglutide and SGLT2i on the composite kidney outcomes without macroalbuminuria was not indicated in the FLOW substudy [50]. This may partly because SGLT2i users at baseline were few (15.6%) and renal events occurred later than MACE in the FLOW. The mechanisms of action of GLP-1RA and SGLT2i are essentially independent [51], and further studies are therefore warranted to assess whether their combination therapy can provide greater clinical benefit.
Table 3.
Impact of GLP-1RAs on clinical outcomes according to baseline combination use with SGLT2is
| Outcomes | Baseline SGLT2i Use | Individual Trials | Meta-analysis [48] | |
|---|---|---|---|---|
| AMPLITUDE-O [47] (Epfegrenatide) | Harmony Outcomes [48] (Albiglutide) | |||
| MACE | With SGLT2i |
0.70 (0.37–1.30) |
0.89 (0.45–1.77) |
0.78 (0.49–1.24) |
| Without SGLT2i |
0.74 (0.58–0.94) |
0.78 (0.67–0.90) |
0.77 (0.68–0.87) |
|
| HHF | With SGLT2i |
0.23 (0.05–0.97) |
0.50 (0.12–2.08) |
0.34 (0.12–0.96) |
| Without SGLT2i |
0.70 (0.42–1.17) |
0.72 (0.54–0.97) |
0.72 (0.55–0.92) |
|
| Composite kidney events* | With SGLT2i |
0.52 (0.33–0.83) |
N/A | N/A |
| Without SGLT2i |
0.70 (0.59–0.83) |
N/A | N/A | |
Data are presented as hazard ratio (95% confidence interval)
GLP-1RA glucagon-like peptide-1 receptor agonist, HHF heart failure hospitalization, MACE major adverse cardiovascular events, N/A not available, SGLT2i sodium-glucose cotransporter 2 inhibitor
*Including macroalubuminuria
Combination with finerenone for cardiorenal protection
Recently, in FIDELIO-DKD [52], FIGARO-DKD [53] and their pooled meta-analysis FIDELITY [11], finerenone has also been shown to reduce cardiovascular and renal events in CKD patients with diabetes. The cardiovascular benefit of finerenone in CKD patients with diabetes was primarily attributable to the reduction in the risk of HHF and was similar in FINEARTS-HF for patients with HFpEF [54]. In the FINE-HEART pooled analysis of these three large clinical trials, finerenone also reduced the risks of HHF, composite kidney outcome without macroalbuminuria, MACE, and all cause death [55]. Among participants, background use of GLP-1RA and SGLT2i was 5.8% and 8.9%, respectively, and treatment effect of finerenone on cardiovascular death was consistent regardless of those medication usages. The clinical benefits of finerenone on cardiovascular and kidney events are also unlikely affected by combination therapy with GLP-1RA or SGLT2i, however, clinical evidence on additive effects of finerenone in combination with GLP-1RA or SGLT2i are still limited [56, 57]. The CONFIDENCE is currently examining the effect of empagliflozin and finerenone combination therapy on kidney outcomes in CKD patients with diabetes [58].
Triple combination therapy
Neuen et al. performed a cross-trial analysis using data from clinical trials of GLP-1RAs (ELIXA, LEADER, SUSTAIN-6, EXSCEL, HARMONY, REWIND, PIONEER-6 [59], and AMPLITUDE-O), SGLT2is (CANVAS and CREDENCE), and finerenone (FIDELIO-DKD and FIGARO-DKD) to estimate the effects of the triple combination therapy of GLP-1RA, SGLT2i, and finerenone using the actuarial method [60]. In patients with diabetes and albuminuria, the HRs for MACE, HHF, and CKD progression with triple combination therapy were estimated to be 0.65 (95% CI 0.55–0.76), 0.45 (95% CI 0.34–0.58), and 0.42 (95% CI 0.31–0.56), respectively, compared with those for conventional therapy alone (renin-angiotensin system inhibitors and classical risk factor management). The mechanisms of action of GLP-1RAs, SGLT2is, and nonsteroidal MRA are generally considered complementary and independent but may overlap in part, and the effects of each drug may not be completely additive when the three drugs are combined. Therefore, when estimated assuming 50% additivity, the HRs were 0.73 (95% CI 0.62–0.87) for MACE, 0.53 (95% CI, 0.41–0.70) for HHF, and 0.51 (95% CI, 0.38–0.68) for CKD progression. Some attenuation in HR was observed, but the impact of triple combination therapy on MACE, HHF, and CKD progression was still consistent, assuming 50% additivity. When patients aged 50 years old start triple therapy, event-free survival was estimated to increase by 3.2 years (95% CI 2.1–4.3 years) for MACE, 3.2 years (95% CI 2.4–4.0 years) for HHF, and 5.5 years (95% CI 4.0–6.7 years) for CKD progression compared with that for conventional therapy alone. Results of this integrated analysis suggest that the triple combination of GLP-1RA, SGLT2i, and finerenone provides stronger cardiorenal protective benefits than either conventional therapy or each alone. Given existing evidence, triple combination therapy can be expected to provide additional cardiorenal protection, although further randomized controlled trials are needed to determine whether triple combination therapy actually has additive or synergetic effects compared with mono or dual therapy.
Summary and future perspectives of GLP-1RA therapy
Thus, we have currently obtained multiple therapeutic tools that reduce the risks of cardiovascular and renal events in patients with diabetes. Moreover, their combined use has the potential to further enhance treatment effects. Appropriate drug choice according to the patient clinical situation will lead to the precision medicine in the field of cardiovascular-kidney-metabolic syndrome.
Given the clinical evidence reviewed above, patients with type 2 diabetes and/or obesity are the most promising candidate population to benefit from GLP-1RA-centered pharmacotherapy to improve cardiovascular and kidney outcomes. GLP-1RAs have reliable hypoglycaemic and weight-loss effects that can intervene in obesity—the basis of type 2 diabetes pathology—and multifaceted effects on other cardiometabolic parameters, such as blood pressure and circulating lipids [21]. Nevertheless, since the risk of cardiovascular and renal events will remain even with the use of GLP-1RA, there is a great deal of clinical interest in its combined use with SGLT2i and finerenone (Table 4). These agents potentially have complementary effects in cardiovascular and renal protection (Graphical Abstract).
Table 4.
Pros and evidence gaps of combination therapy
| Pros | Evidence gaps |
|---|---|
|
Multifactorial favorable effects on cardiometabolic parameters, including glycemia, body weight, blood pressure, and lipid profiles Additive/synergetic cardiovascular and renal benefits, as inferred from clinical trials and mechanistical insights Complementary mechanism of action on residual cardiovascular and renal risks |
Need to identify the suitable patient population who better merit from the combination therapy When and how to implement in the clinical settings Long-term adherence and safety Cost-effectiveness |
To further implement GLP-1RA-centric combination therapy, we need to consider some evidence gaps (Table 4). First, we should identify populations that are suitable or unsuitable for GLP-1RA-centric combination therapy. In recent GLP-1RA trials focusing on patients with obesity, favourable effect on worsening heart failure in HFpEF patients was indicated to be modulated by baseline BMI [32], although the underlying mechanisms that explain the cardiovascular and renal benefits may not necessarily be due to weight loss alone. Since background BMI can be largely different between races, caution is needed when interpreting and applying the results, including combination use with SGLT2is and finerenone. In addition, few studies currently support the use of GLP-1RAs in patients with HFrEF. Second, determining the ideal timing and priority to intensify therapy is also urgently required, since present evidence are not enough to cover this evidence gap. In particular, treatment effect size may differ among each combination and be affected by patient clinical situations and targeting outcomes. Third, long-term adherence and safety of the combination therapy need to be further addressed. Finally, cost-effectiveness is an important issue that is often addressed in multidrug regimen research. Given the tremendous costs of treating cardiovascular disease and continuing renal replacement therapy, the suspected total benefits obtained from therapy should not be overlooked. Therefore, continuous assessment is also required to determine the cost-effectiveness of the combination therapy.
Acknowledgements
We thank Ms. Aya Yamada (Saga University) for her assistance with this work.
Abbreviations
- ASCVD
Atherosclerotic cardiovascular disease
- BMI
Body mass index
- CI
Confidence interval
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- eGFR
Estimated glomerular filtration ratio
- GLP-1RA
Glucagon-like peptide-1 receptor agonist
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- HHF
Heart failure hospitalization
- HR
Hazard ratio
- LDL
Low-density lipoprotein
- LVEF
Left ventricular ejection fraction
- MACE
Major cardiovascular events
- MRA
Mineralocorticoid receptor antagonist
- SGLT2
Sodium-glucose cotransporter 2
Author contributions
KS concepted this work and wrote the draft of the article. AT concepted this work and critically reviewed and edited. KN supervised this work and critically reviewed. All authors approved the final version of manuscript.
Funding
None.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors have read and approved the submission of the manuscript. The manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language. If the manuscript is accepted, we will approve it for publication in Cardiovascular Diabetology.
Competing interests
KS declares nothing to disclose. AT has received honoraria from Boehringer Ingelheim Japan, Mochida, and Amgen; research funding from GlaxoSmithKline, Takeda, Bristol-Myers Squibb, and Novo Nordisk. KN has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim Japan, Daiichi Sankyo, Eli Lilly Japan, Kowa, Mitsubishi Tanabe, MSD, Novartis, Novo Nordisk, and Otsuka; research grant from Astellas, Bayer, Boehringer Ingelheim Japan, Fuji, Mochida, and Novartis; scholarship from Abbott Medical, Boehringer Ingelheim Japan, Daiichi Sankyo Healthcare, Mitsubishi Tanabe, and Teijin.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20(10):685–95. [DOI] [PubMed] [Google Scholar]
- 2.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80(25):2361–71. [DOI] [PubMed] [Google Scholar]
- 3.Cea Soriano L, Johansson S, Stefansson B, Rodriguez LA. Cardiovascular events and all-cause mortality in a cohort of 57,946 patients with type 2 diabetes: associations with renal function and cardiovascular risk factors. Cardiovasc Diabetol. 2015;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, Rosas SE, Rossing P, Bakris G. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association Professional Practice C: 11. Chronic kidney disease and risk management: standards of care in diabetes-2024. Diabetes Care 2024, 47(Suppl 1):S219-S230. [DOI] [PMC free article] [PubMed]
- 6.Bornfeldt KE. Does elevated glucose promote atherosclerosis? Pros and Cons Circ Res. 2016;119(2):190–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–93. [DOI] [PubMed] [Google Scholar]
- 8.Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, Haraguchi M, Morita A, Ohashi K, Hara K, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):951–64. [DOI] [PubMed] [Google Scholar]
- 9.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–91. [DOI] [PubMed] [Google Scholar]
- 10.Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, Kolkhof P, Nowack C, Gebel M, Ruilope LM, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43(6):474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjorkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abel ED, Gloyn AL, Evans-Molina C, Joseph JJ, Misra S, Pajvani UB, Simcox J, Susztak K, Drucker DJ. Diabetes mellitus-progress and opportunities in the evolving epidemic. Cell. 2024;187(15):3789–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. 2020;126(11):1501–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Raalte DH, Bjornstad P, Cherney DZI, de Boer IH, Fioretto P, Gordin D, Persson F, Rosas SE, Rossing P, Schaub JA, et al. Combination therapy for kidney disease in people with diabetes mellitus. Nat Rev Nephrol. 2024;20(7):433–46. [DOI] [PubMed] [Google Scholar]
- 16.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Ryden L, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. [DOI] [PubMed] [Google Scholar]
- 20.Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, Lam CSP, Khurmi NS, Heenan L, Del Prato S, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907. [DOI] [PubMed] [Google Scholar]
- 21.Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. 2023;20(7):463–74. [DOI] [PubMed] [Google Scholar]
- 22.Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, Ceriello A, Chiodini P, Esposito K. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, Lam CSP, Lopes RD, McMurray JJV, Pratley RE, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–62. [DOI] [PubMed] [Google Scholar]
- 24.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, Lawson FC, Ping L, Wei X, Lewis EF, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. [DOI] [PubMed] [Google Scholar]
- 25.Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, Hardt-Lindberg S, Hovingh GK, Kahn SE, Kushner RF, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221–32. [DOI] [PubMed] [Google Scholar]
- 26.Sawami K, Tanaka A, Node K. Anti-obesity therapy for cardiovascular disease prevention: potential expected roles of glucagon-like peptide-1 receptor agonists. Cardiovasc Diabetol. 2022;21(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316(5):500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hanselmann A, Nilsson B, Moller JE, Hjort J, Rasmussen J, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19(1):69–77. [DOI] [PubMed] [Google Scholar]
- 29.Kosiborod MN, Abildstrom SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, Hovingh GK, Kitzman DW, Lindegaard ML, Moller DV, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389(12):1069–84. [DOI] [PubMed] [Google Scholar]
- 30.Kosiborod MN, Petrie MC, Borlaug BA, Butler J, Davies MJ, Hovingh GK, Kitzman DW, Moller DV, Treppendahl MB, Verma S, et al. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N Engl J Med. 2024;390(15):1394–407. [DOI] [PubMed] [Google Scholar]
- 31.Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, Bakris G, Baeres FMM, Idorn T, Bosch-Traberg H, Lausvig NL, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med. 2024;391(2):109–21. [DOI] [PubMed] [Google Scholar]
- 32.Kosiborod MN, Deanfield J, Pratley R, Borlaug BA, Butler J, Davies MJ, Emerson SS, Kahn SE, Kitzman DW, Lingvay I, et al. Semaglutide versus placebo in patients with heart failure and mildly reduced or preserved ejection fraction: a pooled analysis of the SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM randomised trials. Lancet. 2024;404(10456):949–61. [DOI] [PubMed] [Google Scholar]
- 33.Petrie MC, Borlaug BA, Butler J, Davies MJ, Kitzman DW, Shah SJ, Verma S, Jensen TJ, Einfeldt MN, Liisberg K, et al. Semaglutide and NT-proBNP in obesity-related HFpEF. J Am Coll Cardiol. 2024;84(1):27–40. [DOI] [PubMed] [Google Scholar]
- 34.Verma S, Petrie MC, Borlaug BA, Butler J, Davies MJ, Kitzman DW, Shah SJ, Ronnback C, Abildstrom SZ, Liisberg K, et al. Inflammation in obesity-related HFpEF: The STEP-HFpEF Program. J Am Coll Cardiol. 2024;84(17):1646–62. [DOI] [PubMed] [Google Scholar]
- 35.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139(17):2022–31. [DOI] [PubMed] [Google Scholar]
- 36.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24(1):15–30. [DOI] [PubMed] [Google Scholar]
- 38.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110(8):955–61. [DOI] [PubMed] [Google Scholar]
- 39.Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li RK, Volchuk A, Robinson LA, Billia F, Drucker DJ, et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127(1):74–85. [DOI] [PubMed] [Google Scholar]
- 40.Sassoon DJ, Tune JD, Mather KJ, Noblet JN, Eagleson MA, Conteh AM, Sturek JT, Goodwill AG. Glucagon-like peptide 1 receptor activation augments cardiac output and improves cardiac efficiency in obese swine after myocardial infarction. Diabetes. 2017;66(8):2230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen R, Jorsal A, Iversen P, Tolbod LP, Bouchelouche K, Sørensen J, Harms HJ, Flyvbjerg A, Tarnow L, Kistorp C, et al. Effect of liraglutide on myocardial glucose uptake and blood flow in stable chronic heart failure patients: a double-blind, randomized, placebo-controlled LIVE sub-study. J Nucl Cardiol. 2019;26(2):585–97. [DOI] [PubMed] [Google Scholar]
- 42.Lepore JJ, Olson E, Demopoulos L, Haws T, Fang Z, Barbour AM, Fossler M, Davila-Roman VG, Russell SD, Gropler RJ. Effects of the novel long-acting GLP-1 agonist, albiglutide, on cardiac function, cardiac metabolism, and exercise capacity in patients with chronic heart failure and reduced ejection fraction. JACC Heart Fail. 2016;4(7):559–66. [DOI] [PubMed] [Google Scholar]
- 43.Alicic RZ, Neumiller JJ, Tuttle KR. Mechanisms and clinical applications of incretin therapies for diabetes and chronic kidney disease. Curr Opin Nephrol Hypertens. 2023;32(4):377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muskiet MHA, Tonneijck L, Smits MM, van Baar MJB, Kramer MHH, Hoorn EJ, Joles JA, van Raalte DH. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13(10):605–28. [DOI] [PubMed] [Google Scholar]
- 45.Alicic RZ, Cox EJ, Neumiller JJ, Tuttle KR. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol. 2021;17(4):227–44. [DOI] [PubMed] [Google Scholar]
- 46.Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400(10354):757–67. [DOI] [PubMed] [Google Scholar]
- 47.Lam CSP, Ramasundarahettige C, Branch KRH, Sattar N, Rosenstock J, Pratley R, Del Prato S, Lopes RD, Niemoeller E, Khurmi NS, et al. Efpeglenatide and clinical outcomes with and without concomitant sodium-glucose cotransporter-2 inhibition use in type 2 diabetes: exploratory analysis of the AMPLITUDE-O trial. Circulation. 2022;145(8):565–74. [DOI] [PubMed] [Google Scholar]
- 48.Neves JS, Borges-Canha M, Vasques-Novoa F, Green JB, Leiter LA, Granger CB, Carvalho D, Leite-Moreira A, Hernandez AF, Del Prato S, et al. GLP-1 receptor agonist therapy with and without SGLT2 inhibitors in patients with type 2 diabetes. J Am Coll Cardiol. 2023;82(6):517–25. [DOI] [PubMed] [Google Scholar]
- 49.Cahn A, Wiviott SD, Mosenzon O, Murphy SA, Goodrich EL, Yanuv I, Rozenberg A, Wilding JPH, Leiter LA, Bhatt DL, et al. Cardiorenal outcomes with dapagliflozin by baseline glucose-lowering agents: post hoc analyses from DECLARE-TIMI 58. Diabetes Obes Metab. 2021;23(1):29–38. [DOI] [PubMed] [Google Scholar]
- 50.Mann JFE, Rossing P, Bakris G, Belmar N, Bosch-Traberg H, Busch R, Charytan DM, Hadjadj S, Gillard P, Gorriz JL, et al. Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial. Nat Med. 2024;30(10):2849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gourdy P, Darmon P, Dievart F, Halimi JM, Guerci B. Combining glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM). Cardiovasc Diabetol. 2023;22(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29. [DOI] [PubMed] [Google Scholar]
- 53.Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–63. [DOI] [PubMed] [Google Scholar]
- 54.Solomon SD, McMurray JJV, Vaduganathan M, Claggett B, Jhund PS, Desai AS, Henderson AD, Lam CSP, Pitt B, Senni M, et al. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2024;391(16):1475–85. [DOI] [PubMed] [Google Scholar]
- 55.Vaduganathan M, Filippatos G, Claggett BL, Desai AS, Jhund PS, Henderson A, Brinker M, Kolkhof P, Schloemer P, Lay-Flurrie J, et al. Finerenone in heart failure and chronic kidney disease with type 2 diabetes: FINE-HEART pooled analysis of cardiovascular kidney and mortality outcomes. Nat Med. 2024. 10.1038/s41591-024-03264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossing P, Agarwal R, Anker SD, Filippatos G, Pitt B, Ruilope LM, Fonseca V, Umpierrez GE, Caramori ML, Joseph A, et al. Finerenone in patients across the spectrum of chronic kidney disease and type 2 diabetes by glucagon-like peptide-1 receptor agonist use. Diabetes Obes Metab. 2023;25(2):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossing P, Filippatos G, Agarwal R, Anker SD, Pitt B, Ruilope LM, Chan JCN, Kooy A, McCafferty K, Schernthaner G, et al. Finerenone in predominantly advanced CKD and type 2 diabetes with or without sodium-glucose cotransporter-2 inhibitor therapy. Kidney Int Rep. 2022;7(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green JB, Mottl AK, Bakris G, Heerspink HJL, Mann JFE, McGill JB, Nangaku M, Rossing P, Scott C, Gay A, et al. Design of the combinatioN effect of finerenone and empagliflozin in participants with chronic kidney disease and type 2 diabetes using a UACR endpoint study (CONFIDENCE). Nephrol Dial Transplant. 2023;38(4):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51. [DOI] [PubMed] [Google Scholar]
- 60.Neuen BL, Heerspink HJL, Vart P, Claggett BL, Fletcher RA, Arnott C, de Oliveira CJ, Falster MO, Pearson SA, Mahaffey KW, et al. Estimated lifetime cardiovascular, kidney, and mortality benefits of combination treatment with SGLT2 inhibitors, GLP-1 receptor agonists, and nonsteroidal MRA compared with conventional care in patients with type 2 diabetes and albuminuria. Circulation. 2024;149(6):450–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.


