Abstract
Background:
The role of the glymphatic system in multiple sclerosis (MS)-related disability remains underexplored. Diffusion-tensor image analysis along the perivascular space (DTI-ALPS) offers a non-invasive method to assess glymphatic function.
Objective:
To evaluate glymphatic function in MS patients with lower and higher disability.
Methods:
This study included 118 MS patients who underwent structural, diffusion-weighted imaging, and clinical assessment. The participants were divided into lower (MS-L, n = 57) and higher disability (MS-H, n = 61) subgroups. Brain parenchymal fraction (BPF), lesion load (LL), and DTI-ALPS index were measured. Subgroup differences and correlations between DTI-ALPS index and other measures were explored. Logistic regression was performed to evaluate BPF, LL, and DTI-ALPS index in classifying lower and higher disability patients.
Results:
Significant differences in DTI-ALPS index between MS-H and MS-L (d = −0.71, false discovery rate-corrected p-value (p-FDR) = 0.001) were found. The DTI-ALPS index correlated significantly with disease duration (rp = −0.29, p-FDR = 0.002) and EDSS (rsp = −0.35, p-FDR = 0.0002). It also showed significant correlations with BPF and LL. DTI-ALPS index and LL were significant predictors of disability subgroup (DTI-ALPS: odds ratio (OR) = 1.77, p = 0.04, LL: OR = 0.94, p = 0.02).
Conclusion:
Our findings highlight DTI-ALPS index as an imaging biomarker in MS, suggesting the involvement of glymphatic impairment in MS pathology, although further research is needed to elucidate its role in contributing to MS-related disability.
Keywords: Multiple sclerosis, glymphatic system, DTI-ALPS, disability, neurodegeneration, neuroinflammation
Introduction
Multiple sclerosis (MS) is a chronic neuroimmunological disease characterized by central nervous system losses in myelin, oligodendrocytes, and axons. Current understanding of the underlying disease processes implicates elements from auto-reactive adaptive and innate immune systems, oxidative stress, cytotoxicity, as well as mitochondrial and blood–brain barrier dysfunction. 1 Neuroinflammation and neurodegeneration are co-occurring features of MS pathology, 2 with studies highlighting the inception of neurodegeneration prior to the clinical onset of MS. 3 Imaging measures have proved reliability in monitoring MS inflammatory and degenerative outcomes.2,4 MS-related lesions have long been used as an imaging outcome representing the anti-inflammatory effects of MS treatments and have been correlated with clinical disability progression. 4 Measurements of whole-brain volume, such as brain parenchymal fraction (BPF), have demonstrated sensitivity and reliability in quantifying neurodegeneration in MS. 5
Cerebrospinal fluid (CSF) flow dynamics and their role in interstitial fluid (ISF) exchange, collectively comprising the glymphatic system, have been proposed to play a role in various neuroinflammatory and neurodegenerative disease processes. The glymphatic system is purported to play a role in waste clearance in the central nervous system. 6 First observed and proposed in animal models, 7 the glymphatic system represents CSF influx from subarachnoid spaces via periarterial spaces into brain parenchyma facilitated by astroglial aquaporin-4, then into perivenous spaces, finally draining out of the brain into dural and cervical lymphatics. 8
Advances in imaging have enabled the identification of several non-invasive surrogate measures for characterizing glymphatic function. Previously, this characterization was only possible through experiments using intrathecal9,10 or intravenous11,12 tracer agents. A recent study employing positron emission tomography with an intravenous tracer demonstrated CSF clearance deficits in patients with MS (PwMS). 12 In addition, the evaluation of perivascular spaces (PVSs), also known as Virchow–Robin spaces, serves as another surrogate measure for glymphatic function. 13 The literature denotes an increased burden of enlarged PVS in PwMS compared to healthy controls, albeit with inconsistent correlations with MS-related inflammatory and degenerative pathology. 14 Recently developed methods have proposed a non-invasive surrogate measure for the glymphatic system termed diffusion-tensor image analysis along the perivascular space (DTI-ALPS). 15 DTI-ALPS assesses water diffusion along the perivascular space, leveraging the unique architecture at the level of lateral ventricles (LVs), where medullary veins run perpendicular to the LV wall, neighboring projection fibers, and association fibers, allowing for specific assessment of diffusivity parallel to the medullary veins’ perivascular spaces. 15
While studies have shown a correlation between glymphatic impairment and MS-related disability, to date, none have determined whether it predicts disability level. In this study, we aim to assess glymphatic system function, using DTI-ALPS, among other magnetic resonance imaging (MRI) measures of neurodegenerative and neuroinflammatory MS processes in patients with lower and higher MS disability, comparing DTI-ALPS to other established MS imaging markers as a classifier of PwMS with higher versus lower disability.
Methods
Subjects
This retrospective study included 118 PwMS, diagnosed using the 2017 McDonald criteria, seen between 2011 and 2021 at the comprehensive MS care clinic at UT Physicians. Enrolled subjects underwent clinical examination, including the assessment of the Expanded Disability Status Scale (EDSS) score. We used a disability milestone EDSS score of 3.0 (moderate disability but no impairment of walking), 16 to divide the enrolled PwMS into two subgroups. MS-L subgroup included PwMS with EDSS scores lower than 3, while MS-H subgroup included PwMS with EDSS scores greater than or equal to 3. The study was approved by the Institutional Review Board for the University of Texas Health Science Center at Houston—UTHealth. All participants in the study provided written consent following approved procedures by the Committee for the Protection of Human Subjects of McGovern Medical School, UTHealth.
Image acquisition
MRI scans were performed using a 3.0T Philips Ingenia research scanner with a maximum gradient amplitude of 45 mT/m and a 15-channel SENSE-compatible head coil (Philips Medical Systems, Best, The Netherlands). High-resolution 3D-T1 magnetization prepared rapid gradient echo (MPRAGE) images (voxel size: 1 × 1 × 1 mm3, field-of-view (FOV): 256 × 256 mm2, repetition time (TR)/time to echo (TE): 8/3.7 ms), used for anatomical registration, were acquired at the start of each scanning session, as well as 3D T2-fluid-attenuated inversion recovery (FLAIR) images (voxel size: 1 × 1 × 1 mm3, FOV: 256 × 256 mm2, TR/TE = 4800/300 ms), which were used for lesion segmentation. In addition, diffusion-tensor images (DTIs) were obtained using a single-shot spin-echo diffusion-sensitized echo-planar imaging sequence with a balanced Icosa21 tensor encoding scheme. 17 The b-factor was 1000 s mm−2, TR/TE = 7100/65 ms, FOV = 256 × 256 mm, and the slice thickness was 3 mm with a 0-mm gap and a total of 44 slices. DTI quality control throughout data acquisition was assured using the same subject and water phantom tests as detailed previously. 18 Further post-processing is described in these earlier works.18,19
Image analysis
DTI-ALPS processing
The DTI data were processed in FSL (version 6.0.6, FMRIB Software Library; http://www.fmrib.ox.ac.uk/fsl) as described by Taoka et al. 20 DTI-ALPS evaluates water diffusion in the direction of the perivascular spaces, comparing the juxta ventricular projection and association diffusivities along the different three-dimensional axes. This area’s specific incongruent conformation between perivascular spaces, major projection, and association pathways allows for an approximate quantification of the perivascular space diffusivity. 15 The DTI images underwent eddy current correction and tensor fitting using DTIFIT, obtaining the diffusivity maps in the x-axis (Dxx), y-axis (Dyy), and z-axis (Dzz) as well as the FA maps. The FA map of each subject was registered to the FMRIB58_FA standard space using linear and non-linear transformations, and obtained transformations were applied to the diffusivity maps. The subject with the least degree of warping was selected for region-of-interest (ROI) placement. Spherical ROIs with a diameter of 12 pixels were placed in the projection and association areas at the level of the LV body bilaterally. The ROIs were visually inspected on the color-coded FA map in all subjects to confirm their position in their respective areas and the absence of apparently FA hypointense lesions. 21
The ROIs were used to calculate the mean x-axis diffusivity in the projection (Dxxproj) and association (Dxxassoc) areas, the y-axis projection (Dyyproj) area diffusivity, and the z-axis association (Dzzassoc) area diffusivity. The DTI-ALPS index was calculated using the following formula
The DTI-ALPS indices of both hemispheres were calculated and averaged which has been shown to improve reproducibility. 20
Structural image processing
In FSL, T1 and T2-FLAIR images were bias-field corrected and co-registered. In SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/), white matter (WM) hyperintense lesions were segmented using the Lesion Segmentation Toolbox 22 and validated by two trained raters (A.B. and J.A.T.), obtaining the lesion distribution map and the total lesion volumes for each subject. To mitigate the impact of MS lesions on spatial normalization, 23 the produced lesion maps were used to perform lesion filling on the corresponding T1 images. 24
Lesion-filled T1 images were processed in the Computational Anatomy Toolbox (CAT12; https://neuro-jena.github.io/cat), which has been validated in cross-sectional MS studies and shown to provide robust volumetric measures.23,25 The T1 images were first denoised and affine-registered, after which they underwent unified segmentation 26 which was further refined by applying a partial volume estimation; 27 finally, they were spatially normalized using geodesic shooting registrations. 28 Gray matter (GM), total WM, and CSF volumes were obtained as well as the total intracranial volume (TIV) and used to calculate BPF (BPF = GM + WM/TIV), gray matter fraction (GMF = GM/TIV), and total WM fraction (WMF = WM/TIV). On the Neuromorphometrics atlas (Neuromorphometrics, Inc.), the tissue volumes for deep gray matter (DGM) structures and LV were estimated. 29 To account for differences in TIV, lesion, DGM, and LV volumes were divided by TIV and multiplied by 1000, which were eventually used as the lesion load (LL), DGM, and LV volumes in the statistical analysis.
Cortical thickness (CTh) was estimated and reconstructed using a projection-based method, 30 and topological defects were repaired using spherical harmonics. 31 The created surfaces were mapped to the FreeSurfer “FsAverage” template 32 to which the local thickness values were transferred and the mean CTh was obtained for each subject.
Statistical analysis
In JASP (version 0.18.3, https://jasp-stats.org/), with a significance level set at p < 0.05, the demographic and clinical variables were compared between the subgroups MS-L and MS-H using t-test for continuous variables, Mann–Whitney U-test for ordinal variables, and chi-square test for categorical variables. The group differences in MRI measures were estimated using analysis of covariance (ANCOVA), controlling for age and sex. Post hoc comparisons were performed to determine the mean differences, effect sizes as quantified by Cohen’s d (d), and 95% confidence intervals (CIs) calculated from 5000 bootstraps.
The left and right DTI-ALPS index measurements were compared using a paired samples t-test, and Pearson’s correlation coefficient between the mean DTI-ALPS index and age was calculated. To investigate the relationship between the mean DTI-ALPS index and the clinical and structural MRI measures, Pearson’s partial correlation coefficients (rp) were calculated for the continuous variables, while Spearman’s rank correlation (rsp) was used to evaluate correlation with ordinal variables, adjusting for age and sex. The p-values (p) from group differences and correlations were corrected for false discovery rate using the Benjamini–Hochberg procedure (p-FDR).
Multivariable logistic regression was performed to study the effects of the imaging measures and their association with the disability subgroups, MS-L and MS-H. In GraphPad Prism (version 10, GraphPad Inc., San Diego, CA, USA), we used the disability subgroup as a dependent variable and MS-L as the reference level, and BPF, LL, mean DTI-ALPS index, disease duration, age, and sex as covariates. The standardized regression coefficients (β) and odds ratios (ORs) with 95% CI were calculated to assess the relationship between imaging variables and the disability subgroups, and Wald’s test (W) was used to evaluate the significance of individual coefficients in the model. Area under the curve (AUC) was calculated using the receiver-operating characteristic (ROC) curve, to assess the model’s overall performance. Individual ROC analyses were conducted, using the disability subgroup as the dependent variable, to assess the predictive power of the DTI-ALPS index, BPF, and LL in distinguishing between patients with lower and higher disability.
Raw data were generated at UTHealth. Anonymized derived data may be shared upon reasonable request to the corresponding or senior authors with researchers who provide a methodologically sound non-commercial proposal, subject to restrictions according to participant consent and data protection legislation.
Results
A total of 118 PwMS were included in this study (mean age 45 ± 11.8; 65.3% female), divided into two subgroups: MS-L (n = 57; mean age 43.2 ± 12.1; 50.9% female) with EDSS < 3 and MS-H (n = 61; mean age 46.7 ± 11.4; 78.7% female) with EDSS > 3. Their demographic and clinical data are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of PwMS with lower disability (MS-L) and higher disability (MS-H).
| MS (n = 118) | MS-L (n = 57) | MS-H (n = 61) | p | |
|---|---|---|---|---|
| Age, mean (SD) | 45 (11.8) | 43.2 (12.1) | 46.7 (11.4) | 0.1 |
| Sex, (% female) | 65.3% | 50.9% | 78.7% | 0.002 |
| Handedness, (% right-handed) | 90.7% | 94.7% | 86.9% | 0.1 |
| Disease duration, mean (SD) | 12.2 (8.5) | 10.6 (8.1) | 13.6 (8.6) | 0.1 |
| EDSS, median (IQR) | 3 (4) | 2 (1) | 6 (2.5) | <0.001 |
MS-L: lower disability subgroup; MS-H: higher disability subgroup; SD: standard deviation; EDSS: Expanded Disability Status Scale; IQR: interquartile range.
Subject means for the whole group are first provided (MS), and then divided into lower disability (MS-L) and higher disability (MS-H) groups for comparisons. Continuous variables were compared using Student’s t-tests, categorical variables were assessed using the chi-square tests, and EDSS was compared using the Mann–Whitney U-test.
Imaging measures group differences
Compared to the MS-L group, the MS-H group exhibited significantly lower BPF (d = −0.42 (−0.81, −0.03), p-FDR = 0.04) (Figure 1(a)) and GMF (d = −0.41 (−0.79, −0.02), p-FDR = 0.04). The groups did not have significant differences in total WMF. Related to GM, MS-H showed prominent differences in DGM volumes (d = −0.7 (−1.09, −0.30), p-FDR = 0.001) and mean cortical thickness (d = −0.54 (−0.94, −0.15), p-FDR = 0.01). Lesion loads were significantly higher in MS-H compared to MS-L (d = 0.79 (0.39, 1.18), p-FDR = 0.001) (Figure 1(b)). When examining the DTI-ALPS indices, we observed significant differences in mean DTI-ALPS index (d = −0.71 (−1.11, −0.31), p-FDR = 0.001) (Figure 1(c)). Table 2 summarizes group, subgroup, and their differences adjusting for age and sex.
Figure 1.

Group differences in brain parenchymal fraction, lesion load, and mean DTI-ALPS index. Raincloud plots depicting the distribution and differences between the higher (MS-H, green) and the lower (MS-L, orange) disability subgroups. Differences were calculated using ANCOVA adjusting for age and sex. (a) Brain parenchymal fraction (BPF), (b) lesion load, and (c) mean DTI-ALPS.
Table 2.
Group comparisons between PwMS with lower disability (MS-L) and higher disability (MS-H) for MRI measures: structural volumetric measurements and DTI-ALPS indices.
| MS | MS-L | MS-H | Effect size (d) | p-FDR | |
|---|---|---|---|---|---|
| GMF (%) | 41.2 (3.5) | 41.9 (3.2) | 40.5 (3.6) | −0.41 (−0.79, −0.02) | 0.04 |
| Total WMF (%) | 36.5 (3.5) | 36.7 (3.5) | 36.3 (3.4) | −0.19 (−0.57, 0.20) | 0.3 |
| BPF (%) | 77.7 (5.2) | 78.6 (5.4) | 76.8 (4.8) | −0.42 (−0.81, −0.03) | 0.04 |
| CTh (mm) | 2.17 (0.18) | 2.22 (0.16) | 2.12 (0.18) | −0.54 (−0.94, −0.15) | 0.01 |
| DGM (mL) | 18.8 (3.1) | 19.8 (2.7) | 17.9 (3.3) | −0.7 (−1.09, −0.30) | 0.001 |
| LV (mL) | 8.3 (4.2) | 7.3 (3.5) | 9.2 (4.6) | 0.47 (−0.08, 0.86) | 0.03 |
| Lesion load (mL) | 12.8 (14.6) | 7.1 (9.7) | 18.1 (16.3) | 0.79 (0.39, 1.18) | 0.001 |
| Left DTI-ALPS | 1.25 (0.16) | 1.30 (0.16) | 1.20 (0.14) | −0.74 (−1.14, −0.35) | 0.001 |
| Right DTI-ALPS | 1.20 (0.14) | 1.24 (0.14) | 1.17 (0.13) | −0.57 (−0.96, −0.18) | 0.008 |
| Mean DTI-ALPS | 1.22 (0.14) | 1.27 (0.14) | 1.18 (0.13) | −0.71 (−1.11, −0.31) | 0.001 |
MS-L: lower disability group; MS-H: higher disability group; p-FDR: false discovery rate adjusted p-value; GMF: gray matter fraction; WMF: white matter fraction; BPF: brain parenchymal fraction; CTh: cortical thickness; DGM: deep gray matter; LV: lateral ventricle; DTI-ALPS: diffusivity along perivascular spaces index.
Subject means for the whole group are first provided (MS, n = 118), and then divided into lower disability (MS-L, n = 57) and higher disability (MS-H, n = 61) groups for comparisons.
Correlations with DTI-ALPS
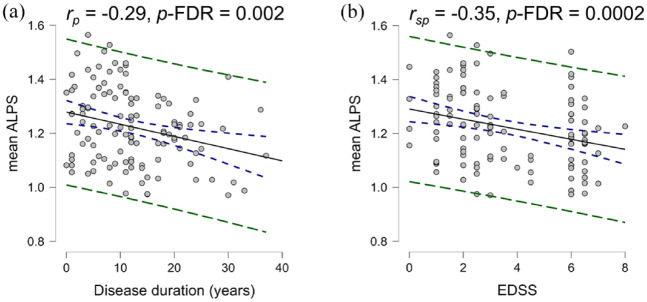
Overall, the left DTI-ALPS indices were higher than the right indices (d = 0.45 (0.26, 0.64), p-FDR = 0.003) (Figure 2(a)) and the mean DTI-ALPS index showed a significant negative correlation with age (rp = −0.27 (−0.46, −0.09), p-FDR = 0.003) (Figure 2(b)). Significant negative correlations were found with disease duration (rp = −0.29 (−0.44, −0.12), p-FDR = 0.002) (Figure 3(a)) and EDSS (rsp = −0.35 (−0.48, −0.19), p-FDR = 0.0002) (Figure 3(b)).
Figure 2.
Paired differences between left and right DTI-ALPS indices and correlation between mean DTI-ALPS index and age. (a) Right and left DTI-ALPS index measurements differences estimated using paired samples t-test and (b) correlation between mean DTI-ALPS index and age. Blue dashes represent the confidence interval, and green dashes represent the prediction intervals.
Figure 3.
Correlations with disease duration and EDSS. (a) Pearson’s correlation calculated between mean DTI-ALPS index and disease duration adjusting for sex and (b) Spearman’s correlation calculated between DTI-ALPS index and EDSS adjusting for age and sex. Blue dashes represent the confidence interval, and green dashes represent the prediction intervals.
For the imaging measures, mean DTI-ALPS index correlated positively with BPF (rp = 0.46 (0.31, 0.59), p-FDR < 0.0001) (Figure 4(a)). Significant correlations were also observed with GMF (rp = 0.35 (0.18, 0.50), p-FDR = 0.0002) (Figure 4(b)), DGM (rp = 0.44 (0.28, 0.57), p-FDR < 0.0001) (Figure 4(c)), and CTh (rp = 0.29 (0.11, 0.45), p-FDR = 0.002) (Figure 4(d)). In the WM, positive correlations were noted with total WMF (rp = 0.31 (0.15, 0.47), p-FDR = 0.0009) (Figure 4(e)) and negative correlations with lesion load (rp = −0.49 (−0.62, −0.35), p-FDR < 0.0001) (Figure 4(f)) (Table 3).
Figure 4.
Correlations with imaging measures. Pearson’s partial correlations adjusting for age and sex between mean DTI-ALPS index and (a) brain parenchymal fraction (BPF), (b) gray matter fraction (GMF), (c) deep gray matter (DGM), (d) mean cortical thickness (CTh), (e) white matter fraction (WMF), and (f) lesion load. Blue dashes represent the confidence interval, and green dashes represent the prediction intervals.
Table 3.
Correlations between mean DTI-ALPS index, disease duration, EDSS, and volumetric MRI measures.
| Correlation coefficient (95% CI) | p-FDR | |
|---|---|---|
| Age a | −0.27 (−0.46, −0.09) | 0.003 |
| Disease duration a | −0.29 (−0.44, −0.12) | 0.002 |
| EDSS b | −0.35 (−0.48, −0.19) | 0.0002 |
| GMF a | 0.35 (0.18, 0.50) | 0.0002 |
| Total WMF a | 0.31 (0.15, 0.47) | 0.0009 |
| BPF a | 0.46 (0.31, 0.59) | <0.0001 |
| Mean CTh a | 0.29 (0.11, 0.45) | 0.002 |
| DGM a | 0.44 (0.28, 0.57) | <0.0001 |
| Lateral ventricle a | −0.52 (−0.63, −0.38) | <0.0001 |
| Lesion load a | −0.49 (−0.62, −0.35) | <0.0001 |
CI: confidence interval; p-FDR: false discovery rate adjusted p-value; EDSS: Expanded Disability Status Scale; GMF: gray matter fraction; WMF: white matter fraction; BPF: brain parenchymal fraction; CTh: cortical thickness; DGM: deep gray matter; LV: lateral ventricle.
Provided values are Pearson’s correlation coefficient (rp).
Provided value is Spearman’s rank correlation coefficient (rsp).
DTI-ALPS discriminative power versus other imaging measures
The results of the multivariable logistic regression, focusing on the relationship of disability subgroup with BPF, LL, and mean DTI-ALPS index, are summarized in Table 4. The model was significantly improved compared to the intercept-only model (log-likelihood ratio (G2) = 33.41, p < 0.0001; AUC = 0.78 (0.70–0.86), p < 0.0001) (Figure 5). The DTI-ALPS index was positively associated with lower disability (OR = 1.77 (1.05, 3.08), W = 4.39, p = 0.04), meanwhile, LL was positively associated with higher disability (OR = 0.94 (0.89, 0.98), W = 5.92, p = 0.01), and BPF was not a significant predictor (OR = 0.95 (0.84, 1.07), W = 0.77, p = 0.38).
Table 4.
Multiple logistic regression parameters showing association between imaging measures and disability subgroups.
| Variable | β | OR (95% CI) | Wald statistic (W) | p |
|---|---|---|---|---|
| Age | −0.20 | 0.98 (0.94, 1.03) | 0.59 | 0.44 |
| Sex (female) | −0.64 | 4.01 (1.57, 11.24) | 0.87 | 0.35 |
| Disease duration | 0.05 | 1.00 (0.95, 1.07) | 0.04 | 0.83 |
| BPF | −0.28 | 0.95 (0.84, 1.07) | 0.77 | 0.38 |
| Lesion load | −0.91 | 0.94 (0.89, 0.98) | 5.92 | 0.02 |
| Mean DTI-ALPS | 0.57 | 1.77 (1.05, 3.08) | 4.39 | 0.04 |
β: standardized regression coefficient; OR: odds ratio; CI: confidence interval; BPF: brain parenchymal fraction; DTI-ALPS: diffusion-tensor image analysis along the perivascular space index.
Figure 5.

Multivariable logistic regression receiver-operating curve. ROC curve assessing the combined performance of the mean DTI-ALPS index, brain parenchymal fraction, and lesion load in predicting disability level.
Finally, individual ROC analyses were conducted for the DTI-ALPS index, LL, and BPF represented in Table 5 and Figure 6. In summary, the largest AUC was observed with LL (AUC = 0.73, p < 0.0001), then the DTI-ALPS index (AUC = 0.67, p = 0.001), followed by BPF (AUC = 0.63, p = 0.01).
Table 5.
Receiver-operating curve analysis results.
| AUC (95% CI) | p | |
|---|---|---|
| DTI-ALPS index | 0.67 (0.57, 0.77) | 0.001 |
| Lesion load | 0.73 (0.63, 0.82) | <0.0001 |
| BPF | 0.63 (0.53, 0.73) | 0.01 |
AUC: area under the curve; CI: confidence interval; DTI-ALPS: diffusion-tensor image analysis along the perivascular space index; BPF: brain parenchymal fraction.
Figure 6.

Independent receiver-operating curves for the mean DTI-ALPS index, brain parenchymal fraction, and lesion load. ROC curves assessing the performance of each imaging measure in predicting disability level.
Discussion
This study evaluated glymphatic function in PwMS using DTI-ALPS and how it relates to clinical disability among other structural imaging including brain atrophy measures and lesion load. PwMS with higher disability exhibited significantly lower DTI-ALPS indices, suggesting more severe glymphatic impairment, compared to PwMS with lower disability. Similar differences were found in the structural imaging measures, with BPF being significantly lower and lesion load being higher in the higher disability group. DTI-ALPS index significantly correlated with disease duration, clinical disability as well as structural imaging measures. We found that DTI-ALPS index and lesion load, but not BPF, were valuable in classifying PwMS into lower and higher disability groups.
Previous studies have investigated glymphatic function in MS using the DTI-ALPS.33,34 Carotenuto et al. 33 reported lower DTI-ALPS indices in PwMS compared to healthy controls and primary progressive PwMS compared to relapsing-remitting PwMS. Another study compared glymphatic function in relapsing-remitting PwMS, secondary progressive PwMS, and healthy controls, showing lower DTI-ALPS indices in the PwMS compared to controls. The authors reported a lack of significant correlation with disease duration. 34
Brain atrophy and lesion load have been long established among the imaging correlates of MS, representing neuroinflammatory and neurodegenerative aspects of MS pathology.2,4,5 Clinically, they have been used in monitoring disease activity, treatment response, and predicting long-term disability.4,5,35 The concordance between DTI-ALPS index and the structural imaging correlates suggests a role for ISF dynamics in the cascade of events contributing to MS pathology. ISF flow is essential to the clearance of solutes including proteins and waste products from the perivascular microenvironment.36,37 The associations of DTI-ALPS-index with other disease measures in addition to its performance as a classifier of PwMS with lower and higher disability might suggest that glymphatic, or ISF dynamics, impairment co-occurs with lesion development and atrophic brain changes.
Glymphatic dysfunction in MS can be explained by several processes including possible impairment in aquaporin-4 expression and polarization. 38 In addition to myelin disruption, MS lesions possibly impair astroglial function, 39 involve perivascular inflammation, and associate with enlarged and leukocyte-infiltrated PVS. 40 It has been shown that the glymphatic system is mostly active during slow-wave sleep due to reduced noradrenaline levels which in turn increases interstitial spaces. 41 PwMS can be disproportionately affected by sleep disorders including sleep-disordered breathing, insomnia, and restless legs syndrome. 42 On the contrary, ISF stasis, or glial dysfunction, could further exacerbate inflammatory and degenerative processes due to the increased time required to clear toxic solutes and waste proteins. 6 While these findings and previous research affirm glymphatic impairment as an epiphenomenon of MS pathology, little is known about whether its involvement is causative or resultative. Further longitudinal research is required to better understand the directionality and significance of its role.
As to the significantly higher DTI-ALPS indices in the left hemisphere compared to the contralateral measurements, similar findings have been previously reported in healthy controls, 43 which can be explained by hemispheric asymmetry and lateralization. Language association pathways, represented in the superior longitudinal fasciculus, can be more developed in the left hemisphere. 44 Our decision to use the average of left and right DTI-ALPS-indices was guided by a previous study that showed higher reproducibility with bilateral measurements. 20
Our study has a few limitations. DTI-ALPS is a newly developed technique with reservations regarding its validity. The DTI-ALPS index specifically quantifies ISF dynamics, that is, water diffusion along perivascular spaces, which might not fully capture the function of the glymphatic system. 45 The retrospective cross-sectional design limits inferences on the role of glymphatic dysfunction in the evolution of MS pathology regarding causality and temporality. This highlights the need for longitudinal studies to better characterize the role of glymphatic function during MS emergence and evolution. Our study lacks healthy controls to act as a referent group, although we aimed to investigate the relationship between glymphatic function, MS-related imaging, and disability outcomes. Finally, we refrained from categorizing the subjects according to their respective MS phenotypes, given the retrospective nature of the study and the bias it could have introduced. To that point, there is growing evidence suggesting that clinical phenotypes may be more of a continuum rather than pathologically distinct conditions. 46
Conclusion
This study elucidates the relationship between glymphatic dysfunction, as quantified by the DTI-ALPS index, MS pathology, and disability. Our results demonstrate that decreased DTI-ALPS indices correlate with higher disability, increased lesion loads, and increased brain atrophy, suggesting a possible role of glymphatic dysfunction in MS progression. The DTI-ALPS index emerges as a possible imaging biomarker, distinguishing between different levels of MS-related disability and correlating with clinical and imaging measures. Despite its limitations, including a cross-sectional design and the absence of healthy controls, this study highlights the potential value of further research on glymphatic function in MS, urging further exploration in longitudinal studies to better understand its role in the disease process.
Acknowledgments
The authors acknowledge the consulting roles of Jerry S. Wolinsky, MD, and Refaat Gabr, PhD, in revising the manuscript.
Footnotes
Data Availability Statement: Raw data were generated at UTHealth. Anonymized derived data may be shared upon reasonable request to the corresponding or senior authors with researchers who provide a methodologically sound non-commercial proposal, subject to restrictions according to participant consent and data protection legislation.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Ahmed Bayoumi  https://orcid.org/0000-0002-7633-5961
https://orcid.org/0000-0002-7633-5961
John A Lincoln  https://orcid.org/0000-0002-4227-3213
https://orcid.org/0000-0002-4227-3213
Contributor Information
Ahmed Bayoumi, Department of Neurology, McGovern Medical School at UTHealth Houston, Houston, TX, USA.
Khader M Hasan, Department of Diagnostic and Interventional Imaging, McGovern Medical School at UTHealth Houston, Houston, TX, USA.
Joseph A Thomas, Department of Neurology, McGovern Medical School at UTHealth Houston, Houston, TX, USA.
Akram Yazdani, Department of Clinical and Translational Sciences, McGovern Medical School at UTHealth Houston, Houston, TX, USA.
John A Lincoln, Department of Neurology, McGovern Medical School at UTHealth Houston, Houston, TX, USA.
References
- 1. Jakimovski D, Bittner S, Zivadinov R, et al. Multiple sclerosis. Lancet 2024; 403: 183–202. [DOI] [PubMed] [Google Scholar]
- 2. Datta G, Colasanti A, Rabiner EA, et al. Neuroinflammation and its relationship to changes in brain volume and white matter lesions in multiple sclerosis. Brain 2017; 140: 2927–2938. [DOI] [PubMed] [Google Scholar]
- 3. Varhaug KN, Torkildsen Ø, Myhr KM, et al. Neurofilament light chain as a biomarker in multiple sclerosis. Front Neurol 2019; 10: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol 2014; 75: 43–49. [DOI] [PubMed] [Google Scholar]
- 5. Barkhof F, Calabresi PA, Miller DH, et al. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 2009; 5: 256–266. [DOI] [PubMed] [Google Scholar]
- 6. Naganawa S, Taoka T, Ito R, et al. The glymphatic system in humans: Investigations with magnetic resonance imaging. Invest Radiol 2024; 59: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cserr HF, Cooper DN, Suri PK, et al. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol 1981; 240: F319–F328. [DOI] [PubMed] [Google Scholar]
- 8. Simon MJ, Iliff JJ. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta 2016; 1862: 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taoka T, Naganawa S. Gadolinium-based contrast media, cerebrospinal fluid and the glymphatic system: Possible mechanisms for the deposition of gadolinium in the brain. Magn Reson Med Sci 2018; 17: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watts R, Steinklein JM, Waldman L, et al. Measuring glymphatic flow in man using quantitative contrast-enhanced MRI. Am J Neuroradiol 2019; 40: 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harada T, Kudo K, Kameda H, et al. Phase I randomized trial of 17O-labeled water: Safety and feasibility study of indirect proton MRI for the evaluation of cerebral water dynamics. J Magn Reson Imaging 2022; 56: 1874–1882. [DOI] [PubMed] [Google Scholar]
- 12. Schubert JJ, Veronese M, Marchitelli L, et al. Dynamic 11C-PiB PET shows cerebrospinal fluid flow alterations in Alzheimer disease and multiple sclerosis. J Nucl Med 2019; 60: 1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramirez J, Berezuk C, McNeely AA, et al. Imaging the perivascular space as a potential biomarker of neurovascular and neurodegenerative diseases. Cell Mol Neurobiol 2016; 36: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Granberg T, Moridi T, Brand JS, et al. Enlarged perivascular spaces in multiple sclerosis on magnetic resonance imaging: A systematic review and meta-analysis. J Neurol 2020; 267: 3199–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: Diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol 2017; 35: 172–178. [DOI] [PubMed] [Google Scholar]
- 16. Beiki O, Frumento P, Bottai M, et al. Changes in the risk of reaching multiple sclerosis disability milestones in recent decades: A nationwide population-based cohort study in Sweden. JAMA Neurol 2019; 76: 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hasan KM, Narayana PA. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: Theoretical analysis and validation. Magn Reson Med 2003; 50: 589–598. [DOI] [PubMed] [Google Scholar]
- 18. Hasan KM. A framework for quality control and parameter optimization in diffusion tensor imaging: Theoretical analysis and validation. Magn Reson Imaging 2007; 25: 1196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hasan KM, Kamali A, Abid H, et al. Quantification of the spatiotemporal microstructural organization of the human brain association, projection and commissural pathways across the lifespan using diffusion tensor tractography. Brain Struct Funct 2010; 214: 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taoka T, Ito R, Nakamichi R, et al. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on Multiple conditiON acquIsition eXperiment (CHAMONIX) study. Jpn J Radiol 2022; 40: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kealey SM, Kim Y, Provenzale JM. Redefinition of multiple sclerosis plaque size using diffusion tensor MRI. Am J Roentgenol 2004; 183: 497–503. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. NeuroImage 2012; 59: 3774–3783. [DOI] [PubMed] [Google Scholar]
- 23. Pirzada S, Uddin MN, Figley TD, et al. Spatial normalization of multiple sclerosis brain MRI data depends on analysis method and software package. Magn Reson Imaging 2020; 68: 83–94. [DOI] [PubMed] [Google Scholar]
- 24. Valverde S, Oliver A, Lladó X. A white matter lesion-filling approach to improve brain tissue volume measurements. NeuroImage Clin 2014; 6: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo C, Ferreira D, Fink K, et al. Repeatability and reproducibility of FreeSurfer, FSL-SIENAX and SPM brain volumetric measurements and the effect of lesion filling in multiple sclerosis. Eur Radiol 2019; 29: 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ashburner J, Friston KJ. Unified segmentation. NeuroImage 2005; 26: 839–851. [DOI] [PubMed] [Google Scholar]
- 27. Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. NeuroImage 2004; 23: 84–97. [DOI] [PubMed] [Google Scholar]
- 28. Ashburner J, Friston KJ. Diffeomorphic registration using geodesic shooting and Gauss–Newton optimisation. NeuroImage 2011; 55: 954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luders E, Cherbuin N, Gaser C. Estimating brain age using high-resolution pattern recognition: Younger brains in long-term meditation practitioners. NeuroImage 2016; 134: 508–513. [DOI] [PubMed] [Google Scholar]
- 30. Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. NeuroImage 2013; 65: 336–348. [DOI] [PubMed] [Google Scholar]
- 31. Yotter RA, Dahnke R, Thompson PM, et al. Topological correction of brain surface meshes using spherical harmonics. Hum Brain Mapp 2011; 32: 1109–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yotter RA, Nenadic I, Ziegler G, et al. Local cortical surface complexity maps from spherical harmonic reconstructions. NeuroImage 2011; 56: 961–973. [DOI] [PubMed] [Google Scholar]
- 33. Carotenuto A, Cacciaguerra L, Pagani E, et al. Glymphatic system impairment in multiple sclerosis: Relation with brain damage and disability. Brain 2021; 145: 2785–2795. [DOI] [PubMed] [Google Scholar]
- 34. Tomizawa Y, Hagiwara A, Hoshino Y, et al. The glymphatic system as a potential biomarker and therapeutic target in secondary progressive multiple sclerosis. Mult Scler Relat Disord 2024; 83: 105437. [DOI] [PubMed] [Google Scholar]
- 35. Popescu V, Agosta F, Hulst HE, et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry 2013; 84: 1082–1091. [DOI] [PubMed] [Google Scholar]
- 36. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012; 4: 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iliff J, Simon M. CrossTalk proposal: The glymphatic system supports convective exchange of cerebrospinal fluid and brain interstitial fluid that is mediated by perivascular aquaporin-4. J Physiol 2019; 597: 4417–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rohr SO, Greiner T, Joost S, et al. Aquaporin-4 expression during toxic and autoimmune demyelination. Cells 2020; 9: 2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aharoni R, Eilam R, Arnon R. Astrocytes in multiple sclerosis—Essential constituents with diverse multifaceted functions. Int J Mol Sci 2021; 22: 5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ge Y, Law M, Herbert J, et al. Prominent perivenular spaces in multiple sclerosis as a sign of perivascular inflammation in primary demyelination. Am J Neuroradiol 2005; 26: 2316–2319. [PMC free article] [PubMed] [Google Scholar]
- 41. Cai X, Qiao J, Kulkarni P, et al. Imaging the effect of the circadian light–dark cycle on the glymphatic system in awake rats. Proc Natl Acad Sci USA 2020; 117: 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Braley TJ. Overview: A framework for the discussion of sleep in multiple sclerosis. Curr Sleep Med Rep 2017; 3: 263–271. [PMC free article] [PubMed] [Google Scholar]
- 43. Wang L, Qin Y, Li X, et al. Glymphatic-system function is associated with addiction and relapse in heroin dependents undergoing methadone maintenance treatment. Brain Sci 2023; 13: 1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Janelle F, Iorio-Morin C, D’Amour S, et al. Superior longitudinal fasciculus: A review of the anatomical descriptions with functional correlates. Front Neurol 2022; 13: 794618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ringstad G. Glymphatic imaging: A critical look at the DTI-ALPS index. Neuroradiology 2024; 66: 157–160. [DOI] [PubMed] [Google Scholar]
- 46. Vollmer TL, Nair KV, Williams IM, et al. Multiple sclerosis phenotypes as a continuum: The role of neurologic reserve. Neurol Clin Pract 2021; 11: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]