Abstract
Background:
Biomarkers of neuronal and axonal damage (serum neurofilament light (sNfL) and serum glial fibrillary acidic protein (sGFAP)) may provide insight into the aetiology of natalizumab wearing-off symptoms (WoSs).
Objectives:
We investigated the longitudinal association between and predictive value of sNfL and sGFAP and the occurrence of WoS in MS patients treated with natalizumab.
Methods:
We performed longitudinal measurements of sNfL and sGFAP in NEXT-MS trial participants who completed a questionnaire about WoS.
Results:
A total of 364 participants were included. In total, 55.5% presented with WoS and 44.5% without WoS during natalizumab treatment. Longitudinal analyses showed no association between sNfL and sGFAP levels and WoS at any timepoint. Biomarker levels at baseline did not predict first-time WoS occurrence.
Conclusion:
Acute and chronic neuronal and axonal damage are most likely not the underlying cause of WoS.
Keywords: Multiple sclerosis, biomarkers, natalizumab, treatment response
Introduction
Wearing-off symptoms (WoSs) are reported by a significant part of multiple sclerosis (MS) patients during treatment with natalizumab. 1 WoS are MS-related symptoms, such as fatigue, that increase towards the next natalizumab dosing and usually disappear shortly afterwards. So far, there is no evidence that MS disease activity is associated with WoS. 2 Only higher body mass index (BMI) and body weight were reported to be associated with WoS.1,3
Blood biomarkers of neuronal and axonal damage such as serum neurofilament light (sNfL) and serum glial fibrillary acidic protein (sGFAP) are increasingly used to monitor disease activity and treatment response.4,5 Biomarkers can also be indicative of smouldering disease, as elevated levels of sNfL are associated with chronic white matter inflammation and microglial activation. 6 The sGFAP levels have been associated with disability, lesion load and neurodegeneration. 7 Measurement of in vivo biomarkers might provide insight into the biological underpinnings of WoS.
The aim of our study was to investigate the association between sNfL and sGFAP and the occurrence of WoS in MS patients treated with natalizumab. In addition, we studied whether these biomarkers can predict the occurrence of WoS during further treatment.
Methods
Study design and participants
Patients with relapsing-remitting MS who received at least six natalizumab infusions were included in the NEXT-MS trial between February 2020 and April 2022.8 The NEXT-MS trial was an investigator-initiated nonrandomized multicenter study on personalized extended dosing based on natalizumab serum concentrations. Participants who completed at least one WoS questionnaire were included in this substudy.
Study procedures and outcomes
Participants received questionnaires about WoS at three time points: start of the NEXT-MS trial, year 1 and year 2. Questions regarding WoS were similar to previous studies on WoS in our centre and captured ever experiencing WoS since natalizumab initiation (baseline) or in the past year (years 1 and 2). 1 Patients were counted as experiencing WoS if they answered these questions positively. Serum was collected after screening and during the study at natalizumab infusions and grouped based on weeks from baseline. Left-over serum after measuring natalizumab concentrations (mean 2 samples per patient, mean time between samples 10.3 months) was used to measure biomarker levels in one batch (Simoa® Neurology 4-Plex E Advantage Kit, Quanterix, Billerica, MA, USA). 4 Biomarker results were expressed as levels and delta values.
Statistical analyses
Patients were divided between those experiencing WoS during treatment with natalizumab and those that did not. Clinical characteristics were compared between groups using chi-square test, t-test and Mann–Whitney U-test. Further analysis into frequency (never WoS vs. often/always) was performed afterwards. The association between longitudinal biomarker levels and WoS was investigated using Generalized Estimating Equations (GEEs).
Next, the predictive values of biomarker levels at baseline for first-time WoS converters (patients experiencing WoS for the first time after baseline) were tested using logistic regression. Corrections for sex, age, BMI and Expanded Disability Status Scale (EDSS) were performed in GEE and logistic regression. A p-value < 0.05 was considered significant. Statistical analyses and visualizations were performed with R software version 4.3.2.
Results
Participants
A total of 364 participants started the NEXT-MS trial and completed at least one WoS questionnaire (mean = 2). We identified 202 (55.5%) participants with WoS and 162 (44.5%) participants without WoS during natalizumab treatment. Thirty-three patients experienced WoS for the first time during follow-up. EDSS at baseline was higher in participants with WoS (Table 1).
Table 1.
Baseline characteristics of included participants.
| Never experienced WoS | Ever experienced WoS | Total | p | |
|---|---|---|---|---|
| (N = 162) | (N = 202) | (N = 364) | ||
| Sex, n (%) | ||||
| Male | 33 (20.4%) | 43 (21.3%) | 76 (20.9%) | .93 |
| Female | 129 (79.6%) | 159 (78.7%) | 288 (79.1%) | |
| Age, years (IQR) | 39.0 (33.0–49.0) | 40.0 (33.0–50.0) | 40.00 (33.0–50.0) | .91 |
| BMI at baseline, kg/m2 (IQR) | 24.44 (21.95–27.70) | 24.86 (21.50–28.18) | 24.69 (21.60–28.04) | .91 |
| EDSS, (IQR) | 2.5 (1.5–4.0) | 3.0 ( 2.0–4.4) | 3.0 (2.0–4.0) | .0022 |
| JCV-status, n (%) | ||||
| Negative | 138 (85.2%) | 169 (83.7%) | 307 (84.3%) | .8 |
| Positive | 24 (14.8%) | 33 (16.3%) | 57 (15.7%) | |
| New/newly enlarged T2 lesions on MRI a , n (%) | ||||
| No activity | 145 (89.5%) | 181 (89.6%) | 326 (89.6%) | 1 |
| Activity | 17 (10.5%) | 21 (10.4%) | 38 (10.4%) | |
| Extended dosing group, n (%) | ||||
| SID group | 18 (11.1%) | 39 (19.3%) | 57 (15.7%) | .098 |
| EID10 group | 113 (69.8%) | 130 (64.4%) | 243 (66.8%) | |
| EID5 group | 31 (19.1%) | 33 (16.3%) | 64 (17.6%) | |
| Duration NTZ treatment, years (IQR) | 4.22 (1.31–8.71) | 3.98 (1.67–7.50) | 4.05 (1.50–8.20) | .85 |
| NfL at baseline, pg/mL (IQR) | 9.51 (7.17–13.02) | 10.22 (7.40–13.16) | 9.81 (7.22–13.1) | .5 |
| GFAP at baseline, pg/mL (IQR) | 66.86 (50.95–90.83) | 70.75 (53.41–94.36) | 68.03 (51.70–92.71) | .71 |
WoS: wearing-off symptom; BMI: body mass index; JCV: John–Cunningham virus; sNfL: serum neurofilament light; sGFAP: serum glial fibrillary acidic protein; EDSS: Expanded Disability Status Scale; NTZ: natalizumab; SID: standard interval dosing (treatment every 4 weeks); EID10: extended interval dosing with an aim drug trough concentration of 10 µg/mL; EID5: extended interval dosing with an aim drug trough concentration of 5 µg/mL. 8
Values are depicted as medians with interquartile ranges or frequencies with percentages. Clinical characteristics were compared between groups using the chi-square test for categorical variables, the t-test for normally distributed continuous variables and the Mann–Whitney U test for non-normally distributed continuous variables.
Baseline MRI scan of the NEXT-MS trial.
Biomarkers and WoSs
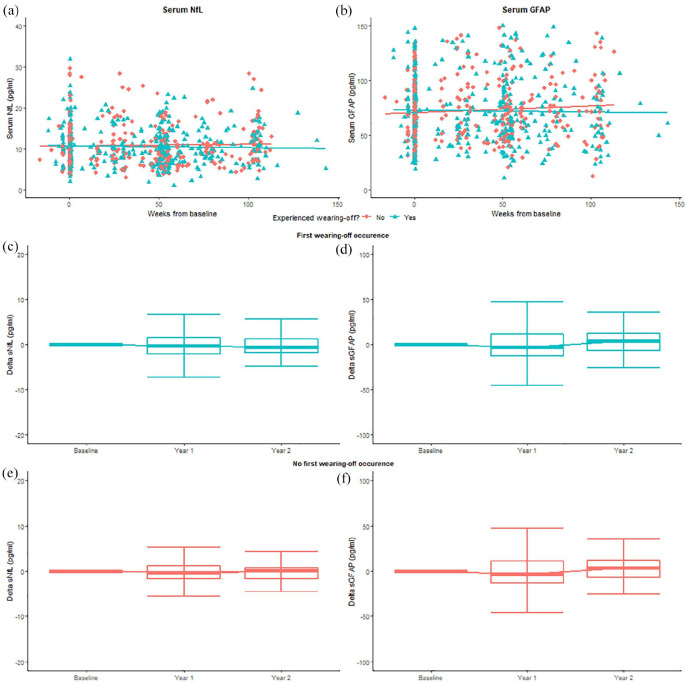
Figure 1 shows the associations between biomarkers and WoS over time. GEE analyses showed no significant associations between sNfL (odds ratio [OR] = 1.00, 95% confidence interval [CI] = [1.00–1.00], p = 0.83) or sGFAP (OR = 1.00, 95% CI = [1.00–1.00], p = 0.45) at any timepoint and the occurrence of WoS. There were no changes after correction for confounders (sNfL: OR = 1.0, 95% CI = [1.00–1.00], p = 0.79; sGFAP: OR = 1.00, 95% CI = [1.00–1.00], p = 0.35). This remained insignificant when comparing never versus often/always WoS.
Figure 1.
sNfL and sGFAP levels over time. (a) and (b) The x-axis displays continuous time points (weeks from baseline). The y-axis displays sNfL levels in pg/mL (a) and sGFAP levels in pg/mL (b). Baseline represents the start of extended interval dosing for each dose. Samples for the current study were retrieved at the start of the study, year 1 and last follow-up. The figures illustrate no significant difference in sNfL and sGFAP levels over time between participants with WoS and without WoS. (c)–(f) The x-axis displays timepoints (baseline, year 1 and year 2). The y-axis displays the absolute difference in sNfL ((c) and (e)) and sGFAP ((d) and (f)) levels compared to baseline. The figures illustrate no significant change in biomarker levels between participants with first-time WoS occurrence during follow-up and those without.
WoS: wearing-off symptom; sNfL: serum neurofilament light; sGFAP: serum glial fibrillary acidic protein.
Furthermore, GEE analyses showed no significant associations between percentual changes in biomarker levels and first-time WoS occurrence. This was observed for both percentual changes in follow-up levels compared to baseline (sNfL: OR = 1.0, 95% CI = [1.00–1.00], p = 0.39; sGFAP: OR = 1.00, 95% CI = [1.00–1.00], p = 0.13), as well in percentual changes between consecutive measurements (sNfL: OR = 1.0, 95% CI = [1.00–1.00], p = 0.99; sGFAP: OR = 1.00, 95% CI = [1.00–1.00], p = 0.10). This remained after correction for confounders.
Logistic regression analyses showed that sNfL at baseline (OR = 1.02, 95% CI = [0.95–1.06], p = 0.70) and sGFAP at baseline (OR = 1.00, 95% CI = [1.00–1.00], p = 0.98) did not predict first-time WoS occurrence. These results did not change after correction for confounders (sNfL: OR = 1.02, 95% CI = [0.96–1.08], p = 0.37; sGFAP: OR = 1.00, 95% CI = [0.99–1.01], p = 0.85).
Discussion
The results of our study could not determine neuronal and axonal damage as an underlying cause for WoS, as we found no evidence that WoS are associated with sNfL and sGFAP levels at any timepoint.
We observed a higher EDSS score in participants with WoS at baseline but no significantly higher BMI. Both findings were not consistently replicated by other studies.1,2,9 Smouldering disease activity is gaining more attention with regard to disease progression independent of relapses, possibly causing MS-related symptoms and disability without radiological activity . Elevated sNfL was associated with chronic white matter inflammation and microglial activation previously, while sGFAP has been associated with higher lesion loads and EDSS scores.6,7 It is reassuring that WoS are likely not reflecting reoccurring neuronal and axonal damage, confirming previous findings in a smaller cohort indicating no elevation of sNfL with WoS. 2
Former studies showed no association with WoS and MS activity.1,2 Other factors such as low natalizumab trough concentrations or presence of serum cytokines were also not associated with WoS.1,9 There was no difference in WoS prevalence between participants with standard- or extended dosing in our study. 10 WoS therefore might reflect a nocebo effect. 1
Limitations of this study include the nonrandomized study design. WoSs were self-reported and remain subjective due to recall and attribution bias. Strengths include evaluation of prospectively collected longitudinal data with measurements of biomarkers in relation to WoS.
In conclusion, longitudinal analyses showed sNfL and sGFAP levels were not associated with natalizumab WoS, indicating that acute and chronic neuronal and axonal damage are most likely not the underlying cause.
Acknowledgments
The authors thank all participants of the NEXT-MS trial and the participating centres for their contribution to the study, inclusion of participants, collection of data, analyses of blood samples and/or other help in conducting the trial.
Footnotes
Author Contributions: A.A.T., M.W., Z.L.E.v.K., JK and C.E.T. contributed to study design. A.A.T., E.H., E.M.P.E.Z., L.C.v.R., C.E.P.v.M., A.V., J.P.M., B.H.A.W., N.F.K., E.L.J.H., J.J.J.E., C.M.R., J.J.K., M.E., J.v.G., J.N., L.G.F.S., M.E.K., E.P.J.A., G.W.v.D., W.H.B., Z.L.E.v.K., J.K. and C.E.T. contributed to study investigators. A.A.T. and M.W. contributed to data analyses. A.A.T. and M.W. contributed to data verification. All authors contributed to data interpretation. A.A.T., M.W., Z.L.E.v.K., J.K. and C.E.T. contributed to manuscript preparation. All authors reviewed and revised the manuscript.
Data Availability Statement: Anonymized data will be shared upon reasonable request from any qualified investigator.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: A.A.T. has nothing to disclose. M.H.J.W. has nothing to disclose. L.B. has nothing to disclose. L.M.Y.G. has nothing to disclose. E.H. has accepted (speaker and congress) fees from Merck Serono, Biogen Idec, Roche and Sanofi Genzyme. E.M.P.E.Z. reports advisory boards/consultancy fees for Merck, Novartis, Genzyme and Roche. L.C.v.R. has nothing to disclose. C.E.P.v.M. has nothing to disclose. A.V. has nothing to disclose. J.P.M. has nothing to disclose. B.H.A.W. has nothing to disclose. N.F.K. has nothing to disclose. E.L.J.H. has nothing to disclose. J.J.J.v.E. reports honoraria for advisory boards and/or speakers fee from Merck Serono, Biogen Idec, Sanofi Genzyme, Roche and Novartis. C.M.R. has nothing to disclose. J.J.K. has nothing to disclose. M.E. has nothing to disclose. J.N. has nothing to disclose. J.v.G. has nothing to disclose. L.G.F.S. has nothing to disclose. M.E.K. has nothing to disclose. E.P.J.A. has nothing to disclose. G.W.v.D. has nothing to disclose. W.H.B. has nothing to disclose. E.M.M.S. has nothing to disclose. B.W.v.O. has nothing to disclose. B.A.d.J. has nothing to disclose. B.M.J.U. reports research support and/or consultancy fees from Genzyme, Biogen Idec, Novartis, Teva Pharmaceutical Industries, Merck Serono, Roche and Immunic Therapeutics. T.R. received funding for research from Genmab and consultancy fees from Novartis. J.K. received research grants for multicentre investigator-initiated trials DOT-MS trial, ClinicalTrials.gov Identifier: NCT04260711 (ZonMW) and BLOOMS trial (ZonMW and Treatmeds) (ClinicalTrials.gov Identifier: NCT05296161); received consulting fees for F. Hoffmann-La Roche Ltd, Biogen, Teva, Merck, Novartis and Sanofi/Genzyme (all payments to institution); reports speaker relationships with F. Hoffmann-La Roche Ltd, Biogen, Immunic, Teva, Merck, Novartis and Sanofi/Genzyme (all payments to institution) and adjudication committee of MS clinical trial of Immunic (payments to institution only). Z.L.E.v.K. has nothing to disclose. C.E.T. reports funding from National MS Society (Progressive MS alliance) and Innovative Medicines Initiatives 3TR (Horizon 2020, grant no. 831434); has a research contract with Celgene; serves on editorial boards of Medidact Neurologie/Springer, Neurology: Neuroimmunology & Neuroinflammation and is editor of a Neuromethods book Springer.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The NEXT-MS trial was kindly funded by the Dutch MS Research Foundation (18-1030), the Brain Foundation Netherlands, the 3TR grant (grand agreement 831434) and Innovation Fund Healthcare insurers. The funding sources had no involvement in the execution of the study.
Standard Protocol Approvals, Registrations and Patient Consents: The study protocol was approved by the medical ethics committee (VUMC Ethics committee number 2019.552). Oral and written informed consent was obtained from all participants. The NEXT-MS trial protocol was registered online (ClinicalTrials.gov: NCT04225312).
ORCID iDs: Alyssa A Toorop  https://orcid.org/0000-0002-7196-9826
https://orcid.org/0000-0002-7196-9826
Mark HJ Wessels  https://orcid.org/0000-0003-2324-0499
https://orcid.org/0000-0003-2324-0499
Beatrijs HA Wokke  https://orcid.org/0000-0002-2616-8464
https://orcid.org/0000-0002-2616-8464
Eva MM Strijbis  https://orcid.org/0000-0001-6705-5864
https://orcid.org/0000-0001-6705-5864
Theo Rispens  https://orcid.org/0000-0001-9600-1312
https://orcid.org/0000-0001-9600-1312
Zoé LE van Kempen  https://orcid.org/0000-0001-9557-5381
https://orcid.org/0000-0001-9557-5381
Contributor Information
Alyssa A Toorop, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Mark HJ Wessels, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Lynn Boonkamp, Neurochemistry Laboratory, Department of Laboratory Medicine, Amsterdam University Medical Center, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Liza MY Gelissen, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
E Hoitsma, Department of Neurology, MS Center Alrijne Hospital, Leiden, The Netherlands.
Esther MPE Zeinstra, Department of Neurology, Isala, Meppel, The Netherlands.
Luuk C van Rooij, Department of Neurology, Maasstad Hospital, Rotterdam, The Netherlands.
Caspar EP van Munster, Department of Neurology, Amphia, Breda, The Netherlands.
Anke Vennegoor, Department of Neurology, Flevoziekenhuis, Almere, The Netherlands.
Jop P Mostert, Department of Neurology, Rijnstate Hospital, Arnhem, The Netherlands.
Beatrijs HA Wokke, Department of Neurology, Erasmus Medical Center, Rotterdam, The Netherlands.
Nynke F Kalkers, Department of Neurology, OLVG, Amsterdam, The Netherlands.
Erwin LJ Hoogervorst, Department of Neurology, St. Antonius Ziekenhuis, Utrecht, The Netherlands.
Jeroen JJ van Eijk, Department of Neurology, Jeroen Bosch Ziekenhuis/Hospital, ‘s-Hertogenbosch, The Netherlands.
Christiaan M Roosendaal, Department of Neurology, Slingeland Hospital, Doetinchem, The Netherlands.
Jolijn J Kragt, Department of Neurology, Reinier de Graaf Hospital, Delft, The Netherlands.
Marijke Eurelings, Department of Neurology, Spaarne Gasthuis, Haarlem, The Netherlands.
Jessie van Genugten, Department of Neurology, Ziekenhuisgroep Twente, Almelo, The Netherlands.
Jessica Nielsen, Department of Neurology, Ommelander Ziekenhuis, Scheemda, The Netherlands.
LGF Sinnige, Department of Neurology, Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands.
Mark E Kloosterziel, Department of Neurology, Wilhelmina Hospital, Assen, The Netherlands.
Edo PJ Arnoldus, Department of Neurology, Elisabeth-TweeSteden Hospital, Tilburg, The Netherlands.
Gert W van Dijk, Department of Neurology, Canisius Wilhelmina Hospital, Nijmegen, The Netherlands.
Willem H Bouvy, Department of Neurology, Diakonessenhuis Hospital, Utrecht, The Netherlands.
Eva MM Strijbis, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Bob W van Oosten, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Brigit A de Jong, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Bernard MJ Uitdehaag, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Theo Rispens, Sanquin Diagnostic Services, Amsterdam, the Netherlands; Department of Immunopathology, Sanquin Research and Landsteiner Laboratory, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Joep Killestein, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Zoé LE van Kempen, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Charlotte E Teunissen, Neurochemistry Laboratory, Department of Laboratory Medicine, Amsterdam University Medical Center, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, Amsterdam, The Netherlands.
References
- 1. Van Kempen ZLE, Doesburg D, Dekker I, et al. The natalizumab wearing-off effect: End of natalizumab cycle, recurrence of MS symptoms. Neurology 2019; 93(17): e1579–e1586. [DOI] [PubMed] [Google Scholar]
- 2. Bringeland GH, Myhr KM, Vedeler CA, et al. Wearing-off at the end of natalizumab dosing interval and risk of MS disease activity: A prospective 1-year follow-up study. J Neurol Sci 2020; 415: 116880. [DOI] [PubMed] [Google Scholar]
- 3. Bringeland GH, Blaser N, Myhr KM, et al. Wearing-off at the end of natalizumab dosing intervals is associated with low receptor occupancy. Neurol Neuroimmunol Neuroinflamm 2020; 7(3): e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018; 14(10): 577–589. [DOI] [PubMed] [Google Scholar]
- 5. Azzolini F, Gilio L, Pavone L, et al. Neuroinflammation is associated with GFAP and sTREM2 levels in multiple sclerosis. Biomolecules 2022; 27(2): 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saraste M, Matilainen M, Vuorimaa A, et al. Association of serum neurofilament light with microglial activation in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2023; 94: 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdelhak A, Foschi M, Abu-Rumeileh S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol 2022; 18(3): 158–172. [DOI] [PubMed] [Google Scholar]
- 8. Toorop AA, Van Lierop ZYGJ, Gelissen LMY, et al. Prospective trial of natalizumab personalized extended interval dosing by therapeutic drug monitoring in relapsing remitting multiple sclerosis (NEXT-MS). J Neurol Neurosurg Psychiatry 2023; 95: 392–400. [DOI] [PubMed] [Google Scholar]
- 9. Cathérine D, Annelien P, Anne S, et al. End of dose interval symptoms in patients treated with natalizumab: A role for serum cytokines. Mult Scler Relat Disord 2020; 41: 102020. [DOI] [PubMed] [Google Scholar]
- 10. Magro G, Barone S, Tosto F, et al. Natalizumab wearing-off symptoms: Effect of extend interval dosing during Sars-CoV-2 pandemic. J Neurol 2023; 270: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]