Abstract
Diabetic kidney disease (DKD) is a prevalent microvascular complication of diabetes mellitus and a primary cause of end-stage renal disease (ESRD). Increasing studies suggest that immune cells are involved in regulating renal inflammation, which contributes to the progression of DKD. Compared with conventional methods, single-cell sequencing technology is more developed technique that has advantages in resolving cellular heterogeneity, parallel multi-omics studies, and discovering new cell types. ScRNA-seq helps researchers to analyze specifically gene expressions, signaling pathways, intercellular communication as well as their regulations in various immune cells of kidney biopsy and urine samples. It is still challenging to investigate the function of each cell type in the pathophysiology of kidney due to its complex and heterogeneous structure and function. Here, we discuss the application of single-cell transcriptomics in the field of DKD and highlight several recent studies that explore the important role of immune cells including macrophage, T cells, B cells etc. in DKD through scRNA-seq analyses. Through combing the researches of scRNA-seq on immune cells in DKD, this review provides novel perspectives on the pathogenesis and immune therapeutic strategy for DKD.
Keywords: Diabetic kidney disease, Single-cell RNA sequencing, Immune system, Dendritic cells and macrophage, T cells, B cells
Introduction
Diabetic kidney disease (DKD), a microvascular complication associated with type I or II diabetes, poses a significant risk to human health. In most countries, more than 50% of individuals receiving renal replacement therapy (RRT) for ESRD are affected by DKD [1]. Although mortality from DKD has decreased over the past three decades owing to improved diabetes management, the absolute risk of renal and cardiovascular morbidity and mortality remains substantial [2–6]. About 30% of patients with type 1 diabetes mellitus (DM1) and 40% of patients with type 2 diabetes mellitus (DM2) turn up microvascular complications [1, 7]. Several independent familial studies in various populations have indicated a genetic predisposition to DKD [8, 9]. Hence, it is crucial to investigate the pathophysiology and gene expression information of DKD in more depth so that innovative therapeutic methods may be developed to prevent, halt, and reverse DKD. Besides, microalbuminuria, as a biomarker for early diabetic nephropathy, has low sensitivity and specificity in predicting DKD [10]. It should be noted that the presence of micro/macroalbuminuria does not always indicate the presence of DKD. Many diabetic patients have declined renal function even without significant proteinuria [11]. Furthermore, renal biopsy is still the method used to diagnose DKD [12]. Yet it is an aggressive procedure related to complications such as infection and hemorrhage [13]. Additionally, it is not feasible to monitor continuously due to the progressive nature of kidney disease and the possibility of sampling errors. Therefore, it is essential to explore noninvasive and highly sensitive immune-associated biomarkers to accurately predict DKD development. The biomarkers provide a promising alternative by offering continuous assessment and early detection of disease progression, addressing the limitations inherent in renal biopsy.
To explain these questions, it is important to explore gene regulatory mechanisms at the cellular level. Fluorescence-activated cell sorting (FACS), as a traditional method for renal cell type studies, characterizes cells based on the expression of surface markers. This approach is crucial for understanding immune responses but imposes stimulus on the cells, potentially altering their expression profiles [14]. The kidney filters and excretes bacterial toxins, circulating cytokines, and inflammatory molecules to maintain the homeostasis of the immune system [15, 16]. The immune system is a complex network of cells, tissues, and organs that work together to defend the body against harmful invaders such as bacteria, viruses, and fungi. Thus, in order to enable detailed characterization of the immune cells, it is necessary to use the sequencing analysis method at the level of single cell. Major progress has been made in exploring the role of immune cells in kidney diseases [15, 16] and applying these findings from laboratory research to clinical practice. However, it is a challenge that determines how these immune cells coordinate kidney immunology in health and disease owing to the small number of these cells and the complex composition of the kidney. So far, we still do not have a panoramic understanding in depth of kidney immunology until now. In this review, we aim to the recent progress made by single-cell studies of DKD. We also discuss recent findings focusing on DKD, an immune-related kidney disease, highlighting the changes in immune cell groups and the potential immune mechanisms revealed by scRNA-seq technology.
Single-cell RNA sequencing technology
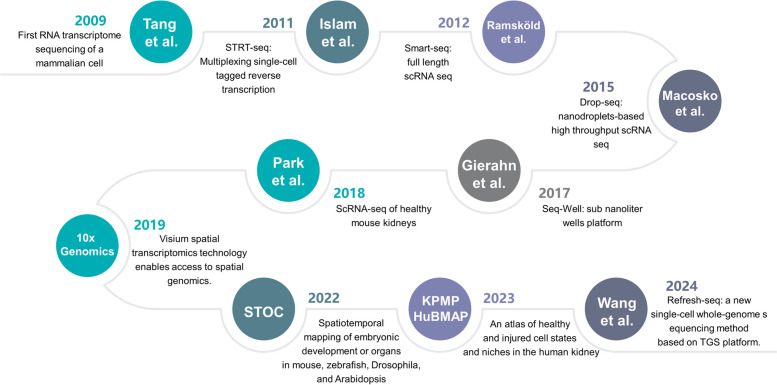
Single-cell sequencing technology is a high-throughput transcriptomics technique used to analyze gene expression at the resolution of individual cells [17, 18]. Unlike traditional whole genome sequencing (WGS), which assesses gene expression at the multicellular level, scRNA-seq provides detailed insights into cellular heterogeneity and gene expression variability. This technique allows for precise measurement of gene expression levels and can identify low-abundance transcripts and rare non-coding RNAs. Since 2009, scRNA-seq technology has been rapidly developed during the last decade (Fig. 1) [19–28], with significantly reduced costs, increased automation, and rising throughput. It has become prevalent in tackling critical questions in biology and medicine.
Fig. 1.
Development of scRNA-seq and its applications in kidney immunology
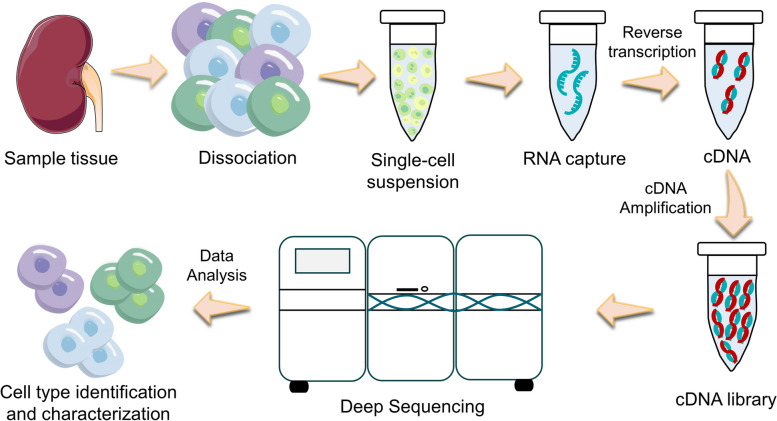
The common workflow of scRNA-seq includes single-cell isolation, cell lysis and RNA capture, reverse transcription, complementary DNA (cDNA) amplification and library construction, high-throughput sequencing, and bioinformatic analysis (Fig. 2) [29, 30]. The core strategies consist of separating individual cells, creating sequencing libraries independently, and identifying single cells based on barcode. Zilionis et al. established a method called inDrops, which used droplet microfluidics to encapsulate individual cells in nanoliter droplets and barcoded (indexed) mRNA for genomic or whole transcriptome analysis [31]. This encapsulation facilitates the simultaneous processing of thousands of cells, significantly enhancing throughput. When RNA levels in a cell are insufficient for sequencing, amplification is necessary to enhance the material available for analysis. For genomic DNA amplification in low-biomass samples, whole genome amplification (WGA) techniques, such as multiple displacement amplification (MDA) using phi29 DNA polymerase, are commonly employed [32–34]. Although MDA effectively addresses nucleic acid concentration issues, it can introduce amplification biases that may affect the reliability of quantitative comparisons [34]. Therefore, when applying these methods to metabolomics analysis of environmental samples, careful selection and validation of MDA kits are crucial [35]. For single-cell transcriptome amplification, reverse transcription (RT) of mRNA to cDNA followed by polymerase chain reaction (PCR) is required. This process enables the conversion of RNA into a more stable form, which can then be amplified for sequencing. Picelli et al. dug out smart-seq2 transcriptome libraries that detect precisely, with better coverage, bias and accuracy compared to smart-seq libraries [36]. A critical aspect of scRNA-seq technology is the bioinformatic analysis that follows sequencing, which is essential for interpreting the vast amounts of data generated. This analysis typically involves several key steps: quality control, normalization, dimensionality reduction, clustering, and differential expression analysis. Quality control checks the integrity and purity of the data to eliminate low-quality reads. Normalization adjusts for variations in sequencing depth and RNA composition across cells, enabling meaningful comparisons. Dimensionality reduction techniques, such as PCA (Principal Component Analysis) or t-SNE (t-distributed Stochastic Neighbor Embedding), reduce the complexity of the data, making it easier to visualize and interpret. Clustering algorithms are then applied to identify distinct cell populations based on gene expression profiles. Finally, differential expression analysis allows researchers to identify genes that are significantly upregulated or downregulated in specific cell types or conditions, providing insights into cellular functions and biological processes.
Fig. 2.
An overview of the single-cell RNA sequencing procedures. The experimental workflow involves isolating cells from tissue samples and dissociating them into a single-cell suspension; individualized RNA capture; reverse transcription and amplification of complementary DNA (cDNA) and library preparation. After performing high-throughput sequencing and conducting bioinformatic analysis, the specific type of cell would be accurately identified and thoroughly characterized
The advent of scRNA-seq technology has enabled the study of kidney disease at unprecedented resolution. The evergrowing compendium of scRNA-seq-based kidney atlases has catapulted our knowledge of not only the immune cells that comprise the microenvironment but also their cell-state heterogeneity. Spatial transcriptomics (ST), with other technologies such as scRNA-seq, has enabled researchers to dissect the organization and interaction of different cell types within the DKD [37]. Recent advancements in methodologies, such as GeoMx Digital Spatial Profiling (DSP) and LightSeq technology, represent significant strides in the field, offering capabilities that classify these approaches into sequencing-based experimental biological techniques (EBTs) and imaging-based nonexperimental biological techniques (NEBTs). These innovative technologies provide spatially resolved transcriptomic and proteomic data, thereby facilitating a more nuanced understanding of the cellular architecture and functional dynamics in the context of DKD [27].
Single-cell RNA sequencing studies in nephrology
The kidney is a highly complex organ for scRNA-seq studies. To date, mapping kidney gene expression is rapidly becoming an irreplaceable method for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)’s Kidney Precision Medicine Project (KPMP) (https://kpmp.org/), the National Institutes of Health (NIH)’s Human BioMolecular Atlas Program (HuBMAP) (https://hubmapconsortium.org/), Susztak lab’s kidney Biobank (KBK) (https://susztaklab.com/), Humphreys lab’s Kidney Interactive Transcriptomics (KIT) (http://humphreyslab.com/) and Kuopio University Hospital (KUH)’s Nephrotic Syndrome Study Network (NEPTUNE) (https://neptune-study.org/). The goal of mapping kidney gene expression is to enhance our comprehension of the prevalent types of kidney diseases [38]. Single cell sequencing techniques were initially created to measure gene expression. However, they have now advanced to enable the simultaneous profiling of other features like chromatin accessibility within the nucleus and protein expression at the cell surface [28, 39–44]. Recently, many studies have been published using scRNA-seq technology in kidney atlas, renal tumors, disease mechanisms, therapeutic targets, and so on (Tables 1 and 3).
Table 1.
Single-cell transcriptomics studies in nephrology
| Article information | Method (s) | Disease/model | Tissue/cell type | Cell number |
|---|---|---|---|---|
| Park et al. (2018) [45] | ScRNA-seq | Healthy mouse | Kidney | 57,979 |
| Fu et al. (2019) [46] | ScRNA-seq | STZ-diabetic eNOS-/- mice | Glomerular cells | 829 |
| Dumas et al. (2020) [47] | ScRNA-seq | A hyperosmolarity model in vitro and dehydrated mice in vivo | renal endothelial cell | 40,662 |
| Chung et al. (2020) [48] | ScRNA-seq | Healthy mouse | Glomerular cells | 75,000 |
| Braun et al. (2021) [49] | ScRNA-seq | ccRCC | Human renal tumors | 164,722 |
| Krishna et al. (2021) [50] | ScRNA-seq | ccRCC | ICB-naïve and ICB-treated patients | 167,283 |
| Obradovic et al. (2021) [51] | ScRNA-seq | ccRCC | Human renal tumors | 200,000 |
| He et al. (2021) [52] | Smart-seq2 | Human living donor renal biopsies and mouse | Podocytes, glomerular endothelial cells, MCs and PECs | 4,332 |
| Pickering et al. (2021) [53] | ScRNA-seq | CMV | Kidney transplant recipients prior to viremia, acutely after viremia, and long-term post-CMV viremia and propensity-matched nonviremic | \ |
| Sheng et al. (2021) [54] | ScRNA-seq & GWAS | Human kidney | Cell-type-eQTLs | 60,661 |
| Li et al. (2022) [55] | ScRNA-seq | Fibrosis mouse | Kidney | 309,666 |
| Wu et al. (2022) [56] | SnRNA-seq & Bulk RNA-seq | db/db (Lepr−/−) | Kidney | 946,660 |
| Lu et al. (2022) [57] | ScRNA-seq | DKD rat model | 10 cell types such as immune cells | \ |
| Li et al. (2022) [58] | ScRNA-seq | RCC | Human renal tumors | 270,000 |
| Kong et al. (2022) [59] | ScRNA-seq | cABMR after renal transplantation | PBMCs | 39,285 |
| Rashmi et al. (2022) [60] | Mux-Seq | Kidney transplants | Human kidney biopsies | 50,275 |
| Lake et al. (2023) [61] | ScRNA-seq | Healthy and diseased kidneys | Kidney | 400,000 |
| McDaniels et al. (2023) [62] | SnRNA-seq | Kidney transplants | Kidney allograft biopsies | 41,893 |
| Wen et al. (2023) [63] | ScRNA-seq | Kidney transplants | Kidney transplantation biopsy cores | 81,139 |
| Aidan et al. (2024) [64] | ScRNA-seq | Kidney transplants | Human kidney transplant biopsies | 31,203 |
| Lu et al. (2024) [65] | ScRNA-seq | Kidney transplant recipients with COVID-19-induced ARDS | PBMCs | 23,980 |
Abbreviations: scRNA-seq single-cell RNA sequencing, Smart-seq2 Switching mechanism at 5’ end of the RNAtranscript sequencing2, GWAS Genome-Wide Association Studies, snRNA-seq single-nucleus RNA sequencing, Bulk RNA-seq bulk RNA sequencing, Mux-Seq Multiplexed droplet single-cell sequencing, STZ streptozotocin, eNOS endothelial nitric oxide synthase, ccRCC clear-cell renal cell carcinoma, CMV cytomegalovirus, DKD diabetic kidney disease, RCC renal cell carcinoma, cABMR chronic antibody-mediated rejection, PBMCs peripheral blood mononuclear cells
Table 3.
The scale and resolution of single-cell transcriptomics studies in nephrology
| Article information | cell number analyzed | Total number of gene | Cell number of DEGa | Public databases |
|---|---|---|---|---|
| Park et al. (2018) [45] | 57,979 | \ |
PC: 870 Trans: 110 IC: 1,729 |
NCBI GEO (GSE107585) |
| Fu et al. (2019) [46] | 644 | 2,226,308 |
EC: 369 MC: 144 Pod: 66 |
NCBI GEO (GSE127235) |
| Dumas et al. (2020) [47] | 40,662 | 15,977 |
gRECs: 15,419 cRECs: 11,762 mRECs: 13,481 |
ArrayExpress (E-MTAB-8145) |
| Chung et al. (2020) [48] | 75,000 | 1,070 |
Pod: 1,930 EC: 1,858 MC: 1,197 |
NCBI GEO (GSE146912) |
| Braun et al. (2021) [49] | 164,722 | 6,260 |
Immune Tumor |
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8138872/ |
| Krishna et al. (2021) [50] | 167,283 | 16,323 |
Immune Tumor |
https://linkinghub.elsevier.com/retrieve/pii/S1535610821001653 |
| Obradovic et al. (2021) [51] | 200,000 | 30,727 |
Immune Tumor |
10.17632/nc9bc8dn4m.1 |
| He et al. (2021) [52] | 4,332 |
973(Pod: 424, GECs: 196, MCs: 353) |
Pod: 224 GECs: 241 MCs: 17 PECs: 44 |
NCBI GEO (GSE160048); EGA (EGAD00001006861); |
| Pickering et al. (2021) [53] | 34,182 | 3,000 | NK and CD8 + T cell | NCBI GEO (GSE168598, PRJNA745955); ImmPort (SDY1600). |
| Sheng et al. (2021) [54] | 60,661 | 19,315 |
PT: 251 Th17: 254 NK: 237 EdnoG: 229 |
NCBI GEO (GSE173343, GSE115098, GSE172008); |
| Li et al. (2022) [55] | 309,666 | \ |
PT: 76,400 TAL: 25,600 DCT: 10,900 CNT: 42,600 |
|
| Wu et al. (2022) [56] | 946,660 | 8,248 |
PCs TAL: PECs PT ECs |
|
| Lu et al. (2022) [57] | \ | 31,077 | 10 cell types (tubular cells, endothelium, …) | https://www.frontiersin.org/articles/10.3389/fcell.2022.798316https://doi.org/10.3389/fcell.2022.798316 |
| Li et al. (2022) [58] | 270,000 | \ |
Immune PT Fibroblasts |
10.17632/g67bkbnhhg.1 https://www.sanger.ac.uk/project/microenvironment-of-kidney-cancer |
| Kong et al. (2022) [59] | 39,285 | 53,664,695 |
T cells B cells |
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE190329 |
| Rashmi et al. (2022) [60] | 50,275 | 20,150 |
Immune: 21,038 PT: 11,887 Endo: 13,294 |
www.kpmp.org |
| Lake et al. (2023) [61] | 400,000 | \ |
PT TAL Immune |
https://www.ncbi.nlm.nih.gov/bioproject/PRJNA671343 |
| Wen et al. (2023) [63] | 81,139 | \ | EC, immune, stromal, Endo | \ |
| Liu et al. (2023) [67] | 70,944 | 740 |
PT Mesan Pod |
NCBI GEO (GSE218563, GSE218086, GSE218413) |
| Tsai et al. (2023) [69] | 6,775 | \ |
Tubular: 5938 Lymphoid: 323 Myeloid: 130 Pod: 19 MC: 21 EC: 202 |
\ |
| Balzer et al. (2023) [70] | 4,821 | 4,265 | PT |
NCBI GEO (GSE209821) |
| Aidan et al. (2024) [64] | 31,203 | >20 |
Immune: 3,117 PT: 1,656 GEC: 9,28 |
NCBI GEO (GSE189536) |
| Lu et al. (2024) [65] | 23,980 | \ | Immune | National Genomics Data Center (HRA004752, HRA005498) |
a The numbers listed in this column represent the number of cells expressing the gene. These cell types are representative part of a broader dataset and not an exhaustive representation of all cell types present in our analysis because of space limitations in table
Abbreviations: DEG differentially expressed genes, PT proximal tubule, IC intercalated cell, Endo endothelial cells, PCs principal cells, TAL thick ascending limb, PECs parietal epithelial cells, ECs endothelial cells, GEO Gene Expression Omnibus, MC mesangial cells, Pod podocytes, RECs renal endothelial cells, GECs glomerular endothelial cells, EndoG glomerular endothelial cells, Th17 T helper 17 cells, NK natural killer cells
Researchers have employed scRNA-seq to analyze human and mouse kidney tissues, accurately characterizing the molecular properties and heterogeneity of mesangial cells (MCs). In contrast to the high expression of genes in podocytes and MCs, glomerular endothelial cells (GECs) have only six human-specific genes and no mouse-specific gene [52]. Dumas et al. inventoried how medullary GECs adapt to hyperosmolarity, as well as the molecular and metabolic adaptations to dehydration [47]. While these studies contribute important knowledge about kidney cellular responses, further investigation with more diverse cohorts is necessary to validate findings and enhance their applicability to human disease. A groundbreaking advancement is the creation of a healthy kidney cell atlas by Park et al., which identified a new type of transitional cell by mapping the atlas of adult mouse kidneys, implicating a more complete molecular characterization of the cell types [45]. Sheng et al. constructed expression profiling data from 659 samples to elucidate the relationship between renal cell type abundances and their specific gene expressions, identifying potential therapeutic targets through two analytical strategies [54]. However, limited kidney eQTL datasets have historically constrained GWAS annotations of renal traits. In addressing this issue, researchers doubled the number of identified eQTLs by accounting for tissue heterogeneity using PEER factors and computational deconvolution. Despite these advancements, the eQTL(ci) approach remains dependent on the quality of single-cell expression data. Co-linearity in cell fractions further complicates analysis, particularly for less abundant cell types, highlighting the need for improved methodologies in future studies. Researchers constructed hitherto the most comprehensive tissue atlas of human kidneys, identifying 51 major cell types by analyzing 45 healthy and 48 diseased human kidneys, which contributed to the exploration of new therapeutic strategies for chronic kidney disease (CKD) and acute kidney injury (AKI) [61]. These atlases serve as a solid foundation for further research into kidney physiology and pathology, especially we should expand human genetic data to support the future study.
The studies utilizing scRNA-seq in kidney disease have demonstrated that differentiated gene expressions correlate with various injury states in glomeruli and proximal tubules, ultimately reflecting distinct pathological changes [48, 55]. By integrating snRNA-seq with a murine model of DKD [56], researchers effectively recapitulated key clinical and histological features of the disease. Analysis across both animal and human models revealed a significant proportion of immune cells in diabetic kidneys, highlighting their crucial role in disease progression [46, 57]. Chung et al. sought to improve cell preparation methods and enhance the quality of data from single-cell sequencing, generating a comprehensive and detailed dataset centered on the glomerulus that facilitates in-depth analysis [48]. However, certain approaches identified only a limited number of glomerular cells, which can be attributed not only to their inherent scarcity but also to the inadequacy of tissue-dissociation methods designed for whole kidney preparations, which are not ideal for isolating glomeruli [45, 46]. Analysis of tubular cells from patients with DKD indicated that histological differences are due to the regulation of tubulointerstitial fibrosis and inflammation pathways. McDaniels et al. elucidated the cellular heterogeneity of allograft fibrogenesis in kidney transplant recipients using the single-cell transcriptome, suggesting that inhibiting interactions between immune cells and parenchymal cells may reverse the progression of renal fibrosis, yet the dynamics process of fibrosis is not documented [62]. This insight is particularly relevant in the context of renal tumors, as the understanding of immune cell interactions in kidney transplantation can inform approaches to immunotherapy. In the renal tumor research field, the tumor immune microenvironment is critical in immunotherapy [49–51, 58]. Single-cell RNA (scRNA) and T cell receptor (TCR) sequencing are used to provide a reference value for the treatment of clear cell renal cell carcinoma (ccRCC) [49, 50]. Based on the atlas, multiple approaches have been established to identify potential targets for immunotherapy in ccRCC [50, 51, 58]. Nonetheless, these approaches may be limited by sample sizes and selection biases, potentially impacting the generalizability of the findings. Future studies could focus on enhancing genetic testing rates to mitigate these limitations and improve the robustness of the conclusions drawn from such research.
What’s more, numerous studies have explored immune mechanisms in chronic antibody-mediated rejection after kidney transplantation [53, 59, 60, 63, 66]. These multi-omics researches revealed that suppression of immune cell upregulation can significantly improve the survival rate of kidney transplant patients. Especially Aidan et al. inferred from scRNA-seq data the transcriptional profiles of physically interacting cells from human kidney transplant biopsies by the method of sequencing physically interacting cells, which complements the estimation of cell-cell physical contact from previous scRNA-seq approaches [64]. On the other hand, Lu et al. innovatively studied the peripheral blood mononuclear cells (PBMCs) of kidney transplant recipients (KTRs) with COVID-19-induced ARDS, which showed significant heterogeneity such as elevated antibody levels, impaired T cell differentiation, and dysregulation of innate immunity [65]. While these studies address different aspects of renal pathology, they collectively advance our understanding of renal diseases and potential therapeutic approaches.
Application of single-cell RNA sequencing in diabetic kidney disease
Single-cell RNA sequencing was performed for DKD, which was among the earliest glomerular diseases to undergo this procedure. It is a hot topic to investigate the pathogenesis at the cellular level by using scRNA-seq while exploring the targets for drug intervention in kidney disease research. Recent advancements in scRNA-seq have significantly enhanced our understanding of cell-specific gene expression in DKD. This chapter examines the currently available studies on single-cell transcriptomics related to DKD (Tables 2 and 3).
Table 2.
Application of single-cell RNA sequencing in diabetic kidney disease
| Article information | Single-cell technique | Disease/model | Tissue type | Sample number | Cell number | |
|---|---|---|---|---|---|---|
| mouse | Fu et al. (2019) [46] | Fluidigm C1 Single-cell Auto Prep System & Illumina NextSeq 500 platform | STZ-induced diabetic eNOS−/− mice | Glomerular cells and immune cells |
Control (3) DKD (3) |
Total: 829 Control:403 DKD:426 |
| Chung et al. (2020) [48] | Chromium Single Cell 3’ Library and Gel Bead Kit; Illumina HiSeq 4,000 |
Nephrotoxic serum nephritis; DKD; Doxorubicin nephropathy; Podocyte-specific genetic disease |
Glomerular cells |
Control (3) Nephritis (4) DKD (6) Doxorubicin (2) CD2AP (2) |
Total: 74,149 Control: 5,488 Nephritis:1,936 |
|
| Fu et al. (2022) [66] | 10x Genomics Chromium and Illumina Novaseq | OVE26 mice | Immune cells |
Control (3) DKD mice (3) |
Total:17,000 Control: 10,500 DKD: 6,500 |
|
| Wu et al. (2022) [56] | 10x Genomics Chromium & Bulk RNA-seq | db/db with uninephrectomy | Kidney | Mice (70) | Total: 946,660 | |
| Wu et al. (2022) [56] | 10×Genomics Chromium and Illumina NovaSeq | db/db | Kidney |
db/db (8) db/db + dapa (8) db/db + irbe (8) db/db + dapa + irbe (8) |
Total: 83,585 | |
| Liu et al. (2023) [67] | ScRNA-seq & Bulk RNA-seq | BTBR ob/ob | Glomerular cells |
Control (8) Ob/ob (8) |
Total: 70,944 | |
| Animal and Human | Li et al. (2022) [68] | ScRNA-seq & snRNA-seq & Bulk RNA-seq | Wild-type mice, db/db mice | Glomerular, tubules cell | Wild-type mice db/db mice | / |
| DKD |
Control (3) DKD (3) |
|||||
| Tsai et al. (2023) [69] | ScRNA-seq | db/m mice, db/db mice | Blood, urine and kidney | db/m mice + db/db mice | Total: 6,775 | |
| Early DKD |
Control (24) T2D (48) |
|||||
| Lu et al. (2022) [57] | Illumina NovaSeq 6000 & Illumina HiSeq 4000 | DKD rat | Kidney |
Control (3) DKD rat (3) |
/ | |
| Early DKD | Control (3) DKD (3) | |||||
| Balzer et al. (2023) [70] | 10X Genomics, Qiagen, Agilent Technologies, Illumina, Meso Scale Discovery and Olink | ZSF1 rats | Kidney |
ZSF1 lean rat (6) ZSF1 obese rat (10) |
Total: 4,821 | |
| DKD |
Control & DKD (991) |
|||||
| Human | Menon et al. (2020) [71] | 10×Genomics Chromium and Illumina | Early DKD | Kidney biopsy |
Control (18) DKD (44) |
Total: 111,035 |
| COVID-19 | Urine | DKD (44) SARS-CoV-2 (13) | Total: 25,791 | |||
| Barwinska et al. (2021) [72] | LMD and sequenced and sn Drop RNA-seq | DKD | Kidney |
Control (9) DKD biopsy (6) |
Total: 24,387 | |
| Stefansson et al. (2022) [73] | ScRNA-seq | Early DKD, Hyperfiltration | Kidney |
Type 2 diabetes with HF (26) or pre-HF (26) |
\ | |
| Wilson et al. (2022) [74] | SnRNA-seq & snATAC-seq | DKD | Kidney cortex | Control (6) DKD (7c) | Total: 107,634 | |
| Hirohama et al. (2023) [75] | ScRNA-seq & Bulk RNA-seq | DKD | Kidney | Control (10) DKD (23) | Total: 64,333 | |
| Schaub et al. (2023) [76] | ScRNA-seq | Type 2 diabetes | Kidney | Control (6) Type 2 diabetes (16) | Total: 40,535 |
Abbreviations: scRNA-seq single-cell RNA sequencing, snRNA-seq single-nucleus RNA sequencing, Bulk RNA-seq bulk RNA sequencing, snATAC-seq single nucleus assay for transposase-accessible chromatin using sequencing, STZ streptozotocin, eNOS endothelial nitric oxide synthase, DKD diabetic kidney disease, BTBR black and tan, brachyuric
Experimental DKD
A study on glomerular cells from streptozotocin-induced diabetic eNOS−/− mouse focused on five distinct populations, including glomerular endothelial cells, mesangial cells, podocytes, immune cells, and tubular cells. They revealed increased immune cell infiltration, primarily macrophages, in diabetic glomeruli, alongside dynamic gene expression changes in endothelial and mesangial cells linked to DKD [46]. Chung et al. further demonstrated the varying dynamics of several glomerular cells in ob/ob mice across different ages. The podocyte injury highlighted the activation of the Hippo pathway, suggesting critical therapeutic targets for diabetic nephropathy [48]. Fu et al. performed single-cell RNA sequencing of CD45-enriched immune cells in the kidneys of OVE26 mice with type 1 diabetes, obtaining approximately 17,000 cells and ultimately yielding 11 cell clusters. In the early stages of CKD, macrophages were shown to be involved in the regulation of renal inflammation. In addition, gene expression analysis highlights the dynamically changing macrophage activation in the early stages of DKD and its potential involvement in disease development [66]. Other researchers analyzed the responses of db/db mouse to five therapeutic regimens: control versus angiotensin-converting enzyme inhibitor (ACEi), Rosiglitazone, sodium-glucose cotransporter two inhibitors (SGLT2i), ACEi + Rosiglitazone, and ACEi + SGLT2i. SGLT2i was implied to contribute to regulating alternative splicing in order to activate a protective metabolic switch [56]. Furthermore, a renal cell transcriptome study found that not only SGLT2i affected mitochondrial function in proximal tubules, but also ARBs have the effects against inflammation and fibrosis in much the same way [77]. A latest research of single-cell and bulk RNA sequencing on renal cells from mice with type 2 diabetes (BTBR ob/ob) at early DKD suggested that mechanosensitive transcriptional pathway MRTF-SRF was mainly activated in mesangial cells, providing a potential novel target for diabetic glomerulopathy [67].
Human DKD
The scRNA-seq results from kidney biopsies of both healthy donors and patients with DKD as well as urine samples from COVID-19 patients revealed that ACE2-coregulated proximal tubular epithelial cell expression program in DKD may interact with the SARS-CoV-2 infection processes and ACE2 expression in proximal tubular epithelial cells does not significantly increase with the use of RAAS inhibitors [71]. Furthermore, investigations across 3 independent cohorts have demonstrated no association between RAAS inhibitors and adverse outcomes in COVID-19 patients [71], suggesting a potentially safer profile for these medications in this context. Schaub et al. elucidated the impact of SGLT2 inhibitors on kidney metabolism in young individuals with type 2 diabetes. By demonstrating alterations in transcriptional profiles across nephron segments and modulation of the mTORC1 signaling pathway, the findings suggest that SGLT2i treatment mitigates diabetes-related metabolic disturbances, offering potential renal protection [76]. However, the generalizability of these findings is contingent upon the size and diversity of the studied cohorts, so larger and more representative studies are essential to validate these results and inform treatment strategies for kidney disease patients during the pandemic. In a research concerning human kidney interstitium, Barwinska D et al. mapped renal interstitial marker genes by combining laser micro dissected (LMD) and single nuclear RNA sequencing (snRNA-seq) [72]. Single cell transcriptional analysis was also used to map hyperfiltration-associated gene expression in early diabetic kidney disease, identifying several putative ligand-receptor pairs with downstream intracellular targets linked to cellular crosstalk between endothelial and mesangial cells [73]. A multimodal single cell sequencing research implicates chromatin accessibility and genetic background in diabetic kidney disease progression, which raises the possibility that glucocorticoid receptor inhibition treating DKD with the adverse metabolic effects [74]. A recent study highlights the critical role of MMP7 as a potential biomarker for kidney fibrosis and function decline in diabetic kidney disease. Hirohama et al. implemented histologic analysis and analyzed single cell transcriptome in 23 patients with diabetic kidney disease. The relatively small cohort size may limit the generalizability of the findings, future research should incorporate larger, more diverse populations and explore longitudinal designs to strengthen the conclusions better [75].
Combined analyses of experimental and human DKD
Some studies have been conducted that integrate experimental methods utilizing both DKD mouse models and patient samples. This approach aims to enhance the accuracy and translational relevance of experimental results, facilitating a more comprehensive understanding of the disease mechanisms and potential therapeutic interventions. Li et al. integrates bulk and single-cell transcriptome analyses to elucidate the mechanisms underlying DKD, focusing on podocytes. Notably, it identifies dysregulation of spermatogenesis-related genes TEKT2 and PIAS2 as key players in DKD progression and they play a role in podocyte cytoskeletal regulation suggests new therapeutic targets [68]. Tsai et al. highlight ferroptosis as a pivotal contributor to DKD progression through immunohistochemistry and other tests, with ceruloplasmin emerging as a key regulator in PT containing AQP4 expression (PTAQP4+). Lower percentages of thick ascending limbs and collecting ducts with impaired metabolism function, as well as SPP1 and SEMA3C causing tubular damage [69]. These findings elucidate essential hub genes that inform the pathophysiological landscape of early DKD and emphasize the need for targeted therapeutic interventions. ScRNA-seq data from human control and diabetic kidney specimens identified immune cells and their marker genes (EIF4B, RICTOR, and PRKCB) as key pathophysiologic factors that might contribute to DKD progression [57]. Another single-cell transcriptomic research on ZSF1 rats suggests pharmacological modulation of soluble guanylate cyclase (sGC) as a promising DKD drug target, which aligns with earlier studies employing immunostaining and in situ hybridization techniques [70]. The ZSF1 rat model effectively mirrors human DKD through its phenotypic traits, including obesity, hypertension, and hyperglycemia. However, the interplay of these comorbidities complicates the analysis of renal function. The pronounced metabolic disturbances, while informative, may mask underlying pathophysiological mechanisms specific to DKD progression [78].
In fact, rodent immune systems differ significantly from those of humans. For example, the types and responses of immune cells, cytokine profiles, and the overall architecture of immune responses can vary greatly. These differences can lead to discrepancies in how diseases are modeled and treated in animals compared to humans. Many treatments that show promise in animal models do not translate effectively to human trials. This phenomenon is particularly pronounced in immunology, where the complexity of human immune responses often leads to unexpected outcomes in clinical settings. Two separate phase 2 clinical trials of CCX140-B, a novel CCR2 antagonist, have demonstrated that targeting CCR2 in mouse models does not yield significant results when compared to outcomes observed in patients with DKD [79, 80]. This discrepancy underscores the necessity for improved models that better replicate human immune responses. Researchers are increasingly looking at more sophisticated models, including genetically engineered mice and humanized models [79], to bridge the gap between preclinical findings and clinical efficacy.
Use of single-cell RNA sequencing to identify immune cell in DKD
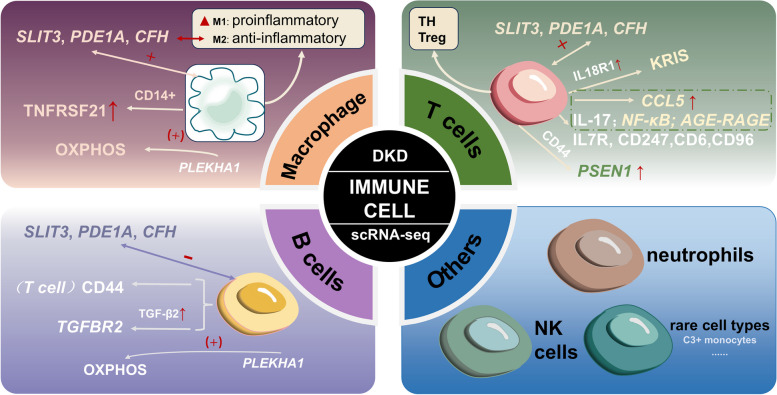
ScRNA-seq has significantly advanced our understanding of the immune landscape in DKD, highlighting the heterogeneity and plasticity of immune cell types involved. These techniques enable comprehensive profiling of gene expression across various renal cell populations, revealing not only traditional immune cells such as macrophages, T cells, and dendritic cells, but also less characterized immune cell types and their functional states in diabetes-related kidney injury. Additionally, sequencing technologies allow for the investigation of immune-related pathways and their transcriptional regulation within the kidney microenvironment, identifying key signaling molecules and inflammatory mediators that may drive DKD progression. By integrating bulk and single-cell sequencing data, researchers can better understand the dynamic interactions between immune cells and renal resident cells, enhancing insights into kidney inflammation, fibrosis, and renal dysfunction progression, thus generating potential immune metabolic therapeutic targets (Fig. 3) [81].
Fig. 3.
Use of scRNA-seq to identify immune cell in DKD. Single-cell RNA sequencing has been employed in immune cell (macrophage, T cells, B cells and others) to improve understanding of normal and disease models
Dendritic cells and macrophage
Dendritic cells (DCs) and macrophages are pivotal components of the innate immune system and are widely distributed in kidney tissue. They play critical roles as sentinels and messengers, continuously monitoring the microenvironment for pathogens and other danger signals [82]. Dendritic cells are particularly adept at antigen presentation and activating T cells, thereby bridging innate and adaptive immunity. Macrophages, on the other hand, are phagocytic cells that not only engulf and digest cellular debris and pathogens but also produce a variety of cytokines that modulate the immune response.
Macrophages play a significant role in the pathogenesis of DKD, with two main subtypes exhibiting distinct functions: M1 macrophages, which are pro-inflammatory, and M2 macrophages, which are anti-inflammatory. In the context of DKD, an inflammatory response leads to an increased presence of immune cells, particularly M1 macrophages. This predominance indicates the critical role of M1 macrophages in the immune response and their contribution to renal injury [83]. For instance, You et al. demonstrated that M1 macrophages disrupt podocyte integrity, suggesting that strategies to mitigate the harmful effects of these macrophages on podocytes could be promising for DKD treatment [84]. Macrophages alter their characteristics in response to the local environment, a process called the M1-M2 transition [85]. Fu et al. investigated the transition of macrophage phenotype within the DKD kidney and confirmed the predominance of M1 macrophages over the M2 type in DKD samples, which is consistent with the results of previous studies showing inflammatory macrophages predominate in DKD glomeruli, suggesting the imbalance of M1/M2 polarization results in renal tissue injury in DKD [46].In contrast to the studies above [46], although Wilson et al. found no significant macrophage infiltration in cryopreserved samples of human diabetic kidneys based on the single-cell transcriptomic landscape, a kidney risk inflammatory signature (KRIS) in CD14 + monocyte was upregulated in the diabetic kidney when involving two publicly available PBMC datasets in comparison analysis [86], and a spatial relationship between a C3 + monocyte subset and pro-inflammatory proximal tubules was confirmed [87], which also indicate that macrophages/monocytes have a significant role in the pathogenesis of DKD. On the one hand, the studies referenced have relatively small patient cohorts, which limits the ability to comprehensively characterize inter-individual variability and fully capture the spectrum of disease severity. On the other hand, Wu et al. utilized the dRNA HybISS approach to address the limitations of scRNA-seq, enabling the detection of transcripts in situ without the need for tissue dissociation or nuclear isolation. But it was constrained by a panel of only 200 genes, which limited the ability to identify specific lymphocyte subpopulations. To further investigate DKD, we required a more extensive panel of immune subtype-specific genes. The Xenium platform now provides the capability to utilize up to 480 custom gene probes, facilitating a more comprehensive analysis of immune cell diversity in DKD. Zhang et al. combined transcriptome data and single-cell analysis to increase the sample size, and found that the biomarker PLEKHA1 has been shown to trigger DKD by activating macrophages, endothelial cells, and other related cells, thereby inducing oxidative phosphorylation (OXPHOS) in renal monocytes [88]. Furthermore, macrophages were regarded as the leading cell in the progression of DKD owing to the correlation between interstitial macrophages and interstitial fibrosis and tubular atrophy, DKD class, and renal function [89]. In flow sorting-assisted scRNA-seq research, macrophages with subtype-specific expression of pro-inflammatory or anti-inflammatory genes contribute to the progression of DKD through immune activation rather than direct mediation [90]. Further analysis showed an increased M1-like inflammatory phenotype, highlighting the dynamic polarization of macrophages in response to the disease environment [66]. Recent high-resolution spatial profiling studies have illustrated the heterogeneity among immune cells in DKD. SnRNA-seq datasets indicate that macrophages and dendritic cells (DCs) are predominant cell types within the renal environment. A reduction in statistical power for identifying differentially expressed genes was noted within immune cell clusters, which may limit the robustness of the findings [56]. Notably, analyzing the relationship between ligands and receptors in the features of single-cell expression profiles revealed that macrophages have a greater potential for interactions with other cell subtypes. Further investigation into the relationship between immune cells and key genes identified three core genes (SLIT3, PDE1A, and CFH), along with macrophage M2 polarization, show a positive correlation with immune cell infiltration [91]. These findings suggest that macrophages and their associated signaling pathways could serve as valuable diagnostic markers and therapeutic targets in the context of DKD.
T cells
T cells exhibit remarkable phenotypic and functional diversity among immune cells. Specialized T helper (TH) subpopulations—including TH1, TH2, TH17, TH9, TH22, and follicular TH cells—and regulatory T (Treg) cells differentiate from CD4 + naïve cells based on the specific cytokine milieu. Similarly, distinct cytotoxic subpopulations—TC1, TC2, TC17, and TC9—as well as CD8 + T regulatory cells arise from the differentiation of CD8 + T cells. These subpopulations respond to various stimuli, including antigens and cytokines, and contribute to the immune response by targeting infected or malignant cells while regulating immune activity to maintain homeostasis.
Recent studies have increasingly highlighted the role of specific genes in modulating T cell behavior and their implications for immune function in DKD. Notably, a comprehensive analysis of five microarray datasets, complemented by advanced techniques such as scRNA-seq, has revealed significant positive interactions between three core genes (SLIT3, PDE1A and CFH) and T cells gamma delta infiltration [91]. This underscores the critical role these genes play in regulating immune cell functions. SLIT3 has been confirmed to influence DKD pathogenesis by activating extracellular matrix receptor interactions and focal adhesion pathways. PDE1A is involved in focal adhesion and lysine degradation pathways, while CFH affects peroxisome-generated succinate, contributing to DKD and other metabolic diseases. DKD’s PBMC dataset and its samples are less, the anticipated publication of larger datasets will significantly enhance our understanding of the disease and facilitate more robust analyses of the associated genes. In further investigations, Li et al. explored ligand-receptor interactions using differentially expressed genes (DEGs) from T lymphocytes. They identified PSEN1, which is up-regulated in glomerular cells, as an important player interacting with CD44 on B and T lymphocytes through autocrine and/or paracrine mechanisms [92].
Another research group observed an approximately 7-to 8-fold increase in leukocyte number consisting of T cells, plasma cells, etc., and IL18R1 was increased in CD4 + and CD8 + T cells in diabetic patients, which are related to the production of KRIS markers due to immune cells infiltration [86]. The finding indicated a potential connection between immune cell activation and inflammation in diabetes, underscoring KRIS could be used as an immunotherapeutic target. One significant area of research focuses on the relationship between interleukins, T cells, and DKD [93]. A significant area of research focuses on the interplay between interleukins, T cells, and DKD. A series of studies have demonstrated that IL-17, produced by CD8 + T cells, is upregulated under pathogenic conditions [94–97]. Elevated levels of IL-17 have been implicated in renal fibrosis and the deposition of complement proteins within the glomeruli [97–99], leading to a cascade of inflammatory responses. This sequence exacerbates leukocyte recruitment, exacerbating proteinuria and albuminuria, while also elevating blood urea nitrogen levels [100]. Several critical signaling pathways, including IL-17, NF-κB, and AGE-RAGE, have been identified in the pathogenesis of DKD. Chen et al. demonstrated that T cells express the chemokine CCL5, which recruits immune cells from the bloodstream to sites of infection and inflammation, first utilizing scRNA-seq and ST for analysis [101]. Understanding the roles of innate immunity pathways in DKD could pave the way for identifying novel therapeutic targets. A comprehensive investigation into the integration and regulation of these innate immunity pathways may yield specific targets with reduced toxicity for the treatment of DKD.
B cells
B cells, derived from lymphoid progenitor cells, play a key role in antigen presentation, antibody production, immune memory generation, and immune tolerance promotion. The presence of B cells is rare in healthy kidneys but their numbers increase during inflammatory reactions, and they are associated directly or indirectly with specific renal diseases which involved in immune response and regulatory processes [82].
Before the discovery of a role for B cells in DKD, several studies had confirmed the contribution of CD20 in the pathogenesis of LN, relapsing focal segmental glomerulosclerosis, and membranous nephropathy [102–104]. DKD is characterized by specific immunological changes in peripheral blood, particularly involving B cells. The count of CD19+ CD38+ B cells in peripheral blood was higher in patients with DKD and positively correlated with 24 h proteinuria level that a critical indicator of kidney damage [105]. Two important markers, TGFBR2 and CD44, have been found to increase in B lymphocytes under diabetic conditions. TGFBR2 activation, driven by upregulated TGF-β2 in endothelial cells, promotes epithelial-mesenchymal transition and subsequent fibrosis, highlighting the contribution of B cells to DKD pathology [92]. B cells are also known to secrete proinflammatory cytokines such as IL-17, which play a dual role: they enhance the activation of effector T cells and stabilize interactions between B and T cells. This interaction is particularly relevant in the context of autoimmune and autoinflammatory diseases, where sustained T cell activation is detrimental [106].
With the growing recognition of B cells in the pathogenesis of DKD, novel therapeutic strategies targeting B cells have emerged. Based on the snRNA-seq dataset, a high-resolution tool created by Wilson et al. [86], DKD samples with high interstitial fibrosis and tubular atrophy were found having more B cells [107]. According to the results of immune infiltration, Zhang et al. suggested naive B cells have a negative correlation with three key genes (SLIT3, PDE1A and CFH) and DKD patients have high expression of these three genes [91]. The phenomenon raises the prospect of these genes being leveraged as therapeutic targets, especially since targeting the B cell response could provide a dual benefit: modulating the immune landscape while directly influencing pathways associated with fibrosis and inflammation. A biomarker screening research based on single-cell analysis implied PLEKHA1, as an important biomarker, also a possible therapeutic target, might initiate DKD by activating B cells, proximal tubular cells, distal tubular cells, macrophages, and endothelial cells, thereby inducing OXPHOS in renal monocytes [88]. Future research should aim to employ single-cell sequencing techniques to elucidate the precise mechanisms through which B cells contribute to diabetic kidney disease DKD pathology and explore the potential synergies of combined immunotherapy approaches.This comprehensive approach may ultimately lead to more effective interventions, such as personalized treatment regimens that mitigate B cell-mediated damage and address the underlying fibrotic processes in the kidneys, resulting in improved outcomes for patients suffering from DKD.
Clinical therapies targeting the immune system for the treatment of DKD
As has been noted, the microenvironments of DKD are infiltrated with the immune cells including DCs, macrophages, T cells, B cells, neutrophils, NK cells, NKT cells and so on. Thus immunotherapy of DKD is naturally explored for potential targets in immune cells [108]. Zhang et al. identified several immunotherapeutic targets, notably the gene SLIT3, which has been shown to alleviate abnormalities induced by advanced glycation end products (AGEs) in human renal mesangial cells (HRMCs) associated with DKD. In addition to SLIT3, the genes PDE1A and CFH were identified through scRNA-seq, DEGs, and weighted correlation network analysis (WGCNA). These findings underscore the potential for targeting these genes in therapeutic interventions for DKD [91]. Moreover, other studies utilizing WGCNA have revealed several gene signatures linked to DKD, including CD36, ITGB2, SLC1A3, ADI1, PTGS2, DGKH, POLR2B, LCK and HCK which are closely associated with immune cells [109–111]. Future studies should investigate how these gene signatures interact within the renal microenvironment and their potential as biomarkers for disease progression or therapeutic targets.
Many researches highlight the complex interplay between immune responses and DKD, emphasizing the potential of targeted therapies to modulate inflammatory mechanisms and improve clinical outcomes. Kaempferol and quercetin were suggested in Ma’s research as the potential drugs to improve the immune and inflammatory mechanisms of DKD by affecting ferroptosis [14]. Kong et al. presented the potential therapeutic advantages of targeting the adaptive immune system (T cells) to impede disease progression [112]. Neutrophil extracellular traps (NETs) was also implicated being involved in DKD [113]. NET-induced sterile inflammation promotes diabetes-associated endothelial dysfunction and inhibition of NETs (PAD4 inhibitor) ameliorate endothelial dysfunction and renal injury in DKD [113]. The higher level of the systemic immune-inflammation index (SII), a novel and integrated inflammatory biomarker, is associated with DKD in T2DM patients, implying the SII could be a cost-effective and straightforward approach to detecting DKD [114]. In an experimental diabetic animal research, the interleukin-6 (IL-6), a pleiotropic cytokine was demonstrated to be an effective target, which was potently inhibited in the experiment by the recombinant anti-IL-6R fusion proteins (VHH-0031) in DKD [115]. It has been demonstrated that mesenchymal stem cells (MSCs) could prevent kidney damage in a diabetes model by modulating the immune system, which is achieved by suppressing the responses of CD8 + T cell [116] and the transcription factor EB (TFEB)-dependent M1/M2 macrophages (Mφ) switch [117]. On the whole, the immune response’s role in DKD pathogenesis is intricate and varied. scRNA-seq is going to act as a potent tool for technique application in the future exploration of immunotherapy for DKD.
Limitations of single-cell RNA sequencing in DKD
Single-cell RNA sequencing has emerged as one of the most transforming technologies in the life sciences nowadays. Despite the technology has taken a great leap forward, there are still several limitations. ScRNA-seq captures either entire individual cells or a portion of the molecules physically presenting in the cell, with capture rates varying from 5 to 20% in high-throughput protocols [118] to as high as 30–40% in certain well-based assays [119]. Thus, limited cell sequences accentuate the restrictions and uncertainties of the experiment, something that bulk counterparts do not encounter. Conversely, scRNA-seq is more likely to reflect the differences of gene expression on the cellular level, by examining genomic and transcriptomic changes within individual cells [120]. Although laboratory mice are extensively used in biomedical research, their immune systems differ functionally from those of humans. For instance, laboratory mice are more resistant to certain toxins than humans while differences even exist between species and their respective responses to treatment [121]. In addition, laboratory mice are susceptible to external environmental influences which result in changes in the physiological status of mice due to the breeding environment or inadequate experimental design by researchers [121]. When conducting research on human kidney specimens, the primary challenges are the difficulty in obtaining them and the ethical issues that they pose [74, 91]. The storing technique for human kidney tissue need to be improved while the availability of such tissue is limited. The renal stroma of adults is relatively dense, which makes the isolation of cells and capturing of RNA for individualized analysis challenging. Moreover the transcriptional status of an individual cell before dissociation is highly dependent on the quality of single-cell suspensions since enzymatic digestion protocols usually reduce the activity of cells [122].
ScRNA-seq has significantly advanced our comprehension of kidney immunity and has become a highly influential instrument in the investigation of kidney diseases nowadays. Studies on the immunity of DKD are comparatively limited when compared to studies of lupus nephritis, renal tumors, renal transplantation, and other diseases. Immune cells are much less than parenchymal cells, which makes it difficult to characterize immune cell heterogeneity in DKD and cost highly on scRNA-seq studies [56, 86]. The process of single-cell dissociation has been reported to damage cell health, leading to artifactual dissociation-induced transcriptional stress responses and the risk of RNA degradation [123]. Also immune cells in DKD were not comprehensively identified, most likely due to technical limitations in the single-cell transcriptome dataset. To address these challenges, future research should focus on developing improved dissociation protocols that minimize cell stress and enhance RNA quality. Additionally, integrating advanced computational methods to analyze multi-omic data could help characterize immune cell heterogeneity more effectively. The application of scRNA-seq in the immunity of DKD will become more widespread, providing insight into the role of the immune system in health and disease. Achieving a comprehensive spatiotemporal immune landscape of DKD will necessitate the development of innovative strategies to analyze multiple single-cell profiles and their molecular signatures. Integrating diverse molecular data from various studies is crucial for advancing our understanding of DKD. However, the complexity inherent in synthesizing these diverse findings into a coherent framework poses significant challenges, particularly in this rapidly evolving field. Addressing these challenges will be essential for unlocking new insights and therapeutic approaches in DKD research.
Conclusions
ScRNA-seq is a revolutionary technology. At present, our understanding of kidney immunology is being rapidly advanced by scRNA-seq. Transcriptomic profiling of individual cells from DKD kidneys has substantially improved the resolution with which we understand the immune cell infiltrates in DKD, overviewing numerous macrophages, T cell, and B cell subsets that accumulate in DKD. These studies applied single-cell sequencing to investigate the immunology of DKD by examining renal biopsy samples, urine cells, and/or blood samples, leading to the identification of novel immune cell populations, gene regulation, and signaling pathways. Although interpreting scRNA-seq data comes with important limitations, utilizing these methods on informative cohorts of patients with DKD and other kidney diseases might provide clinically effective evaluation for prognosis and treatment response.
In this review, we summarize the study and application of single-cell transcriptomics in DKD immunology. Although there are still some limitations and challenges, scRNA-seq has a wide range of potential applications in the fields of kidney immunology, disease mechanism research, and drug therapeutic targets.
Abbreviations
- scRNA-seq
single cell RNA sequencing
- DKD
Diabetic kidney disease
- ESRD
End-stage renal disease
- RRT
Renal replacement therapy
- DM1
Type 1 diabetes mellitus
- DM2
Type 2 diabetes mellitus
- FACS
Fluorescence-activated cell sorting
- WGS
Whole genome sequencing
- cDNA
Complementary DNA
- WGA
Whole genome amplification
- MDA
Multiple displacement amplification
- PCR
Polymerase chain reaction
- MCs
Mesangial cells
- GECs
Glomerular endothelial cells
- CKD
Chronic kidney disease
- AKI
Acute kidney injury
- TCR
T cell receptor
- ccRCC
Clear cell renal cell carcinoma
- PBMCs
Peripheral blood mononuclear cells
- KTRs
Kidney transplant recipients
- ACEi
Angiotensin-converting enzyme inhibitor
- SGLT2i
Sodium-glucose cotransporter two inhibitors
- LMD
Laser micro dissected
- snRNA-seq
single nuclear RNA sequencing
- MMP7
Matrix metalloprotease 7
- PT
Proximal tubule
- PTAQP4+
PT containing AQP4 expression
- DCs
Dendritic cells
- KRIS
Kidney risk inflammatory signature
- OXPHOS
Oxidative phosphorylation
- TH
T helper
- Treg
Regulatory T
- DEGs
Differentially expressed genes
- TGF-β2
Transforming growth factor-β2
- AGEs
Advanced glycation end products
- HRMCs
Human renal mesangial cells
- WGCNA
Weighted correlation network analysis
- NETs
Neutrophil extracellular traps
- SII
systemic immune-inflammation index
- IL-6
Interleukin-6
- MSCs
Mesenchymal stem cells
- TFEB
Transcription factor EB
- eQTL
Expression quantitative trait locus
- ST
Spatial transcriptomics
Authors’ contributions
All literatures were reviewed by F.Y and C.D., Materials were collected by M.W, N.C, L.D, S.Z, T.W, J.Y., The final manuscript was drafted by C.D and F.Y., reviewed by C.D and approved by all authors.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This work was supported by Funds for Guiding Local Scientific and Technological Development by the Central Government of China (No. 236Z7716G, 236Z7737G), National Natural Science Foundation of China (No. 82000773), the Natural Science Foundation of Hebei province (No. H2022206463, H2022206468).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mengjia Wang and Fang Yao should be considered joint first author.
References
- 1.Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KAM, Zoungas S, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1(1):15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bojestig M, Arnqvist HJ, Hermansson G, Karlberg BE, Ludvigsson J. Declining incidence of Nephropathy in insulin-dependent diabetes Mellitus. N Engl J Med. 1994;330(1):15–8. [DOI] [PubMed] [Google Scholar]
- 3.Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, et al. Decreasing incidence of severe Diabetic Microangiopathy in Type 1 diabetes. Diabetes Care. 2003;26(4):1258–64. [DOI] [PubMed] [Google Scholar]
- 4.Burrows NR, Li Y, Geiss LS. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care. 2010;33(1):73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoccali C, Kramer A, Jager K. The databases: renal replacement therapy since 1989—The European Renal Association and European Dialysis and Transplant Association (ERA-EDTA). Clin J Am Soc Nephrol. 2009;4(1 Suppl):S18–22. [DOI] [PubMed] [Google Scholar]
- 6.Ogurtsova K, Da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. [DOI] [PubMed] [Google Scholar]
- 7.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, Progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of Diabetic kidney disease. N Engl J Med. 1989;320(18):1161–65. [DOI] [PubMed] [Google Scholar]
- 9.Borch-Johnsen K, Nørgaard K, Hommel E, Mathiesen ER, Jensen JS, Deckert T, et al. Is diabetic nephropathy an inherited complication? Kidney Int. 1992;41(4):719–22. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Tsogbadrakh B, Yang S, Ryu H, Kang E, Kang M, et al. Klotho ameliorates protection. Biochem Biophys Res Commun. 2021;534:1040–46. [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, Group ftUS. Risk factors for renal dysfunction in type 2 diabetes. Diabetes. 2006;55(6):1832–39. [DOI] [PubMed] [Google Scholar]
- 12.Hasan I, Brifkani Z, Heilig C, Nahman N, Atta M, Heilig K et al. Diabetic Nephropathy on Renal Biopsy in the absence of clinical diabetes Mellitus. Am J Kidney Dis. 2020;75(4):583.
- 13.Li Y, Pan Y, Cao S, Sasaki K, Wang Y, Niu A, et al. Podocyte EGFR inhibits Autophagy through Upregulation of Rubicon in type 2 Diabetic Nephropathy. Diabetes. 2021;70(2):562–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, Li C, Liu T, Zhang L, Wen X, Liu X, et al. Identification of markers for diagnosis and treatment of Diabetic kidney Disease based on the ferroptosis and Immune. Oxid Med Cell Longev. 2022;2022:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurts C, Panzer U, Anders H-J, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13(10):738–53. [DOI] [PubMed] [Google Scholar]
- 16.Donnan MD, Kenig-Kozlovsky Y, Quaggin SE. The lymphatics in kidney health and disease. Nat Rev Nephrol. 2021;17(10):655–75. [DOI] [PubMed] [Google Scholar]
- 17.Eberwine J, Sul J-Y, Bartfai T, Kim J. The promise of single-cell sequencing. Nat Methods. 2014;11(1):25–7. [DOI] [PubMed] [Google Scholar]
- 18.Pennisi E. Chronicling embryos, cell by cell, gene by gene. Science. 2018;360(6387):367–67. [DOI] [PubMed] [Google Scholar]
- 19.Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6(5):377–82. [DOI] [PubMed] [Google Scholar]
- 20.Islam S, Kjällquist U, Moliner A, Zajac P, Fan J-B, Lönnerberg P, et al. Characterization of the single-cell transcriptional landscape by highly multiplex RNA-seq. Genome Res. 2011;21(7):1160–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsköld D, Luo S, Wang Y-C, Li R, Deng Q, Faridani OR, et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat Biotechnol. 2012;30(8):777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macosko Evan Z, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, et al. Highly parallel genome-wide expression profiling of individual cells using Nanoliter droplets. Cell. 2015;161(5):1202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gierahn TM, Wadsworth MH, Hughes TK, Bryson BD, Butler A, Satija R, et al. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat Methods. 2017;14(4):395–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asp M, Bergenstråhle J, Lundeberg J. Spatially resolved transcriptomes—Next Generation Tools for tissue exploration. BioEssays. 2020;42(10):1900221. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Li R, Li Y, Lin X, Zhao K, Liu Q, et al. Spatiotemporal mapping of gene expression landscapes and developmental trajectories during zebrafish embryogenesis. Dev Cell. 2022;57(10):1284–e985. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Chen Y, Gao J, Xie H, Guo Y, Yang J, et al. Mapping crossover events of mouse meiotic recombination by restriction fragment ligation-based Refresh-Seq. Cell Discov. 2024;10(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isnard P, D Humphreys B. Spatial transcriptomics: integrating morphology and molecular mechanisms of kidney diseases. Am J Pathol. 2024. S0002-9440(24)00276-1. [DOI] [PubMed]
- 28.Muto Y, Wilson PC, Ledru N, Wu H, Dimke H, Waikar SS, et al. Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat Commun. 2021;12(1):2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489(7416):391–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegenhain C, Vieth B, Parekh S, Reinius B, Guillaumet-Adkins A, Smets M, et al. Comparative analysis of single-cell RNA sequencing methods. Mol Cell. 2017;65(4):631–e434. [DOI] [PubMed] [Google Scholar]
- 31.Zilionis R, Nainys J, Veres A, Savova V, Zemmour D, Klein AM, et al. Single-cell barcoding and sequencing using droplet microfluidics. Nat Protoc. 2017;12(1):44–73. [DOI] [PubMed] [Google Scholar]
- 32.Dupont CL, Rusch DB, Yooseph S, Lombardo M-J, Alexander Richter R, Valas R, et al. Genomic insights to SAR86, an abundant and uncultivated marine bacterial lineage. ISME J. 2012;6(6):1186–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aad G, Abbott B, Abbott DC, Abud AA, Abeling K, Abhayasinghe DK, et al. Measurements of jet observables sensitive to b-quark fragmentation in tt¯ events at the LHC with the ATLAS detector. Phys Rev D. 2022;106(3):032008. [Google Scholar]
- 34.Spadaccini R, Crescenzi O, Tancredi T, De Casamassimi N, Saviano G, Scognamiglio R, et al. Solution structure of a sweet protein: NMR study of MNEI, a single chain monellin. J Mol Biol. 2001;305(3):505–14. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz S, Allgaier M, Hugenholtz P. Multiple displacement amplification compromises quantitative analysis of metagenomes. Nat Methods. 2010;7(12):943–44. [DOI] [PubMed] [Google Scholar]
- 36.Picelli S, Björklund ÅK, Faridani OR, Sagasser S, Winberg G, Sandberg R. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat Methods. 2013;10(11):1096–98. [DOI] [PubMed] [Google Scholar]
- 37.Mao ZH, Gao ZX, Liu Y, Liu DW, Liu ZS, Wu P. Single-cell transcriptomics: a new tool for studying diabetic kidney disease. Front Physiol. 2023;13:1053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong E, Wang LL, Schaub J, O’Toole JF, Steck B, Rosenberg AZ, et al. Modelling kidney disease using ontology: insights from the kidney Precision Medicine Project. Nat Rev Nephrol. 2020;16(11):686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen S, Lake BB, Zhang K. High-throughput sequencing of the transcriptome and chromatin accessibility in the same cell. Nat Biotechnol. 2019;37(12):1452–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pijuan-Sala B, Wilson NK, Xia J, Hou X, Hannah RL, Kinston S, et al. Single-cell chromatin accessibility maps reveal regulatory programs driving early mouse organogenesis. Nat Cell Biol. 2020;22(4):487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu H, Kirita Y, Donnelly EL, Humphreys BD. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and Novel Cell States revealed in fibrosis. J Am Soc Nephrol. 2019;30(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo J, Grow EJ, Yi C, Mlcochova H, Maher GJ, Lindskog C, et al. Chromatin and single-cell RNA-Seq profiling reveal Dynamic Signaling and metabolic transitions during human spermatogonial stem Cell Development. Cell Stem Cell. 2017;21(4):533–e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Zhang Y, Zhang B, Fu Y, Zhao X, Zhang J, et al. Single-cell chromatin accessibility landscape in kidney identifies additional cell-of-origin in heterogenous papillary renal cell carcinoma. Nat Commun. 2022;13(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gisch DL, Brennan M, Lake BB, Basta J, Keller MS, Ferreiraet RM, et al. The chromatin landscape of healthy and injured cell types in the human kidney. Nat Commun. 2024;15(1):433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science. 2018;360(6390):758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu J, Akat KM, Sun Z, Zhang W, Schlondorff D, Liu Z, et al. Single-cell RNA profiling of glomerular cells shows dynamic changes in Experimental Diabetic kidney disease. J Am Soc Nephrol. 2019;30(4):533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumas SJ, Meta E, Borri M, Goveia J, Rohlenova K, Conchinha NV, et al. Single-cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to Water Deprivation. J Am Soc Nephrol. 2020;31(1):118–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung J-J, Goldstein L, Chen Y-JJ, Lee J, Webster JD, Roose-Girma M, et al. Single-cell transcriptome profiling of the kidney Glomerulus identifies key cell types and reactions to Injury. J Am Soc Nephrol. 2020;31(10):2341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braun DA, Street K, Burke KP, Cookmeyer DL, Denize T, Pedersen CB, et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell. 2021;39(5):632–e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishna C, DiNatale RG, Kuo F, Srivastava RM, Vuong L, Chowell D, et al. Single-cell sequencing links multiregional immune landscapes and tissue-resident T cells in ccRCC to tumor topology and therapy efficacy. Cancer Cell. 2021;39(5):662–e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obradovic A, Chowdhury N, Haake SM, Ager C, Wang V, Vlahos L, et al. Single-cell protein activity analysis identifies recurrence-associated renal tumor macrophages. Cell. 2021;184(11):2988–e300516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He B, Chen P, Zambrano S, Dabaghie D, Hu Y, Möller-Hackbarth K, et al. Single-cell RNA sequencing reveals the mesangial identity and species diversity of glomerular cell transcriptomes. Nat Commun. 2021;12(1):2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pickering H, Sen S, Arakawa-Hoyt J, Ishiyama K, Sun Y, Parmar R, et al. NK and CD8 + T cell phenotypes predict onset and control of CMV viremia after kidney transplant. JCI Insight. 2021;6(21):e153175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheng X, Guan Y, Ma Z, Wu J, Liu H, Qiu C, et al. Mapping the genetic architecture of human traits to cell types in the kidney identifies mechanisms of disease and potential treatments. Nat Genet. 2021;53(9):1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, Dixon EE, Wu H, Humphreys BD. Comprehensive single-cell transcriptional profiling defines shared and unique epithelial injury responses during kidney fibrosis. Cell Metab. 2022;34(12):1977–e989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu H, Gonzalez Villalobos R, Yao X, Reilly D, Chen T, Rankin M, et al. Mapping the single-cell transcriptomic response of murine diabetic kidney disease to therapies. Cell Metab. 2022;34(7):1064–e786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu X, Li L, Suo L, Huang P, Wang H, Han S, et al. Single-cell RNA sequencing profiles identify important pathophysiologic factors in the Progression of Diabetic Nephropathy. Front Cell Dev Biol. 2022;10:798316. [DOI] [PMC free article] [PubMed]
- 58.Li R, Ferdinand JR, Loudon KW, Bowyer GS, Laidlaw S, Muyas F, et al. Mapping single-cell transcriptomes in the intra-tumoral and associated territories of kidney cancer. Cancer Cell. 2022;40(12):1583–e9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong F, Ye S, Zhong Z, Zhou X, Zhou W, Liu Z, et al. Single-cell transcriptome analysis of chronic antibody-mediated rejection after renal transplantation. Front Immunol. 2022;12:767618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rashmi P, Sur S, Sigdel TK, Boada P, Schroeder AW, Damm I, et al. Multiplexed droplet single-cell sequencing (Mux-Seq) of normal and transplant kidney. Am J Transpl. 2022;22(3):876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lake BB, Menon R, Winfree S, Hu Q, Ferreira RM, Kalhor K, et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature. 2023;619(7970):585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDaniels JM, Shetty AC, Kuscu C, Kuscu C, Bardhi E, Rousselle T, et al. Single nuclei transcriptomics delineates complex immune and kidney cell interactions contributing to kidney allograft fibrosis. Kidney Int. 2023;103(6):1077–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wen N, Wu J, Li H, Liao J, Lan L, Yang X, et al. Immune landscape in rejection of renal transplantation revealed by high-throughput single-cell RNA sequencing. Front Cell Dev Biol. 2023;11:1208566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leckie-Harre A, Silverman I, Wu H, Humphreys BD, Malone AF. Sequencing of physically interacting cells in human kidney allograft rejection to Infer Contact-dependent Immune cell transcription. Transplantation. 2024;108(2):421–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu J, Chen Y, Zhou K, Ling Y, Qin Q, Lu W, et al. Immune characteristics of kidney transplant recipients with acute respiratory distress syndrome induced by COVID-19 at single-cell resolution. Respir Res. 2024;25(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu J, Sun Z, Wang X, Zhang T, Yuan W, Salem F, et al. The single-cell landscape of kidney immune cells reveals transcriptional heterogeneity in early diabetic kidney disease. Kidney Int. 2022;102(6):1291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu S, Zhao Y, Lu S, Zhang T, Lindenmeyer MT, Nair V, et al. Single-cell transcriptomics reveals a mechanosensitive injury signaling pathway in early diabetic nephropathy. Genome Med. 2023;15(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Lin H, Shu S, Sun Y, Lai W, Chen W, et al. Integrative transcriptome analysis reveals TEKT2 and PIAS2 involvement in diabetic nephropathy. FASEB J. 2022;36(11):e22592. [DOI] [PubMed] [Google Scholar]
- 69.Tsai Y-C, Kuo M-C, Huang J-C, Chang W-A, Wu L-Y, Huang Y-C, et al. Single-cell transcriptomic profiles in the pathophysiology within the microenvironment of early diabetic kidney disease. Cell Death Dis. 2023;14(7):442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balzer MS, Pavkovic M, Frederick J, Abedini A, Freyberger A, Vienenkötter J, et al. Treatment effects of soluble guanylate cyclase modulation on diabetic kidney disease at single-cell resolution. Cell Rep Med. 2023;4(4):100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menon R, Otto EA, Sealfon R, Nair V, Wong AK, Theesfeld CL, et al. SARS-CoV-2 receptor networks in diabetic and COVID-19–associated kidney disease. Kidney Int. 2020;98(6):1502–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barwinska D, El-Achkar TM, Melo Ferreira R, Syed F, Cheng Y-H, Winfree S, et al. Molecular characterization of the human kidney interstitium in health and disease. Sci Adv. 2021;7(7):eabd3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stefansson VTN, Nair V, Melsom T, Looker HC, Mariani LH, Fermin D, et al. Molecular programs associated with glomerular hyperfiltration in early diabetic kidney disease. Kidney Int. 2022;102(6):1345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson PC, Muto Y, Wu H, Karihaloo A, Waikar SS, Humphreys BD. Multimodal single cell sequencing implicates chromatin accessibility and genetic background in diabetic kidney disease progression. Nat Commun. 2022;13(1):5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hirohama D, Abedini A, Moon S, Surapaneni A, Dillon ST, Vassalotti A, et al. Unbiased human kidney tissue Proteomics identifies Matrix Metalloproteinase 7 as a kidney Disease Biomarker. J Am Soc Nephrol. 2023;34(7):1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schaub JA, AlAkwaa FM, McCown PJ, Naik AS, Nair V, Eddy S, et al. SGLT2 inhibitors mitigate kidney tubular metabolic and mTORC1 perturbations in youth-onset type 2 diabetes. J Clin Invest. 2023;133(5):e164486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu J, Sun Z, Yang S, Fu J, Fan Y, Wang N, et al. Kidney single-cell transcriptome profile reveals distinct response of proximal tubule cells to SGLT2i and ARB treatment in diabetic mice. Mol Ther. 2022;30(4):1741–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sembach FE, Østergaard MV, Vrang N, et al. Rodent models of diabetic kidney disease: human translatability and preclinical validity. Drug Discov Today. 2021;26(1):200–17. [DOI] [PubMed] [Google Scholar]
- 79.Sullivan T, Miao Z, Dairaghi DJ, Krasinski A, Wang Y, Zhao BN, et al. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am J Physiol Ren Physiol. 2013;305(9):F1288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeeuw Dd, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diab Endocrinol. 2015;3(9):687–96. [DOI] [PubMed]
- 81.Zeng H, Yang X, Luo S, Zhou Y. The advances of single-cell RNA-Seq in kidney immunology. Front Physiol. 2021;12:752679 [DOI] [PMC free article] [PubMed]
- 82.Stewart BJ, Ferdinand JR, Young MD, Mitchell TJ, Loudon KW, Riding AM, et al. Spatiotemporal immune zonation of the human kidney. Science. 2019;365(6460):1461–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams JW, Giannarelli C, Rahman A, Randolph GJ, Kovacic JC. Macrophage Biology, classification, and phenotype in Cardiovascular Disease. J Am Coll Cardiol. 2018;72(18):2166–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.You H, Gao T, Cooper TK, Brian Reeves W, Awad AS. Macrophages directly mediate diabetic renal injury. Am J Physiol Ren Physiol. 2013;305(12):F1719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang PM-K, Nikolic-Paterson DJ, Lan H-Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15(3):144–58. [DOI] [PubMed] [Google Scholar]
- 86.Wilson PC, Wu H, Kirita Y, Uchimura K, Ledru N, Rennke HG, et al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci U S A. 2019;116(39):19619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu H, Dixon EE, Xuanyuan Q, Guo J, Yoshimura Y, Debashish C, et al. High resolution spatial profiling of kidney injury and repair using RNA hybridization-based in situ sequencing. Nat Commun. 2024;15(1):1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang C, Li H, Wang S. Common gene signatures and molecular mechanisms of diabetic nephropathy and metabolic syndrome. Front Public Health. 2023;11:1150122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, et al. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transpl. 2016;32(8):1322–29. [DOI] [PubMed] [Google Scholar]
- 90.Wu H, Humphreys BD. Immune cell heterogeneity in a mouse model of diabetic kidney disease. Kidney Int. 2022;102(6):1215–16. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X, Chao P, Zhang L, Xu L, Cui X, Wang S, et al. Single-cell RNA and transcriptome sequencing profiles identify immune-associated key genes in the development of diabetic kidney disease. Front Immunol. 2023;14:1030198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li T, Shen K, Li J, Leung SWS, Zhu T, Shi Y. Glomerular endothelial cells are the coordinator in the Development of Diabetic Nephropathy. Front Med (Lausanne). 2021;8:655639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng Z, Zheng F. A complex auxiliary: IL-17/Th17 signaling during type 1 diabetes progression. Mol Immunol. 2019;105:16–31. [DOI] [PubMed] [Google Scholar]
- 94.Yen H-R, Harris TJ, Wada S, Grosso JF, Getnet D, Goldberg MV, et al. Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol. 2009;183(11):7161–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ciric B, El-behi M, Cabrera R, Zhang G-X, Rostami A. IL-23 drives pathogenic IL-17-Producing CD8 + T cells. J Immunol. 2009;182(9):5296–305. [DOI] [PubMed] [Google Scholar]
- 96.Marwaha AK, Crome SQ, Panagiotopoulos C, Berg KB, Qin H, Ouyang Q, et al. Cutting Edge: increased IL-17–Secreting T cells in children with New-Onset type 1 diabetes. J Immunol. 2010;185(7):3814–18. [DOI] [PubMed] [Google Scholar]
- 97.Chan AJ, Alikhan MA, Odobasic D, Gan PY, Khouri MB, Steinmetz OM, et al. Innate IL-17A–Producing leukocytes promote acute kidney Injury via Inflammasome and Toll-Like receptor activation. Am J Pathol. 2014;184(5):1411–18. [DOI] [PubMed] [Google Scholar]
- 98.Krohn S, Nies JF, Kapffer S, Schmidt T, Riedel J-H, Kaffke A, et al. IL-17 C/IL-17 receptor E signaling in CD4 + T cells promotes TH17 cell-driven glomerular inflammation. J Am Soc Nephrol. 2018;29(4):1210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koga T, Ichinose K, Tsokos GC. T cells and IL-17 in lupus nephritis. Clin Immunol. 2017;185:95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng X, Xiao Z, Zhang J, Li Y, Dong Y, Du J. IL-17A produced by both γδ T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J Pathol. 2015;235(1):79–89. [DOI] [PubMed] [Google Scholar]
- 101.Chen D, Shao M, Song Y, Ren G, Guo F, Fan X, et al. Single-cell RNA-seq with spatial transcriptomics to create an atlas of human diabetic kidney disease. FASEB J. 2023;37(6):e22938. [DOI] [PubMed] [Google Scholar]
- 102.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3(85):85ra46. [DOI] [PMC free article] [PubMed]
- 103.Gregersen JW, Jayne DRW. B-cell depletion in the treatment of lupus nephritis. Nat Rev Nephrol. 2012;8(9):505–14. [DOI] [PubMed] [Google Scholar]
- 104.Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al. Rituximab or Cyclosporine in the treatment of Membranous Nephropathy. N Engl J Med. 2019;381(1):36–46. [DOI] [PubMed] [Google Scholar]
- 105.Smith MJ, Simmons KM, Cambier JC. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat Rev Nephrol. 2017;13(11):712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15(7):441–51. [DOI] [PubMed] [Google Scholar]
- 107.Wei Y, Gao X, Li A, Liang M, Jiang Z. Single-nucleus transcriptomic analysis reveals important cell cross-talk in Diabetic kidney disease. Front Med (Lausanne). 2021;8:657956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16(4):206–22. [DOI] [PubMed] [Google Scholar]
- 109.Xu M, Zhou H, Hu P, Pan Y, Wang S, Liu L, et al. Identification and validation of immune and oxidative stress-related diagnostic markers for diabetic nephropathy by WGCNA and machine learning. Front Immunol. 2023;14:1084531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang H, Hu J, Zhu J, Li Q, Fang L. Machine learning-based metabolism-related genes signature and immune infiltration landscape in diabetic nephropathy. Front Endocrinol (Lausanne). 2022;13:1026938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu K, Wang L, Fu Y, Li G, Zhang X, Cao M. Bioinformatics analysis identifies immune-related gene signatures and subtypes in diabetic nephropathy. Front Endocrinol (Lausanne). 2022;13:1048139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kong L, Andrikopoulos S, MacIsaac RJ, Mackay LK, Nikolic-Paterson DJ, Torkamani N, et al. Role of the adaptive immune system in diabetic kidney disease. J Diabetes Investig. 2022;13(2):213–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gupta A, Singh K, Fatima S, Ambreen S, Zimmermann S, Younis R, et al. Neutrophil Extracellular traps promote NLRP3 inflammasome activation and glomerular endothelial dysfunction in Diabetic kidney disease. Nutrients. 2022;14(14):2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guo W, Song Y, Sun Y, Du H, Cai Y, You Q, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in type 2 diabetes mellitus patients: evidence from NHANES 2011–2018. Front Endocrinol (Lausanne). 2022;13:1071465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang N, Zheng Q, Wang Y, Lin J, Wang H, Liu R, et al. Renoprotective Effect of the recombinant Anti-IL-6R Fusion proteins by inhibiting JAK2/STAT3 signaling pathway in Diabetic Nephropathy. Front Pharmacol. 2021;12:681424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang F, Wang C, Wen X, Chen Y, Mao R, Cui D, et al. Mesenchymal stem cells alleviate rat diabetic nephropathy by suppressing CD103 + DCs-mediated CD8 + T cell responses. J Cell Mol Med. 2020;24(10):5817–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yuan Y, Li L, Zhu L, Liu F, Tang X, Liao G, et al. Mesenchymal stem cells elicit macrophages into M2 phenotype via improving transcription factor EB-mediated autophagy to alleviate diabetic nephropathy. Stem Cells. 2020;38(5):639–52. [DOI] [PubMed] [Google Scholar]
- 118.Ding J, Adiconis X, Simmons SK, Kowalczyk MS, Hession CC, Marjanovic ND, et al. Systematic comparison of single-cell and single-nucleus RNA-sequencing methods. Nat Biotechnol. 2020;38(6):737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hagemann-Jensen M, Ziegenhain C, Chen P, Ramsköld D, Hendriks G-J, Larsson AJM, et al. Single-cell RNA counting at allele and isoform resolution using Smart-seq3. Nat Biotechnol. 2020;38(6):708–14. [DOI] [PubMed] [Google Scholar]
- 120.Kharchenko PV. The triumphs and limitations of computational methods for scRNA-seq. Nat Methods. 2021;18(7):723–32. [DOI] [PubMed] [Google Scholar]
- 121.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu H, Humphreys BD. The promise of single-cell RNA sequencing for kidney disease investigation. Kidney Int. 2017;92(6):1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Adam M, Potter AS, Potter SS. Psychrophilic proteases dramatically reduce single cell RNA-seq artifacts: a molecular atlas of kidney development. Development. 2017;144(19):3625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.