Abstract
Orally consumed nicotine pouches that contain no tobacco are marketed as a less harmful alternative to tobacco products. This study aims to assess nicotine in pouches and potential risks for health damage. 31 samples of nicotine pouches were collected and analyzed. The median nicotine uptake from single pouches was calculated to be 65 µg/kg bw. Consumption of more than one pouch per day would lead to a strikingly higher nicotine dose over the day. Health effects after nicotine exposure are, among others, cardiovascular effects. An acute reference dose (ARfD) of 0.8 µg/kg bw was proposed for risk assessment purposes based on an observed increased heart rate in a human study after acute nicotine uptake. The ARfD was exceeded by at least 20-fold, even when considering the consumption of a single pouch with the lowest detected nicotine level. With higher nicotine contents in pouches or with an increasing number of pouches per day, vast ARfD exceedances are possible. Therefore, a clinically relevant elevation of heart rate is very likely to occur in consumers after acute consumption of nicotine pouches.
Keywords: Nicotine, Pouches, Risk assessment, Heart rate, Health damage
Highlights
-
•
Nicotine pouches are marketed as “safe” alternative to tobacco products.
-
•
However, nicotine may cause health damage, e. g. by cardio-vascular effects.
-
•
Nicotine in pouches was determined and resulting health risks assessed.
-
•
All investigated pouches pose health risks.
Nomenclatures
- ARfD
Acute reference dose
- ATE
Acute toxicity estimate
- BfR
Federal Institute for Risk Assessment (Bundesinstitut für Risikobewertung)
- CLP
Classification, Labelling and Packaging of substances and mixtures
- EFSA
European Food Safety Authority
- EU
European Union
- GC-FID
Gas chromatography analysis with flame-ionization detection
- GHS
Globally Harmonized System
- LD50
Median lethal dose
- LOAEL
Lowest observed adverse effect level
1. Introduction
Smoking tobacco products is a leading threat to public health due to addictive properties and associations with various diseases like cancer and cardiovascular dysfunction [1]. Tobacco-free nicotine-containing cessation products may have the potential to help people stop or reduce smoking.
Nicotine pouches are flavored sachets containing crystalline nicotine, various salts, and cellulose. For application, these pouches are usually placed between lips and gum [2]. With respect to the application method and the containing nicotine doses, pouches are similar to Swedish snus products, which are also pouches but contain tobacco leaves. By using pouches, nicotine is released after contact with saliva in the oral cavity. As nicotine pouches are tobacco-free and are not combusted, they are often considered to pose a lower health risk compared to traditional tobacco-products [3], [4], [5]. Regarding the users of nicotine pouches several surveys investigated consumer behavior revealing that only a minority of users (20–30 % of total participants) indicate the lower health risk of these products as primary motivation for their use [6]. Additionally, several current users were indeed non-smokers or even never-smokers [7]. Hence, while nicotine pouches may hold promise as harm reduction tools, they might instead pose an extra health risk at least for some consumers.
While snus products are prohibited within the European Union (EU) except in Sweden, the legal status of nicotine pouches varies significantly ranging from a complete ban to their unrestricted availability for adult consumers in some countries [8]. In Germany, there is no dedicated legislation governing the regulation of nicotine pouches at present. Since the ingredients of nicotine pouches (nicotine, flavorings, and sweetener) are ultimately orally ingested by humans, these products are currently classified as food by the federal state authorities in Germany. Food, however, must not cause any sort of detrimental effects on human health according to the general food law (Regulation (EC) No. 178/2002). Therefore, in contrast to other products for smoking-cessation often regulated as medicinal products, a risk-benefit assessment is not considered for nicotine pouches.
Hence, the question arises regarding the potential health risks associated with the consumption of nicotine pouches. Thus, in the present study a risk assessment of nicotine pouches with respect to the general food law is presented based on an appropriate health-based guidance value after evaluation of its relevance. For this purpose, various commercially available nicotine pouches in Germany were analyzed for their nicotine content as a basis for a reliable estimation of the nicotine exposure of consumers using different consumption scenarios.
2. Materials and methods
2.1. Nicotine pouches
From 2019–2023, 31 different nicotine pouches were commercially purchased in various parts of Bavaria as part of the official food control procedure. These samples were then analyzed at the Bavarian Health and Food Safety Authority for their nicotine levels. Although these samples reflect available products for consumers in Bavaria, analyzed samples may not necessarily be considered as representative for the whole marked of nicotine pouches in Germany. Product labeling was checked for information on nicotine content, GHS pictograms, or specific warnings for certain demographic groups, like children or pregnant women.
2.2. Nicotine analytics
The analysis of nicotine in pouches was done using gas chromatography analysis with flame-ionization detection (GC-FID, Agilent 6890 N; liquid injection). Briefly, 0.38–1.27 g of the content of each pouch sample was mixed with 20 ml of water, 40 ml of n-hexane (containing 0.5 mg/ml heptadecane as an internal standard), and 10 ml of an aqueous sodium hydroxide solution (32 %). Following continuous stirring of the alkaline solution for 1 hour, the organic phase was separated. Each sample was extracted in duplicate. After filtration of the organic phase with a 0.45 µm membrane filter, an aliquot of 1 µl (each extraction two-fold) was injected into the GC-FID system (carrier gas: helium 5.0, inlet: split: 1:20, 250 °C, oven temperature program: 140 °C for 5 min, then with 20 °C/minutes to 210 °C, hold till 33.5 min, constant pressure: 0.53 bar, detector: gases hydrogen (40 ml/min) and air (450 ml/min) with make-up gas nitrogen (45 ml/min), 280 °C) for analysis. For gas chromatographic separation and quantification purposes, a GC column with a nonpolar phenyl-methyl-polysiloxane stationary phase (HP 5, 30 m x 0.32 mm x 0.25 µm, Agilent, Waldbronn, Germany) was used, and for confirmation purposes, a second GC-FID with another GC column (DB Wax, 30 m x 0.32 mm x 1 µm) was performed using the following parameters: carrier gas: helium, 5.0, inlet: split 1:40, 240 °C, oven temperature program: 150 °C for 2 min, then with 4.375 °C/minutes to 220 °C, hold till 36.0 min, constant pressure: 0.56 bar, detector: gases hydrogen (40 ml/min) and air (450 ml/min) with make-up gas nitrogen (45 ml/min), 240 °C. For calibration, six calibration standards in the range of 0.026 mg nicotine/ml to 1.056 mg nicotine/ml were prepared in n-hexane, each containing 0.5 mg heptadecane/ml. Quality control material was prepared by spiking tobaccos (naturally containing 0.48 g nicotine/100 g or 0.65 g nicotine/100 g) with nicotine at concentration levels of 2.6–2.8 g/100 g. The recovery rates were 95.6–103.7 %. At least one quality control sample was analyzed during each analytical series. The coefficient of correlation of the linear calibration curve was found to be ≥ 0.999. The limit of detection and limit of quantification were determined to be 0.0056 g nicotine/100 g and 0.0184 g nicotine/100 g, respectively.
2.3. Statistical analysis
All data evaluation, calculation of metrics and confidence intervals were conducted using Microsoft Excel 2016. Results of nicotine analytics were presented as means of duplicates.
3. Results and discussion
3.1. Toxicology of nicotine
3.1.1. Hazard identification and dose description for nicotine
Nicotine is a major alkaloid of tobacco (Nicotiana tabacum) that contains nicotine levels of 2–8 % of the plant. It has formerly also been used as an insecticide in the EU until 2010 as nicotine acts as an agonist on acetylcholine receptors of insects leading to neurotoxicity. A similar mechanism in humans and other mammals is considered responsible for many of the observed toxic effects like the symptoms occurring after severe poisonings: convulsions, tremors and disturbances of coordination and autonomic functions [9], [10].
The European Chemicals Agency's Committee for Risk Assessment derived an acute toxicity estimate (ATE) for the classification of nicotine-containing mixtures of 5 mg/kg bw as a point estimate that was also used in a report of the European Commission to explain health risks caused by accidental ingestion of nicotine-containing cartridges by children [9], [11].
Based on this ATE, the Bundesinstitut für Risikobewertung (BfR, German Federal Institute for Risk Assessment) derived a maximal amount for nicotine pouches of 16.7 mg/g, at which the product was still classifiable as acute toxicity hazard category 3 (highly toxic with a median lethal dose (LD50) in animals of 50–300 mg/kg bw) when the Classification, Labelling and Packaging of substances and mixtures (CLP) scheme is applied [2]. Thus, a correct labeling would require a pictogram with an exclamation mark instead of a skull in cases of nicotine levels above 16.7 mg/g pouch. However, this approach of the German BfR is not equivalent to an informed toxicological assessment with respect to consumer health and should not be misinterpreted as such, as the CLP regulation is not suitable for food or products for oral use like nicotine pouches.
A toxicological risk assessment of nicotine-containing products for oral use classified as food requires the derivation of a health-based guidance value for nicotine. This was already done by the European Food Safety Authority (EFSA), who used a human intervention study: 16 healthy volunteers, who were regular smokers, received single intravenous infusions of nicotine in doses ranging from 3.5 to 28 µg/kg bw [12]. The lowest dose of 3.5 µg/kg bw was identified as the lowest observed adverse effect level (LOAEL) based on a slight transient increase in the heart rate of approximately 4 beats per minute for 20 minutes [13]. Considering the oral human bioavailability of nicotine of 44 % of the applied dose and by application of an toxicological assessment factor of 10 for intraspecies variability, an acute reference dose (ARfD) of 0.8 µg/kg bw was derived by the EFSA in 2009 [10], [13]. It was noted that in the critical study a 4–8-fold higher dose than the identified LOAEL already led to an elevation of the mean heart rate by approximately 10–14 beats per minute [12].
A more recent study investigating effects after use of nicotine pouches by healthy snus-experienced adults for 60 minutes (supposedly resulting in similar systemic nicotine levels as in the study by Lindgren et al. [12]) observed an increase in the heart rate of some volunteers up to 22 beats per minute, while other volunteers in this study experienced only minor changes in heart rates, demonstrating a distinct interindividual variability, while only minor dose-dependent differences were observed, suggesting a saturation of the receptor mediated nicotine effect [14]. For another study with active smokers, nicotine pouches at concentrations of (measured) 4.8, 16.3 and 27.1 mg nicotine were used for 20 min. Although a time- and dose-dependent increase in heart rate was monitored, no significance was reached except for one time point at the highest dose while significance could not be observed either after consumption of additionally investigated tobacco cigarettes [15]. In another study with young, occasional smokers receiving tobacco-containing chewing bags containing 8.8 mg nicotine, an increase in heart rate and blood pressure was observed that was similar to the observed effects after consumption of traditional cigarettes [16]. Similar effects were also observed after snus consumption or cessation products like nicotine gums with 4 mg nicotine [17], [18], [19], [20].
Altogether, the available data demonstrate a strong association between nicotine uptake and an increased heart rate – regardless of the application form. As an appropriate health based guidance value for this effect, the ARfD value for nicotine intake set by the EFSA can be used.
3.1.2. Relevance of an increased heart rate for human health
While there is strong evidence for the association between nicotine exposure and cardiovascular effects, the question of the clinical relevance of these effects is not yet fully answered. Based on the observed maximum increase in heart rate of 22.5 beats per minute after consumption of a pouch containing 6 mg nicotine, a transient tachycardia (heart rate > 100 beats per minute) may manifest in some individuals [14], [21]. As a heart rate elevation was observed even in healthy adults with a history of snus consumption, even more prominent elevation rates may result for sensitive and unexperienced persons following nicotine uptake. This in in line with known side effects of oral nicotine cessation products like palpitations or arrhythmia (beside various gastro-intestinal side effects or headache and dizziness) observed in a randomized control trial [22]. An analysis of 12 randomized controlled trials found an increased risk for heart palpitations and chest pains (Odds ratio: 2.06, 95 % CI: 1.51–2.82) in association with nicotine replacement therapy (mostly patches and gums), although no significant differences were observed for serious adverse events. However, no dose-response association can be derived based on these data [23]. Severe effects of poisonings like tremor, convulsions or disturbances of coordination, known e.g. from the misuse of nicotine patches or animal experiments, were so far, to the best of our knowledge, not associated with nicotine uptake by the use of nicotine pouches.
Although long-term studies regarding nicotine pouches consumption are not yet available, the possible consequences of a continuously increased heart rate are well established: Clinical studies have shown that an elevated resting heart rate is a risk factor for heart failure in patients with heart disease [25], [24]. But even for healthy individuals, several comprehensive meta-analyses came to the conclusion that the relative extra risk for cardiovascular disease and mortality is elevated by 9–17 % per 10 beats per minute increase of the resting heart rate [26], [27], [28]. It has been shown in numerous further epidemiological studies that a persistent elevation of the heart rate is associated with cardiovascular mortality and morbidity [29]. As a consequence, an increased heart rate (resting values > 80 bpm) is an established factor that influences cardiovascular risk in patients with hypertension [30]. Similar adverse effects are known after consumption of nicotine-containing tobacco products:
Due to the use of snus for an extended period of time, at least in Sweden, studies on snus provide valuable insights into the health consequences of long-term oral nicotine exposure. A recent analysis of eight Swedish cohort studies showed hazard ratios above 1 for all-cause mortality and deaths due to cardiovascular diseases (hazard ratios of 1.28 (95 % CI: 1.16–1.41) and 1.44 (95 % CI: 1.23–1.69), respectively) even after consumption of low amounts of snus, which was defined as < 4 cans/week, compared to never-users of tobacco [31]. This is in line with former studies on snus users demonstrating increased mortality amongst consumers compared to non-users [33], [32]. However, other studies focusing on the incidence of specific diseases among snus users did not observe a significant association between snus consumption and stroke or atrial fibrillation incidents [34], [35]. Only for snus users consuming high amounts of snus with ≥ 7 cans/week, a slightly increased hazard ratio for acute myocardial infarction was observed, however not of statistical significance [36]. A more recent cohort study on snus users, in contrast, did not find an association with myocardial infarction, heart failure, atrial fibrillation or CVD mortality after adjustment for confounders like smoking. However, there was a statistically significant increased risk of total and ischemic stroke in snus users, who did not smoke [37].
It is difficult to assess, if and to what extent the observed effects of long-term snus consumption are mainly caused by the chronic nicotine uptake or other ingredients. However, the observed effects are well in line with the known health consequences of a persistent elevated heart rate. Smoking is also a known major risk factor for cardiovascular diseases, but the regular smokers are exposed to a higher variety of toxic substances in comparison to consumers of nicotine pouches [38]. The mode of action of nicotine exposure regarding its impact on vascular function has already been described: a dysfunction is caused by nicotine via various mechanisms leading to vascular remodeling and changes in the structure and function of blood vessels observed in animals, which might thus contribute to the development of cardiovascular diseases [39]. There is evidence that arterial stiffness is altered after usage of nicotine pouches indicating vascular effects of nicotine [15].
However, further studies are needed to allow for a more precise estimation of the long-term-effects of a chronic nicotine exposure from pouches, particularly with respect to the cardiovascular system.
3.1.3. Other health-related effects related to nicotine uptake
A systematic review of available studies addressing effects on pregnancy after consumption of non-combustible tobacco and nicotine products showed that smoke-free products likely pose a lower risk for health damage during pregnancy than smoking [40]. However, a reliable quantification of these effects was not feasible mainly due to the variety of existing confounding factors. For the severe toxicological endpoint of stillbirth, results from a cohort study indicate an increased risk for smokers or snus consumers compared to non-smokers [41]. Given the known association of nicotine intake and developmental toxicity, it appears plausible that the contained nicotine in these products is at least partially responsible for the observed effect. Therefore, nicotine consumption during pregnancy poses a risk to the unborn child and should be avoided.
In addition, an association between nicotine intake and diabetes is often discussed, but the derivation of threshold levels is difficult [42]. Meta-analysis of five cohort studies investigating snus consumption revealed an increased incidence of type 2 diabetes after intake of ≥ 7 cans per week, but no significant effects were observed after mild to moderate snus consumption up to 4 cans/week [43]. Possibly, the formation of type 2 diabetes is a less sensitive effect of nicotine compared to its effects on the cardiovascular system.
A major health problem with regard to nicotine is its high addiction potential. Though, threshold levels for nicotine addiction are not yet established. The reduction of nicotine levels in cigarettes during a clinical trial led to a decrease in addiction among users [44]. It may therefore be assumed that nicotine in pouches may also lead to addiction issues for users.
In conclusion, the use of the ARfD of 0.8 µg/kg bw and day as threshold dose based on the endpoint heart rate elevation is a suitable available quantitative approach to assess the risk from nicotine uptake. It refers to the most sensitive endpoint of nicotine uptake and is thus supposedly protective for other adverse health effects of nicotine, too. The ARfD has already been used for the risk description of nicotine in various food matrices during several assessments performed by the EFSA [45], [46], [47].
3.2. Estimation of exposure to nicotine from pouches
Overall, 31 nicotine pouches were analyzed in the present study with a median pouch weight of 0.55 g per pouch ranging from 0.38 to 0.85 g per pouch. Nicotine content was labeled on 48 % of the products only and ranged from 2.58 to 36.9 mg per pouch (Table 1). Analysis of the nicotine content in pouches showed a median nicotine level of 1.6 g/100 g (range: 0.46–7.86 g/100 g), corresponding to a median nicotine level of 9.04 mg (mean: 13.80 mg/pouch, 95 % CI: 8.74–18–87 mg/pouch per pouch) with high variability ranging from 2.20 to 56.00 mg per pouch. Regarding the 15 samples with labeled nicotine content, we usually analyzed lower nicotine levels in the pouches with median deviation of 14.7 % compared to labeled levels (cf. Table 1). Similar nicotine contents were found in another study with 44 pouches [48].
Table 1.
Overview of the investigated nicotine pouches. Labeled information (nicotine content, warning labels and recommendations on intake, if available) as well as results of nicotine analytics were depicted and, in case of nicotine levels, compared with each other. GHS - Globally Harmonized System. GHS symbol 06: skull, GHS symbol 07: exclamation mark.
| No. | Pouch weight [g] | Labeled nicotine | Nicotine/pouch according to label [mg] | Detected nicotine [g/100 g] |
Uncertainty (± g/100 g) |
Detected nicotine/pouch [mg] | Difference between labeled and detected nicotine/pouch [mg] | Relative difference labeled/detected nicotine/pouch [%] | Warning for children (C) or pregnant women (P) | GHS pictogram | Recommended intake |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.49 | - | - | 1.80 | 0.13 | 9.0 | - | - | C | 07 | |
| 2 | 0.73 | 16 mg/g | 11.7 | 1.63 | 0.12 | 11.9 | −0.2 | 2 | C | 07 | |
| 3 | 0.49 | 24 mg/g | 11.8 | 2.38 | 0.17 | 11.7 | 0.1 | 1 | C | 07 | |
| 4 | 0.54 | 22.5 mg/g | 12.2 | 1.52 | 0.12 | 8.2 | 4 | 33 | C, P | 07 | |
| 5 | 0.58 | 24 mg/g | 13.9 | 1.57 | 0.12 | 9.1 | 4.8 | 35 | C | 07 | |
| 6 | 0.4 | 1.5 % | 6 | 1.42 | 0.11 | 5.7 | 0.3 | 5 | C | - | |
| 7 | 0.69 | - | - | 0.46 | 0.04 | 3.2 | - | - | C | 07 | |
| 8 | 0.57 | 15 mg/g | 8.6 | 1.04 | 0.08 | 5.9 | 2.7 | 31 | C, P | 07 | |
| 9 | 0.49 | 18 mg/g | 8.8 | 1.34 | 0.1 | 6.6 | 2.2 | 25 | C | 07 | |
| 10 | 0.52 | 50 mg/g | 26 | 3.45 | 0.23 | 17.9 | 8.1 | 31 | C | 07 | |
| 11 | 0.71 | - | - | 1.46 | 0.11 | 10.4 | - | - | C | 07 | |
| 12 | 0.55 | - | - | 1.79 | 0.13 | 9.8 | - | - | C | 07 | |
| 13 | 0.85 | 1.6 % | 13.6 | 1.58 | 0.12 | 13.4 | 0.2 | 1 | C | - | |
| 14 | 0.83 | 1.2 % | 10 | 1.16 | 0.09 | 9.6 | 0.4 | 4 | C | - | |
| 15 | 0.59 | 10 mg/pouch | 10 | 1.45 | 0.11 | 8.6 | 1.4 | 14 | C | 07 | 20 min |
| 16 | 0.76 | - | - | 7.37 | 1.13 | 56 | - | - | C, P | 06 | |
| 17 | 0.39 | - | - | 3.32 | 0.51 | 12.9 | - | - | C, P | 06 | 30 min |
| 18 | 0.44 | 16 mg/g | 7 | 1.48 | 0.23 | 6.5 | 0.5 | 7 | C, P | 07 | 30 min |
| 19 | 0.43 | 6 mg/g | 2.58 | 0.52 | 0.08 | 2.2 | 0.38 | 15 | C, P | 07 | 30 min |
| 20 | 0.38 | - | - | 0.85 | 0.13 | 3.2 | - | - | C, P | 06 | 30 min |
| 21 | 0.49 | 8 mg/pouch | 8 | 1.15 | 0.09 | 5.6 | 2.4 | 30 | C | 07 | |
| 22 | 0.69 | - | - | 1.31 | 0.1 | 9.04 | - | - | - | - | |
| 23 | 0.65 | - | - | 0.48 | 0.04 | 3.1 | - | - | C | 07 | |
| 24 | 0.4 | - | - | 1.99 | 0.31 | 7.96 | - | - | C, P | 06 | 30 min |
| 25 | 0.74 | - | - | 6.37 | 0.98 | 47.1 | - | - | C, P | 06 | |
| 26 | 0.39 | - | - | 2.14 | 0.33 | 8.3 | - | - | C, P | 06 | 30 min |
| 27 | 0.42 | - | - | 4.34 | 0.67 | 18.2 | - | - | C, P | 06 | 30 min |
| 28 | 0.74 | 49.9 mg/g | 36.9 | 4.12 | 0.63 | 30.5 | 6.4 | 17 | C,P | - | |
| 29 | 0.56 | - | - | 5.17 | 0.78 | 29.0 | - | - | C,P | 06, 07 | |
| 30 | 0.62 | - | - | 7.86 | 1.21 | 48.7 | - | - | C, P | 07 | |
| 31 | 0.48 | - | - | 1.48 | 0.23 | 7.1 | - | - | C, P | 07 | 30 min |
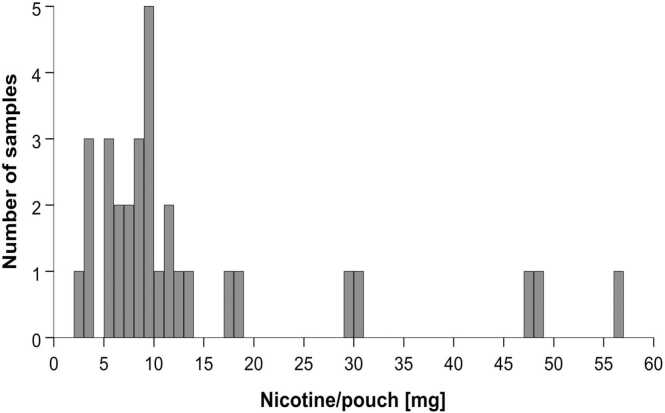
84 % of the samples showed nicotine levels of less than 20 mg per pouch (Fig. 1). All samples but one were labeled with an appropriate warning for children and 15 samples provided additional warnings for pregnant women. 19 samples depicted the Globally Harmonized System (GHS)-pictogram GHS07 (exclamation mark) and 8 samples depicted GHS06 (skull); one sample had both pictograms.
Fig. 1.
Histogram of detected nicotine levels in pouches. Analysis was conducted using GC-FID with 31 samples. Most pouches contained less than 20 mg nicotine, however, few samples had very high levels up to more than 56 mg nicotine/pouch.
Based on a study investigating nicotine concentration in the blood plasma of volunteers following pouch consumption (exposure time of 60 minutes), bioavailability of nicotine pouch consumption was identified as to range between 50 % and 60 % of the total nicotine content [14], [2]. In another study, a nicotine extraction rate after 60 minutes of application was determined to be 62 % of total nicotine content [49]. As in reality shorter durations of consumption may appear, a recent study investigated nicotine extraction after 20 minutes of consumption and found independent of nicotine levels rates of 24–52 % of total nicotine content [15]. Thus, in the present exposure assessment, an extraction rate of 50 % of total nicotine content was applied for estimating the oral nicotine uptake dose as a conservative, but still realistic approach considering application times of up to 60 minutes (and more) for some users [4]. Based on the median nicotine content in the analyzed pouches of 9.04 mg, this results in a median nicotine uptake of 65 µg/kg bw (range 20 – 400 µg/kg bw) from one pouch for an adult consumer with 70 kg bodyweight (Table 2).
Table 2.
Exposure estimation of nicotine pouches. Metrics of nicotine analytics of all investigated nicotine pouch samples and resulting nicotine exposure after consumption of pouches based on an extraction ratio of 50 % of the total nicotine content considering single and multiple use consumption patterns. Scenario 1: 5 pouches/day, scenario 2: 20 pouches/day.
| Minimum | percentile | Mean | Median | percentile | Maximum | |
|---|---|---|---|---|---|---|
| Nicotine content [mg] | 2.20 | 3.15 | 14.08 | 9.04 | 47.90 | 56.00 |
| Consumption pattern | ||||||
| Single use [µg/kg bw] | 16 | 23 | 101 | 65 | 342 | 400 |
| Scenario 1 [µg/kg bw and day] | 79 | 113 | 503 | 323 | 1711 | 2000 |
| Scenario 2 [µg/kg bw and day] | 314 | 450 | 2011 | 1291 | 6843 | 8000 |
Nicotine pouches are known to be often consumed more than just once a day. Swedish market survey data indicate an average nicotine pouch consumption of 8.6 pouches per day that is in line with another survey stating an average daily consumption of 8.4 and 8 pouches for Sweden and Denmark, respectively [50], [4]. A recent Dutch survey came to the conclusion that about 42 % of all ever users consume less than 5 pouches per day, while 8.3 % of all users consume even more than 20 pouches/day [6]. Due to the observed variability in consumption patterns among users, two scenarios for exposure assessment were applied in the here presented risk assessment, whereby scenario 1 with an estimated consumption of 5 pouches/day (reflecting the largest fraction of current users in the survey by Havermans et al. [6]) is used to assess the risk for low to moderate consumers, while scenario 2 with an estimated consumption of 20 pouches per day (reflecting the minor fraction of current users with the highest consumption based on the survey by Havermans et al. [6]) considers high consumers. This approach has already been used in a previous risk assessment regarding flavorings and other ingredients in nicotine pouches [51].
Based on these scenarios, a daily consumption of 5 pouches (scenario 1) with a median nicotine content of 9.04 mg per pouch results in a daily nicotine uptake of 323 µg/kg bw, while the consumption of pouches with higher nicotine levels of 47.90 mg per pouch (95th percentile of the here analyzed samples considering expectable consumption of pouches with high nicotine content) leads to an uptake of 1711 µg/kg bw (Table 2). In scenario 2, a daily consumption of 20 pouches per day with a median nicotine content results in a daily uptake of 1291 µg/kg, whereas pouches with a 95th percentile nicotine content yield a nicotine uptake of 6843 µg/kg bw and day.
3.3. Risk characterization
For risk characterization, the ARfD value of the EFSA for oral nicotine uptake of 0.8 µg/kg bw was used (Table 3). Even after a single use of just one pouch with the lowest nicotine content of 2.20 mg/pouch, the ARfD is exceeded by a factor of 20. The corresponding oral LOAEL of 8 µg/kg bw (derived from the intravenous LOAEL from the study by Lindgren et al. [12] after correction for bioavailability) was also exceeded for all exposure scenarios and even after single consumption of only one pouch with the lowest analyzed nicotine content of 2.20 mg per pouch (Table 3). Median analyzed nicotine levels lead to an 80-fold exceedance of the ARfD and a LOAEL exceedance by a factor of 8. Considering the observed significant increase of the heart rate of 10–14 bpm at doses 4- to 8-fold higher than the LOAEL, the here determined nicotine levels in the analyzed samples supposedly lead to a relevant and significant heart rate increase in consumers even after the use of just one pouch.
Table 3.
Risk description of nicotine pouch consumption. Factors of exceedance of estimated nicotine uptake based on different exposure scenarios with the ARfD value and the corresponding LOAEL for nicotine. Scenario 1: 5 pouches/day, scenario 2: 20 pouches/day.
| Minimum | percentile | Mean | Median | percentile | Maximum | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | ARfD | LOAEL | ARfD | LOAEL | ARfD | LOAEL | ARfD | LOAEL | ARfD | LOAEL | ARfD | LOAEL |
| Single use | 20 | 2 | 28 | 3 | 126 | 13 | 81 | 8 | 428 | 43 | 500 | 50 |
| Scenario 1 | 98 | 10 | 141 | 14 | 628 | 63 | 404 | 40 | 2138 | 214 | 2500 | 250 |
| Scenario 2 | 393 | 39 | 563 | 56 | 2514 | 251 | 1614 | 161 | 8554 | 855 | 10000 | 1000 |
The ARfD usually refers to a dose ingested within 24 hours. Thus, apart from single exposure, also the above described scenarios for multiple consumption of pouches on a single day were considered leading to ARfD exceedances of factor 404 and 1614 (using the median analyzed nicotine level) for scenarios 1 and 2, respectively. Considering the 95th percentile nicotine level, ARfD exceedances of factor 2138 and 8554 can be calculated for scenarios 1 and 2, respectively. However, the use of these scenarios has some limitations:
The ARfD was derived based on data after single administration of nicotine to human volunteers. Thus, the kinetics of nicotine have to be considered for repeated consumption of nicotine pouches within a single day. The half-life of nicotine is known to range between 100 and 150 minutes [2]. Hence, an accumulation effect will be of some relevance, but the repetitive nicotine uptake over a day will not result in a complete additive accumulation of nicotine in the body [14], [52], [53]. As the action of nicotine on the cardiovascular system is receptor-mediated, saturation processes may further limit a linear increase of effects. This is in line with observations by Lindgren et al., where no linear correlation was found between nicotine plasma levels and corresponding effects on heart rate [12]. To our knowledge, there are no human studies available investigating possible effects after consumption of more than one pouch within short time periods; therefore, the cumulative effects of nicotine pouch consumption remain uncertain. However, it can be assumed that repetitive nicotine uptake leads to constantly elevated nicotine levels in plasma with the respective consequences for the heart rate. Studies on nicotine pharmacokinetics demonstrated repetitively increased peak nicotine levels after smoking one cigarette every 60 minutes over several hours, while the corresponding heart rate elevation was indeed observed after each cigarette consumption, however, to a lower extent compared to the first cigarette indicating an adaptive effect [52], [53]. However, nicotine kinetics following nicotine pouch consumption are characterized by a prolonged resorption period leading to prolonged elevated nicotine levels in blood as well.
Taken all together, it can safely be assumed that nicotine uptake from pouches results in an acute and, based on typical consumption patterns, usually even repetitive elevation of the heart rate, which might in turn result in adverse health effects like palpitations and arrhythmias as has been observed after usage of oral nicotine containing cessation products. Though, the available data with respect to the consequences of nicotine uptake on the cardiovascular system, particularly following long-term consumption of nicotine pouches and other nicotine containing cessation products, are of low quantity. The already known consequences of nicotine exposure, e. g. regarding heart rate elevation, have to be considered as sufficient cause for concern, especially with respect to the basic principles of food law.
4. Conclusion
In conclusion, it could be shown that the here analyzed nicotine pouches have to be considered as probably harmful to health following acute exposure based on expected heart rate elevations due to nicotine uptake. Despite the observed high variations in nicotine levels between the individual analyzed pouches, all, even the products with the lowest nicotine levels, have to be assessed as injurious to health according to general food law based on a significant ARfD exceedance. However, in consideration of a risk-benefit assessment, usage of nicotine pouches may nevertheless be preferable in comparison to smoking traditional cigarettes or using other combustible tobacco products, as many classical tobacco products are known to cause greater health damages (e.g. cancer) in particular due to the presence of the manifold by-products of tobacco. Nicotine pouches are thus often lauded as an aid to reduce smoking and therefore improving the health status of the consumers. However, keeping in mind that nicotine pouches are also used by non-smokers and even never-smokers, that claim has to be critically questioned.
In Germany, nicotine pouches are currently classified as food due to the absence of an appropriate legislative framework, rendering a risk-benefit assessment unfeasible. An appropriate framework could be the regulation of nicotine pouches as approved medicinal products, like nicotine gums, taking into account a risk-benefit analysis. As such, nicotine pouches are already available in several countries, e.g. in Finland, Spain or Portugal. In contrast, many other countries regulate nicotine pouches as tobacco products or under similar classification that may, dependent on national child protection laws, offer some protection against health damage particularly for children [54]. Regardless of the applied regulatory framework, comprehensive studies are necessary not only for the assessment of a valid risk-benefit analysis but also for addressing long-term health effects after consumption of nicotine pouches.
CRediT authorship contribution statement
Hauke Reimann: Writing – original draft, Visualization, Data curation, Conceptualization. Matthias Berger: Writing – review & editing, Validation, Methodology, Investigation. Katja Merches: Writing – review & editing, Data curation, Conceptualization. Elisabeth Eckert: Writing – review & editing, Data curation, Conceptualization. Frederik Börnke: Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Data will be made available on request.
References
- 1.Abrams D.B., Glasser A.M., Pearson J.L., Villanti A.C., Collins L.K., Niaura R.S. Harm Minimization and Tobacco Control: Reframing Societal Views of Nicotine Use to Rapidly Save Lives. Annu Rev. Public Health. 2018;39:193–213. doi: 10.1146/annurev-publhealth-040617-013849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.BfR. (2022). Health risk assessment of nicotine pouches. Updated BfR Opinion no. 023/2022, 7 October 2022. https://mobil.bfr.bund.de/cm/349/health-risk-assessment-of-nicotine-pouches.pdf.
- 3.Nutt D.J., Phillips L.D., Balfour D., Curran H.V., Dockrell M., Foulds J., Fagerstrom K., Letlape K., Milton A., Polosa R., Ramsey J., Sweanor D. Estimating the harms of nicotine-containing products using the MCDA approach. Eur. Addict. Res. 2014;20(5):218–225. doi: 10.1159/000360220. [DOI] [PubMed] [Google Scholar]
- 4.Prasad K., Shetty M., Kanitscheider C., Szentes B.L., Nassar R., Edward L. Assessing consumer use and behaviour patterns of oral nicotine pouches in a multi-country study. Int. J. Sci. Rep. 2022 [Google Scholar]
- 5.Robichaud M.O., Seidenberg A.B., Byron M.J. Tobacco companies introduce 'tobacco-free' nicotine pouches. Tob. Control. 2020;29(e1):e145–e146. doi: 10.1136/tobaccocontrol-2019-055321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havermans A., Pennings J.L.A., Hegger I., Elling J.M., de Vries H., Pauwels C., Talhout R. Awareness, use and perceptions of cigarillos, heated tobacco products and nicotine pouches: A survey among Dutch adolescents and adults. Drug Alcohol Depend. 2021;229(Pt B) doi: 10.1016/j.drugalcdep.2021.109136. [DOI] [PubMed] [Google Scholar]
- 7.Plurphanswat N., Hughes J.R., Fagerström K., Rodu B. Initial Information on a Novel Nicotine Product. Am. J. Addict. 2020;29(4):279–286. doi: 10.1111/ajad.13020. [DOI] [PubMed] [Google Scholar]
- 8.TobaccoTactics. (2023). Nicotine Pouches. Retrieved 02.10.2024 from https://tobaccotactics.org/article/nicotine-pouches/.
- 9.ECHA. (2015). Committee for Risk Assessment, Opinion proposing harmonised classification and labelling at EU level of Nicotine (ISO); 3-[(2S)-1-methylpyrrolidin-2-yl]pyridine. 〈https://echa.europa.eu/documents/10162/23665416/clh_opinion_nicotine_5579_en.pdf/0103fadb-e945-4839-c4f4-17d20854adf0〉.
- 10.EFSA Potential risks for public health due to the presence of nicotine in wild mushrooms. EFSA J. 2009;7(5):286r. doi: 10.2903/j.efsa.2009.286r. [DOI] [Google Scholar]
- 11.European Commission . 2016. Report from the Commission to the European Parliament and the Council on the potential risks to the public health associated with the use of refillable electronic cigarettes.〈https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52016DC0269〉 [Google Scholar]
- 12.Lindgren M., Molander L., Verbaan C., Lunell E., Rosén I. Electroencephalographic effects of intravenous nicotine--a dose-response study. Psychopharmacol. (Berl. ) 1999;145(3):342–350. doi: 10.1007/s002130051067. [DOI] [PubMed] [Google Scholar]
- 13.BfR. (2009). Nikotin in getrockneten Steinpilzen: Ursache der Belastung muss geklärt werden. Stellungnahme 009/2009 des BfR vom 28. Februar 2009. 〈https://www.bfr.bund.de/cm/343/nikotin_in_getrockneten_steinpilzen_ursache_der_belastung_muss_geklaert_werden.pdf〉.
- 14.Lunell E., Fagerström K., Hughes J., Pendrill R. Pharmacokinetic comparison of a novel non-tobacco-based nicotine pouch (ZYN) with conventional, tobacco-based Swedish Snus and American Moist Snuff. Nicotine Tob. Res. 2020;22(10):1757–1763. doi: 10.1093/ntr/ntaa068. [DOI] [PubMed] [Google Scholar]
- 15.Mallock-Ohnesorg N., Rabenstein A., Stoll Y., Gertzen M., Rieder B., Malke S., Burgmann N., Laux P., Pieper E., Schulz T., Franzen K., Luch A., Rüther T. Small pouches, but high nicotine doses-nicotine delivery and acute effects after use of tobacco-free nicotine pouches. Front Pharm. 2024;15 doi: 10.3389/fphar.2024.1392027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauck A.S., Buchwald I., Watz H., Trinkmann F., Söling C., Rabenstein A., Ruether T., Mortensen K., Drömann D., Franzen K.F. Impact of chewing bags, E-cigarettes, and combustible cigarettes on arterial stiffness and small airway function in healthy students. Toxics. 2023;11(1) doi: 10.3390/toxics11010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cobb C.O., Weaver M.F., Eissenberg T. Evaluating the acute effects of oral, non-combustible potential reduced exposure products marketed to smokers. Tob. Control. 2010;19(5):367–373. doi: 10.1136/tc.2008.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunell E., Curvall M. Nicotine delivery and subjective effects of Swedish portion snus compared with 4 mg nicotine polacrilex chewing gum. Nicotine Tob. Res. 2011;13(7):573–578. doi: 10.1093/ntr/ntr044. [DOI] [PubMed] [Google Scholar]
- 19.Nyberg G., Panfilov V., Sivertsson R., Wilhelmsen L. Cardiovascular effects of nicotine chewing gum in healthy non-smokers. Eur. J. Clin. Pharmacol. 1982;23(4):303–307. doi: 10.1007/BF00613610. [DOI] [PubMed] [Google Scholar]
- 20.Ozga J.E., Felicione N.J., Elswick D., Blank M.D. Acute effects of snus in never-tobacco users: a pilot study. Am. J. Drug Alcohol Abus. 2018;44(1):113–119. doi: 10.1080/00952990.2016.1260581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woolf A., Burkhart K., Caraccio T., Litovitz T. Childhood poisoning involving transdermal nicotine patches. Pediatrics. 1997;99(5) doi: 10.1542/peds.99.5.e4. E4. [DOI] [PubMed] [Google Scholar]
- 22.Haleon Fachinf. Nicotinell Lutschtabletten 2 mg Mint. 2024 [Google Scholar]
- 23.Mills E.J., Wu P., Lockhart I., Wilson K., Ebbert J.O. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob. Induc. Dis. 2010;8(1):8. doi: 10.1186/1617-9625-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Böhm M., Swedberg K., Komajda M., Borer J.S., Ford I., Dubost-Brama A., Lerebours G., Tavazzi L. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376(9744):886–894. doi: 10.1016/s0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 25.Fox K., Komajda M., Ford I., Robertson M., Böhm M., Borer J.S., Steg P.G., Tavazzi L., Tendera M., Ferrari R., Swedberg K. Effect of ivabradine in patients with left-ventricular systolic dysfunction: a pooled analysis of individual patient data from the BEAUTIFUL and SHIFT trials. Eur. Heart J. 2013;34(29):2263–2270. doi: 10.1093/eurheartj/eht101. [DOI] [PubMed] [Google Scholar]
- 26.Aune D., Sen A., ó'Hartaigh B., Janszky I., Romundstad P.R., Tonstad S., Vatten L.J. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality - A systematic review and dose-response meta-analysis of prospective studies. Nutr. Metab. Cardiovasc Dis. 2017;27(6):504–517. doi: 10.1016/j.numecd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Jensen M.T., Suadicani P., Hein H.O., Gyntelberg F. Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart. 2013;99(12):882–887. doi: 10.1136/heartjnl-2012-303375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D., Shen X., Qi X. Resting heart rate and all-cause and cardiovascular mortality in the general population: a meta-analysis. Cmaj. 2016;188(3) doi: 10.1503/cmaj.150535. E53-e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perret-Guillaume C., Joly L., Benetos A. Heart Rate as a Risk Factor for Cardiovascular Disease. Prog. Cardiovasc. Dis. 2009;52(1):6–10. doi: 10.1016/j.pcad.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Mancia G., Kreutz R., Brunström M., Burnier M., Grassi G., Januszewicz A., Muiesan M.L., Tsioufis K., Agabiti-Rosei E., Algharably E.A.E., Azizi M., Benetos A., Borghi C., Hitij J.B., Cifkova R., Coca A., Cornelissen V., Cruickshank J.K., Cunha P.G., Kjeldsen S.E. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA) J. Hypertens. 2023;41(12):1874–2071. doi: 10.1097/hjh.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 31.Byhamre M.L., Araghi M., Alfredsson L., Bellocco R., Engström G., Eriksson M., Galanti M.R., Jansson J.H., Lager A., Lundberg M., Östergren P.O., Pedersen N.L., Trolle Lagerros Y., Ye W., Wennberg P., Magnusson C. Swedish snus use is associated with mortality: a pooled analysis of eight prospective studies. Int J. Epidemiol. 2021;49(6):2041–2050. doi: 10.1093/ije/dyaa197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolinder G., Alfredsson L., Englund A., de Faire U. Smokeless tobacco use and increased cardiovascular mortality among Swedish construction workers. Am. J. Public Health. 1994;84(3):399–404. doi: 10.2105/ajph.84.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roosaar A., Johansson A.L., Sandborgh-Englund G., Axéll T., Nyrén O. Cancer and mortality among users and nonusers of snus. Int J. Cancer. 2008;123(1):168–173. doi: 10.1002/ijc.23469. [DOI] [PubMed] [Google Scholar]
- 34.Hansson J., Galanti M.R., Hergens M.P., Fredlund P., Ahlbom A., Alfredsson L., Bellocco R., Engström G., Eriksson M., Hallqvist J., Hedblad B., Jansson J.H., Pedersen N.L., Trolle Lagerros Y., Ostergren P.O., Magnusson C. Snus (Swedish smokeless tobacco) use and risk of stroke: pooled analyses of incidence and survival. J. Intern Med. 2014;276(1):87–95. doi: 10.1111/joim.12219. [DOI] [PubMed] [Google Scholar]
- 35.Hergens M.P., Galanti R., Hansson J., Fredlund P., Ahlbom A., Alfredsson L., Bellocco R., Eriksson M., Fransson E.I., Hallqvist J., Jansson J.H., Knutsson A., Pedersen N., Lagerros Y.T., Ostergren P.O., Magnusson C. Use of Scandinavian moist smokeless tobacco (snus) and the risk of atrial fibrillation. Epidemiology. 2014;25(6):872–876. doi: 10.1097/ede.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 36.Hansson J., Galanti M.R., Hergens M.P., Fredlund P., Ahlbom A., Alfredsson L., Bellocco R., Eriksson M., Hallqvist J., Hedblad B., Jansson J.H., Nilsson P., Pedersen N., Trolle Lagerros Y., Ostergren P.O., Magnusson C. Use of snus and acute myocardial infarction: pooled analysis of eight prospective observational studies. Eur. J. Epidemiol. 2012;27(10):771–779. doi: 10.1007/s10654-012-9704-8. [DOI] [PubMed] [Google Scholar]
- 37.Titova O.E., Baron J.A., Michaëlsson K., Larsson S.C. Swedish snuff (snus) and risk of cardiovascular disease and mortality: prospective cohort study of middle-aged and older individuals. BMC Med. 2021;19(1):111. doi: 10.1186/s12916-021-01979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondo T., Nakano Y., Adachi S., Murohara T. Effects of Tobacco Smoking on Cardiovascular Disease. Circulation Journal. 2019;83(10):1980–1985. doi: 10.1253/circj.CJ-19-0323. [DOI] [PubMed] [Google Scholar]
- 39.Whitehead A.K., Erwin A.P., Yue X. Nicotine and vascular dysfunction. Acta Physiologica. 2021;231(4):e13631. doi: 10.1111/apha.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glover M., Phillips C.V. Potential effects of using non-combustible tobacco and nicotine products during pregnancy: a systematic review. Harm Reduct. J. 2020;17(1):16. doi: 10.1186/s12954-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wikström A.K., Cnattingius S., Stephansson O. Maternal use of Swedish snuff (snus) and risk of stillbirth. Epidemiology. 2010;21(6):772–778. doi: 10.1097/EDE.0b013e3181f20d7e. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z., Liu X.-a, Kenny P.J. Central and peripheral actions of nicotine that influence blood glucose homeostasis and the development of diabetes. Pharmacol. Res. 2023;194 doi: 10.1016/j.phrs.2023.106860. [DOI] [PubMed] [Google Scholar]
- 43.Carlsson S., Andersson T., Araghi M., Galanti R., Lager A., Lundberg M., Nilsson P., Norberg M., Pedersen N.L., Trolle-Lagerros Y., Magnusson C. Smokeless tobacco (snus) is associated with an increased risk of type 2 diabetes: results from five pooled cohorts. J. Intern Med. 2017;281(4):398–406. doi: 10.1111/joim.12592. [DOI] [PubMed] [Google Scholar]
- 44.Front Office Voedsel- en Productveiligheid . 2021. Beoordeling van de verslavende werking van nicotinezakjes.〈https://www.rivm.nl/documenten/beoordeling-van-verslavende-werking-van-nicotinezakjes〉 [Google Scholar]
- 45.EFSA Setting of temporary MRLs for nicotine in tea, herbal infusions, spices, rose hips and fresh herbs. EFSA J. 2011;9(3):2098. doi: 10.2903/j.efsa.2011.2098. [DOI] [Google Scholar]
- 46.EFSA Statement on the short-term (acute) dietary risk assessment for the temporary maximum residue levels for nicotine in rose hips, teas and capers. EFSA J. 2022;20(9) doi: 10.2903/j.efsa.2022.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.EFSA Statement on the revised targeted risk assessment for certain maximum residue levels for nicotine. EFSA J. 2023;21(3) doi: 10.2903/j.efsa.2023.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mallock N., Schulz T., Malke S., Dreiack N., Laux P., Luch A. Levels of nicotine and tobacco-specific nitrosamines in oral nicotine pouches. Tob. Control. 2024;33(2):193–199. doi: 10.1136/tc-2022-057280. [DOI] [PubMed] [Google Scholar]
- 49.Azzopardi D., Ebajemito J., McEwan M., Camacho O.M., Thissen J., Hardie G., Voisine R., Mullard G., Cohen Z., Murphy J. A randomised study to assess the nicotine pharmacokinetics of an oral nicotine pouch and two nicotine replacement therapy products. Sci. Rep. 2022;12(1):6949. doi: 10.1038/s41598-022-10544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azzopardi D., Liu C., Murphy J. Chemical characterization of tobacco-free "modern" oral nicotine pouches and their position on the toxicant and risk continuums. Drug Chem. Toxicol. 2022;45(5):2246–2254. doi: 10.1080/01480545.2021.1925691. [DOI] [PubMed] [Google Scholar]
- 51.Mallock-Ohnesorg N., Rinaldi S., Malke S., Dreiack N., Pieper E., Laux P., Schulz T., Zimmermann R., Luch A. Oral nicotine pouches with an aftertaste? Part 1: screening and initial toxicological assessment of flavorings and other ingredients. Arch. Toxicol. 2023;97(9):2357–2369. doi: 10.1007/s00204-023-03538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mello N.K. Hormones, nicotine, and cocaine: clinical studies. Horm. Behav. 2010;58(1):57–71. doi: 10.1016/j.yhbeh.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendelson J.H., Goletiani N., Sholar M.B., Siegel A.J., Mello N.K. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;33(4):749–760. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- 54.Duren M., Atella L., Welding K., Kennedy R.D. Nicotine pouches: a summary of regulatory approaches across 67 countries. Tob. Control. 2024;33(e1) doi: 10.1136/tc-2022-057734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.