Abstract
Background:
While standard clinical assessments provide great value for people with multiple sclerosis (PwMS), they are limited in their ability to characterize patient perspectives and individual-level symptom heterogeneity.
Objectives:
To identify PwMS subgroups based on patient reported outcomes (PROs) of physical, cognitive, and emotional symptoms. We also sought to connect PRO-based subgroups with demographic variables, functional impairment, hypertension and smoking status, traditional qualitative MS symptom groupings, and neuroperformance measurements.
Methods:
Using a cross-sectional design, we applied latent profile analysis (LPA) to a large database of patient reported outcomes (PROs; analytic sample N=6,619).
Results:
We identified nine distinct MS subtypes based on PRO patterns. The subtypes were primarily categorized into low, moderate, and high mobility impairment clusters. Approximately 70% of participants were classified in a low mobility impairment group, 10% in a moderate mobility impairment group, and 20% in a high mobility impairment group. Within these subgroups, several unexpected patterns were observed, such as high mobility impairment clusters reporting low non-mobility impairment.
Conclusions:
The present study highlights an opportunity to advance precision medicine approaches in MS. Combining PROs with data-driven methodology allows for a cost-effective and personalized characterization of symptom presentations. that can inform clinical practice and future research designs.
Keywords: Multiple Sclerosis (MS), Patient-Reported Outcomes (PROs), Latent Profile Analysis (LPA), Health Disparities
In the United States, over 900,000 people live with multiple sclerosis (PwMS).1 The multiple sclerosis (MS) incidence rate continues to increase,1 and MS symptoms are associated with reduced quality of life (QOL)2 along with increases in overall healthcare costs.3 Among PwMS, symptom presentation can be very heterogeneous. In addition to nervous system and muscle dysfunction, PwMS can experience cognitive deficits, emotional and sensory disturbances, and chronic pain.4 Domain-specific impairments can occur alone or in combination, can vary in severity, and can severely limit QOL for patients.2 The observed heterogeneity in MS symptom presentations creates several challenges for clinicians, especially for identifying diagnostic subgroups that can be aligned with clinical recommendations and outcomes.5 Identifying subgroups with shared symptom patterns would provide a foundation for a precision medicine approach to MS, with patient-specific interventions tailored to individual phenotypes.
When identifying subgroups of PwMS, many clinical symptoms can form the basis for patient clusters. Given the absence of a single assessment for MS classification and the variability of symptoms among individuals, clinicians employ multiple tools including the Expanded Disability Status Scale (EDSS), magnetic resonance imaging, evoked potentials, and the Timed 25-Foot Walk (T25FW) test.6 Although these measures provide insight into functioning, they do not fully capture patient experiences, and they are labor intensive and require expert providers to administer them. To complement clinician-administered assessments, patient reported outcomes (PROs) have received increased focus. PROs are self-reported measures that emphasize patients’ experiences with the disorder. They consider treatment, intervention side effects, QOL, and overall health outcomes.7 PROs can enhance communication with providers, aid patients in decision making, and support disease self-management.8 They have been used to predict health related QOL, patient satisfaction, and treatment effects in MS9 and other neurological disorders such as neurofibromatosis10 and Huntington’s disease.11 PROs provide a valuable, cost effective, and patient-centered approach for informing assessment and treatment related decisions.
Much MS research has focused on clinical evaluation by expert providers and researchers, with less attention on PROs. While this approach has provided key insights, it does not fully account for symptom course and treatment outcomes. Newly available data-driven modeling approaches provide a complementary approach. Previous data-driven approaches in MS have successfully predicted immune system response evolution based on biological, genetic, environmental, and therapeutic intervention data.12, 13 These approaches have been instrumental in creating clinical decision support systems, guiding MS diagnosis, testing for additional risk factors,13 predicting pharmacological intervention effects,12–14 and identifying subgroups experiencing change in MS symptoms over time.14
While previous research has generated important insights, there has not been a comprehensive data-driven approach to identify symptom-based subgroups of PwMS based on PROs.15 This knowledge gap provides an opportunity to identify patient-specific treatment targets in a cost-effective manner that reflects patient perspectives and increases clarity about MS symptomology for both clinicians and researchers. To address this gap, we applied latent profile analysis (LPA) to PROs collected from electronic health records (EHRs). We considered a variety of PROs that reflect physical, cognitive, and emotional symptoms. We hypothesized that multiple subgroups would emerge from the PRO data that signify distinct clinical clusters of patients. Additionally, we hypothesized that the identified subgroups would be differentially associated with demographic variables, functional impairment, hypertension and smoking status, traditional qualitative MS symptom groupings, and neuroperformance assessments such as T25FW and the 9-Hole Peg Test (9HPT).
Methods
Study Design and Sample
This is a cross-sectional study of a real-world patient population from the Mellen Center for Multiple Sclerosis Treatment and Research (MCMS). The MCMS, a tertiary MS referral center at the Cleveland Clinic in Cleveland, Ohio, conducts >20,000 clinical encounters annually with >8,000 patients, including 1,000 new patients. The Knowledge Program © (KP) was launched in 2007, electronically capturing PROs and clinician-entered outcomes at each encounter.16 Patients aged 18 years or older with at least two clinical encounters six or more months apart between 1/2008–6/2016 were included. To establish the study population as patients seeking long-term care, we restricted the study to patients 18 or older who had at least two clinical encounters six or more months apart. This study was approved by the institutional review boards at Cleveland Clinic, Case Western Reserve University, and The MetroHealth System, Cleveland, Ohio. Among available patients (N=9,084), we analyzed data from a subset (analytic sample N=6,619) that had recorded responses to any of the 11 items on the MS-PRO scales (see Measures section for detail). All subsequent analyses and results refer to this analytic sample, covering baseline MS-PRO measurements spanned 1/2008–12/2015.
Measures
Our principal focus was on 11 Multiple Sclerosis Patient Report Outcome (MS-PRO) scales from the NARCOMS registry.17 These MS-PROs included the 8 items of the MS Performance Scales© measuring specific domains of function (mobility, hand function, vision, fatigue, cognition, bladder/bowel, sensory, and spasticity), and the MS Functional Scales© which include three additional items assessing disability associated with pain, depression, and tremor/coordination.17 Reliability, criterion and construct validity have been demonstrated for these 11 scales in PwMS.18, 19 Respondents rated their current disability compared to pre-MS onset. The mobility scale uses a seven-level severity measure, while the other MS-PRO scales include six ordinal categories.
Standard assessment procedures recorded disease course as relapsing-remitting, progressive (including primary-progressive, progressive-relapsing, secondary-progressive without relapses, and secondary-progressive with relapses), clinically isolated syndrome, or other/missing. Other variables included time from MS onset, age (years), race (Black, White, Other), tobacco usage (current, former, never [includes <1% who reported second-hand smoke exposure or had missing data]), and a diagnosis of hypertension. Available measures included T25-FW and dominant hand 9HPT (objective timed measures of walking impairment and upper extremity fine motor skills, respectively),6 the EQ5D (a general daily health-related QoL measure validated in diverse populations),20 and the PHQ-9 (an empirically-supported measure of depression symptom severity in the past two weeks).21 Lastly, ZIP-code mapped to the area deprivation index (ADI) national ranking served as a proxy for neighborhood socioeconomic status, with higher values indicating greater deprivation.22
Statistical Approach
To identify PwMS clusters with similar self-reported impairment profiles across domains, we applied LPA to responses on the 11 MS-PRO scales. The objective was to phenotype a real-world MS patient population to discern if there are distinct subpopulations and identify correlates of cluster assignment. LPA identifies clusters using latent (unobserved) variables based on the assumption that responses come from multiple underlying distributions that underlie observed responses.
To enumerate the number of clusters, we used an iterative process. We first fit a one-cluster model to the data, and we then fit a two-cluster model and compared its fit to the one-cluster model. If the two-cluster model provided a better fit, then we subsequently conducted a three-cluster model. This sequence continued comparing the k cluster model to the previous k-1 cluster model until a 12-cluster model was fitted. The key model fit summary metric was the Bayesian Information Criterion (BIC). Lower values of BIC reflect better fit, with a BIC difference of 10 suggesting an improved model.23 Lo-Mendell-Rubin (LMR)24 and Vuong-Lo-Mendell-Rubin (VLMR)25 tests were also conducted. When selecting our final model, we also considered both qualitative interpretability26 and entropy between the clusters.27 Values of entropy range from 0 to 1, where values of 0.4, 0.6, and 0.8 have been described as low, medium, and high degrees of cluster separation respectively.28 Models were estimated in Mplus version 829 using full-information maximum likelihood estimation and robust standard errors.
Once we established the number of patient clusters based on responses to the 11 MS-PRO scales, covariate values associated with each cluster were identified using the Bolck–Croon–Hagenaars (BCH) method.30 The BCH method utilizes class-specific weights to correct for asymptotic bias in estimates due to uncertainty in these membership classifications. Baseline attributes were compared across cluster membership.
Results
Participants in our analytic sample were adults age 18 or older (mean=45.61, SD=12.07) who experienced symptoms of MS for an average of 10.3 years (SD=9.9). 73.3% were female, and 1.75% ethnically identified as Hispanic. Racially, 86.31% of participants identified as White, 10.70% identified as Black, 0.42% identified as part of another racial group (including Asian/Pacific Islander, Native Hawaiian/Pacific Islander, and Native American), 1.04% identified as multiracial, and 1.55% did not self-identify a racial group. 9.03% of participants experienced hypertension and 19.6% were current smokers. Regarding socioeconomic status, the mean ADI national rank among participants was 57.04 (SD=23.77). 3.5% of data were missing across all PROs, and no individual PRO item was missing more than 5% of data.
Cluster Enumeration
Based on quantitative fit metrics and qualitative cluster interpretation, the 9-cluster model was chosen as the best fitting model (see Supplementary Table S1). In our sequence of models, the 9-cluster model was the last one to show a significant improvement in model fit on VLMR and LMR tests. While BIC values continued to decrease in models with ≥10 clusters, the rate of change in BIC was decreasing by the time a 9-cluster model was identified, and qualitative inspection found that additional clusters did not provide clinically important information. A 9-cluster model also showed strong entropy (entropy=0.84), reflecting clear cluster separation.
Among the nine clusters, distinct PRO patterns emerged (see Table 1 for numerical values and qualitative cluster descriptors, and Figure 1 for a graphical depiction via profile plot). A predominant pattern centered on mobility, with three major groupings: Clusters 1–4 reflected low mobility impairment scores, Cluster 5 reflected moderate mobility impairment scores, and Clusters 6–9 reflected high mobility impairment scores. Among the low mobility impairment clusters, the largest overall cluster (Cluster 1; 31.7% of sample) showed relatively low impairment across all PRO measures. Other low mobility clusters exhibited moderate or substantial impairment in specific PRO domains, including fatigue (Clusters 2, 3, and 4), sensory symptom (Cluster 3), and pain (Clusters 3 and 4). Together, these low mobility impairment clusters represented 68.4% of the sample. The moderate mobility cluster (Cluster 5; 10.2% of the sample) showed relatively moderate impairment across all PROs. Among the clusters with high mobility impairment scores, the largest cluster (Cluster 7; 8.3% of sample) only showed elevations on mobility impairment, and otherwise showed relatively low impairment across the other PROs measured. Cluster 6 (4.1% of sample) showed moderate mobility impairment, along with moderate or substantial impairment on all other PROs measured. Collectively, high mobility impairment clusters accounted for 21.4% of the sample. Between-cluster statistical comparisons of PRO response levels can be found in Supplementary Tables S2–S12. Supplementary Figure S1 contains an additional profile plot that places all nine classes on a single figure, and Supplementary Figures S2–S12 contain scatterplots of cluster mean values for each PRO plotted against years since symptom onset.
Table 1.
Descriptive Characteristics of Observed Patient Clusters Using the Domains of the MS Performance and Functional Scales©
| Patient Reported Impairment | Overall Sample Mean (SD) | Low Mobility Impairment | Moderate Mobility Impairment | High Mobility Impairment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 (Normal Functioning) | Cluster 2 (Mild Fatigue) | Cluster 3 (Sensory Symptoms) | Cluster 4 (Somatic Symptoms) | Cluster 5 (Somatic and Cognitive) | Cluster 6 (Severe Disability) | Cluster 7 (Mobility-Specific) | Cluster 8 (Moderate Disability) | Cluster 9 (Physical Symptoms) | ||
| Mobility | 1.83 (1.83) | 0.34 | 0.96 | 1.58 | 1.86 | 2.42 | 4.33 | 4.45 | 4.73 | 4.77 |
| Hand Function | 1.18 (1.27) | 0.30 | 0.78 | 1.31 | 1.44 | 2.16 | 3.66 | 1.27 | 2.37 | 2.49 |
| Tremor | 1.29 (1.37) | 0.30 | 0.87 | 1.15 | 1.70 | 2.44 | 3.87 | 1.21 | 2.95 | 3.23 |
| Spasticity | 1.40 (1.41) | 0.38 | 0.89 | 1.45 | 1.76 | 2.68 | 3.81 | 1.63 | 3.37 | 2.55 |
| Bladder/Bowel | 1.21 (1.29) | 0.37 | 0.92 | 0.93 | 1.51 | 1.76 | 3.19 | 1.74 | 2.67 | 2.78 |
| Pain | 1.71 (1.50) | 0.57 | 1.42 | 2.18 | 2.67 | 3.40 | 3.96 | 1.13 | 2.80 | 2.15 |
| Sensory Sx | 1.72 (1.39) | 0.72 | 1.13 | 3.52 | 1.60 | 3.52 | 4.19 | 0.97 | 3.65 | 1.25 |
| Vision | 1.13 (1.15) | 0.64 | 1.09 | 0.87 | 1.65 | 1.99 | 2.52 | 0.72 | 1.19 | 1.72 |
| Fatigue | 2.18 (1.48) | 0.69 | 2.36 | 2.26 | 3.38 | 3.69 | 4.24 | 1.95 | 3.26 | 3.37 |
| Cognitive Sx | 1.29 (1.27) | 0.42 | 1.21 | 0.94 | 2.41 | 2.79 | 3.49 | 0.60 | 0.99 | 2.24 |
| Depression | 1.10 (1.21) | 0.35 | 1.00 | 0.83 | 1.93 | 2.19 | 3.17 | 0.64 | 1.19 | 1.95 |
| Na | 2096.6 | 1241.7 | 525.3 | 664.2 | 673.8 | 271.5 | 549.2 | 339.9 | 256.9 | |
| % of Sample | 31.7% | 18.8% | 7.9% | 10.0% | 10.2% | 4.1% | 8.3% | 5.1% | 3.9% | |
Because the number of participants in each class reflect model-based estimates, the results are allowed to take on fractional values
Figure 1.
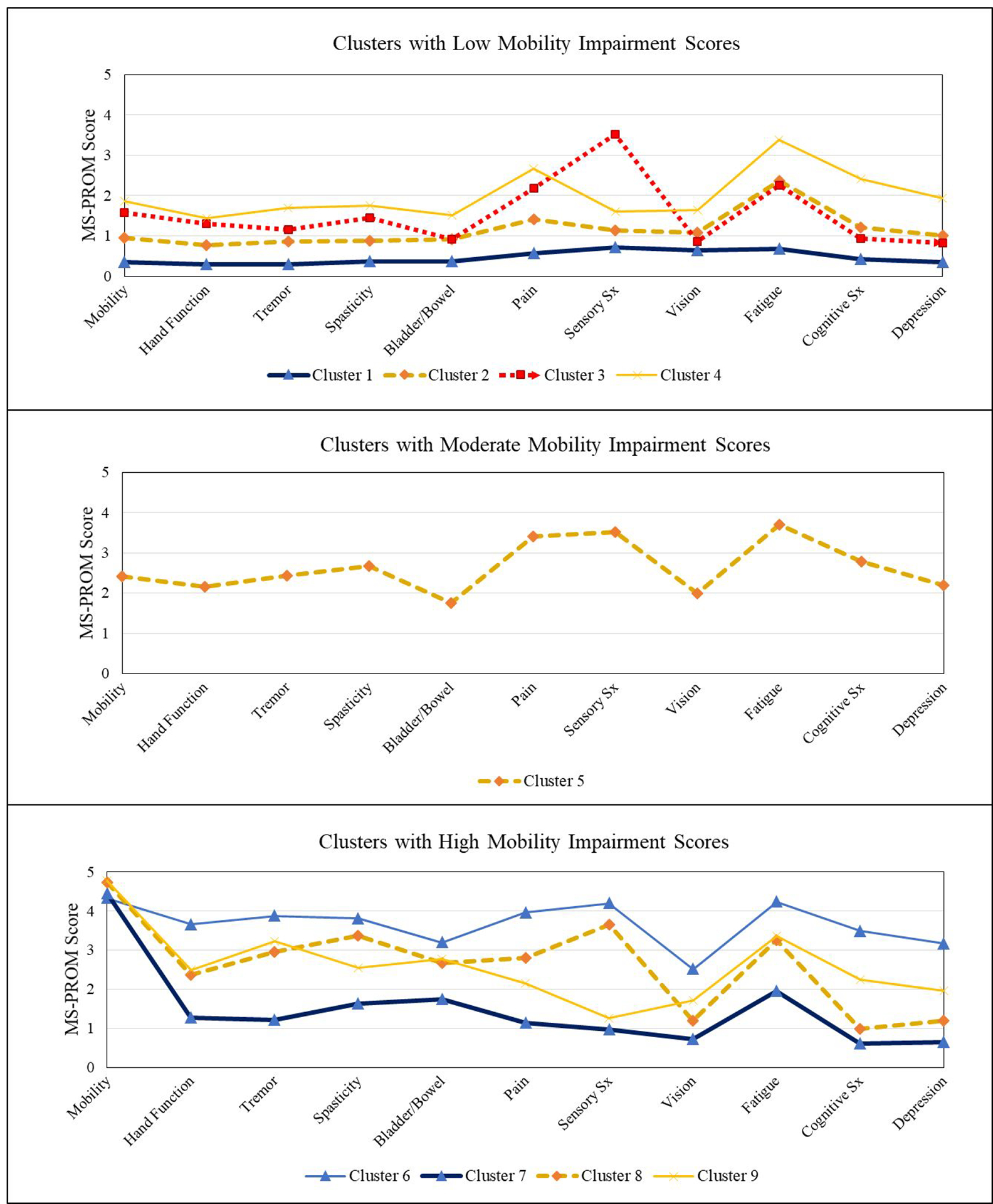
Profile Plots of Patient Reported Outcomes for Each Observed Cluster
Demographic and Clinical Correlates of Cluster Membership
Values for each cluster on relevant demographic and clinical variables are detailed in Table 2. Distinctions across groups were most pronounced in clinical variables. Specifically, observed MS clinical course was markedly different across the clusters, where a relapsing remitting pattern was more prevalent in clusters that had low mobility impairment scores, while progressive MS was more prevalent in participants who were members of clusters associated with high mobility impairment scores. QOL demonstrated a consistent pattern, where higher EQ5D scores were generally associated with lower mobility impairment scores. An exception was observed in Cluster 7, which exhibited high mobility impairment yet displayed relatively high EQ5D scores. This cluster also showed relatively healthy functioning on all non-mobility PROs. Participants in higher mobility impairment clusters generally had longer time since their initial symptom onset, though even those in low mobility impairment clusters showed extended symptom durations (range of 6.30–11.34 years across Clusters 1–4). Predictably, T25FW and 9HPT performance was poorer in clusters with high mobility impairment scores. While current tobacco use rates were lowest in the overall highest functioning group (Cluster 1; 10.2% current smokers) and relatively high in the overall lowest functioning cluster (Cluster 6; 26.6% current smokers), smoking did not exhibit clear trends related to mobility impairment across clusters.
Table 2.
Covariates Associated with Cluster Membership
| Attribute | Total Population Mean (SD) or % | Low Mobility Impairment | Moderate Mobility Impairment | High Mobility Impairment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 (Normal Functioning) | Cluster 2 (Mild Fatigue) | Cluster 3 (Sensory Symptoms) | Cluster 4 (Somatic Symptoms) | Cluster 5 (Somatic and Cognitive) | Cluster 6 (Severe Disability) | Cluster 7 (Mobility-Specific) | Cluster 8 (Moderate Disability) | Cluster 9 (Physical Symptoms) | ||
| Demographic Variables | ||||||||||
| Age (Years) | 45.6 (12.1) | 42.3 | 44.8 | 44.0 | 45.8 | 45.0 | 47.7 | 54.4 | 53.6 | 51.2 |
| Years since onset | 10.3 (9.9) | 7.25 | 9.51 | 6.30 | 11.34 | 9.87 | 12.7 | 18.0 | 15.9 | 17.4 |
| Years since-Not Available | 21.0% | 19.5% | 20.8% | 25.1% | 24.7% | 25.1% | 21.9% | 17.4% | 13.6% | 20.8% |
| Sex (Male) | 26.7% | 29.9% | 22.6% | 27.0% | 23.2% | 21.6% | 25.0% | 30.5% | 32.1% | 28.9% |
| White American | 86.3% | 87.1% | 88.0% | 88.1% | 86.9% | 86.8% | 77.7% | 82.5% | 86.4% | 81.9% |
| Black American | 10.7% | 9.30% | 9.40% | 8.80% | 11.6% | 10.3% | 17.9% | 14.8% | 11.0% | 14.1% |
| Other Race | 1.50% | 1.80% | 1.40% | 1.90% | 0.80% | 1.30% | 2.00% | 0.50% | 0.80% | 3.30% |
| Race-Not Available | 1.50% | 1.70% | 1.20% | 1.20% | 0.70% | 1.60% | 2.40% | 2.20% | 1.90% | 0.70% |
| ADI National Rank | 57.0 (23.8) | 52.3 | 57.4 | 56.9 | 60.7 | 62.7 | 67.1 | 55.9 | 61.1 | 58.7 |
| ADI-Not Available | 0.30% | 0.50% | 0.50% | 0.20% | 0.30% | 0.10% | 0.00% | 0.20% | 0.00% | 0.00% |
| MS Clinical Course | ||||||||||
| Relapsing-Remitting | 56.1% | 66.1% | 69.5% | 56.8% | 64.6% | 64.3% | 39.7% | 16.8% | 17.8% | 15.6% |
| Progressive | 24.8% | 7.30% | 14.9% | 14.7% | 18.3% | 18.9% | 46.1% | 76.5% | 70.8% | 76.1% |
| CIS | 9.00% | 17.7% | 6.50% | 15.6% | 3.30% | 3.30% | 2.30% | 0.90% | 2.20% | 0.60% |
| Other | 3.50% | 3.30% | 2.70% | 5.90% | 3.60% | 4.70% | 4.20% | 1.40% | 3.60% | 3.90% |
| Not Available | 6.60% | 5.80% | 6.40% | 7.0% | 10.2% | 8.80% | 7.70% | 4.4% | 5.6% | 3.80% |
| Symptoms and Functioning | ||||||||||
| Timed 25-Foot Walk | 7.31 (6.02) | 5.05 | 5.58 | 6.31 | 7.06 | 8.06 | 12.2 | 17.7 | 16.2 | 14.4 |
| Timed 25-Not Available | 47.6% | 38.1% | 48.6% | 37.4% | 47.5% | 47.3% | 65.1% | 59.1% | 71.9% | 68.0% |
| 9-Hole Peg Test | 25.2 (10.8) | 21.0 | 23.5 | 23.6 | 25.1 | 27.0 | 38.6 | 32.3 | 42.1 | 36.6 |
| 9-Hole-Not Available | 66.3% | 61.8% | 66.1% | 64.3% | 68.0% | 66.7% | 73.8% | 68.9% | 76.2% | 77.3% |
| EQ5D | 0.71 (0.21) | 0.88 | 0.75 | 0.69 | 0.63 | 0.53 | 0.34 | 0.68 | 0.49 | 0.53 |
| EQ5D-Not Available | 2.70% | 1.90% | 0.90% | 1.20% | 2.80% | 3.10% | 9.30% | 4.50% | 6.00% | 5.10% |
| PHQ-9 | 7.65 (6.34) | 2.50 | 7.80 | 7.51 | 12.7 | 14.9 | 19.1 | 3.89 | 9.17 | 11.9 |
| PHQ-9-Not Available | 18.3% | 16.5% | 20.1% | 13.7% | 19.3% | 17.0% | 19.8% | 24.2% | 18.5% | 20.2% |
| Health Conditions | ||||||||||
| Hypertension | 9.00% | 6.80% | 7.60% | 7.80% | 12.0% | 10.3% | 10.0% | 16.0% | 10.7% | 7.30% |
| Tobacco - Current | 16.6% | 10.2% | 16.1% | 18.2% | 22.7% | 31.4% | 26.6% | 12.8% | 16.2% | 11.6% |
| Tobacco - Former | 26.5% | 22.6% | 27.1% | 27.9% | 30.0% | 25.3% | 27.4% | 32.8% | 25.9% | 34.4% |
| Tobacco - Never | 41.5% | 51.6% | 41.9% | 40.4% | 35.5% | 28.7% | 24.4% | 39.9% | 39.2% | 32.0% |
| Tobacco - Not Available | 15.4% | 15.6% | 14.9% | 13.5% | 11.8% | 14.6% | 21.6% | 14.6% | 18.7% | 22.0% |
Note: Percentages are reported for dichotomous variables and means are reported for continuous variables. For the overall sample, standard deviations are also reported for continuous variables. ADI=Area deprivation index; MS=Multiple sclerosis; CIS=Clinically isolated syndrome.
Demographic differences were observed across clusters, though they were generally less pronounced than those seen for clinical variables. No strong sex difference patterns were evident (males ranged from 21.6% to 32.1% across all clusters). The most impaired cluster across all PROs (Cluster 6) had the highest proportion of patients who were Black (17.9%). High mobility impairment clusters generally had slightly higher proportions of Black patients, but remained within a 10% range compared to the clusters associated with low mobility impairment (Clusters 1–4). Clusters with higher levels of mobility impairment were also generally associated with higher ADI index scores. As a sensitivity analysis, we evaluated several correlates of cluster membership using propensity score matching, where clusters were conditioned on years since onset and type of MS. Results remained stable (see Supplementary Table S13), providing evidence of the robustness of our results.
Discussion
Using LPA, we found nine MS subtypes based on PROs. Within these nine subtypes, we found three high-level groupings based on mobility, where participants experienced low, moderate, and high levels of mobility impairment. Approximately 70% of participants were classified in a low mobility impairment group (Clusters 1–4), 10% in the moderate mobility impairment group (Cluster 5), and 20% in a high mobility impairment group (Clusters 6–9). These subgroups complement traditional clinical classifications,31 where they provide a greater breadth of information. Among the nine MS subtypes, notable variability was observed in traditional MS classification and assessment, hypertension and smoking status, and demographic distributions. These findings provide novel clinical characterizations that are relevant for clinical care, where clusters are anchored in mobility but also reflect alternative but common presentations (e.g., proportion of patients who express primary problems with fatigue or sensory symptoms in the absence of mobility impairment). The clusters we identified can also inform future precision medicine approaches, through linkage to differential symptom progression trajectories and response to specific interventions.
When examining subgroups associated with low mobility impairment, several patterns were notable. The largest group (Cluster 1, 31.7% of sample) experienced relatively low impairment across all PRO domains. Other clusters associated with low mobility impairment, often marked by moderate to high elevations in several specific symptoms, including fatigue (Clusters 2, 3, and 4), sensory symptoms (Cluster 3), and pain (Clusters 3 and 4). The second-largest cluster (Cluster 2, 18.8% of sample) showed low mobility impairment and moderate elevations in fatigue and pain, providing a common symptomatic phenotype not distinguished by movement impairments. Among participants with higher mobility impairment, a substantial proportion had self-reported impairment largely confined to mobility. Specifically, Cluster 7 represented 8.3% of the sample, which was nearly double any other clusters associated with high mobility impairment. Patients in Cluster 7 has limited elevations on other MS symptoms, and they reported relatively high QOL. In contrast, a smaller group (Cluster 9, 3.9% of sample) reported substantial symptoms across all domains. Except for Cluster 1, all clusters showed at least moderate levels of fatigue, and a higher proportion of patients were members of a subgroup that showed elevations in fatigue compared to elevations in mobility impairment.
Connecting outside factors to PRO-based clusters revealed additional insights. Regarding clinical course, higher rates of relapsing-remitting MS occurred in clusters with lower mobility impairment, while progressive MS was more prevalent in subgroups with higher mobility impairment. Clinical test results (T25FW, 9HPT) aligned with expected patterns,32 showing lower performance in subgroups with higher mobility impairment. Time since symptom onset was also longer in clusters associated with greater mobility problems. These findings underscore the consistency of PROs with widely-used clinical measures33 and supports their ability to serve as an easily administered proxy for more labor intensive clinical assessments. Demographically, higher rates of Black patients were observed in subgroups with greater mobility impairment (11.0–17.9% Black patients in Clusters 6–9, vs. 8.8–11.6% in Clusters 1–4). The proportion of male patients was relatively constant across groups, with slightly lower proportions of males in Cluster 5 (moderate mobility impairment). While hypertension rates were elevated in two of the high mobility impairment groups, no single group had an overall high proportion of participants with hypertension. Tobacco use varied across groups but did not show a consistent pattern related to mobility-related impairment or other PROs. While QOL was usually lower in the high mobility impairment groups Cluster 7 (EQ5D score=0.68) reported QOL levels consistent with the low mobility impairment clusters despite high mobility impairment. Conversely, the moderate mobility Cluster 5 (EQ5D score=0.53) mirrored EQ5D averages in more impaired Clusters 8 and 9. These findings suggest that while mobility consistently relates to QOL, it is not the single predominant factor. Certain PwMS can experience relatively high QOL despite significant mobility impairments.
Several limitations are noted. First, participants were drawn from a single healthcare system, potentially limiting population-based inference. However, data were drawn from an international leader and referral center for MS which encompasses a diverse array of MS phenotypes in its database. As part of this large database, patients were drawn from different ages and stages of disease. While these variables could have influenced class formation, we found multiple classes with similar mean ages and years since diagnosis, suggesting that these factors are not a primarily sole influence on results. Second, while our retrospective sample was comprehensive, prospective research would benefit from increased inclusion of participants across diverse demographic groups. Clusters with the highest levels of across-the-board impairment (Cluster 6) showed higher proportions of Black patients in higher-ADI locations. Finally, varying amounts of missing data were present in our sample. While our main analysis addressed missing data using gold-standard methodology (full-information maximum likelihood estimation),29 secondary analyses considering relevant covariates might be affected by this issue.
We found notable heterogeneity in PwMS, both within the total sample and within groupings based on mobility. While impairments in patient-reported mobility can indicate poorer functioning in other domains,34 we found a surprising amount of cases where this relationship did not hold. For example, nearly half of patients in the high mobility impairment clusters showed relatively low impairment on other PRO domains. Further, fatigue was a consistent symptom across nearly all identified patient clusters and it may serve as a particularly consistent marker of MS. Through our patient-centered approach, PROs corresponded to traditional clinical measures and provided unique information that complements them.
Supplementary Material
Acknowledgments
We thank Mr. Nicolas Thompson and Mr. Scott Husak for their assistance with data curation and management.
Funding Statement
This work was supported by the NIH/NINR (Grant No. R56NR019306) to PIs De Nadai, Gunzler, and Briggs, NIH/NIDA (Grant No. R01DA058315) to PI De Nadai, and NIH/NIMH (Grant No. R01MH137075) to PI De Nadai.
Author Disclosures
The institution of Dr. De Nadai has received research support from NIH.
The institution of Dr. Miller has received research support from NIH and NMSS.
Dr. Ontaneda has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Novartis. Dr. Ontaneda has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Genentech/Roche. Dr. Ontaneda has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Biogen Idec. Dr. Ontaneda has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Janssen. Dr. Ontaneda has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Genentech/Roche. The institution of Dr. Ontaneda has received research support from NIH, PCORI, NMSS, and Genentech.
Dr. Conway has received research support paid to his institution by Biogen, Bristol Myers Squibb, EMD Serono, Horizon Therapeutics, Novartis, and the Department of Defense. He has received consulting fees from Alexion (range $500-$4,999), Bristol Myers Squibb (range $5,000-$9,999), Novartis (range $500-$4,999), and TG Therapeutics (range $500-$4,999), and speaking fees from Biogen (range $10,000-$49,999).
The institution of Dr. Gunzler has received research support from NIH and Michael J. Fox Foundation. Dr. Gunzler reports a book royalty agreement with Taylor and Francis Publishers.
Dr. Briggs has received personal compensation in the range of $10,000-$49,999 for serving as a Consultant for iConquerMS™. Dr. Briggs has received personal compensation in the range of $500-$5,000 for serving as a Consultant for Michael J. Fox Foundation. The institution of Dr. Briggs has received research support from NIH, National MS Society, and Michael J. Fox Foundation.
Footnotes
Conflict of Interest Statement
The authors have no conflicts to report.
References
- 1.Briggs FB, Hill E. Estimating the prevalence of multiple sclerosis using 56.6 million electronic health records from the United States. Mult Scler 2020; 26(14), 1948–1952. [DOI] [PubMed] [Google Scholar]
- 2.Gil-González I, Martín-Rodríguez A, Conrad R, et al. Quality of life in adults with multiple sclerosis: A systematic review. BMJ Open 2020; 10(11): e041249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartung DM. Economics and cost-effectiveness of multiple sclerosis therapies in the USA. Neurotherapeutics 2017; 14(4): 1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matejčíková Z, Mareš J, Přikrylová Vranová H, et al. Cerebrospinal fluid inflammatory markers in patients with multiple sclerosis: a pilot study. J Neural Transm 2015; 122(2): 273–7. [DOI] [PubMed] [Google Scholar]
- 5.Dobson R, Chico D, Evans R, et al. 120 The impact of socioeconomic status and comorbidities on emergency admissions in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2022; 93(6): A138–A. [Google Scholar]
- 6.Meyer-Moock S, Feng YS, Maeurer M, et al. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol 2014; 14: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Amico E, Haase R, and Ziemssen T. Review: Patient-reported outcomes in multiple sclerosis care. Mult Scler Relat Disord 2019; 33: 61–6. [DOI] [PubMed] [Google Scholar]
- 8.Noonan VK, Lyddiatt A, Ware P, et al. Montreal Accord on Patient-Reported Outcomes (PROs) use series - Paper 3: patient-reported outcomes can facilitate shared decision-making and guide self-management. J Clin Epidemiol 2017; 89: 125–35. [DOI] [PubMed] [Google Scholar]
- 9.Nickerson M and Marrie RA. The multiple sclerosis relapse experience: patient-reported outcomes from the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry. BMC Neurology. 2013; 13(1): 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai JS, Jensen SE, Charrow J, et al. Patient reported outcomes measurement information system and quality of life in neurological disorders measurement system to evaluate quality of life for children and adolescents with neurofibromatosis type 1 associated plexiform neurofibroma. J Pediatr 2019; 206: 190–6. [DOI] [PubMed] [Google Scholar]
- 11.Carlozzi NE, Ready RE, Frank S, et al. Patient-reported outcomes in Huntington’s disease: Quality of life in neurological disorders (Neuro-QoL) and Huntington’s disease health-related quality of life (HDQLIFE) physical function measures. Mov Disord 2017; 32(7): 1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappalardo F, Russo G, Pennisi M, et al. The potential of computational modeling to predict disease course and treatment response in patients with relapsing multiple sclerosis. Cells 2020; 9(3): 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosseini A, Asadi F, Arani LA. Development of a knowledge-based clinical decision support system for multiple sclerosis diagnosis. J Med Life. 2020; 13(4): 612–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Mahony J, Salter A, Ciftci B, et al. Physical and mental health-related quality of life trajectories among people with multiple sclerosis. Neurology 2022; 99(14): e1538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briggs FBS, Conway DS, De Nadai AS, Ontaneda D, Gunzler DD. Integrating patient-reported outcomes and quantitative timed tasks to identify relapsing remitting multiple sclerosis patient subgroups: a latent profile analysis. Mult Scler Relat Disord 2021; 51: 102912. [DOI] [PubMed] [Google Scholar]
- 16.Katzan I, Speck M, Dopler C, et al. The Knowledge Program: An innovative, comprehensive electronic data capture system and warehouse. AMIA Annu Symp Proc 2011; 2011: 683–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Chamot E, Kister I and Cutter GR. Item response theory-based measure of global disability in multiple sclerosis derived from the Performance Scales and related items. BMC Neurol 2014; 14: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrie RA and Goldman M. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler 2007; 13(9): 1176–82. [DOI] [PubMed] [Google Scholar]
- 19.Marrie RA and Goldman M. Validation of the NARCOMS Registry: Tremor and Coordination Scale. Int J MS Care 2011; 13(3): 114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabin R and de Charro F. EQ-5D: A measure of health status from the EuroQol Group. Ann Med 2001; 33(5): 337–43. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL and Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9): 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kind AJH and Buckingham WR. Making neighborhood-disadvantage metrics accessible - The Neighborhood Atlas. N Engl J Med 2018; 378(26): 2456–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kass RE and Raftery AE. Bayes Factors. J Am Stat Assoc 1995; 90(430): 773–95. [Google Scholar]
- 24.Lo Y, Mendell NR and Rubin DB. Testing the number of components in a normal mixture. Biometrika 2001; 88(3): 767–78. [Google Scholar]
- 25.Vermunt JK. The Vuong-Lo-Mendell-Rubin Test for latent class and latent profile analysis: A note on the different implementations in Mplus and latentGOLD. Methodology. 2024;20(1):72–83. [Google Scholar]
- 26.Masyn KE. Latent class analysis and finite mixture modeling. In: The Oxford handbook of quantitative methods: Statistical analysis, Vol 2. Oxford library of psychology. New York, NY, US: Oxford University Press; 2013, pp. 551–611. [Google Scholar]
- 27.Asparouhov T and Muthén B Variable-specific entropy contribution. https://www.statmodel.com/download/UnivariateEntropy.pdf. 2014.
- 28.Clark S and Muthén B. Relating latent class analysis results to variables not included in the analysis. 2009.
- 29.Muthén B and Muthén L. Mplus. 1st ed. CRC Press; 2018. p, 507–18. [Google Scholar]
- 30.Bakk Z and Vermunt JK. Robustness of stepwise latent class modeling with continuous distal outcomes. Struct Equ Model 2016; 23: 20–31. [Google Scholar]
- 31.Klineova S and Lublin FD. Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med 2018; 8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motl RW, Cohen JA, Benedict R, et al. Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Mult Scler 2017; 23(5): 704–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hulst HE, Thompson AJ and Geurts JJ. The measure tells the tale: Clinical outcome measures in multiple sclerosis. Mult Scler 2017; 23(5): 626–7. [DOI] [PubMed] [Google Scholar]
- 34.Thrue C, Riemenschneider M, Hvid L, et al. Time matters: Early-phase multiple sclerosis is accompanied by considerable impairments across multiple domains. Mult Scler 2021; 27(10): 1477–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


