Abstract
Based on the experimentally reported stable and conductive two-dimensional covalent organic frameworks with copper phthalocyanine (CuPc) as building block and cyan substituted phenyl as connector (CuCOF-CN) as an electrocatalyst for CO2 reduction reaction (RR), first principle calculations were performed on CuCOF-CN and its analog with the CN being replaced by H (CuCOF). Comparatively studied on the crystal structures, electronic properties, and CO2RR performance of the two catalysts found that CuCOF has reduced crystal unit size, more positive charge on Cu and CuPc segments, smaller band gap, and lower reaction barrier for CO2 RR than CuCOF-CN. CuCOF is proposed to be good potential electrocatalyst with good environment friendliness. The substituent effect and structure-property-performance relationship would help for designing and fabricating new electrocatalysts.
Keywords: COF, CO2 reduction reaction, Copper phthalocyanine, Electrocatalyst, First principle calculation
Subject terms: Coordination chemistry, Materials chemistry, Physical chemistry, Theoretical chemistry
Introduction
Increasing energy crisis and global warming problems from carbon dioxide (CO2) emission have been caused by the utilization of non-renewable fossil fuels and mankind’s living requirements1. Reducing the CO2 concentration in the atmosphere therefore is highly desirable by transferring CO2 to other sustainable energies2–6. The thermodynamically stable molecule CO2 is highly inert and the electrochemical CO2 reduction (CO2RR) process is kinetically sluggish requiring very high potential (−1.90V vs.NHE)7,8 Many different kind of materials have been developed to seek highly efficient, selective and stable electrocatalyst that can reduce the activation energy barrier and expedite the kinetics of CO2RR9–11. Cheap transition metal copper-based catalysts had many advantages and were widely developed12–17.
Among the catalysts for CO2 RR, N4-macrocyclic complexes based molecular electrocatalysts including metal porphyrins, phthalocyanines and their related derivatives have emerged as the promising candidates18–22. To solve the problem of the relatively high over-potential due to the poor conductivity and sluggish electron transfer22, efforts have been tried to either coupling metal phthalocyanines or porphyrins with high conductive carbon nanomaterials or designing highly conjugated system20,22,23. A number of two-dimensional (2D) conductive covalent-organic frameworks (COFs) with extensive planar π-conjugation have been shown high electrical conductivity and developed for electrocatalysts24–27.
Series of metal phthalocyanine based 2D-COFs have been developed recently. Liao et al. reported a stable and conductive 2D-COF with copper phthalocyanine as building block and cyan substituted phenyl as connector (CuCOF-CN) in 202228. CuCOF-CN show good electrocatalytic performance for CO2 RR to acetate with a single-product Faradaic efficiency (FE) of 90.3(2)% at − 0.8 V (vs. RHE) and a current density of 12.5 mA cm−2 in 0.1 M KHCO3solution. They also performed theoretical calculation to explain why CuCOF-CN show different catalysis property from other Cu-based catalyst including copper porphyrin and single-atom copper catalyst. However, the synthesis of CuCOF-CN was time-costing and not economy (it was synthesized by a condensation reaction at 150 ℃ for 3 days under vacuum). The crystal structures of the CuCOF-CN was also not discussed. In addition, the phenyl connector contain two -CN group, which is extremely toxic substance and has great harm to the environment and to human beings. Jiang et al. developed a solvent-free, facile, and fast synthesis strategy for fabricating two fully conjugated Pc-based COFs under ionothermal synthesis for 8 h29. One of the COF reported by Jiang et al. show very similar structure as CuCOF-CN reported by Liao et al.28, with the center metal of CuCOF-CN being changed to zinc and the CN substituents replaced with H (ZnCOF). Despite that the zinc center was not good candidate for catalyst, these COFs show high-performance K+storage in potassium-ion batteries in terms of the large reversible capacities, excellent rate performance, and long-term cycling stability. Recently and during the preparation of the present work, Chen et al. further improved the synthesis strategy of 2D-COF and synthesized series metal (M = Fe, Co, Ni, Cu, Zn) phthalocyanine based COFs (MCOFs) by a facile trace-solvent-assisted one-pot self-condensation method30. Among these MCOFs, NiCOF show excellent sensing properties for various analytes including neurotransmitters, organic pollutants, and heavy metal ions, with high sensitivity and low detection limit of 0.53 to 25.66 nM. However, the catalysis performance of these MCOFs were not reported though CuCOF-CN show good CO2 RR performance. The influence of CN substitution on crystal structure, electronic properties, and catalytic performance were not clear. Clarifying these questions with a theoretical prospective work would therefore have great significance.
To further clarify the structures and rule of CN on the CO2 reduction performance, in the present work, we comparatively studied the crystal structures, electronic properties, and CO2 RR performance of CuCOF-CN and CuCOF through first principle calculations. The substituent effect and structure-property-performance relationship would help for designing and fabricating new electrocatalysts.
Computational methods
The initial crystal structures of CuCOF and CuCOF-CN were built with Device Studio program31. Device Studio program provides a number of functions for performing visualization, modeling and simulation. The first principle simulation were performed using DS-PAW software32integrated in Device Studio program. The PBE exchange-correlation functional33and D3BJ Van der Waals correction34 were chosen.
Results and discussion
Structures
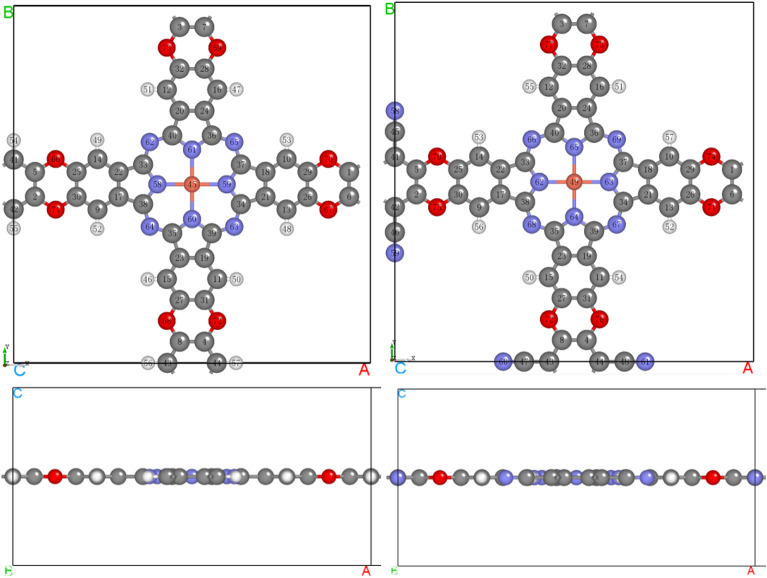
Figure 1 shows the optimized crystal structures of CuCOF and CuCOF-CN. Their main structure parameters are tabulated in Table 1. Both CuCOF and CuCOF-CN are 2D planar layer structure without any deviation from the 2D plane. The crystals are both P4/mmm symmetry with a = b and α = β = γ = 90.0º. To avoid interactions between layers, the c-axis are set to 10.000 Å after involving the vacumm layer. The a and b values of CuCOF-CN is somewhat longer than those of CuCOF (20.197 vs. 20.192 Å), indicating that the substitution of H on the phenyl connector in CuCOF with electron-withdrawing CN group slightly enlarge the cell size of the COF crystals. More detail, the Cu-N bonds and the width of phenyl ring of phthalocyanine and the neighbour C4O2 ring all reduce, while the pyrrole ring of Pc and phenyl connector enlarge largely. In addition, CN substitution also induces the increase of height of phenyl connector. The different changes suggest that the electron density in the COF is redistributed due to the involving of electron-withdrawing CN groups in CuCOF-CN, which in turn would induce their different electrocatalytic performance.
Fig. 1.
Top (top) and side view (bottom) of the optimized crystal structures of CuCOF (left) and CuCOF-CN (right). (Red: O, gray: C, blue: N, white: H, coral: Cu).
Table 1.
Main structure parameters of the optimized crystal of CuCOF and CuCOF-CN (length in Å, angle in º).
| Parameter | CuCOF | CuCOF-CN |
|---|---|---|
| Symmetry group | P4/mmm | P4/mmm |
| a | 20.192 | 20.197 |
| b | 20.192 | 20.197 |
| c | 10.000 | 10.000 |
| α | 90.0 | 90.0 |
| β | 90.0 | 90.0 |
| γ | 90.0 | 90.0 |
| Cu-N | 1.963 | 1.961 |
| Width(Py) | 2.195 | 2.197 |
| Width(Ph of Pc) | 2.381 | 2.373 |
| Width(C4O2) | 2.356 | 2.351 |
| Width(Ph connector) | 2.403 | 2.432 |
| Height(Ph connector) | 2.799 | 2.815 |
Bader charge
The introduction of electron-withdrawing CN groups would induce the redistribution of electrons. Table 2 shows the calculated Bader charge on each atom of CuCOF and CuCOF-CN as well as the change of Bader charge upon CN substitution. The H atoms of phenyl connector in CuCOF have Bader charge of 0.112 e, while the CN substituents in CuCOF-CN have Bader charge of −0.435 e. The Bader charge on C connected with H or CN changes from negative − 0.179 in CuCOF to positive 1.272 e in CuCOF-CN. The phenyl connector (in a or b direction) and its neighbour four O atoms in CuCOF totally contain Bader charge of −2.068 e. Since there are two connector in each crystal cell and the crystal is neutral, the remaining CuPc segment in CuCOF thus has Bader charge of 4.136 e. For CuCOF-CN, the Bader charge on each connector and CuPc segment increases to −2.452 and 4.904 e, respectively. These results indicate that electron transfer from CuPc segment to the connector and CN substitution on connector increases the electron transfer extent. Considering the Barder charge on Cu atom, Cu in CuCOF however has larger value than that in CuCOF-CN, 1.101 vs. 0.913 e. Large charge on Cu is advantage for CO2 RR reaction.
Table 2.
Calculated bader charge (in e) on each atom of CuCOF and CuCOF-CN (see Fig. 1 for atomic label).
| CuCOF | CuCOF-CN | Change of bader charge | ||||
|---|---|---|---|---|---|---|
| Atomic number | Element | Bader charge | Atomic number | Element | Bader charge | |
| 1 | C | 0.59784 | 1 | C | −0.34203 | −0.93987 |
| 2 | C | 0.59784 | 2 | C | −0.33591 | −0.93375 |
| 3 | C | 0.3899 | 3 | C | −0.34191 | −0.73181 |
| 4 | C | 0.38819 | 4 | C | −0.33602 | −0.72421 |
| 5 | C | 0.38819 | 5 | C | 0.67422 | 0.28603 |
| 6 | C | 0.3899 | 6 | C | 0.67433 | 0.28443 |
| 7 | C | 0.59785 | 7 | C | 0.67422 | 0.07637 |
| 8 | C | 0.59785 | 8 | C | 0.67433 | 0.07648 |
| 9 | C | 0.00882 | 9 | C | 0.64228 | 0.63346 |
| 10 | C | 0.00882 | 10 | C | 0.64225 | 0.63343 |
| 11 | C | 0.00882 | 11 | C | 0.64223 | 0.63341 |
| 12 | C | 0.00882 | 12 | C | 0.64228 | 0.63346 |
| 13 | C | 0.00882 | 13 | C | 0.64227 | 0.63345 |
| 14 | C | 0.00882 | 14 | C | 0.64223 | 0.63341 |
| 15 | C | 0.00882 | 15 | C | 0.64223 | 0.63341 |
| 16 | C | 0.00882 | 16 | C | 0.64226 | 0.63344 |
| 17 | C | −0.10487 | 17 | C | −1.00724 | −0.90237 |
| 18 | C | −0.10487 | 18 | C | −0.09079 | 0.01408 |
| 19 | C | −0.10487 | 19 | C | −0.09079 | 0.01408 |
| 20 | C | 0.10195 | 20 | C | −1.00724 | −1.10919 |
| 21 | C | 0.10196 | 21 | C | −1.00724 | −1.1092 |
| 22 | C | 0.10196 | 22 | C | −0.09079 | −0.19275 |
| 23 | C | 0.10196 | 23 | C | −1.00724 | −1.1092 |
| 24 | C | −0.10487 | 24 | C | −0.09079 | 0.01408 |
| 25 | C | 0.59277 | 25 | C | −0.38101 | −0.97378 |
| 26 | C | 0.39143 | 26 | C | −0.38116 | −0.77259 |
| 27 | C | 0.59277 | 27 | C | −0.38116 | −0.97393 |
| 28 | C | 0.39143 | 28 | C | −0.38101 | −0.77244 |
| 29 | C | 0.59278 | 29 | C | 0.58038 | −0.0124 |
| 30 | C | 0.39144 | 30 | C | 0.58022 | 0.18878 |
| 31 | C | 0.39144 | 31 | C | 0.58037 | 0.18893 |
| 32 | C | 0.59278 | 32 | C | 0.58022 | −0.01256 |
| 33 | C | 0.99682 | 33 | C | 1.37397 | 0.37715 |
| 34 | C | 0.99682 | 34 | C | 1.37433 | 0.37751 |
| 35 | C | 0.99682 | 35 | C | 1.37397 | 0.37715 |
| 36 | C | 0.99682 | 36 | C | 1.37397 | 0.37715 |
| 37 | C | 0.99682 | 37 | C | 1.37436 | 0.37754 |
| 38 | C | 0.99682 | 38 | C | 1.37436 | 0.37754 |
| 39 | C | 0.99682 | 39 | C | 1.374 | 0.37718 |
| 40 | C | 0.99682 | 40 | C | 1.37436 | 0.37754 |
| 41 | C | −0.01794 | 41 | C | 1.2717 | 1.28964 |
| 42 | C | −0.01794 | 42 | C | 1.2717 | 1.28964 |
| 43 | C | −0.01793 | 43 | C | 1.27171 | 1.28964 |
| 44 | C | −0.01794 | 44 | C | 1.2717 | 1.28964 |
| 45 | Cu | 1.1009 | 49 | Cu | 0.9131 | −0.1878 |
| 46 | H | 0.070138 | 50 | H | 0.356329 | 0.286191 |
| 47 | H | 0.07014 | 51 | H | 0.356392 | 0.286252 |
| 48 | H | 0.070142 | 52 | H | 0.356217 | 0.286075 |
| 49 | H | 0.070137 | 53 | H | 0.356631 | 0.286494 |
| 50 | H | 0.070138 | 54 | H | 0.356769 | 0.286631 |
| 51 | H | 0.070143 | 55 | H | 0.356355 | 0.286212 |
| 52 | H | 0.070143 | 56 | H | 0.356657 | 0.286514 |
| 53 | H | 0.070141 | 57 | H | 0.356594 | 0.286453 |
| 58 | N | −1.13474 | 62 | N | −1.35308 | −0.21834 |
| 59 | N | −1.13474 | 63 | N | −1.35302 | −0.21828 |
| 60 | −1.13474 | 64 | N | −1.35304 | −0.2183 | |
| 61 | N | −1.13474 | 65 | N | −1.35295 | −0.21821 |
| 62 | −1.23937 | 66 | N | −1.49621 | −0.25684 | |
| 63 | N | −1.23937 | 67 | N | −1.49621 | −0.25684 |
| 64 | −1.23937 | 68 | N | −1.49621 | −0.25684 | |
| 65 | N | −1.23937 | 69 | N | −1.49621 | −0.25684 |
| 66 | O | −1.05733 | 70 | O | −1.19894 | −0.14161 |
| 67 | O | −1.05733 | 71 | O | −1.19894 | −0.14161 |
| 68 | O | −1.05733 | 72 | O | −1.19894 | −0.14161 |
| 69 | O | −1.05733 | 73 | O | −1.19894 | −0.14161 |
| 70 | O | −1.05733 | 74 | O | −1.19894 | −0.14161 |
| 71 | O | −1.05733 | 75 | O | −1.19894 | −0.14161 |
| 72 | O | −1.05733 | 76 | O | −1.19894 | −0.14161 |
| 73 | O | −1.05733 | 77 | O | −1.19894 | −0.14161 |
| 54 | H | 0.111745 | 45 | C | 0.7974 | |
| 55 | H | 0.111748 | 46 | C | 0.7974 | |
| 56 | H | 0.111757 | 47 | C | 0.7974 | |
| 57 | H | 0.111753 | 48 | C | 0.7974 | |
| 78 | 58 | −1.2327 | ||||
| 79 | 59 | N | −1.23243 | |||
| 80 | 60 | N | −1.23239 | |||
| 81 | 61 | N | −1.23252 | |||
Band structure
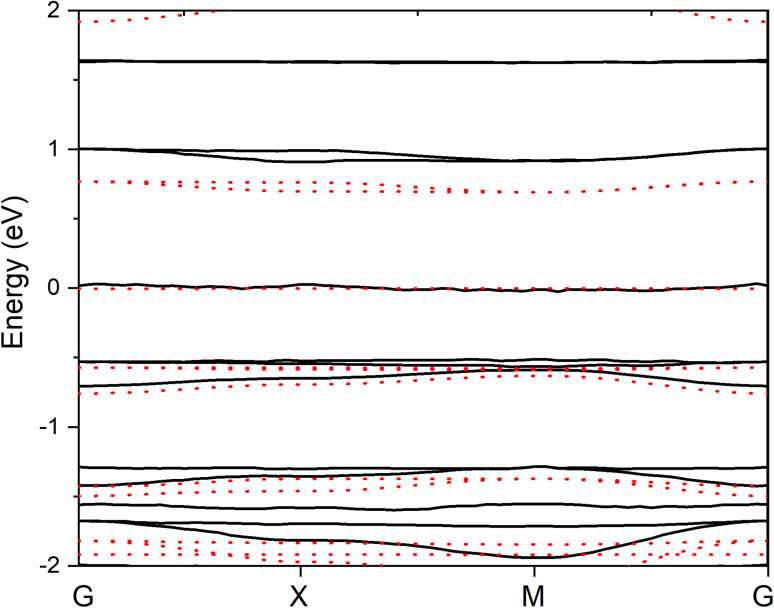
Figure 2 shows the calculated projected band structure of CuCOF and CuCOF-CN between − 2 and 2 eV. The calculated Fermi energies of CuCOF and CuCOF-CN are − 3.04 and − 3.40 eV, respectively. The lower Fermi energy upon electron-withdrawing CN substitution is consistent with the common knowledge that electron-withdrawing groups would lower the orbital energy for molecule. Worth noting that when aligning the Fermi energy of both CuCOF and CuCOF-CN to 0 eV, the bands of CuCOF-CN appear above the corresponding bands of CuCOF. For CuCOF, the band around 0 eV is mainly composed from the p orbital of N atoms and d orbital of Cu. The band around − 0.5 eV of CuCOF including the p orbital of both C and O atoms, while that below it is mainly from C p orbital. Band around − 1.5 eV have large contribution from O p orbital in addition to major content from C p orbital. The bands around − 1.7 eV are composed from p orbital of both C and N. Similarly, the conduction band around 0.7 eV are also composed from p orbital of both C and N atoms. The shape and composition of band structures of CuCOF-CN are very similar to those of CuCOF. Some new bands appear around 1.6 eV in CuCOF-CN, which are composed of p orbitals of C and N and may due to the contribution of the CN substituents. The band gap from conducting band bottom to valence band top for CuCOF is 0.69 eV, much smaller than the gap for CuCOF-CN (0.88 eV), indicating that the conductivity of CuCOF is better than CuCOF-CN. Good conductivity is advantage for improving the electrocatalytic performance.
Fig. 2.
Projected band structure of CuCOF (red dot line) and CuCOF-CN (black line).
Density of states
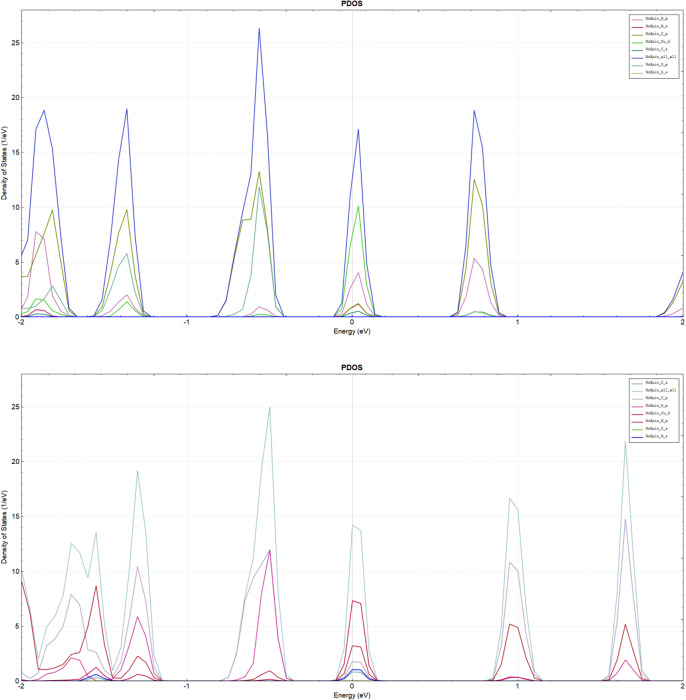
The projected density of states (PDOS) of CuCOF and CuCOF-CN are shown in Fig. 3. In line with the assignments in Band structure section, PDOS peak around 0 eV are mainly contributed from the Cu d orbital and N p orbital, while the other peaks are attributed from the p orbital of C, N, and O atoms. The PDOS peaks of CuCOF are stronger than those of CuCOF-CN, indicating CN substitution reduces the electron density in the range of −2 to 2 eV. Large electron density on CuPc segment is advantage for CO2RR reaction.
Fig. 3.
Projected density of states of CuCOF (top) and CuCOF-CN (bottom).
CO2 RR
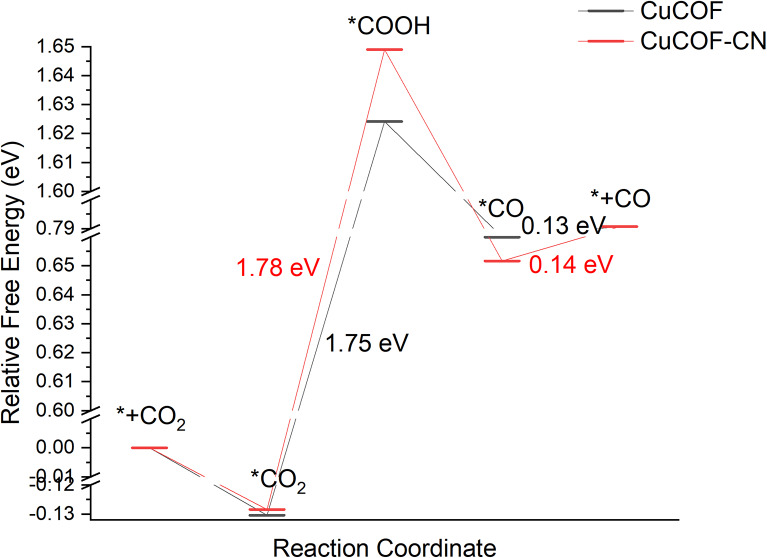
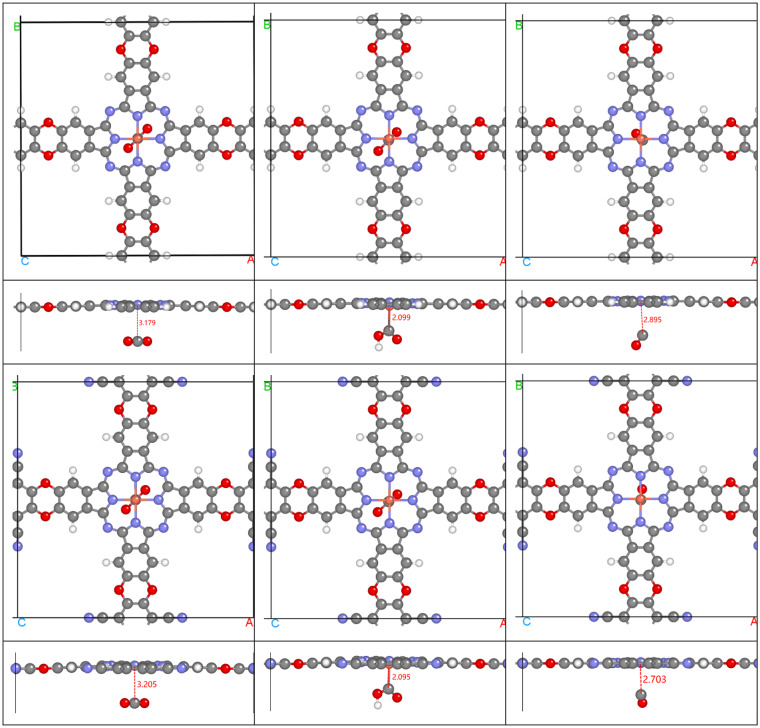
Figure 4 shows the free energy diagram of CO2 RR on CuCOF and CuCOF-CN surface at U = 0 V. The structures of the main intermediates are given in Fig. 5. For both catalysts, the formation of *COOH is the rate determine step, with reaction energy of 1.75 and 1.78 eV for CuCOF and CuCOF-CN, respectively. The smaller reaction energy for CuCOF than CuCOF-CN indicates that changing CN groups to H is advantage for CO2 RR. The desorption of CO need very small energy of only 0.13 and 0.14 eV for CuCOF and CuCOF-CN, respectively. The smaller reaction energy for CO desorption in CuCOF comparing to CuCOF-CN is also advantage for the whole CO2 RR process. The different free energy of the intermediates for CuCOF and CuCOF-CN could be explained from the main structure parameter of the intermediates. The Cu-C distance of *CO2 for CuCOF is shorter than that for CuCOF-CN (Fig. 5), well explaining the better stability of the former than the latter. Similarly, the longer Cu-C distance of *CO for CuCOF than CuCOF-CN reasons the less stable fact for the former than the latter. Though the Cu-C length of *COOH for CuCOF is slightly longer than that of CuCOF-CN, the more positive charge of Cu in the former than the latter render CuCOF-COOH more stable than CuCOF-CN-COOH.
Fig. 4.
The free energy diagram of CO2RR on CuCOF (black) and CuCOF-CN (red) at U = 0 V.
Fig. 5.
Optimized crystal structure of the intermediates (*CO2, left; *COOH, middle; *CO, right; * being the catalyst) during CO2 RR with CuCOF (top two lines for top and side views, respectively) and CuCOF-CN (bottom two lines) as catalyst. (Red: O, gray: C, blue: N, white: H, coral: Cu).
Conclusion
The crystal structures, electronic properties, and CO2RR performance of CuCOF and CuCOF-CN were comparatively studied through first principle calculations. CuCOF was found to have reduced crystal unit size, more positive charge on Cu, smaller band gap, and lower reaction barrier for CO2 RR, in comparing with CuCOF-CN. The good CO2 RR catalytic performance and lower poisonousness for CuCOF than CuCOF-CN renders it good potential electrocatalyst with good environment friendliness.
Acknowledgements
Financial supports from National Natural Science Foundation of China (52460023, 22478366), Natural Science Foundation of Shandong Province (2023TSGC0018), Natural Science Foundation of Jianxi Province (20224BAB213031), and Dezhou University (2023xjrc111) are acknowledged. This work was also supported by funding from Shandong Provincial Key Laboratory of Monocrystalline Silicon Semiconductor Materials and Technology (2023KFKT012). We gratefully acknowledge HZWTECH for providing computation facilities.
Author contributions
Yuexing Zhang conceived the idea, performed the DFT calculations and wrote the original draft. Junhao Peng, Guangsong Zhang, and Xingguo Zhang performed addition DFT calculations, analyzed calculation data, and revised the manuscript. Shuai Zhang, Qing Li, Guanfeng Tian, Xiaoli Wang, and Ping Wu discussed the results and revised the manuscript. Yuexing Zhang and Xue-Li Chen supervised and directed the projects and revised the manuscript. All authors reviewed the manuscript.
Data availability
Data availability The data of this work including the input and output calculation files are available upon requirement from the corresponding authors.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally to this work.
Contributor Information
Yuexing Zhang, Email: zhangyuexing@sdu.edu.cn.
Xue-Li Chen, Email: xueli089@foxmail.com.
References
- 1.Forkel, M. et al. Enhanced seasonal CO2 exchange caused by amplified plant productivity in northern ecosystems. Science. 351, 696–699 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Seneviratne, S. I., Donat, M. G., Pitman, A. J., Knutti, R. & Wilby, R. L. Allowable CO2 emissions based on regional and impact-related climate targets. Nature. 529, 477–483 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Varghese, O. K., Paulose, M., LaTempa, T. J. & Grimes, C. A. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett.9, 731–737 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Liu, B. et al. Phosphorus-Doped Graphitic Carbon Nitride nanotubes with amino-rich surface for efficient CO2 capture, enhanced photocatalytic activity, and product selectivity. ACS Appl. Mater. Interfaces. 10 (4), 4001–4009 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Wang, W. H., Himeda, Y., Muckerman, J. T., Manbeck, G. F. & Fujita, E. CO 2 hydrogenation to formate and methanol as an alternative to photo- and Electrochemical CO 2 reduction. Chem. Rev.115 (23), 12936–12973. 10.1021/acs.chemrev.5b00197 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Wang, T. et al. Central Site Regulation of Cobalt Porphyrin Conjugated polymer to give highly active and selective CO2 reduction to CO in aqueous solution. Appl. Catal. B Environ.291, 120128. 10.1016/j.apcatb.2021.120128 (2021). [Google Scholar]
- 7.Shen, J. et al. Electrocatalytic Reduction of Carbon Dioxide to Carbon Monoxide and methane at an immobilized cobalt protoporphyrin. Nat. Commun.6 (1), 8177. 10.1038/ncomms9177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen, J., Kolb, M. J., Göttle, A. J. & Koper, M. T. M. DFT Study on the mechanism of the Electrochemical Reduction of CO 2 catalyzed by Cobalt Porphyrins. J. Phys. Chem. C. 120 (29), 15714–15721. 10.1021/acs.jpcc.5b10763 (2016). [Google Scholar]
- 9.Wang, Y. R. et al. Oriented Electron Transmission in Polyoxometalate-Metalloporphyrin Organic Framework for highly selective electroreduction of CO2. Nat. Commun.9 (1), 4466. 10.1038/s41467-018-06938-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasileff, A., Zheng, Y. & Qiao, S. Z. Carbon solving Carbon’s problems: recent progress of Nanostructured Carbon-based catalysts for the Electrochemical Reduction of CO 2. Adv. Energy Mater.7 (21). 10.1002/aenm.201700759 (2017).
- 11.Elgrishi, N., Chambers, M. B., Wang, X. & Fontecave, M. Molecular polypyridine-based metal complexes as catalysts for the reduction of CO 2. Chem. Soc. Rev.46 (3), 761–796. 10.1039/C5CS00391A (2017). [DOI] [PubMed] [Google Scholar]
- 12.Nitopi, S. et al. Progress and perspectives of Electrochemical CO 2 reduction on copper in Aqueous Electrolyte. Chem. Rev.119 (12), 7610–7672. 10.1021/acs.chemrev.8b00705 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Wei, X. et al. Highly selective reduction of CO 2 to C 2 + hydrocarbons at Copper/Polyaniline Interfaces. ACS Catal.10 (7), 4103–4111. 10.1021/acscatal.0c00049 (2020). [Google Scholar]
- 14.Ma, W. et al. Electrocatalytic reduction of CO2 to Ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal.3 (6), 478–487. 10.1038/s41929-020-0450-0 (2020). [Google Scholar]
- 15.Chen, D. et al. A Tandem Strategy for Enhancing Electrochemical CO 2 reduction activity of single-atom Cu‐S 1 N 3 catalysts via integration with Cu Nanoclusters. Angew Chemie Int. Ed.60 (45), 24022–24027. 10.1002/anie.202109579 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Yang, B. et al. CO 2 electroreduction to Multicarbon products via Synergistic Electric–Thermal Field on Copper Nanoneedles. J. Am. Chem. Soc.144 (7), 3039–3049. 10.1021/jacs.1c11253 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Creissen, C. E. & Fontecave, M. Keeping Sight of copper in single-atom catalysts for Electrochemical Carbon Dioxide Reduction. Nat. Commun.13 (1), 2280. 10.1038/s41467-022-30027-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han, N. et al. Supported Cobalt Polyphthalocyanine for High-Performance Electrocatalytic CO2 reduction. Chem. 2017, 3 (4), 652–664. 10.1016/j.chempr.2017.08.002
- 19.Wu, Y., Jiang, Z., Lu, X., Liang, Y. & Wang, H. Domino electroreduction of CO2 to methanol on a Molecular Catalyst. Nature. 575 (7784), 639–642. 10.1038/s41586-019-1760-8 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Sun, L. et al. Conjugated N 4 -Macrocyclic Cobalt Complex for Heterogeneous Electrocatalytic CO2 reduction with high activity. Angew Chemie Int. Ed.59 (39), 17104–17109. 10.1002/anie.202007445 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Wang, R., Kapteijn, F. & Gascon, J. Engineering Metal–Organic frameworks for the Electrochemical reduction of CO 2: a Minireview. Chem. – Asian J.14 (20), 3452–3461. 10.1002/asia.201900710 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Wang, J., Huang, X., Xi, S., Xu, H. & Wang, X. Axial Modification of Cobalt Complexes on heterogeneous surface with enhanced Electron transfer for Carbon Dioxide Reduction. Angew Chemie Int. Ed.59 (43), 19162–19167. 10.1002/anie.202008759 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Zhang, X. et al. Highly selective and active CO2 reduction Electrocatalysts based on Cobalt Phthalocyanine/Carbon Nanotube Hybrid structures. Nat. Commun.8 (1), 14675. 10.1038/ncomms14675 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, N. et al. Stable and conductive metallophthalocyanine Framework for Electrocatalytic Carbon Dioxide Reduction in Water. Angew Chemie Int. Ed.59 (38), 16587–16593. 10.1002/anie.202005274 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Lu, M. et al. Stable dioxin-linked Metallophthalocyanine Covalent Organic frameworks (COFs) as photo‐coupled electrocatalysts for CO 2 reduction. Angew Chemie Int. Ed.60 (9), 4864–4871. 10.1002/anie.202011722 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Diercks, C. S., Liu, Y., Cordova, K. E. & Yaghi, O. M. The role of Reticular Chemistry in the design of CO2 reduction catalysts. Nat. Mater.17 (4), 301–307. 10.1038/s41563-018-0033-5 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Diercks, C. S. & Yaghi, O. M. The atom, the Molecule, and the Covalent Organic Framework. Science. 355 (6328), eaal1585. 10.1126/science.aal1585 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Qiu, X. F. et al. A stable and conductive covalent Organic Framework with isolated active sites for highly selective electroreduction of Carbon Dioxide to acetate. Angew. Chemie - Int. Ed.61 (36), e202206470. 10.1002/anie.202206470 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Yang, X. et al. Ionothermal synthesis of fully conjugated covalent Organic frameworks for High-Capacity and Ultrastable Potassium‐Ion batteries. Adv. Mater.34 (50), 2207245. 10.1002/adma.202207245 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Liu, Q. et al. Universal Synthesis of Metallophthalocyanine Covalent Organic frameworks as Ultra-sensitive Multifaceted Electrochemical Sensor. Sci. China Chem.67 (6), 2092–2101. 10.1007/s11426-024-1998-5 (2024). [Google Scholar]
- 31.Hongzhiwei, Technology & China Device Studio, Version 2023A, Available online: (2023). https://cloud.hzwtech.com/web/product-service?id=6
- 32.Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B. 50 (24), 17953 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradi- ent approximation made simple. Phys. Rev. Lett.77, 3865–3868. 10.1103/PhysRevLett.77.3865 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in Dispersion Corrected Density Functional Theory. J. Comput. Chem.32 (7), 1456–1465. 10.1002/jcc.21759 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability The data of this work including the input and output calculation files are available upon requirement from the corresponding authors.