Abstract
The long-term health impacts of niacin are still debated, and the association between dietary niacin and mortality risk in populations hasn’t been extensively explored. This study included 26,746 US adults aged 20 years or older from the National Health and Nutrition Examination Survey 2003–2018, with a median follow-up of 9.17 years. During this period, there were 3,551 all-cause deaths, including 1,096 cardiovascular deaths. Cox models were used to compare hazard ratios (HRs) for mortality among participants grouped into different dietary niacin intake quartiles. Participants with the highest dietary niacin intake had a lower risk of all-cause mortality (HR 0.74, 95%CI 0.63–0.86) compared to those in the lowest intake quartile. For cardiovascular mortality, the HR was 0.73 (95%CI 0.57–0.95) in the highest niacin intake quartile. A dose-response relationship was revealed between dietary niacin intake and mortality by restricted cubic spline. Subgroup analysis showed a significant interaction between dietary niacin intake and diabetes concerning all-cause mortality (P = 0.046). In this population-based cohort study, higher dietary niacin intake correlates with lower risk of all-cause and cardiovascular mortality among US adults. The influence of niacin intake on all-cause mortality appears to be more significant in non-diabetic individuals compared to those with diabetes.
Keywords: Cardiovascular risk, Dietary niacin intake, Mortality, NHANES
Subject terms: Health care, Medical research, Risk factors
Introduction
Niacin, vitamin B3, is a vital water-soluble nutrient crucial for various physiological processes in the human body. Niacin deficiency has been linked to pellagra, a severe condition marked by dermatitis, diarrhea, dementia, and fatality1. Some nations have adopted fortification measures to niacin fortification of wheat flour and cereals, specifically targeting the prevention of pellagra2. And many common foods are rich in niacin, such as beef, pork, chicken, coffee, and tea3,4. Therefore, niacin is prevalent in modern western diets, with many individuals consuming niacin levels far exceeding the recommended dietary allowance, particularly in the US where intake surpasses the recommended dietary allowance by threefold5.
Niacin is one of the earliest medications employed for dyslipidemia6. It was widely utilized, especially prior to the introduction of statins. Niacin can significantly reduce low density lipoprotein cholesterol and triglycerides, and raise high density lipoprotein cholesterol. The cardiovascular benefits of niacin were first demonstrated in the Coronary Drug Program (a classic randomized controlled trial), where niacin modestly reduced cardiovascular events in high-risk populations and reduced mortality at long-term follow-up after the randomized treatment period7,8. However, two randomized controlled trials in recent years have found that niacin does not reduce the risk of cardiovascular events on the basis of statin therapy, even with improvements in lipid levels9,10.And a recent meta-analysis even found that niacin increased the risk of all-cause mortality11. This leads to what’s known as the “niacin paradox”, which highlights the discrepancy between niacin’s favorable lipid profile changes and its failure to reduce cardiovascular events risk. Recent advancement has further complicated the understanding of niacin’s role in cardiovascular health. A study involving over 4300 stable coronary artery disease patients revealed that niacin-derived end products of Nicotinamide adenine dinucleotide (NAD) metabolism, 2PY and 4PY, promote vascular inflammation, thereby increasing the risk of cardiovascular disease12. This underscores the need for continued evaluation of niacin’s impact on cardiovascular disease risk and long-term health outcomes, particularly concerning dietary niacin intake.
Niacin is a key precursor of NAD. NAD plays a crucial role in numerous biological processes and functions, including but not limited to cell aging, cell death, cell metabolism, DNA repair, mitochondrial function, redox reactions, and inflammation13. Recent research has demonstrated that niacin can modulate NAD metabolism, thereby improving the aging process and mitigating related diseases, including neurodegenerative disorders, cardiovascular conditions, cancer, and diabetes14–17.
The intricate impact of niacin on cardiovascular disease risk and long-term health remains contentious, with limited research exploring the association between dietary niacin intake and long-term health outcomes in various populations. The purpose of this study was to assess the association of dietary niacin intake and all-causes and cardiovascular mortality, providing valuable insights into recommended dietary niacin intake for the general population.
Methods
Study population
Study participants aged 20 years or older included in the National Health and Nutrition Examination Survey (NHANES) 2003–2018 were analyzed for this study (n = 44790). Then, participants with missing follow-up information were excluded (n = 1965). Additionally, participants with missing information on dietary niacin intake were excluded (n = 7849). Further, participants with missing covariates were excluded (n = 8230). Finally, total 26,746 participants were included for analysis, with a follow-up period from their survey participation date until December 31, 201920. All open data used in this study are sourced from the official NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). US National Center for Health Statistics Research Ethics Review Board approved NHANES protocol, and each participant signed the informed consent21.
Dietary niacin intake
Since 2003, every NHANES participants have been subject to two 24-hour dietary recall interviews. These interviews are conducted to assess the types and quantities of beverages (including water) and foods consumed in the 24-hour period (midnight to midnight). Detailed niacin content data for various beverages and foods items were sourced from the Food and Nutrient Database for Dietary Studies22. Based on the quartiles of the average intake of dietary niacin over two days, participants were divided into four groups.
Outcome assessment
The study focused on two outcomes: all-cause mortality (deaths from all cause) and cardiovascular mortality (the International Classification of Diseases 10th revision codes I00-I09, I11, I13, I20-I51, and I60-I69). NHANES-linked mortality information have been updated through December 31, 201920.
Covariate assessment
The interview questionnaire provided data on the following variables: age, sex, ethnicity, educational level, smoking (yes, no), alcohol consumption (less than 12 alcohol drinks/year, at least 12 alcohol drinks/year), medical conditions (including hypertension, diabetes, dyslipidemia, cardiovascular disease, and cancer) and medication use. According to standard protocols, the following variables were measured: blood pressure, body mass index (BMI), glycohemoglobin, serum creatinine. The serum creatinine based Chronic Kidney Disease Epidemiology Collaboration equation was employed to calculate the estimated glomerular filtration rate (eGFR)23. The definition of hypertension encompassed a systolic blood pressure (SBP) of ≥ 140 mmHg, or a diastolic blood pressure (DBP) of ≥ 90 mmHg, or a hypertension history, or treatment with antihypertensive medication. The definition of diabetes encompassed a glycohemoglobin level of ≥ 6.5%, a diabetes history, or treatment with anti-diabetes medication.
Statistical analysis
Based on the guidelines provided by the Centers for Disease Control and Prevention regarding the NHANES data (21), The weights utilized in each analysis were tailored to the selected variables. Mean ± standard error (SE) was used to represent continuous variables, with group comparisons analyzed through analysis of variance. Percentages were utilized for categorical variables, with group comparisons assessed using the chi-square test. Cox models were employed to examine the hazard ratios (HRs) and 95% confidence intervals (CIs) of mortality among dietary niacin intake quartiles. Model 1 was adjusted for age, sex, ethnicity, educational level, smoking, alcohol consumption and BMI. Model 2 was further adjusted for disease conditions (including hypertension, diabetes, dyslipidemia, cardiovascular disease, and cancer) and eGFR. Subgroup analyses stratified by age, sex, ethnicity, educational level, smoking, alcohol consumption, BMI, disease conditions and eGFR were conducted. Restricted cubic spline (RCS) analysis with three knots was employed to investigate dose-response relationship between dietary niacin intake and mortality outcome. Statistical analyses were conducted using R version 4.3.2 (R Project for Statistical Computing), with a significance level set at P < 0.05 for all tests.
Results
Baseline characteristics
The study involved 26,746 US adults aged 20 years or older, with a median follow-up of 9.17 years (interquartile range, 6.0–12.5). A total of 3551 deaths from all causes occurred, including 1096 cardiovascular deaths. Baseline information of participants grouped according to dietary niacin intake quartiles were listed in Table 1. Participants with higher dietary niacin intake, as opposed to those with lower intake, were characterized by being younger, more predominantly male and non-Hispanic white, smokers, and drinkers. Additionally, they demonstrated higher education and eGFR levels, along with a lower prevalence of hypertension, diabetes, dyslipidemia, cardiovascular disease, and cancer.
Table 1.
Baseline characteristics of participants according to dietary niacin intake quartiles.
| Characteristic | Total | Dietary niacin intake, mg/day | P value | |||
|---|---|---|---|---|---|---|
| Quintile 1 (< 16.51) |
Quintile 2 (16.51–22.45) |
Quintile 3 (22.46–30.15) |
Quintile 4 (≥ 30.16) |
|||
| Unweighted sample | 26,746 | 6688 | 6686 | 6685 | 6687 | |
| Age, years | 47.31 ± 0.26 | 50.54 ± 0.38 | 49.16 ± 0.38 | 47.56 ± 0.38 | 43.03 ± 0.33 | < 0.001 |
| Gender, n (%) | < 0.001 | |||||
| Male | 12,951 (48.42) | 1825 (27.29) | 2634 (39.4) | 3550 (53.1) | 4942 (73.9) | |
| Female | 13,795 (51.58) | 4863 (72.71) | 4052 (60.6) | 3135 (46.9) | 1745 (26.1) | |
| Race, n (%) | < 0.001 | |||||
| Mexican American | 4333 (16.2) | 1185 (17.72) | 1089 (16.29) | 1024 (15.32) | 1035 (15.48) | |
| Other Hispanic | 2273 (8.5) | 647 (9.67) | 557 (8.33) | 561 (8.39) | 508 (7.6) | |
| Non-Hispanic White | 12,727 (47.58) | 2885 (43.14) | 3163 (47.31) | 3324 (49.72) | 3355 (50.17) | |
| Non-Hispanic Black | 5312 (19.86) | 1523 (22.77) | 1326 (19.83) | 1217 (18.2) | 1246 (18.63) | |
| Other Race | 2101 (7.86) | 448 (6.7) | 551 (8.24) | 559 (8.36) | 543 (8.12) | |
| Education level, n (%) | < 0.001 | |||||
| Less than high school | 6401 (23.93) | 2152 (32.18) | 1634 (24.44) | 1409 (21.08) | 1206 (18.03) | |
| High school graduation/GED | 6209 (23.21) | 1553 (23.22) | 1591 (23.8) | 1505 (22.51) | 1560 (23.33) | |
| More than high school | 14,136 (52.85) | 2983 (44.6) | 3461 (51.76) | 3771 (56.41) | 3921 (58.64) | |
| Body mass index, kg/m2 | 28.83 ± 0.09 | 28.86 ± 0.13 | 28.89 ± 0.13 | 28.77 ± 0.14 | 28.80 ± 0.15 | 0.873 |
| Body mass index, n (%) | 0.012 | |||||
| <25 | 7579 (28.34) | 1830 (27.36) | 1902 (28.45) | 1915 (28.65) | 1932 (28.89) | |
| 25–29.9 | 9080 (33.95) | 2232 (33.37) | 2161 (32.32) | 2328 (34.82) | 2359 (35.28) | |
| ≥30 | 10,087 (37.71) | 2626 (39.26) | 2623 (39.23) | 2442 (36.53) | 2396 (35.83) | |
| Smoking, n (%) | 0.013 | |||||
| Yes | 12,193 (45.59) | 2939 (43.94) | 2928 (43.79) | 3041 (45.49) | 3285 (49.13) | |
| No | 14,553 (54.41) | 3749 (56.06) | 3758 (56.21) | 3644 (54.51) | 3402 (50.87) | |
| Alcohol consumption, n (%) | < 0.001 | |||||
| less than 12 alcohol drinks/year | 7646 (28.59) | 2650 (39.62) | 2090 (31.26) | 1697 (25.39) | 1209 (18.08) | |
| at least 12 alcohol drinks/year | 19,100 (71.41) | 4038 (60.38) | 4596 (68.74) | 4988 (74.61) | 5478 (81.92) | |
| Hypertension, n (%) | < 0.001 | |||||
| Yes | 11,390 (42.59) | 3310 (49.49) | 2956 (44.21) | 2727 (40.79) | 2397 (35.85) | |
| No | 15,356 (57.41) | 3378 (50.51) | 3730 (55.79) | 3958 (59.21) | 4290 (64.15) | |
| Diabetes, n (%) | < 0.001 | |||||
| Yes | 4052 (15.15) | 1230 (18.39) | 1114 (16.66) | 942 (14.09) | 766 (11.46) | |
| No | 22,694 (84.85) | 5458 (81.61) | 5572 (83.34) | 5743 (85.91) | 5921 (88.54) | |
| dyslipidemia, n (%) | < 0.001 | |||||
| Yes | 9294 (34.75) | 2481 (37.1) | 2408 (36.02) | 2360 (35.3) | 2045 (30.58) | |
| No | 17,452 (65.25) | 4207 (62.9) | 4278 (63.98) | 4325 (64.7) | 4642 (69.42) | |
| eGFR | 93.50 ± 0.34 | 90.87 ± 0.53 | 92.31 ± 0.51 | 93.71 ± 0.46 | 96.36 ± 0.42 | < 0.001 |
| eGFR, n (%) | < 0.001 | |||||
| <60 | 2468 (9.23) | 908 (13.58) | 710 (10.62) | 522 (7.81) | 328 (4.91) | |
| ≥60 | 24,278 (90.77) | 5780 (86.42) | 5976 (89.38) | 6163 (92.19) | 6359 (95.09) | |
| Cardiovascular disease, n (%) | < 0.001 | |||||
| Yes | 2857 (10.68) | 936 (14) | 812 (12.14) | 621 (9.29) | 488 (7.3) | |
| No | 23,889 (89.32) | 5752 (86) | 5874 (87.86) | 6064 (90.71) | 6199 (92.7) | |
| Cancer, n (%) | < 0.001 | |||||
| Yes | 2608 (9.75) | 722 (10.8) | 722 (10.8) | 674 (10.08) | 490 (7.33) | |
| No | 24,138 (90.25) | 5966 (89.2) | 5964 (89.2) | 6011 (89.92) | 6197 (92.67) | |
|
eGFR, estimated glomerular filtration rate. Continuous variables are expressed as means and standard error and categorical variables as unweighted sample and percentages. Means and standard errors are weighted. | ||||||
Association of dietary niacin Intake and mortality outcome
Multiple Cox models have shown negative associations of dietary niacin intake with all-cause and cardiovascular mortality (Table 2). For all-cause mortality, Model 2 have shown that participants in the highest quartile of dietary niacin intake have a HR of 0.74 [95%CI, 0.63–0.86] compared to participants in the lowest quartile. For cardiovascular mortality, Model 2 have shown that participants in the highest quartile of dietary niacin intake have a HR of 0.73 [95%CI, 0.57–0.95] compared to participants in the lowest quartile.
Table 2.
HRs (95% CIs) for all-cause and cardiovascular mortality according to dietary niacin intake quartiles.
| Outcomes | Dietary niacin intake, mg/day | P value for trend | |||
|---|---|---|---|---|---|
| Quintile 1 (< 16.51) |
Quintile 2 (16.51–22.45) |
Quintile 3 (22.46–30.15) |
Quintile 4 (≥ 30.16) |
||
| All-cause mortality | |||||
| Unadjusted HR | 1 [Ref] | 0.82(0.71–0.95) | 0.65(0.58–0.74) | 0.42(0.36–0.49) | < 0.001 |
| P value | 0.007 | < 0.001 | < 0.001 | ||
| Model 1 h | 1 [Ref] | 0.89(0.79–1.01) | 0.78(0.69–0.89) | 0.71(0.61–0.83) | < 0.001 |
| P value | 0.065 | < 0.001 | < 0.001 | ||
| Model 2 h | 1 [Ref] | 0.88(0.79–0.99) | 0.80(0.71–0.91) | 0.74(0.63–0.86) | < 0.001 |
| P value | 0.039 | 0.001 | < 0.001 | ||
| Cardiovascular mortality | |||||
| Unadjusted HR | 1 [Ref] | 0.79(0.62–1.01) | 0.63(0.51–0.77) | 0.34(0.25–0.45) | < 0.001 |
| P value | 0.063 | < 0.001 | < 0.001 | ||
| Model 1 h | 1 [Ref] | 0.89(0.71–1.11) | 0.81(0.67–0.98) | 0.68(0.52–0.88) | 0.001 |
| P value | 0.299 | 0.029 | 0.004 | ||
| Model 2 h | 1 [Ref] | 0.90(0.72–1.12) | 0.86(0.71–1.05) | 0.73(0.57–0.95) | 0.011 |
| P value | 0.328 | 0.136 | 0.017 | ||
|
HR, Hazard ratio; Ref, reference; BMI, body mass index; eGFR, estimated glomerular filtration rate. Model 1 was adjusted for age, sex, race/ethnicity, educational level, smoking, alcohol consumption and BMI. Model 2 was further adjusted for disease conditions (hypertension, diabetes, dyslipidemia, cardiovascular disease, and cancer) and eGFR. P value for trend was obtained from Cox models with the medians of each dietary niacin intake quartile as a continuous variable. | |||||
Dose-response curves for dietary niacin intake and mortality
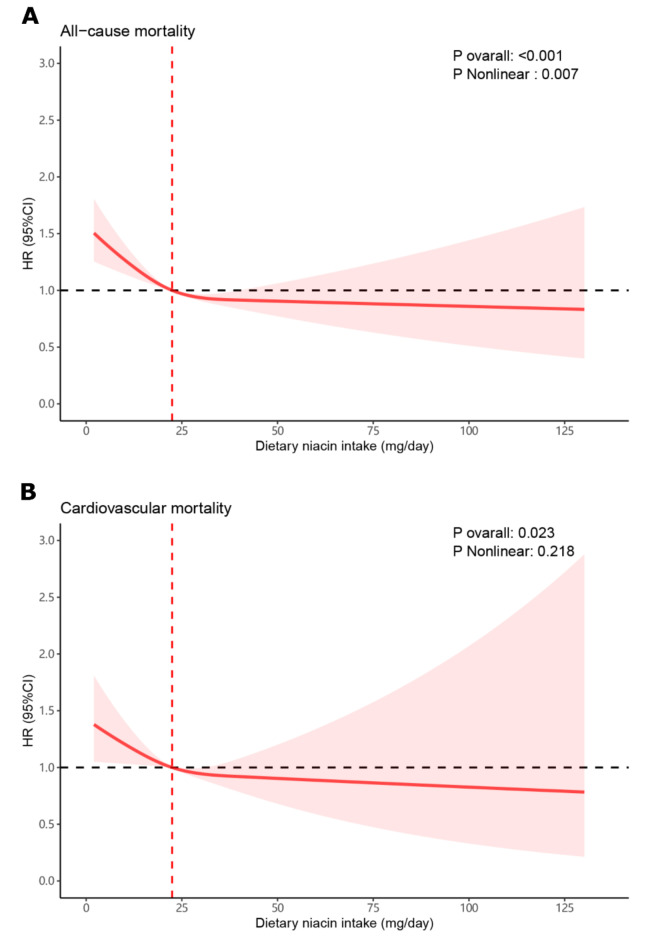
A dose-response connection between dietary niacin intake and all-cause as well as cardiovascular mortality was observed through restricted cubic spline analysis: As dietary niacin intake increased, the risk of all-cause and cardiovascular mortality decreased, while dietary niacin intake exceeds the median (22.45 mg/day), the risk of all-cause and cardiovascular mortality risk reduced slowed down (Fig. 1).
Fig. 1.
Dose-response curves for dietary niacin intake and mortality. (A) dietary niacin intake and all-cause mortality. (B) dietary niacin intake and cardiovascular mortality.
Subgroup Analysis
Subgroup analysis based on Model 2 showed that a significant association between dietary niacin intake and all-cause mortality was observed in most subgroups (Table 3). In addition, a significant interaction of dietary niacin intake and diabetes on all-cause mortality was observed (P = 0.046). Compared to participants with the lowest quartile of dietary niacin intake, HRs were 0.66 (95%CI, 0.56–0.79) and 1.01 (95%CI, 0.76–1.35) for participants with and without diabetes, respectively. Subgroup analysis based on Model 2 showed that dietary niacin intake associated with cardiovascular mortality in some subgroups, including the older, female, white, more than high school, obesity, smoking, non-hypertension, non-diabetes, non-dyslipidemia, non-cardiovascular disease, and high eGFR subgroups (Table 4). Despite the lack of significant interaction, relatively lower HR values of cardiovascular mortality were observed in these subgroups.
Table 3.
Subgroup analyses for the association of dietary niacin intake with all-cause mortality.
| Outcomes | Total | Cases | Dietary niacin intake, mg/day | P value for trend | P value for interaction | |||
|---|---|---|---|---|---|---|---|---|
| Quintile 1 (< 16.51) |
Quintile 2 (16.51–22.45) |
Quintile 3 (22.46–30.15) |
Quintile 4 (≥ 30.16) |
|||||
| Age | 0.598 | |||||||
| < 65 | 20,204 | 1022 | 1 [Ref] | 1.00(0.75–1.33) | 0.84(0.64–1.09) | 0.69(0.51–0.92) | 0.002 | |
| P value | 0.986 | 0.185 | 0.011 | |||||
| ≥ 65 | 6542 | 2529 | 1 [Ref] | 0.80(0.69–0.93) | 0.74(0.63–0.87) | 0.58(0.49–0.68) | < 0.001 | |
| P value | 0.003 | < 0.001 | < 0.001 | |||||
| Sex | 0.486 | |||||||
| Male | 12,951 | 2013 | 1 [Ref] | 0.97(0.80–1.17) | 0.86(0.71–1.05) | 0.73(0.59–0.90) | < 0.001 | |
| P value | 0.731 | 0.145 | 0.004 | |||||
| Female | 13,795 | 1538 | 1 [Ref] | 0.83(0.69–1.01) | 0.76(0.63–0.91) | 0.81(0.61–1.08) | 0.025 | |
| P value | 0.057 | 0.003 | 0.156 | |||||
| White | 0.405 | |||||||
| Yes | 12,727 | 2297 | 1 [Ref] | 0.87(0.76–0.99) | 0.75(0.65–0.88) | 0.67(0.56–0.79) | < 0.001 | |
| P value | 0.038 | < 0.001 | < 0.001 | |||||
| No | 14,019 | 1254 | 1 [Ref] | 0.85(0.70–1.04) | 0.97(0.78–1.19) | 1.04(0.77–1.41) | 0.695 | |
| P value | 0.109 | 0.763 | 0.780 | |||||
| Education | 0.680 | |||||||
| Less than high school | 6401 | 1236 | 1 [Ref] | 0.90(0.76–1.06) | 0.77(0.62–0.96) | 0.72(0.53–0.98) | 0.021 | |
| P value | 0.213 | 0.022 | 0.038 | |||||
| High school graduation/GED | 6209 | 954 | 1 [Ref] | 0.87(0.71–1.07) | 0.82(0.65–1.04) | 0.85(0.64–1.12) | 0.239 | |
| P value | 0.196 | 0.101 | 0.244 | |||||
| More than high school | 14,136 | 1361 | 1 [Ref] | 0.87(0.72–1.06) | 0.78(0.64–0.96) | 0.69(0.55–0.86) | 0.001 | |
| P value | 0.165 | 0.020 | 0.001 | |||||
| BMI | 0.537 | |||||||
| < 25 | 7579 | 1049 | 1 [Ref] | 0.97(0.79–1.20) | 0.72(0.56–0.92) | 0.88(0.68–1.13) | 0.103 | |
| P value | 0.782 | 0.008 | 0.303 | |||||
| 25–29.9 | 9080 | 1244 | 1 [Ref] | 0.89(0.71–1.12) | 0.81(0.66–1.00) | 0.71(0.55–0.91) | 0.005 | |
| P value | 0.317 | 0.053 | 0.007 | |||||
| ≥ 30 | 10,087 | 1258 | 1 [Ref] | 0.82(0.66–1.03) | 0.88(0.69–1.13) | 0.68(0.51–0.90) | 0.012 | |
| P value | 0.083 | 0.318 | 0.008 | |||||
| Smoking | 0.309 | |||||||
| Yes | 12,193 | 2172 | 1 [Ref] | 0.85(0.74–0.98) | 0.77(0.67–0.89) | 0.74(0.64–0.87) | < 0.001 | |
| P value | 0.028 | 0.001 | < 0.001 | |||||
| No | 14,553 | 1379 | 1 [Ref] | 0.93(0.79–1.11) | 0.87(0.71–1.07) | 0.71(0.53–0.95) | 0.020 | |
| P value | 0.435 | 0.183 | 0.021 | |||||
| Alcohol consumption | 0.879 | |||||||
| < 12 alcohol drinks/year | 19,100 | 2396 | 1 [Ref] | 0.92(0.79–1.07) | 0.81(0.69–0.97) | 0.73(0.62–0.87) | < 0.001 | |
| P value | 0.285 | 0.020 | < 0.001 | |||||
| ≥ 12 alcohol drinks/year | 7646 | 1155 | 1 [Ref] | 0.84(0.69–1.02) | 0.81(0.64–1.02) | 0.78(0.57–1.07) | 0.073 | |
| P value | 0.080 | 0.073 | 0.123 | |||||
| Hypertension | 0.349 | |||||||
| Yes | 11,390 | 2564 | 1 [Ref] | 0.90(0.78–1.05) | 0.78(0.66–0.92) | 0.79(0.65–0.96) | 0.004 | |
| P value | 0.175 | 0.003 | 0.016 | |||||
| No | 15,356 | 987 | 1 [Ref] | 0.82(0.67–1.01) | 0.82(0.68–1.00) | 0.63(0.49–0.81) | < 0.001 | |
| P value | 0.061 | 0.049 | < 0.001 | |||||
| Diabetes | 0.046 | |||||||
| Yes | 4052 | 1030 | 1 [Ref] | 0.90(0.74–1.10) | 0.96(0.75–1.23) | 1.01(0.76–1.35) | 0.841 | |
| P value | 0.310 | 0.753 | 0.938 | |||||
| No | 22,694 | 2521 | 1 [Ref] | 0.87(0.76–1.00) | 0.76(0.66–0.87) | 0.66(0.56–0.79) | < 0.001 | |
| P value | 0.047 | < 0.001 | < 0.001 | |||||
| Dyslipidemia | 0.576 | |||||||
| Yes | 9294 | 1712 | 1 [Ref] | 0.92(0.76–1.12) | 0.82(0.68–0.99) | 0.84(0.67–1.05) | 0.075 | |
| P value | 0.423 | 0.035 | 0.117 | |||||
| No | 17,452 | 1839 | 1 [Ref] | 0.86(0.73–1.01) | 0.79(0.66–0.95) | 0.66(0.53–0.82) | < 0.001 | |
| P value | 0.059 | 0.013 | < 0.001 | |||||
| Cancer | 0.330 | |||||||
| Yes | 2608 | 834 | 1 [Ref] | 0.77(0.59–0.99) | 0.85(0.66–1.08) | 0.70(0.54–0.89) | 0.014 | |
| P value | 0.043 | 0.187 | 0.004 | |||||
| No | 24,138 | 2717 | 1 [Ref] | 0.93(0.80–1.07) | 0.78(0.68–0.90) | 0.75(0.62–0.91) | 0.001 | |
| P value | 0.320 | 0.001 | 0.004 | |||||
| Cardiovascular disease | 0.735 | |||||||
| Yes | 2857 | 1173 | 1 [Ref] | 0.88(0.74–1.04) | 0.79(0.66–0.95) | 0.84(0.65–1.08) | 0.106 | |
| P value | 0.136 | 0.013 | 0.174 | |||||
| No | 23,889 | 2378 | 1 [Ref] | 0.89(0.77–1.03) | 0.81(0.69–0.96) | 0.70(0.58–0.85) | < 0.001 | |
| P value | 0.127 | 0.015 | < 0.001 | |||||
| eGFR | 0.723 | |||||||
| < 60 | 2468 | 1175 | 1 [Ref] | 0.81(0.66–0.99) | 0.81(0.65–1.01) | 0.76(0.56–1.03) | 0.049 | |
| P value | 0.042 | 0.062 | 0.080 | |||||
| ≥ 60 | 24,278 | 2376 | 1 [Ref] | 0.92(0.80–1.06) | 0.80(0.68–0.94) | 0.72(0.61–0.86) | < 0.001 | |
| P value | 0.236 | 0.005 | < 0.001 | |||||
|
HR, Hazard ratio; Ref, reference; BMI, body mass index; eGFR, estimated glomerular filtration rate. Model 2 was adjusted for age, sex, race/ethnicity, educational level, smoking, alcohol consumption, BMI, disease conditions (hypertension, diabetes, dyslipidemia, cardiovascular disease, and cancer) and eGFR. P value for trend was obtained from Cox models with the medians of each dietary niacin intake quartile as a continuous variable. | ||||||||
Table 4.
Subgroup analyses for the association of dietary niacin intake with cardiovascular mortality.
| Outcomes | Total | Cases | Dietary niacin intake, mg/day | P value for trend | P value for interaction | |||
|---|---|---|---|---|---|---|---|---|
| Quintile 1 (< 16.51) |
Quintile 2 (16.51–22.45) |
Quintile 3 (22.46–30.15) |
Quintile 4 (≥ 30.16) |
|||||
| Age | 0.723 | |||||||
| < 65 | 20,204 | 236 | 1 [Ref] | 1.06(0.64–1.77) | 1.04(0.63–1.72) | 0.74(0.44–1.25) | 0.162 | |
| P value | 0.823 | 0.874 | 0.265 | |||||
| ≥ 65 | 6542 | 860 | 1 [Ref] | 0.80(0.60–1.05) | 0.75(0.59–0.95) | 0.56(0.42–0.74) | < 0.001 | |
| P value | 0.111 | 0.017 | < 0.001 | |||||
| Sex | 0.096 | |||||||
| Male | 12,951 | 625 | 1 [Ref] | 1.10(0.80–1.51) | 0.99(0.74–1.33) | 0.90(0.65–1.26) | 0.324 | |
| P value | 0.555 | 0.963 | 0.550 | |||||
| Female | 13,795 | 471 | 1 [Ref] | 0.79(0.56–1.11) | 0.82(0.60–1.13) | 0.42(0.24–0.75) | 0.008 | |
| P value | 0.176 | 0.220 | 0.003 | |||||
| White | 0.483 | |||||||
| Yes | 12,727 | 727 | 1 [Ref] | 0.87(0.68–1.11) | 0.81(0.65–0.99) | 0.65(0.49–0.88) | 0.002 | |
| P value | 0.260 | 0.044 | 0.004 | |||||
| No | 14,019 | 369 | 1 [Ref] | 0.93(0.64–1.35) | 1.05(0.68–1.63) | 1.09(0.61–1.92) | 0.731 | |
| P value | 0.704 | 0.827 | 0.780 | |||||
| Education | 0.347 | |||||||
| Less than high school | 6401 | 393 | 1 [Ref] | 1.06(0.81–1.39) | 1.04(0.76–1.43) | 0.78(0.49–1.25) | 0.416 | |
| P value | 0.654 | 0.802 | 0.305 | |||||
| High school graduation/GED | 6209 | 292 | 1 [Ref] | 0.85(0.58–1.26) | 0.94(0.63–1.39) | 0.97(0.65–1.43) | 0.977 | |
| P value | 0.423 | 0.748 | 0.870 | |||||
| More than high school | 14,136 | 411 | 1 [Ref] | 0.78(0.57–1.08) | 0.65(0.45–0.94) | 0.55(0.36–0.83) | 0.004 | |
| P value | 0.136 | 0.024 | 0.005 | |||||
| BMI | 0.181 | |||||||
| < 25 | 7579 | 298 | 1 [Ref] | 0.88(0.59–1.31) | 0.65(0.41–1.02) | 0.89(0.56–1.41) | 0.401 | |
| P value | 0.530 | 0.063 | 0.615 | |||||
| 25–29.9 | 9080 | 379 | 1 [Ref] | 1.11(0.75–1.63) | 0.98(0.74–1.30) | 0.90(0.61–1.32) | 0.446 | |
| P value | 0.602 | 0.882 | 0.589 | |||||
| ≥ 30 | 10,087 | 419 | 1 [Ref] | 0.79(0.55–1.12) | 0.97(0.67–1.42) | 0.52(0.30–0.88) | 0.035 | |
| P value | 0.182 | 0.893 | 0.015 | |||||
| Smoking | 0.131 | |||||||
| Yes | 12,193 | 614 | 1 [Ref] | 0.72(0.56–0.94) | 0.76(0.58–0.99) | 0.71(0.53–0.96) | 0.044 | |
| P value | 0.014 | 0.045 | 0.027 | |||||
| No | 14,553 | 482 | 1 [Ref] | 1.14(0.79–1.64) | 1.01(0.74–1.38) | 0.70(0.46–1.09) | 0.095 | |
| P value | 0.474 | 0.934 | 0.114 | |||||
| Alcohol consumption | 0.707 | |||||||
| < 12 alcohol drinks/year | 19,100 | 711 | 1 [Ref] | 0.90(0.71–1.13) | 0.90(0.70–1.17) | 0.78(0.58–1.05) | 0.127 | |
| P value | 0.347 | 0.438 | 0.105 | |||||
| ≥ 12 alcohol drinks/year | 7646 | 385 | 1 [Ref] | 0.92(0.62–1.35) | 0.78(0.52–1.17) | 0.62(0.37–1.03) | 0.050 | |
| P value | 0.661 | 0.235 | 0.064 | |||||
| Hypertension | 0.342 | |||||||
| Yes | 11,390 | 853 | 1 [Ref] | 0.88(0.68–1.14) | 0.85(0.67–1.08) | 0.82(0.62–1.10) | 0.163 | |
| P value | 0.328 | 0.186 | 0.186 | |||||
| No | 15,356 | 243 | 1 [Ref] | 0.89(0.56–1.42) | 0.86(0.58–1.27) | 0.46(0.24–0.87) | 0.007 | |
| P value | 0.635 | 0.441 | 0.016 | |||||
| Diabetes | 0.125 | |||||||
| Yes | 4052 | 353 | 1 [Ref] | 1.07(0.80–1.44) | 1.16(0.81–1.65) | 0.85(0.53–1.36) | 0.557 | |
| P value | 0.652 | 0.419 | 0.497 | |||||
| No | 22,694 | 743 | 1 [Ref] | 0.82(0.63–1.08) | 0.76(0.61–0.94) | 0.68(0.50–0.93) | 0.007 | |
| P value | 0.164 | 0.012 | 0.015 | |||||
| Dyslipidemia | 0.122 | |||||||
| Yes | 9294 | 569 | 1 [Ref] | 0.93(0.69–1.25) | 0.99(0.77–1.26) | 0.78(0.54–1.11) | 0.188 | |
| P value | 0.622 | 0.908 | 0.169 | |||||
| No | 17,452 | 527 | 1 [Ref] | 0.84(0.62–1.14) | 0.70(0.53–0.93) | 0.67(0.48–0.94) | 0.012 | |
| P value | 0.266 | 0.014 | 0.021 | |||||
| Cancer | 0.120 | |||||||
| Yes | 2608 | 219 | 1 [Ref] | 0.54(0.33–0.90) | 0.80(0.52–1.24) | 0.59(0.33–1.05) | 0.201 | |
| P value | 0.017 | 0.322 | 0.074 | |||||
| No | 24,138 | 877 | 1 [Ref] | 1.06(0.83–1.34) | 0.88(0.69–1.11) | 0.78(0.58–1.06) | 0.054 | |
| P value | 0.650 | 0.273 | 0.114 | |||||
| Cardiovascular disease | 0.630 | |||||||
| Yes | 2857 | 465 | 1 [Ref] | 0.99(0.73–1.35) | 0.93(0.67–1.28) | 0.94(0.64–1.37) | 0.687 | |
| P value | 0.958 | 0.653 | 0.744 | |||||
| No | 23,889 | 631 | 1 [Ref] | 0.83(0.61–1.13) | 0.83(0.61–1.12) | 0.61(0.42–0.89) | 0.007 | |
| P value | 0.243 | 0.223 | 0.010 | |||||
| eGFR | 0.563 | |||||||
| < 60 | 2468 | 433 | 1 [Ref] | 0.90(0.65–1.26) | 1.02(0.74–1.41) | 0.73(0.45–1.19) | 0.328 | |
| P value | 0.555 | 0.887 | 0.211 | |||||
| ≥ 60 | 24,278 | 663 | 1 [Ref] | 0.87(0.69–1.09) | 0.76(0.57–1.00) | 0.69(0.49–0.97) | 0.027 | |
| P value | 0.227 | 0.050 | 0.034 | |||||
|
HR, Hazard ratio; Ref, reference; BMI, body mass index; eGFR, estimated glomerular filtration rate. Model 2 was adjusted for age, sex, race/ethnicity, educational level, smoking, alcohol consumption, BMI, disease conditions (hypertension, diabetes, dyslipidemia, cardiovascular disease, and cancer) and eGFR. P value for trend was obtained from Cox models with the medians of each dietary niacin intake quartile as a continuous variable. | ||||||||
Sensitivity analysis
To evaluate the robust association between dietary niacin intake and mortality, sensitivity analysis was carried out, such as excluding participants with CVD or cancer, NHANES 2003–2016, and further adjustment for low density lipoprotein cholesterol. The significant negative associations consistent in sensitivity analysis (Table 5).
Table 5.
Sensitivity analyses for the association of dietary niacin intake with all-cause and cardiovascular mortality.
| Outcomes | Total | Cases | Dietary niacin intake, mg/day | P value for trend | |||
|---|---|---|---|---|---|---|---|
|
Quintile 1
(< 16.51) |
Quintile 2
(16.51–22.45) |
Quintile 3
(22.46–30.15) |
Quintile 4
(≥ 30.16) |
||||
| All-cause mortality | |||||||
| Removing participants with CVD or cancer at baseline | 21,903 | 1860 | 1 [Ref] | 0.92(0.78–1.09) | 0.80(0.67–0.95) | 0.69(0.67–0.95) | < 0.001 |
| P value | 0.346 | 0.012 | 0.002 | ||||
| NHANES 2003–2016 | 23,018 | 3392 | 1 [Ref] | 0.88(0.78–0.99) | 0.79(0.69–0.90) | 0.71(0.61–0.83) | < 0.001 |
| P value | 0.032 | < 0.001 | < 0.001 | ||||
| Further adjustment for low density lipoprotein cholesterol | 11,941 | 1582 | 1 [Ref] | 0.83(0.70–0.98) | 0.76(0.65–0.90) | 0.64(0.51–0.81) | < 0.001 |
| P value | 0.026 | 0.001 | < 0.001 | ||||
| Cardiovascular mortality | |||||||
| Removing participants with CVD or cancer at baseline | 21,903 | 528 | 1 [Ref] | 0.82(0.67–1.26) | 0.81(0.59–1.10) | 0.60(0.39–0.92) | 0.008 |
| P value | 0.600 | 0.177 | 0.018 | ||||
| NHANES 2003–2016 | 23,018 | 1060 | 1 [Ref] | 0.90(0.72–1.12) | 0.83(0.69–1.01) | 0.69(0.53–0.89) | 0.002 |
| P value | 0.347 | 0.066 | 0.005 | ||||
| Further adjustment for low density lipoprotein cholesterol | 11,941 | 500 | 1 [Ref] | 1.06(0.82–1.38) | 0.85(0.64–1.14) | 0.67(0.46–0.97) | 0.013 |
| P value | 0.638 | 0.276 | 0.033 | ||||
|
CVD, cardiovascular diseases; Ref, reference; NHANES, National Health and Nutrition Examination Survey; BMI, body mass index; eGFR, estimated glomerular filtration rate. Model 2 was adjusted for age, sex, race/ethnicity, educational level, smoking, alcohol consumption, BMI, disease conditions (hypertension, diabetes, dyslipidemia, cardiovascular disease, and cancer) and eGFR. P value for trend was obtained from Cox models with the medians of each dietary niacin intake quartile as a continuous variable. | |||||||
Discussion
We found a negative correlation between dietary niacin intake and all-cause and cardiovascular mortality in the US population. Additionally, we identified a significant interaction for dietary niacin intake and diabetes on all-cause mortality. The potential benefits of dietary niacin intake on all-cause mortality may be more prominent in non-diabetic individuals compared to those with diabetes. No significant interaction was observed between dietary niacin intake and stratification factors on cardiovascular mortality, the relationship between dietary niacin intake and cardiovascular mortality may differ among diverse populations.
Since niacin is one of the earliest lipid-regulating drugs, many studies have explored the effect of niacin on patients with coronary heart disease (CHD). Several older studies from the Coronary Drugs Program have demonstrated the efficacy of niacin in reducing the risk of cardiovascular events and mortality7,8. This was also confirmed in the Stockholm Ischemic Heart Disease Secondary Prevention Study24. A meta-analysis based on these older studies proposed that niacin reduce the risk of certain cardiovascular events among patients without statin treatment25. Due to the widespread use of statins, most studies in recent years have explored the effects of niacin on cardiovascular events and prognosis on the basis of statin therapy, suggesting that niacin does not reduce the risk of cardiovascular events and mortality in patients with CHD26–29. Recently, a meta-analysis suggested that niacin supplementation increased all-cause death risk of patients treated with statins11. This may be due to the effective control of lipids by statin therapy, and the improvement of lipids brought by niacin cannot further affect the prognosis of patients with CHD. However, in the general population or those in the early stages of CHD, dietary niacin intake is likely to have cardiovascular benefits. Few previous studies have assessed the relationship between dietary niacin intake and mortality. Ying et al. observed more dietary niacin intake associated with lower risk of all-cause and cancer-related mortality in cancer patients30. Pan et al. found a correlation between higher dietary niacin intake and reduced risk of all-cause mortality in patients with nonalcoholic fatty liver disease31. Although these two studies suggest potential benefits of dietary niacin intake in specific populations. The effect of dietary niacin intake on the overall population and on other specific populations remains unclear.
This study based on a population-based cohort study investigated the association of dietary intake with mortality in the general US population. We also conducted a number of stratified analyses to assess differences in the association of dietary niacin intake and mortality among various populations. This study contributes to the understanding of the relationship between dietary niacin intake and mortality across different populations. It is suggested that moderate dietary niacin intake may help reduce mortality risk in populations, especially in those without disease risk.
The potential benefits of dietary niacin intake on mortality may stem from the improvement of NAD metabolism. As a precursor of NAD, niacin can increase the level of NAD and improve cell capacity metabolism, DNA damage, inflammation, mitochondrial function, cell aging, and cell death through multiple mechanisms13,32. In recent years, more and more studies have focused on the mechanisms associated with NAD to improve disease. The research by Beltrà et al. indicated that niacin can effectively restore tissue NAD levels, improve mitochondrial metabolism, and alleviate cancer-related cachexia resulting from chemotherapy16. Pirinen et al.‘s research demonstrates that nicotinic acid treatment alleviates systemic NAD deficiency and enhances muscle performance in adult-onset mitochondrial myopathy by promoting mitochondrial biogenesis and respiratory chain activity19. According to Mouchiroud et al., the NAD (+)/Sirtuin pathway regulates longevity by activating mitochondrial UPR and FOXO signaling33. These findings offer partial insight into how dietary niacin intake reduces the risk of mortality. However, it has also been suggested that the extent or efficacy of NAD depletion may diminish the disease-modifying effect of NAD elevation methods, thus limiting data interpretation34. As for the role of niacin in the pathogenesis of cardiovascular diseases, previous studies have mainly attributed to its lipid-lowering effect. Adipocyte lipolysis is regulated by a number of G protein-coupled receptors (GPR). Niacin is a potent GPR109A agonist, which can effectively inhibit lipolysis and reduce free fatty acids35. In addition, niacin also affects cholesterol synthesis and transport by affecting key enzymes of lipid metabolism and cholesteryl ester transfer protein36,37. But recent research indicates that elevated niacin end metabolites could elevate the risk of cardiovascular disease by activating inflammatory pathways, such as directly boosting VCAM-1 expression12. Overall, niacin is more likely to function as an NAD modulator rather than lipid-lowering medication to improve disease and long-term health risks, warranting further exploration.
This study also found that the effect of dietary niacin intake on all-cause mortality is more pronounced in non-diabetic participants than in diabetic participants. Previous studies have found that niacin may raise blood sugar and increase the risk of diabetes38–40. Niacin may impair insulin sensitivity through several pathways41–43. Therefore, higher dietary niacin is recommended to reduce the risk of all-cause death in non-diabetic people, but not in diabetic patients. And further research is needed to clarify this result and explore the specific mechanisms.
This study has some limitations. Firstly, the estimation of dietary niacin intake relied on self-reported data, introducing inherent measurement inaccuracies. Secondly, a two-day average of niacin intake may not fully capture long-term dietary patterns. Lastly, despite extensive adjustments for confounding variables, a notable risk of bias from confounding factors persists.
Conclusion
In this population-based cohort study, increased dietary niacin intake is associated with reduced all-cause and cardiovascular mortality in US adults. The impact of niacin intake on all-cause mortality is more pronounced in non-diabetic individuals than in those with diabetes. Although no significant interaction was found between dietary niacin intake and stratification factors regarding cardiovascular mortality, the association between niacin intake and cardiovascular mortality may vary across different populations. Further research is needed to clarify the variations in the impact of dietary niacin intake on cardiovascular mortality across different populations.
Acknowledgements
The authors thank the participants and staff of the National Health and Nutrition Examination Survey for their valuable contributions.
Author contributions
YF and LL conceived and designed research. SJC, LL, ZCH, LL and CY processed data and performed statistical analysis. LL and CSJ wrote the initial paper; YF, LDL, CQR and ZXY reviewed and corrected the article. All authors read and approved the final manuscript. LL and SJC have contributed equally to this work and share first authorship.
Funding
This research was funded by the National Natural Science Foundation of China (82003046), the Natural Science Foundation of Chongqing Municipal Science and Technology Bureau (CSTB2022NSCQ-MSX0561), the Young and Middle-aged Project of Chongqing Municipal Health Commission (2024GDRC011), the Medical and Industrial Integration Project of Chongqing University (2023CDJYGRH-YB10) and the Natural Science Foundation of Fujian Province (2020J05212).
Data availability
The data used in this study are openly available in the NHANES website: NHANES Questionnaires, Datasets, and Related Documentation (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
Declarations
Ethics approval and consent to participate
This study is an analysis derived from publicly available NHANES data. The National Center for Health Statistics Research Ethics Review Board approved the NHANES protocol (https://www.cdc.gov/nchs/nhanes/irba98.htm). The NHANES has obtained written informed consent from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ling Lin and Shuaijie Chen have contributed equally to this work and share first authorship.
References
- 1.Makarov, M. V., Trammell, S. A. J. & Migaud, M. E. The chemistry of the vitamin B3 metabolome. Biochem. Soc. Trans.47, 131–147. 10.1042/bst20180420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan, P., Moat, S. & Anstey, A. Pellagra: a review with emphasis on photosensitivity. Br. J. Dermatol.164, 1188–1200. 10.1111/j.1365-2133.2010.10163.x (2011). [DOI] [PubMed] [Google Scholar]
- 3.Guyton, J. R. & Boden, W. E. Niacin, food intake and cardiovascular effects. Nat. Med.30, 2444–2445. 10.1038/s41591-024-03220-2 (2024). [DOI] [PubMed] [Google Scholar]
- 4.BLOCK, G. et al. : I. VITAMINS AND MINERALS. Am. J. Epidemiol.122, 13–26. 10.1093/oxfordjournals.aje.a114072 (1985). [DOI] [PubMed] [Google Scholar]
- 5.Stierman, B. et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of files and Prevalence estimates for selected Health outcomes. NCHS Natl. Health Stat. Rep. (2021). [DOI] [PMC free article] [PubMed]
- 6.Carlson, L. A. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med.258, 94–114. 10.1111/j.1365-2796.2005.01528.x (2005). [DOI] [PubMed] [Google Scholar]
- 7.Clofibrate and Niacin in Coronary Heart Disease. Jama231, 360–381, doi:10.1001/jama.1975.03240160024021 (1975). [PubMed] [Google Scholar]
- 8.Canner, P. L. et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J. Am. Coll. Cardiol.8, 1245–1255. 10.1016/s0735-1097(86)80293-5 (1986). [DOI] [PubMed] [Google Scholar]
- 9.Boden, W. E. et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med.365, 2255–2267. 10.1056/NEJMoa1107579 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Landray, M. J. et al. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med.371, 203–212. 10.1056/NEJMoa1300955 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Jenkins, D. J. A. et al. Supplemental vitamins and minerals for Cardiovascular Disease Prevention and Treatment: JACC Focus Seminar. J. Am. Coll. Cardiol.77, 423–436. 10.1016/j.jacc.2020.09.619 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Ferrell, M. et al. A terminal metabolite of niacin promotes vascular inflammation and contributes to cardiovascular disease risk. Nat. Med.30, 424–434. 10.1038/s41591-023-02793-8 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu, X. & Raju, R. P. Regulation of NAD(+) metabolism in aging and disease. Metab. Clin. Exp.12610.1016/j.metabol.2021.154923 (2022). [DOI] [PMC free article] [PubMed]
- 14.Chini, C. C. S., Cordeiro, H. S., Tran, N. L. K. & Chini, E. N. NAD metabolism: role in senescence regulation and aging. Aging cell.23, e13920. 10.1111/acel.13920 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdellatif, M., Sedej, S. & Kroemer, G. NAD(+) metabolism in Cardiac Health, Aging, and Disease. Circulation. 144, 1795–1817. 10.1161/circulationaha.121.056589 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Beltrà, M. et al. NAD(+) repletion with niacin counteracts cancer cachexia. Nat. Commun.14, 1849. 10.1038/s41467-023-37595-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wuerch, E., Urgoiti, G. R. & Yong, V. W. The Promise of Niacin in Neurology. Neurotherapeutics: J. Am. Soc. Experimental Neurother.20, 1037–1054. 10.1007/s13311-023-01376-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zapata-Pérez, R., Wanders, R. J. A., van Karnebeek, C. D. M. & Houtkooper, R. H. NAD(+) homeostasis in human health and disease. EMBO Mol. Med.13, e13943. 10.15252/emmm.202113943 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirinen, E. et al. Niacin cures systemic NAD(+) Deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metabol.31, 1078–1090e1075. 10.1016/j.cmet.2020.04.008 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. 2019 Public-Use Linked Mortality Files, <https://www.cdc.gov/nchs/data-linkage/mortality-public.htm> (2022).
- 21.Centers for Disease Control and Prevention. NCHS Research Ethics Review Board Approval, <https://www.cdc.gov/nchs/nhanes/irba98.htm> (2022).
- 22.United States Department of Agriculture. What’s In The Foods You Eat Search Tool, <https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/whats-in-the-foods-you-eat-search-tool/> (2022).
- 23.Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med.150, 604–612. 10.7326/0003-4819-150-9-200905050-00006 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson, L. A. & Rosenhamer, G. Reduction of mortality in the Stockholm Ischaemic Heart Disease secondary Prevention Study by combined treatment with clofibrate and nicotinic acid. Acta Med. Scand.223, 405–418. 10.1111/j.0954-6820.1988.tb15891.x (1988). [DOI] [PubMed] [Google Scholar]
- 25.D’Andrea, E., Hey, S. P., Ramirez, C. L. & Kesselheim, A. S. Assessment of the role of Niacin in Managing Cardiovascular Disease outcomes: a systematic review and Meta-analysis. JAMA Netw. open.2, e192224. 10.1001/jamanetworkopen.2019.2224 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keene, D., Price, C., Shun-Shin, M. J. & Francis, D. P. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ (Clinical Res. ed.). 349, g4379. 10.1136/bmj.g4379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaughnessy, A. F. Niacin does not decrease mortality in patients with coronary artery disease or low HDL. Am. Family Phys.96, 129 (2017). [PubMed] [Google Scholar]
- 28.Schandelmaier, S. et al. Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst. Rev.6 (Cd009744). 10.1002/14651858.CD009744.pub2 (2017). [DOI] [PMC free article] [PubMed]
- 29.Garg, A. et al. Role of Niacin in current clinical practice: a systematic review. Am. J. Med.130, 173–187. 10.1016/j.amjmed.2016.07.038 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Ying, H. et al. Association between Niacin and mortality among patients with cancer in the NHANES retrospective cohort. BMC cancer. 22, 1173. 10.1186/s12885-022-10265-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan, J., Zhou, Y., Pang, N. & Yang, L. Dietary niacin intake and mortality among individuals with nonalcoholic fatty liver disease. JAMA Netw. open.7, e2354277. 10.1001/jamanetworkopen.2023.54277 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munk, S. H. N. et al. NAD(+) regulates nucleotide metabolism and genomic DNA replication. Nat. Cell Biol.25, 1774–1786. 10.1038/s41556-023-01280-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouchiroud, L. et al. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO Signaling. Cell. 154, 430–441. 10.1016/j.cell.2013.06.016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niño-Narvión, J., Camacho, M., Julve, J. & NAD + Precursors Physiological Reboot? Nutrients15, doi:10.3390/nu15204479 (2023). [DOI] [PMC free article] [PubMed]
- 35.Javaid, A. & Mudavath, S. L. Niacin-induced flushing: mechanism, pathophysiology, and future perspectives. Arch. Biochem. Biophys.761, 110163. 10.1016/j.abb.2024.110163 (2024). [DOI] [PubMed] [Google Scholar]
- 36.Ganji, S. H. et al. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J. Lipid Res.45, 1835–1845. 10.1194/jlr.M300403-JLR200 (2004). [DOI] [PubMed] [Google Scholar]
- 37.van der Hoorn, J. W. et al. Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice. Arterioscler. Thromb. Vasc. Biol.28, 2016–2022. 10.1161/atvbaha.108.171363 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Goldie, C. et al. Niacin therapy and the risk of new-onset diabetes: a meta-analysis of randomised controlled trials. Heart (British Cardiac Society). 102, 198–203. 10.1136/heartjnl-2015-308055 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke, P. et al. Relationship between dietary niacin intake and diabetes mellitus in the National Health and Nutrition Examination Survey (NHANES) 2003–2018. Eat. Weight Disorders: EWD. 27, 2425–2434. 10.1007/s40519-021-01347-6 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Elam, M. B. et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial disease multiple intervention trial. Jama. 284, 1263–1270. 10.1001/jama.284.10.1263 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Choi, S. et al. Widespread effects of nicotinic acid on gene expression in insulin-sensitive tissues: implications for unwanted effects of nicotinic acid treatment. Metab. Clin. Exp.60, 134–144. 10.1016/j.metabol.2010.02.013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heemskerk, M. M. et al. Long-term niacin treatment induces insulin resistance and adrenergic responsiveness in adipocytes by adaptive downregulation of phosphodiesterase 3B. Am. J. Physiol. Endocrinol. Metab.306, E808–813. 10.1152/ajpendo.00641.2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montastier, E. et al. Niacin induces mir-502-3p expression which impairs insulin sensitivity in human adipocytes. Int. J. Obes.43, 1485–1490. 10.1038/s41366-018-0260-5 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are openly available in the NHANES website: NHANES Questionnaires, Datasets, and Related Documentation (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).