Abstract
Microplastics (MPs)-induced changes in soil nutrient cycling and microbial activity may pose a potential risk to soil ecosystem. Although some studies have explored these topics, there is still a large space for exploration and a relative lack of research on the mechanism by which soil health and its functions are affected by these changes. Thus, this study investigated the effects of polyethylene (PE) MPs with two particle sizes (13 μm and 130 μm) at five concentrations (0%, 1%, 3%, 6% and 10%, w/w) on soil biochemical properties and ecosystem function. The findings revealed that the exposure to 13 μm MPs significantly reduced soil respiration (Res) rate, β-glucosidase (Glu) and catalase (CAT) activity, which accompanied with enhanced urease activity and decreased soil pH, available phosphorus (AP), dissolved reactive phosphorus (DRP), dissolved organic carbon (DOC) and available potassium (AK) content in most cases. However, 130 μm MPs exerted negligible influence on the DOC and DRP content, Glu and CAT activity. High concentrations of 130 μm MPs significantly reduced soil pH, total dissolved nitrogen (TDN), AP and AK content, but significantly increased soil Res rate. Overall, soil ecosystem function was significantly reduced by the addition of MPs. The Res rate, soil AP and DRP content and Glu activity were the most important predictors of soil ecosystem function. We found that the risk posed by MPs to soil ecosystem function was dose-dependent and size-dependent. These findings underscore that MPs can alter soil functions related to soil nutrient cycling and provide further insights into MPs behavior in agroecosystems.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80124-8.
Keywords: Microplastics, Polyethylene, Soil biochemical properties, Soil ecosystem function
Subject terms: Ecology, Environmental sciences
Introduction
Microplastics (MPs), defined as plastic particles smaller than 5 mm, enter agro-ecosystems through various pathways. It is estimated that the abundance of MPs in agriculture soils has far exceed that in the ocean, and soil has become a larger reservoir for environmental MPs pollution1,2. Their widespread presence and potential impacts on ecosystems make them “pollutants of importance and agents of global change”3,4. Due to their small particle size and large surface area, MPs can absorb various organic and inorganic pollutants, rendering them resistant to bio-ingestion and degradation5,6. This resistance allows MPs to persist in the soil for a long time, resulting in rapid accumulation in the global terrestrial environment, potentially leading to long-term effects on soil ecosystems7. Therefore, MPs in terrestrial ecosystems are of increasing concern.
Recent studies have revealed that a relatively high concentration of MPs in soils8 significantly altered the physicochemical properties of the soil9, and directly and adversely affected soil fauna10, plants11 and microorganisms12. Furthermore, there was a potential risk to human health through the accumulation and transmission of MPs via the food chain13. After infiltrating the soil, MPs were bound with organic matter or minerals and incorporated into the soil matrix, thereby inducing alterations in soil aggregate structure, bulk density, porosity, permeability, water holding capacity, as well as other physical and chemical properties14. For instance, the addition of high-density polyethylene (HDPE) at a concentration of 0.1% significantly decreased soil pH, whereas polylactic acid (PLA) did not exhibit a significant impact on soil pH at the same concentration15. However, Qi et al.16 found that PLA significantly increased soil pH and alleviated soil acidification. The contents of soil available nitrogen and phosphorus were reduced by 10–13% and approximately 30% in a rice paddy soil amended with 1% polyvinyl chloride (PVC) MPs, respectively17. The changes in soil microhabitats induced by MPs may affect the structure and diversity of local microbial community15. The addition of 1% and 5% of polyethylene (PE) and 5% of PVC significantly declined the richness and diversity of the bacterial communities, but significantly increased the abundance of betaproteobacteriales, including the Burkholderiaceae, which were closely related to nitrogen fixation18. Soil microorganisms and the enzymes they produce were sensitive to soil stresses that could be used as indicators of microbial activity and as environmental biomarkers19. However, it was found that the addition of low-density polyethylene (LDPE) and HDPE had no obvious influence on the activities of urease, CAT and invertase20. In conclusion, MPs can directly or indirectly affect the soil properties in most cases, varying with MPs type, dose, size, shape, and soil type.
The impact of MPs on soil physicochemical and biological properties is likely to influence the soil functions, which refers to the capacity of soil ecosystems to provide and maintain multiple ecosystem functions and services simultaneously21. For instance, under well-watered conditions, the presence of MPs fibers led to a decrease in soil multifunctionality, indicating their negative effects on the ecosystem22. Furthermore, the activities of α/β-1, 4-glucosidase, urease, protease and NH4+-N content were the most important predictors of ecosystem multifunctionality23. Zhou et al.24 revealed that adding 0.03% polystyrene (PS) improved soil ecosystem multifunctionality by 4–12%, but decreased by 4–11% by adding 0.3% PS. However, the influence of MPs on soil functions remains unclear, which is critical to elucidate the ecological consequences of MPs in agroecosystems.
The persistence and non-natural properties of MPs in soils might qualify these particles to be drivers of soil function change. However, how the presence of MPs shaping soil functions is still an open question, not to mention the studies on the effects of different concentrations and particle sizes of MPs on soil functions. Therefore, in this study, we evaluated on how the concentration and particle size of PE MPs influenced soil biochemical properties and soil function related to soil nutrient cycling to enhance the comprehension of soil ecosystem responses to MPs as a global change factor. We hypothesized that (i) the presence of MPs may affect the availability and cycling of soil nutrients; (ii) high concentrations and small particle sizes of MPs may have greater negative impacts on soil system; and (iii) MPs may decrease soil function related to soil nutrient cycling, with their size mediating the differentiation of soil function.
Materials and methods
Soil and MPs
The topsoil (0–20 cm) used in this study was collected from an experimental field (113°50′24″ E, 35°12′26″N) established by Henan Xinlianxin Chemicals Group Co. in cooperation with local universities. The experimental field sampled were control plots without any treatment and were not covered with mulch films in history; thus there is no concern regarding MPs pre-contamination of the soil. The average sand, silt and clay contents in the 0–20 cm soil were 35.81%, 44.62% and 19.57%, respectively. The tested soil is a loamy fluvo-aquic soil, which is relatively widespread globally, particularly in alluvial plains adjacent to rivers, lakes, and oceans, as well as in regions with high groundwater levels, including North America, Europe and Southeast Asia. The following are the physicochemical properties of the tested soil: pH 7.65 (soil-water ratio 1:2.5), organic matter 10.68 g·kg− 1, available nitrogen 123.90 mg·kg− 1, available phosphorus (AP) 26.41 mg·kg− 1 and available potassium (AK) 277.0 mg·kg− 1. The air-dried soil was sieved through a 2 mm mesh and mixed homogenously.
Plastic film, mainly composed of PE, is one of the direct sources of MPs in farmland soils25. Therefore, PE MPs were chosen because they are commonly detected in farmland soil26. PE MPs with average diameters of 130 μm and 13 μm, respectively, were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China) and were white powders composed of spherical particles.
Experimental setup
As reported, the environmentally relevant concentration of MPs in soil was found to be 1%, with 5% presenting a high level of MPs23,26,27. Therefore, 1% and 3% were designed to simulate different levels of MPs pollution under environmental conditions, and 6% and 10% to simulate the condition of extreme MPs pollution based on the previous studies26,28. In this study, two size of MPs with diameters of 13 μm and 130 μm were selected, and five concentrations were established. Nine treatments with three replicates for each were considered for cultivation: (1) CK: 0% (w/w) MPs (sharing in both MP particle sizes); (2) T1-130: 1% (w/w) 130 μm MPs; (3) T2-130: 3% (w/w) 130 μm MPs; (4) T3-130: 6% (w/w) 130 μm MPs; (5) T4-130: 10% (w/w) 130 μm MPs; (6) T1-13: 1% (w/w) 13 μm MPs; (7) T2-13: 3% (w/w) 13 μm MPs; (8) T3-13: 6% (w/w) 13 μm MPs; and (9) T4-13: 10% (w/w) 13 μm MPs. Approximately 0, 1.5, 4.5, 9.0 and 15 g of MPs of both sizes were weighed and added to 150 g of soil (dry weight). The samples were incubated at 25 ± 1 °C under a natural photoperiod for 30 days and the soil water content was maintained at 60% of the maximum water holding capacity throughout the experiment. After 30 days of exposure, soil was collected and separated into two subsamples. One subsample was stored at 4 °C for the determination of the activities of CAT, Glu and urease, Res rate, and the contents of dissolved organic carbon (DOC) and total dissolved nitrogen (TDN); while the other was air-dried at room temperature, ground and passed through a 1 mm sieve for the determination of soil pH, the contents of AP, AK and dissolved reactive phosphorus (DRP).
Measurement of soil biochemical properties
Soil pH was measured with a pH meter at a ratio of 1:2.5 (w/w soil: water)29. Soil AP30 and DRP31 were extracted by 0.5 M NaHCO3 solution and 0.01 M CaCl2 solution respectively, followed by analysis using the molybdenum blue colorimetric method. Soil AK was extracted by 1 M ammonium acetate solution, and its concentration was subsequently measured via flame photometry32. TDN content was quantified at both 220 nm and 275 nm using a UV spectrophotometer following potassium persulfate oxidation33. DOC content was determined using a TOC analyzer (Vario TOC, Elementar Analysensysteme GmbH, Germany) in a 1: 3 (w/v) soil: water suspension according to the Kalbitz et al.34. Soil Res rate was determined using the indoor-incubation alkali absorption method35. Briefly, 20 g fresh soil was incubated in a sealed container at 25 °C for 7 days. The CO2 produced was trapped in an excess of 0.05 M NaOH, and the residual NaOH was titrated with 0.05 M HCl. The amount of CO2 is then determined by titrating the remaining NaOH. Soil Res rate was expressed in mg CO2-C·kg− 1 soil·d− 1.
Urease activity was measured by indophenol blue colorimetry according to the Kandeler and Gerber36. Briefly, 5 g fresh soil was incubated with 10 mL 10% urea solution and 10 mL citrate buffer solution (pH 6.7) at 37 °C for 24 h, followed by quick filtration. 1 mL of the filtrate was then diluted to 20 mL, and treated with 4 mL sodium phenol solution and 3 mL sodium hypochlorite solution. The released ammonium was measured by colorimetry at 578 nm. Urease activity was expressed in mg NH4+-N·g− 1 soil·d− 1. CAT activity was measured using a titrimetric method37. Briefly, 2 g fresh soil was homogenized with 40 mL distilled water and 5 mL 0.3% H2O2 for 20 min. Then, 5 mL 1.5 M H2SO4 was added to stop the reaction, and the reactants were filtrated. The amount of surplus H2O2 from 20 mL of the filtrate was measured by titration using 20 mM KMnO4. CAT activity was expressed in mg H2O2·g− 1 soil·20 min− 1. Glu activity was measured using the method of Asensio et al.38. In brief, 2 g fresh soil was incubated with 6 mL sodium acetate buffer (0.2 M, pH 6.2) and 2 mL 2% hydroquinone-β-D-glucoside (as substrate) at 37 °C for 24 h, then diluted with distilled water to 50 mL. 5 mL filtrate was diluted to 10 mL, and treated with 3 mL 3, 5-dinitrosalicylic acid solution. The amount of glucose was measured by colorimetry at 578 nm. Glu activity was expressed in mg glucose·g− 1 soil·d− 1.
Assessment of soil ecosystem function
Referring to the soil multi-nutrient cycling index proposed by Jiao et al.39, which is analogous to the multifunctionality, we employed the soil function index in this study to quantify the impact of MPs on the nutrient cycling within soil ecosystems. Ten soil properties including soil pH, DOC, TDN, AK, AP, DRP, Res rate, CAT, Glu and urease were considered to assess soil function index. These variables are closely related to soil nutrient cycling and microbial activity, and can well reflect multiple functions of soil ecosystem, such as nutrient retention and utilization, soil fertility and biogeochemical cycles40. The soil function index was calculated by the common averaging approach. Firstly, each function was standardized separately by Z-score transformation ranging from 0 to 1, and then averaged to obtain the function index values using the averaging approach41.
Statistical analysis
All statistical analyses were performed using SPSS 26.0 (IBM SPSS Statistics 26). Data were first tested for normality using the Shapiro-Wilk test (P < 0.05) and homogeneity of variance using the Levene test (P < 0.05). Then the data were analyzed using one-way ANOVA with particle size as a fixed factor to evaluate the differences between different MPs concentrations. Mean values were then compared using the Duncan test at P < 0.05. Significant differences between particle sizes for a given MP concentration were determined via a t test with 95% confidence intervals. A detrended correspondence analysis (DCA) was conducted on ten soil biochemical properties in relation to MPs concentration and particle size. Given that all the ordination axes were less than 3, the redundancy analysis (RDA) was performed by Canoco 5.0 to reveal the correlation between soil biochemical properties and microplastic concentration and particle size. Random forest analysis was conducted using the “randomForest” package in R (version 4.3) to evaluate the credible predictors of soil function related to the cycling of multiple nutrients. Soil biochemical properties were served as predictors for soil function index in random forest analysis. The mean decrease in accuracy (IncMSE%) was used to estimate the importance of these predictors. And the cluster heat map analysis was conducted using the “pheatmap” package in R (version 4.3). All figures were generated using Origin 2018.
Results
Soil chemical properties
In the present study, no significant changes of soil DOC content were observed in 130 μm MPs treatments relative to the CK (Fig. 1a). In contrast, except for T1-13, the addition of 13 μm MPs led to a significant decrease in DOC content (9.15–59.07% difference, P < 0.05) with the increase of MPs concentration. Soil TDN was significantly affected by 130 μm MPs (Fig. 1b), specifically, compared to CK, TDN content was significantly higher in T1-130 treatment (increased by 5.34%, P < 0.05), but was significantly lower in other treatments (decreased by 5.39–8.55%, P < 0.05). For the 13 μm group, significant decrease in TDN content was observed only in T4-13 with the highest concentration, compared to CK (19.26% decrease, P < 0.05). Soil AK was not significantly altered by low-dose MPs of both sizes (P > 0.05), but significantly reduced by high-dose MPs (average decreased by 9.21% and 55.51% for 130 μm MPs and 13 μm MPs, respectively, P < 0.05 in both groups; Fig. 1c). AP content was decreased by both sizes of MPs, with the highest decrement observed in T4-13 (58.53% decrease, P < 0.05; Fig. 1d). Similarly, soil pH was significantly reduced in both the 130 μm and 13 μm treatments (average reduction 10.59%, P < 0.05; and 11.47%, P < 0.05 respectively; Fig. 1e). DRP content did not exhibit significant changes due to the addition of 130 μm MPs (P > 0.05), but decreased significantly after the addition of 13 μm MPs (5.01–41.85% difference, P < 0.05; Fig. 1f). Overall, there were significant difference in the influence of MPs of both sizes on soil chemical properties at the concentrations of 3%, 6% and 10% in addition to soil pH (P > 0.05, Fig. 1). Among these chemical properties, TDN, AK, AP and pH were negatively correlated with the concentration of 130 μm MPs (Fig. S1). In 13 μm group, all measured soil properties except pH did decrease with increasing MPs concentration (Fig. S2).
Fig. 1.
The effect of MPs addition on soil physicochemical properties. DOC: dissolved organic carbon (a); TDN: total dissolved nitrogen (b); AK: available potassium (c); AP: available phosphorus (d); pH: soil pH (e); DRP: dissolved reactive phosphorus (f). Data are represented as mean ± SD (n = 3). Different letters indicate significant difference among MPs concentrations with same particle size, and asterisks indicate significant difference between two particle sizes of MPs for a given concentration at the level of P < 0.05.
Soil biological properties
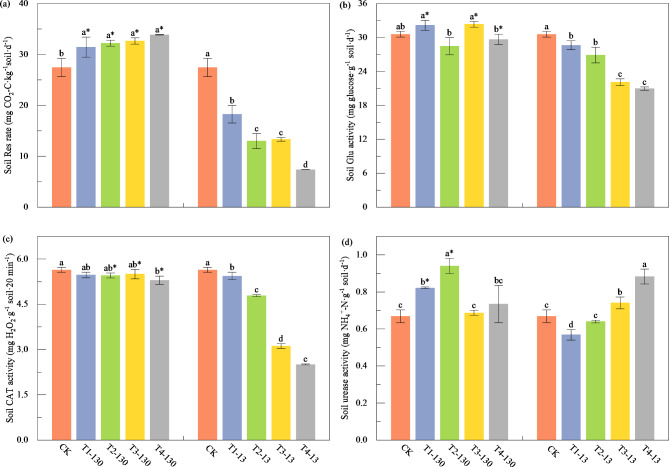
The effects of MPs of two sizes on soil Res were completely different (Fig. 2a). Specifically, Res rate was promoted by the addition of 130 μm MPs (average increased by 18.55%, P < 0.05), and increased with the increase of MPs concentration, but significantly suppressed with the increase of 13 μm MPs concentration (average decreased by 52.69%, P < 0.05). The activity of Glu in the soil was decreased significantly with increasing 13 μm MPs concentration (6.30-31.35% difference, P < 0.05), however, no obvious change was observed by adding 130 μm MPs (Fig. 2b). Similarly, CAT activity was significantly suppressed as the concentration of 13 μm MPs increased (decreased by 3.56–55.59%, P < 0.05; Fig. 2c). For 130 μm MPs, a significant decrease of 6.04% in CAT activity was observed only at the highest concentration (P < 0.05, Fig. 2d). Compared with CK, urease activity was significantly promoted by low concentration of 130 μm MPs (increased by 23.05% in T1-130 and 40.65% in T2-130, respectively, P < 0.05; Fig. 2d). Urease activity showed an increasing trend with the increase of 13 μm MPs concentration, however, significant increase was observed when concentration was reached to 6% (P < 0.05). In addition, the biological properties in 130 μm MPs treatments were significantly higher than those in 13 μm MPs treatments under the same concentration (P < 0.05, Fig. 2). Generally, the Res rate and CAT activity were positively and negatively correlated with 130 μm MPs concentration, respectively. However, 13 μm MPs decreased the soil Res rate, Glu and CAT activities, showing a decreasing trend with increasing MPs concentration, which was exactly the opposite of urease (Fig. 2d).
Fig. 2.
The effect of MPs addition on soil respiration (a) and enzyme activity. Res: soil respiration (a); Glu: β-glucosidase (b); CAT: catalase (c); urease (d). Data are represented as mean ± SD (n = 3). Different letters indicate significant difference among MPs concentrations with same particle size, and asterisks indicate significant difference between two particle sizes of MPs for a given concentration at the level of P < 0.05.
Key factor determining soil biochemical properties in the presence of MPs
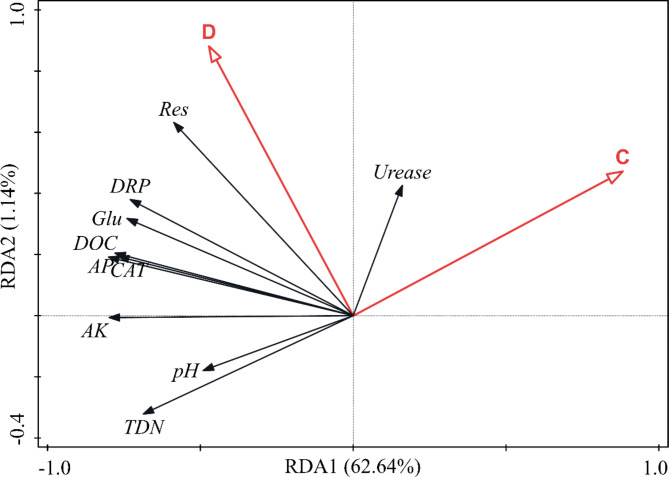
Both MPs concentration and particle size significantly influenced soil biochemical properties (Table S1), however, it remained unclear which factor was the primary driver of these changes. To address this, the RDA was conducted, which accounted for 63.8% of the total variation in soil biochemical properties (Fig. 3, P = 0.002). The percentages of variance explained by the first and second axes were 62.64% and 1.14%, respectively. The contribution rates of MPs concentration and particle size to the variations in soil biochemical properties were 76.7% and 23.3%, respectively, indicating that MPs concentration was the primary factor affecting these properties. As shown in Fig. 3, except for urease, other indicators were negatively correlated with MPs concentration. Similarly, most of these indicators were also negatively correlated with the MPs size, which were consistent with the correlation analysis (Fig. S1 and Fig. S2).
Fig. 3.
Redundancy analysis (RDA) of soil properties and the concentration and particle size. C: MP concentration; D: MP particle size; AK: available potassium; AP: available phosphorus; CAT: catalase; DOC: dissolved organic carbon; DRP: dissolved reactive phosphorus; Glu: β-glucosidase; pH: soil pH; Res: soil respiration; TDN: total dissolved nitrogen. The length and direction of the arrow represent the degree of influence of particle size and concentration of MPs on soil properties, and the positive and negative correlation between the two.
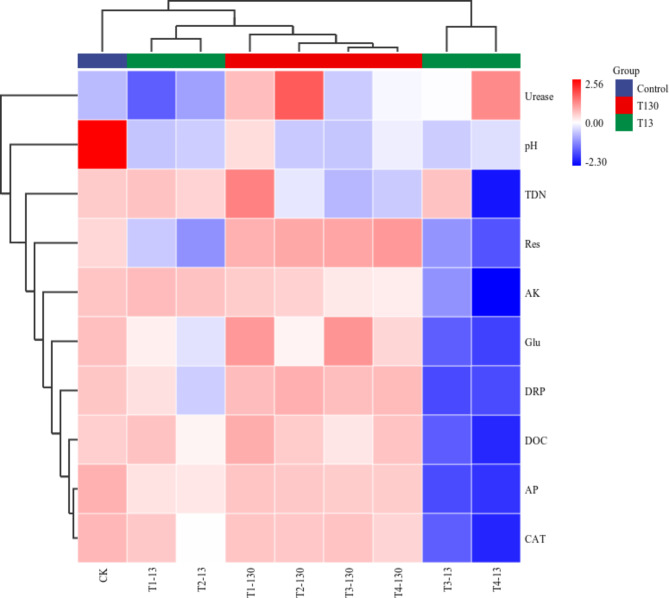
To further investigate the impact of MPs concentration on soil properties, we constructed a cluster heat map, which revealed that the nine treatments could be grouped into two major clusters (Fig. 4). The first major cluster, consisted of CK, T1-13, T2-13, T1-130, T2-130, T3-130 and T4-130, was further subdivided into two minor clusters. One minor cluster was composed exclusively of CK, in which soil pH, contents of AK and AP, and CAT activity were the highest among the two major clusters. The other minor cluster comprised T1-13, T2-13, T1-130, T2-130, T3-130 and T4-130, in which soil biochemical properties (except urease) were lower than CK but higher than the second major cluster. Similarly, all the biochemical indicators besides urease in the second major cluster composed of T3-13 and T4-13 were lower than the first major cluster. Overall, soil biochemical indicators in the second major cluster were generally low, indicating that high concentrations of small-sized MPs had a greater negative impact on soil nutrient content and cycling.
Fig. 4.
Cluster heat map analysis of soil properties under different MPs treatments. AK: available potassium; AP: available phosphorus; CAT: catalase; DOC: dissolved organic carbon; DRP: dissolved reactive phosphorus; Glu: β-glucosidase; pH: soil pH; Res: soil respiration; TDN: total dissolved nitrogen.
Soil function index
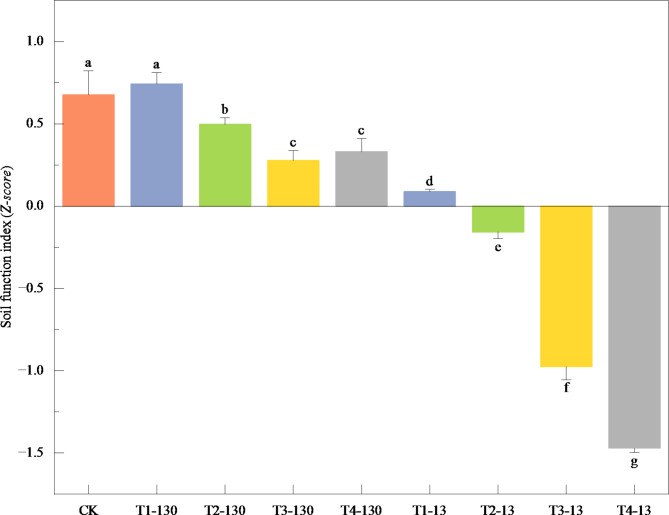
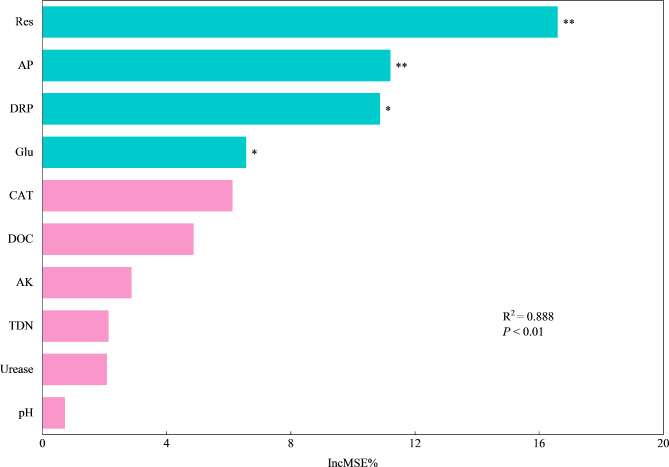
Soil function index varied significantly across different MPs concentrations and sizes (P < 0.05, Fig. 5). Except for the T1-130, other concentrations of MPs significantly reduced the soil function index, which decreased significantly with the increasing concentration of MPs (Fig. 5). Furthermore, the soil function index in 13 μm MPs group was significantly lower than that in 130 μm MPs group. The random forest model explained 88.8% of the overall variance in soil function index and soil Res rate was the most prominent predictor (Fig. 6). Soil AP, DRP content and Glu activity were also reliable indicators (P < 0.05).
Fig. 5.
Changes of soil function index across different MPs concentrations. Data are shown as mean ± SD. Different lowercase letters indicate significant differences among treatments (P < 0.05).
Fig. 6.
Random forest analysis to identify relative importance of soil variables drivers on soil function index. AK: available potassium; AP: available phosphorus; CAT: catalase; DOC: dissolved organic carbon; DRP: dissolved reactive phosphorus; Glu: β-glucosidase; pH: soil pH; Res: soil respiration; TDN: total dissolved nitrogen. The mean decrease in accuracy (IncMSE%) was used to indicate the relative importance of each variable for predicting soil function index. Significance levels of each predictor are as follows: *P < 0.05 and **P < 0.01.
Discussion
In this study, the results demonstrated that MPs exposure altered soil biochemical properties and function index, and these impacts depended on the MPs concentrations and particle sizes. As hypothesized, soil ecological functions linked with nutrient content and cycling were affected by MPs with different concentrations and particle sizes. These results suggested that high MPs concentrations, especially small sizes, had significant negative impact on soil functions.
Impacts of MPs addition on soil chemical properties
Soil DOC is the most chemically bio-available and easily influenced carbon by microorganism in soil. A minor effect of 130 μm MPs on soil DOC content was observed at all concentrations, however, significant reductions were found in 13 μm treatments (Fig. 1a). This negative effect may be attributing the inhibition of Glu activity by 13 μm MPs, which attenuated the decomposition of carbohydrate and finally decreased soil DOC content26. This was consistent with our speculation that MPs with smaller particle sizes have a greater impact on the soil. Due to the addition of 13 μm MPs, soil bioavailable carbon (such as DOC, Fig. 1a) content was reduced, which inhibited microbial activity and consequently suppressed the consumption of soil TDN. Conversely, 130 μm MPs had no significant effects on soil DOC content. However, as shown by the significant activation of soil Res rate (Fig. 2a), it could be hypothesized that microbial activity was promoted, which might lead to the microbial consumption of TDN19. Therefore, the TDN content was significantly lower than that of CK (Fig. 1b). High application rates of 13 and 130 μm MPs at 6% and 10% significantly reduced the AK content (Fig. 1c). Interestingly, the smaller size of MPs also significantly reduced the AK content, which might suggest that high application rates and small sizes of MPs would produce negative influences on soil fertility42. MPs could also influence biochemical cycle of P by altering microbial processes43. For instance, Feng et al.44 reported that the addition of biodegradable MPs (e.g. PLA and polyhydroxybutyate) diminished the availability of soil AP, which may be attributed to inhibition of soil phosphatase activity by MPs43. Furthermore, the increase in soil carbon-to-nitrogen ratio caused by degradable MPs might stimulate microbial assimilation of inorganic P, thus exacerbating P deficiency45. Two particle size MPs with different concentrations significantly reduced the AP content (except for T1-130), which might be attributed to the changes in microbial abundance and activity, and the inhibition of soil enzyme activity caused by MPs43. Due to their relatively large, possibly reactive surface area and charged properties, MPs could influence cation exchange in soil, selectively adsorb substances with negatively or positively charges, and allow free proton exchange in soil water, ultimately causing changes in soil pH15. Boots et al.15 found that soil pH experienced a significant decrease when exposed to HDPE, which is in line with our results. The smaller the MPs size, the larger the specific surface area, resulting in an increased number of unoccupied adsorption sites on the surface46. This enhances the probability of solid-liquid two-phase contact and facilitates improved adsorption capacity of DRP in solution by MPs46. Consequently, there were significant reductions in DRP content in soil solution under high 13 μm MPs concentrations. Overall, the presence of MPs could elicit declines in the availability of soil inherent nutrients, which is consistent with our hypothesis.
Impacts of MPs addition on soil biological properties
For 130 μm MPs, the soil Res rate was significantly increased, potentially attributed to the enhanced soil aeration that stimulated microbial activity by supplying oxygen content47. Instead, the addition of 130 μm MPs did not produce a statistically significant impact on the activities of Glu and CAT. This could be attributed to the functional resistance of microbial communities to the exposure of 130 μm MPs that exhibited no significant harmful effects on microbes48. Conversely, significant suppression in Res rate by 13 μm MPs may be due to the lower DOC content (Fig. 1a), which may serve as a potential nutrient and/or substrate for microorganisms22. The result of the significant inhibition of Res rate by 13 μm MPs was contrary to the 130 μm MPs and the results of most existing studies48–51. Due to the current preliminary understanding of the impact of MPs on soil Res, the exact mechanisms leading to the opposite effects of different MPs sizes on soil Res remain unclear and require further investigation. Similarly, the microbial activity was inhibited due to the low DOC concentration, leading to a significant inhibition in the activities of Glu and CAT involved in the soil carbon cycle52. The significant and positive correlations between the Glu activity and DOC content, as well as CAT activity and DOC content, further confirm this (Fig. S3, R = 0.94, P < 0.001 and R = 0.98, P < 0.001, respectively). In contrast to the suppression effect of Glu and CAT activities, the urease activity was stimulated by 13 μm MPs. The presence of MPs in soil has been found to enhance the abundance of diazotrophs, which play a major role in stimulating urease activity18. This may account for the positive effect of 13 μm MPs on urease activity. In addition, several parameters (such as TDN, AK, AP, Res rate, CAT) exhibited a dose-effect relationship with MPs concentration (Fig. S1, Fig. S2). This indicated that the effect of MPs on soil ecological environment might have a cumulative effect53, which means that as the MPs concentration increases, their effects on the soil ecosystem may become increasingly significant. In particular, except for soil pH, all other indicators demonstrated a significant dose-effect relationship with 13 μm MPs (Fig. S2), suggesting that the cumulative effect (negative impact) of small-sized MPs on soil biochemical properties might be more pronounced. However, the influence of MPs size on the soil ecological environment remains poorly understood and necessitates more research in the future.
Impacts of MPs addition on soil function index
Soil function index has been designated to evaluate soil ecosystem functions and services under various external disturbances20; however, research on the impact of MPs on soil ecosystem functioning is still in its infancy. In this study, soil function index was found to be diminished by the addition of MPs. This trend was consistent with the observations in nutrient availability (i.e. AP, DRP) and nutrient cycling function (i.e. soil Res, Glu), as they were the primary contributors to soil function index. This finding highlighted that the soil ecosystem had the ability to tolerate a certain amount of MPs pollution, albeit with a negative impact on soil functions. MPs influences on soil ecosystem functions can be related with their concentration and particle size. Small-sized MPs, due to their large active surface area, provided more adsorption sites for soluble nutrients, thereby directly reducing the content of available nutrients46. As the substrate of soil microorganisms, this decrease in available nutrients would inhibit the activity of enzymes involved in nutrient recycling and transformation by suppressing the abundance and activity of soil microorganisms19,21,22,25. This further intensifies the obstruction of soil nutrients cycling and transform processes ultimately diminishes soil functions. Indeed, adding MPs leads to a decline in soil DOC, available N and AP contents due to the inhibition of biochemical process driven by associated microorganisms upon MPs incorporation17,43. Yi et al.54 reported that the addition of PE and PP MPs decreased the bacteria abundance and changed the physicochemical properties of soils, subsequently reducing the resistance of soil microorganisms against pollutants. Similarly, the richness and diversity of soil bacterial communities were reduced by the addition of PE and PVC MPs, and PE had more severe effects than PVC19. Soil Res largely depended on soil microbial activity and was highly susceptible to variations in soil conditions, which explained why soil Res was the most significant predictor of soil function index in the presence of MPs19.
Key factors affecting the impacts of MPs on soil properties and soil function index
Our findings demonstrated that MPs had an impact not only on single soil property but also on soil function index. Specially, MPs concentration was the primary influencing factor for soil properties and also had significant impacts on the soil function index, with higher MPs concentrations associated with lower soil function index. MPs size also had significant influence on soil function index. The soil function index treated with small particle size MPs was significantly lower than that of large particle size MPs, which indicated that under the same concentration, small particle size MPs might have more serious negative effects on soil nutrients content, availability and cycling. Previous studies have demonstrated that the presence of MPs significantly affected the ecosystem multiple functions, with this effect being highly dependent on MPs concentration23. Specially, the addition of relatively low-concentration MPs promoted soil biological properties; however, this impact was shifted from positive to negative as MPs concentration increase23,24,28. Instead, our RDA analysis indicated that soil biochemical properties were primarily influenced by MPs concentration, whereas the influence of MPs size was relatively minor. Furthermore, based on the clustering analysis results, when MPs concentration falls below a certain threshold (3% in this study), there was no significant difference in their impact on soil biochemical properties between the two MPs sizes. A significant size effect occurred when MPs concentration was reached to 6%; specifically, smaller-sized MPs exhibited more substantial negative effects on soil ecosystem function. It is important to note that 6% and 10% are not environmentally relevant concentrations for MPs in soils; however, such high concentrations can be observed in certain highly polluted areas, including urban and industrial discharge zones, oceans, and regions surrounding municipal wastewater treatment plants, where MPs concentrations may reach or even exceed 6%27,55. Therefore, although MPs concentrations of 6% and 10% are relatively rare under normal circumstances, they remain plausible in specific contaminated environments. These two high concentrations were selected as hypothetical extreme scenarios to explore the potential impacts of high MPs loads on soil ecosystems and determine the threshold at which soil ecosystem functions may be affected, which is crucial for understanding the potentially serious harm that MPs can inflict on soil ecosystems. Overall, the influence of MPs on the soil ecosystem may be cumulative with a more pronounced negative effect observed for small-sized MPs. It should be noted that these outcomes may be attributed to the limited selection of only one soil type and two MPs sizes in this study. Different soil types vary in physical, chemical, and biological properties, which can significantly influence the behavior of MPs in soil and their ecological effects. In future research, it would be beneficial to include multiple types of soil and MPs to comprehensively evaluate the ecological impacts of MPs in different soil environments.
Conclusions
The presence of MPs can affect soil biochemical properties and ecosystem function. In this study, 13 μm MPs treatment reduced soil pH, soil DOC, AP and DRP contents, as well as soil Res rate, Glu and CAT activities. However, the addition of high-concentration MPs promoted the urease activity, while reduced the soil AK content. Differently, 130 μm MPs treatment had no significant effect on soil DOC, DRP contents, Glu and CAT activities. Meanwhile, it reduced soil pH, TDN and AP contents but significantly promoted the soil Res rate. MPs significantly decreased soil function index. These findings highlight the profound influence of MPs on soil biochemical properties and ecosystem function, emphasizing the pressing need to address and control the MPs pollution in agroecosystems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded by the Henan Provincial Science and Technology Research Project (242102320092), the National Natural Science Foundation of China (31872184), and the Natural Science Foundation of Henan Province (222300420044).
Author contributions
Yanan Cheng contributed to conceptualization, data curation, methodology, writing – original draft, writing – review & editing. Fei Wang contributed to conceptualization, methodology, writing – review & editing. Wenwen Huang contributed to conceptualization, formal analysis, writing– review & editing. Yongzhuo Liu contributed to data curation, formal analysis, writing – review & editing. All authors have read and agreed to the published version of the manuscript.
Data availability
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bläsing, M. & Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ.612, 422–435. 10.1016/j.scitotenv.2017.08.086 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Horton, A. A., Walton, A., Spurgeon, D. J., Lahive, E. & Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ.586, 127–141. 10.1016/j.scitotenv.2017.01.190 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt, E. S., Rosi, E. J. & Gessner, M. O. Synthetic chemicals as agents of global change. Front. Ecol. Environ.15, 84–90. 10.1002/fee.1450 (2017). [Google Scholar]
- 4.Rillig, M. C., Ryo, M. & Lehmann, A. Classifying human influences on terrestrial ecosystems. Global Change Biol.27, 2273–2278. 10.1111/gcb.15577 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Seidensticker, S., Zarfl, C., Cirpka, O. A., Fellenberg, G. & Grathwohl, P. Shift in mass transfer of wastewater contaminants from microplastics in the presence of dissolved substances. Environ. Sci. Technol.51, 12254–12263. 10.1021/acs.est.7b02664 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Yeo, B. G. et al. PCBs and PBDEs in microplastic particles and zooplankton in open water in the Pacific Ocean and around the coast of Japan. Mar. Pollut Bull.151, 110806. 10.1016/j.marpolbul.2019.110806 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Mbachu, O., Jenkins, G., Kaparaju, P. & Pratt, C. The rise of artificial soil carbon inputs: Reviewing microplastic pollution effects in the soil environment. Sci. Total Environ.780, 146569. 10.1016/j.scitotenv.2021.146569 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Li, S. et al. Macro- and microplastic accumulation in soil after 32 years of plastic film mulching. Environ. Pollut. 300, 118945. 10.1016/j.envpol.2022.118945 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Rillig, M. C. & Lehmann, A. Microplastic in terrestrial ecosystems. Science368, 1430–1431. 10.1126/science.abb5979 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, X. Y. et al. Prominent toxicity of isocyanates and maleic anhydrides to Caenorhabditis elegans: Multilevel assay for typical organic additives of biodegradable plastics. J. Hazard. Mater.442, 130051. 10.1016/j.jhazmat.2022.130051 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Shafea, L. et al. Microplastics in agroecosystems: A review of effects on soil biota and key soil functions. J. Plant. Nutr. Soil. Sci.186, 5–22. 10.1002/jpln.202200136 (2023). [Google Scholar]
- 12.Ren, X. W., Tang, J. C., Liu, X. M. & Liu, Q. L. Effects of microplastic on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 256, 113347. 10.1016/j.envpol.2019.113347 (2020). [DOI] [PubMed] [Google Scholar]
- 13.He, L. Y., Li, Z. B., Jia, Q. & Xu, Z. C. Soil microplastics pollution in agriculture. Science379, 547. 10.1126/science.adf6098 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Dissanayake, P. D. et al. Effects of microplastics on the terrestrial environment: A critical review. Environ. Res.209, 112734. 10.1016/j.envres.2022.112734 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Boots, B., Russell, C. W. & Green, D. S. Effects of microplastics in soil ecosystems: Above and below ground. Environ. Sci. Technol.53, 11496–11506. 10.1021/acs.est.9b03304 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Qi, Y. L. et al. Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J. Hazard. Mater.387, 121711. 10.1016/j.jhazmat.2019.121711 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Yan, Y. et al. Effect of polyvinyl chloride microplastics on bacterial community and nutrient status in two agricultural soils. Bull. Environ. Contam. Toxicol.107, 602–609. 10.1007/s00128-020-02900-2 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Fei, Y. F. et al. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ.707, 135634. 10.1016/j.scitotenv.2019.135634 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Wang, F. Y., Wang, Q. L., Adams, C. A., Sun, Y. H. & Zhang, S. W. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater.424, 127531. 10.1016/j.jhazmat.2021.127531 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Liu, Z. Q. et al. Effect of polyethylene microplastics and acid rain on the agricultural soil ecosystem in Southern China. Environ. Pollut. 303, 119094. https://doi.org/10.1016/j. envpol.2022.119094 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Manning, P. et al. Redefining ecosystem multifunctionality. Nat. Ecol. Evol.2, 427–436. 10.1038/s41559-017-0461-7 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Lozano, Y. M. et al. Effects of microplastics and drought on soil ecosystem functions and multifunctionality. J. Appl. Ecol.58, 988–996. 10.1111/1365-2664.13839 (2021). [Google Scholar]
- 23.Liu, Z. Q., Wen, J. H., Liu, Z. X., Wei, H. & Zhang, J. E. Polyethylene microplastics alter soil microbial community assembly and ecosystem multifunctionality. Environ. Int.183, 108360. 10.1016/j.envint.2023.108360 (2024). [DOI] [PubMed] [Google Scholar]
- 24.Zhou, Y. F. et al. Nanoplastics alter ecosystem multifunctionality and may increase global warming potential. Global Change Biol.29, 3895–3909. 10.1111/gcb.16734 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Müller, A., Goedecke, C., Eisentraut, P., Piechotta, C. & Braun, U. Microplastic analysis using chemical extraction followed by LC-UV analysis: A straightforward approach to determine PET content in environmental samples. Environ. Sci. Eur.32, 85. 10.1186/s12302-020-00358-x (2020). [Google Scholar]
- 26.Huang, S. Y. et al. Polyethylene and polyvinyl chloride microplastics promote soil nitrification and alter the composition of key nitrogen functional bacterial groups. J. Hazard. Mater.453, 131391. 10.1016/j.jhazmat.2023.131391 (2023). [DOI] [PubMed] [Google Scholar]
- 27.de Souza Machado, A. A. et al. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol.52, 9656–9665. 10.1021/acs.est.8b02212 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nayab, G. et al. Climate warming masks the negative effect of microplastics on plant-soil health in a silt loam soil. Geoderma425, 116083. 10.1016/j.geoderma.2022.116083 (2022). [Google Scholar]
- 29.Kerley, S. J., Shield, I. F. & Huyghe, C. Specific and genotypic variation in the nutrient content of lupin species in soils of neutral and alkaline pH. Crop Pasture Sci.52, 93–102. 10.1071/AR00060 (2000). [Google Scholar]
- 30.Olsen, S. R. & Sommers, L. E. Phosphorus, in Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd ed. (1982).
- 31.Daly, K. & Casey, A. Environmental aspects of soil phosphorus testing. Ir. J. Agric. Food Res.44, 261–279. 10.1590/S1413-70542005000100030 (2005). [Google Scholar]
- 32.Lu, D. J. et al. Crop yield and soil available potassium changes as affected by potassium rate in rice-wheat systems. Field Crops Res.214, 38–44. 10.1016/j.fcr.2017.08.025 (2017). [Google Scholar]
- 33.Doyle, A., Weintraub, M. N. & Schimel, J. P. Persulfate digestion and simultaneous colorimetric analysis of carbon and nitrogen in soil extracts. Soil. Sci. Soc. Am. J.68, 669–676. 10.2136/sssaj2004.6690 (2004). [Google Scholar]
- 34.Kalbitz, K., Schmerwitz, J., Schwesig, D. & Matzner, E. Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma113, 273–291. 10.1016/s0016-7061(02)00365-8 (2003). [Google Scholar]
- 35.Xue, S. et al. Effects of elevated CO2 and drought on the microbial biomass and enzymatic activities in the rhizospheres of two grass species in Chinese loess soil. Geoderma286, 25–34. 10.1016/j.geoderma.2016.10.025 (2017). [Google Scholar]
- 36.Kandeler, E. & Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils. 6, 68–72. 10.1007/bf00257924 (1988). [Google Scholar]
- 37.Stępniewska, A., Wolińska, A. & Ziomek, J. Response of soil catalase activity to chromium contamination. J. Environ. Sci.21, 1142–1147. 10.1016/S1001-0742(08)62394-3 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Asensio, D. et al. Soil biomass-related enzyme activity indicates minimal functional changes after 16 years of persistent drought treatment in a Mediterranean Holm oak forest. Soil. Biol. Biochem.189, 109281. 10.1016/j.soilbio.2023.109281 (2024). [Google Scholar]
- 39.Jiao, S. et al. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome6, 146. 10.1186/s40168-018-0526-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu, W. G. et al. Aridity-driven shift in biodiversity-soil multifunctionality relationships. Nat. Commun.12, 5350. 10.1038/s41467-021-25641-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maestre, F. T. et al. Plant species richness and ecosystem multifunctionality in global drylands. Science335, 214–218. 10.1126/science.1215442 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, M. et al. Influences of different source microplastics with different particle sizes and application rates on soil properties and growth of Chinese cabbage (Brassica chinensis L). Ecotox Environ. Safe. 222, 112480. 10.1016/j.ecoenv.2021.112480 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Dong, Y., Gao, M., Qiu, W. & Song, Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotox Environ. Safe. 211, 111899. 10.1016/j.ecoenv.2021.111899 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Feng, X. Y., Wang, Q. L., Sun, Y. H., Zhang, S. W. & Wang, F. Y. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater.424, 127364. 10.1016/j.jhazmat.2021.127364 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Chang, N. et al. Unveiling the impacts of microplastic pollution on soil health: A comprehensive review. Sci. Total Environ.951, 175643. 10.1016/j.scitotenv.2024.175643 (2024). [DOI] [PubMed] [Google Scholar]
- 46.Liu, P. et al. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ. Sci. Technol.53, 3579–3588. 10.1021/acs.est.9b00493 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Gao, B., Gao, F. Y., Zhang, X. F., Li, Y. Y. & Yao, H. Y. Effects of different sizes of microplastic particles on soil respiration, enzyme activities, microbial communities, and seed germination. Sci. Total Environ. 933, 173100. 10.1016/j.scitotenv.2024.173100 (2024). [DOI] [PubMed]
- 48.Blöcker, L., Watson, C. & Wichern, F. Living in the plastic age- different short-term microbial response to microplastics addition to arable soils with contrasting soil organic matter content and farm management legacy. Environ. Pollut.267, 115468. 10.1016/j.envpol.2020.115468 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Klimek, B., Grzyb, D., Łukiewicz, B. & Niklińska, M. Microplastics increase soil respiration rate, decrease soil mesofauna feeding activity and change enchytraeid body length distribution in three contrasting soils. Appl. Soil. Ecol.201, 105463. 10.1016/j.apsoil.2024.105463 (2024). [Google Scholar]
- 50.Liu, X. H., Li, Y. Y., Yu, Y. X. & Yao, H. Y. Effect of nonbiodegradable microplastics on soil respiration and enzyme activity: A meta-analysis. Appl. Soil. Ecol.184, 104770. 10.1016/j.apsoil.2022.104770 (2023). [Google Scholar]
- 51.Zhao, T. T., Lozano, Y. M. & Rilling, M. C. Microplastics increase soil pH and decrease microbial activities as a function of microplastic shape, polymer type, and exposure time. Front. Environ. Sci.9, 675803. 10.3389/fenvs.2021.675803 (2021). [Google Scholar]
- 52.Wang, H. Y., Wu, J. Q., Li, G. & Yan, L. J. Changes in soil carbon fractions and enzyme activities under different vegetation types of the northern Loess Plateau. Ecol. Evol.10, 12211–12223. 10.1002/ece3.6852 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, J. R. et al. Effects of plastic residues and microplastics on soil ecosystems: A global meta-analysis. J. Hazard. Mater.435, 129065. 10.1016/j.jhazmat.2022.129065 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Yi, M. L., Zhou, S. H., Zhang, L. L. & Ding, S. Y. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res.93, 24–32. 10.1002/wer.1327 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Rillig, M. C. Microplastic disguising as soil carbon storage. Environ. Sci. Technol.52, 6079–6080. 10.1021/acs.est.8b02338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding author on reasonable request.