Abstract
Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) is characterized by the abrupt onset of significant obsessive-compulsive symptoms (OCS) and/or severe food restriction, together with other neuropsychiatric manifestations. An autoimmune pathogenesis triggered by infection has been proposed for at least a subset of PANS. The older diagnosis of Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcus (PANDAS) describes rapid onset of OCD and/or tics associated with infection with Group A Streptococcus. The pathophysiology of PANS and PANDAS remains incompletely understood. We recently found serum antibodies from children with rigorously defined PANDAS to selectively bind to cholinergic interneurons (CINs) in the striatum. Here we examine this binding in children with relapsing and remitting PANS, a more heterogeneous condition, collected in a distinct clinical context from those examined in our previous work, from children with a clinical history of Streptococcus infection. IgG from PANS cases showed elevated binding to striatal CINs in both mouse and human brain. Patient plasma collected during symptom flare decreased a molecular marker of CIN activity, phospho-riboprotein S6, in ex vivo brain slices; control plasma did not. Neither elevated antibody binding to CINs nor diminished CIN activity was seen with plasma collected from the same children during remission. These findings replicate what we have seen previously in PANDAS and support the hypothesis that at least a subset of PANS cases have a neuroimmune pathogenesis. Given the critical role of CINs in modulating basal ganglia function, these findings confirm striatal CINs as a locus of interest in the pathophysiology of both PANS and PANDAS.
Keywords: Antibody binding, Cholinergic interneurons, Dorsal striatum, Neuronal activity, PANDAS, PANS, Phospho-rpS6, Plasma autoantibodies, OCD
1. Introduction
Some children develop obsessive-compulsive disorder (OCD) and other neuropsychiatric symptoms extremely rapidly, even overnight. This has been described as ‘pediatric acute-onset neuropsychiatric syndrome’, or PANS (Chang et al., 2015; Murphy et al., 2015; Singer et al., 2012; Swedo et al., 2012). Clinical manifestations of PANS include abrupt onset of significant OCS and/or severely restricted food intake, together with other symptoms such as emotional lability, anxiety, motor abnormalities, cognitive regression, sleep disruption, and urinary urgency. Symptoms typically follow a relapsing and remitting course (Chang et al., 2015; Frankovich et al., 2015a; Murphy et al., 2015; Swedo et al., 2012; Toufexis et al., 2015). PANS is often associated with evidence of immune dysregulation, and it has been proposed that many cases derive from an autoimmune process triggered by infection. While the diagnosis does not require temporal association with a documented infection, it has been associated with various pathogens, including Streptococcus and mycoplasma (Allen et al., 1995; Ercan et al., 2008; Frankovich et al., 2015b; Gerentes et al., 2019; Rhee and Cameron, 2012).
The association between pediatric infection and rapid onset of neuropsychiatric symptoms was first described in conjunction with Streptococcus. A recent population-based study reported that children positive for Streptococcus are at increased risk of developing OCD (Orlovska et al., 2017). Rapid onset of OCD and/or tics in a child with evidence of Streptococcal infection has been named Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcus, or PANDAS (Swedo, 2002; Swedo et al., 1998). An autoimmune pathophysiology based on molecular mimicry has been hypothesized, by analogy to the well-established postinfectious neuropsychiatric condition Sydenham chorea (Swedo and Williams, 2017; Williams and Swedo, 2015). Several autoantibodies to Streptococcal antigens that cross-react with various basal ganglia epitopes have been reported (Ben-Pazi et al., 2013; Dale et al., 2012; Kirvan et al., 2007; Kirvan et al., 2003; Kirvan et al., 2006a). Immunomodulatory therapies have been recommended for for PANS and PANDAS, though controlled trials have been small and have had mixed results (as is the case with SC) (Allen et al., 1995; Frankovich et al., 2017; Garvey et al., 2005; Hajjari et al., 2022; Johnson et al., 2021; Perlmutter et al., 1999; Sigra et al., 2018; Williams et al., 2016).
PANS and PANDAS overlap phenomenologically with pediatric obsessive-compulsive disorder (OCD) and Tourette syndrome (TS); comorbidity is common (Calaprice et al., 2017; Gromark et al., 2019; Murphy et al., 2015; O’Dor et al., 2022). The symptoms of PANS and PANDAS overlap in that OCD symptoms are typically prominent in both, though PANDAS has stricter requirements for clearly documented temporal association with a Streptococcal infection and an episodic course. Cases where the core symptom is eating restriction (PANS) or tics (PANDAS), in the absence of prominent OCD symptoms, may look quite distinct. Inflammation and microstructural differences have been described in the basal ganglia during the acute phase of both PANDAS and PANS (Giedd et al., 2000; Giedd et al., 1996; Kumar et al., 2015; Zheng et al., 2020).
Numerous studies over the past 25 years have sought to characterize the causes of PANDAS, and in particular to identify the hypothesized pathogenic autoantibodies; however, the pathophysiology remains incompletely understood. Binding of PANDAS serum antibodies to basal ganglia tissue has been reported (Frick et al., 2013; Morer et al., 2008; Pavone et al., 2004; Singer et al., 2004; Xu et al., 2021). Several autoantibody targets have also been described, including lysoganglioside GM1, tubulin, and dopamine D1 and D2 receptors (Ben-Pazi et al., 2013; Chain et al., 2020; Cox et al., 2013; Dale et al., 2012; Kirvan et al., 2003; Kirvan et al., 2006b; Singer et al., 2015). However, these findings have not been consistently replicated (Brilot et al., 2011; Morris et al., 2009; Singer et al., 2005; Xu et al., 2021). Few studies have examined the pathophysiology of PANS.
In recent work (Frick et al., 2018; Xu et al., 2021), we focused on the binding of PANDAS-associated antibodies to specific cell types in the basal ganglia; this contrasts with earlier work examining candidate molecular targets. We have described, and replicated, elevated binding of IgG from children with strictly defined PANDAS to striatal cholinergic interneurons (CINs), relative to age- and gender-matched healthy controls. This antibody binding leads to reduced CIN activity in vitro; it is reduced, in parallel with symptom improvement, after IVIG treatment (Frick et al., 2018; Xu et al., 2021).
It is unclear whether this finding extends to PANS, a more heterogeneous diagnostic entity. Here, we addressed this question in a cohort of patients with relapsing/remitting PANS and clinical evidence of Streptococcal infection, treated at the Immune Behavioral Health Clinic at Stanford University. We confirm elevated binding to striatal CINs by IgG in plasma drawn during symptom flare, compared to plasma drawn from matched control subjects. PANS IgG drawn at flare reduces activity of striatal CINs; neither elevated IgG binding to CINs nor the inhibition of CIN activity are seen in plasma drawn from the same children during symptom recovery/remission. These data suggest that IgG binding to striatal CINs contributes to the pathophysiology of PANS.
2. Materials and methods
2.1. Specimen collection
These investigations of human plasma samples were approved by the Human Investigations Committees of Yale and Stanford Universities. Plasma was collected at the Stanford Immune Behavioral Health Clinic and Research Program, from local patients and controls (living in the 7 counties surrounding Stanford University). Patients were classified as meeting PANS criteria, with a relapsing and remitting course, by a child psychiatrist (MT or MS). Patients were selected who had a positive test for Streptococcus, though many did not meet full diagnostic criteria for PANDAS. Parents gave written informed consent to participate in the study, and competent subjects gave assent prior to blood collection. Serum samples were collected during symptom flare; in a subset of subjects, samples were also collected during a period of symptom recovery/remission, either before or after the flare (time between symptom onset and remission/recovery draws: 4.15 ± 0.85 years). Mean IgG titers were 956.53 ± 76.59 mg/dL in the healthy control subjects and 756.75 ± 46.63 mg/dL in the flare subjects. In the subset of subjects who had recovery draws, IgG titer were 717.27 ± 55.35 mg/dL and 756.61 ± 91.08 mg/dL during flare and recovery, respectively. All samples were aliquoted prior to storage at the Stanford Biobank, re-aliquoted into smaller volumes upon arrival at Yale, and stored at −80 °C until use. Demographic data for all subjects are shown in Supplementary Table 1 and Table 1. All samples were anonymized before being sent from Stanford to Yale for analysis; all analyses were performed blind to diagnosis and condition. Samples/disease status classification was based on the data collected by the Clinician Encounter Form which clinicians/research staff complete at the end of the clinic visit after reviewing patient/parent questionnaires, psychometrics, and visit notes.
Table 1. Cohort Characteristics - Patients and Healthy Controls.
Demographic and Clinical Characteristics of 25 Patients with Pediatric Acute-onset Neuropsychiatric Syndrome and 25 Healthy Controls.
| Patients (N = 25) | Healthy Controls (N = 25) | |
|---|---|---|
| Characteristic | No. (%) | No. (%) |
| Age of First neuropsychiatric symptom onset, mean (SD), years | 8.4 (2.9) | |
| Age of meeting PANS criteria | 9.9 (3.8) | |
| Age of first IBH PANS clinic visit | 11.2 (3.6) | |
| Age at “flare”/Control blood draw chosen for this study | 12.2 (4.0) | 12.2 (4.2) |
| Age at “recovery” blood draw chosen for this study | 12.1 (4.4) | |
| Biological Male Sex | 12 (48.0) | |
| Race and ethnicity, n (%) | ||
| Non-Hispanic White | 19 (76.0) | 11 (44.0) |
| Other | 6 (24.0) | 14 (56.0) |
| PANS symptoms at clinic presentation, n (%) | ||
| OCD | 25 (100.0) | |
| Eating restriction | 15 (60.0) | |
| Anxiety | 25 (100.0) | |
| Emotional lability and/or depression | 19 (76.0) | |
| Irritability, aggression, and/or severely oppositional behaviors | 22 (88.0) | |
| Behavioral/developmental regression | 10 (40.0) | |
| Deterioration in school performance | 11(44.0) | |
| Sensory dysregulationa | 16 (64.0) | |
| Motor abnormalitiesb | 12 (48.0) | |
| Sleep disturbance | 19 (76.0) | |
| Increased urinary frequency and/or enuresis | 10 (40.0) | |
| Homicidal or suicidal ideation/attempts | 9 (36.0) | |
| Global Impairment from psychiatric symptoms, mean (SD)c, d | 52.8 (18.9) | 0.0 (0.2) |
| Caregiver Burden Inventory, mean (SD)c, e | 39.1(14.8) | 4.2 (6.5) |
Hyperacusis, photophobia, pain amplification, inability to feel pain, etc.
Tics, motoric hyperactivity, chorea, etc.
As these measures vary with the relapsing-remitting course of PANS, the score recorded during the visit closest to the blood draw was used for analysis.
Global impairment score is a parent-rated score of global functioning validated for the PANS population. The score ranges from 0 to 100 (the higher the worse).
Caregiver burden inventory is a parent-rated score of caregiver burden, and ranges from 0 to 96 (the higher the worse). A cut-off point of 36 represents a need for respite care. It has been validated in the PANS population.
ADHD, attention-deficit/hyperactivity disorder; IQR, interquartile range; OCD, obsessive compulsive disorder; PANS, Pediatric Acute-onset Neuropsychiatric Syndrome; SD, standard deviation.
2.2. Mouse and human brain tissues
All experimental procedures were approved by the Yale University Institutional Animal Care and Use Committee, in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Male and female C57BL/6J mice (adults 3–6 months old and juveniles 4–5 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, Maine, http://jaxmice.jax.org/strain/013636.html). Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and transcardially perfused with cold 4% paraformaldehyde in 1×PBS (pH 7.4). Brains were fixed overnight in 4% PFA at 4 °C, followed by equilibration in 30% sucrose for 48 h at 4°C. Striatal slices were cut at 20 μm using a Leica CM3050S cryostat (Leica, Buffalo Grove, IL). Slices were stored in a cryoprotectant solution (30% glycerin, 30% ethylene glycol in 1×PBS pH 7.4) at −20°C until use.
Slices of human basal ganglia were collected and prepared as part of an unrelated study, as described previously (Kataoka et al., 2010). Subject demographics are shown in Supplementary Table 2.
2.3. Reagents and antibodies
Ketamine (Ketaset) was obtained from Zoetis (Madison, NJ). Xylazine (Anased) was obtained from Akorn, Inc. (Decatur, IL). Reagents for immunohistochemical staining (Fig. 1–3) were obtained from Sigma (St. Louis, MO: Sudan Black B, Triton X-100, sodium fluoride) or from JT Baker (Phillipsburg, NJ: paraformaldehyde). Normal donkey serum in blocking buffer was obtained from Jackson Immunoresearch (West Grove, PA). Chemicals used for acute brain slice analysis (Fig. 4) were obtained from Sigma (N-methyl-D-glucamine (NMDG), glucose, thiourea, sodium pyruvate, sodium ascorbate and HEPES) or JT Baker (KCl, NaCl, NaH2PO4, NaHCO3, MgSO4 and CaCl2). Antibodies used in immunohistochemical staining are listed in Supplementary Table 3.
Fig. 1. Elevated binding of PANS IgG to striatal cholinergic interneurons in adult mouse brains.
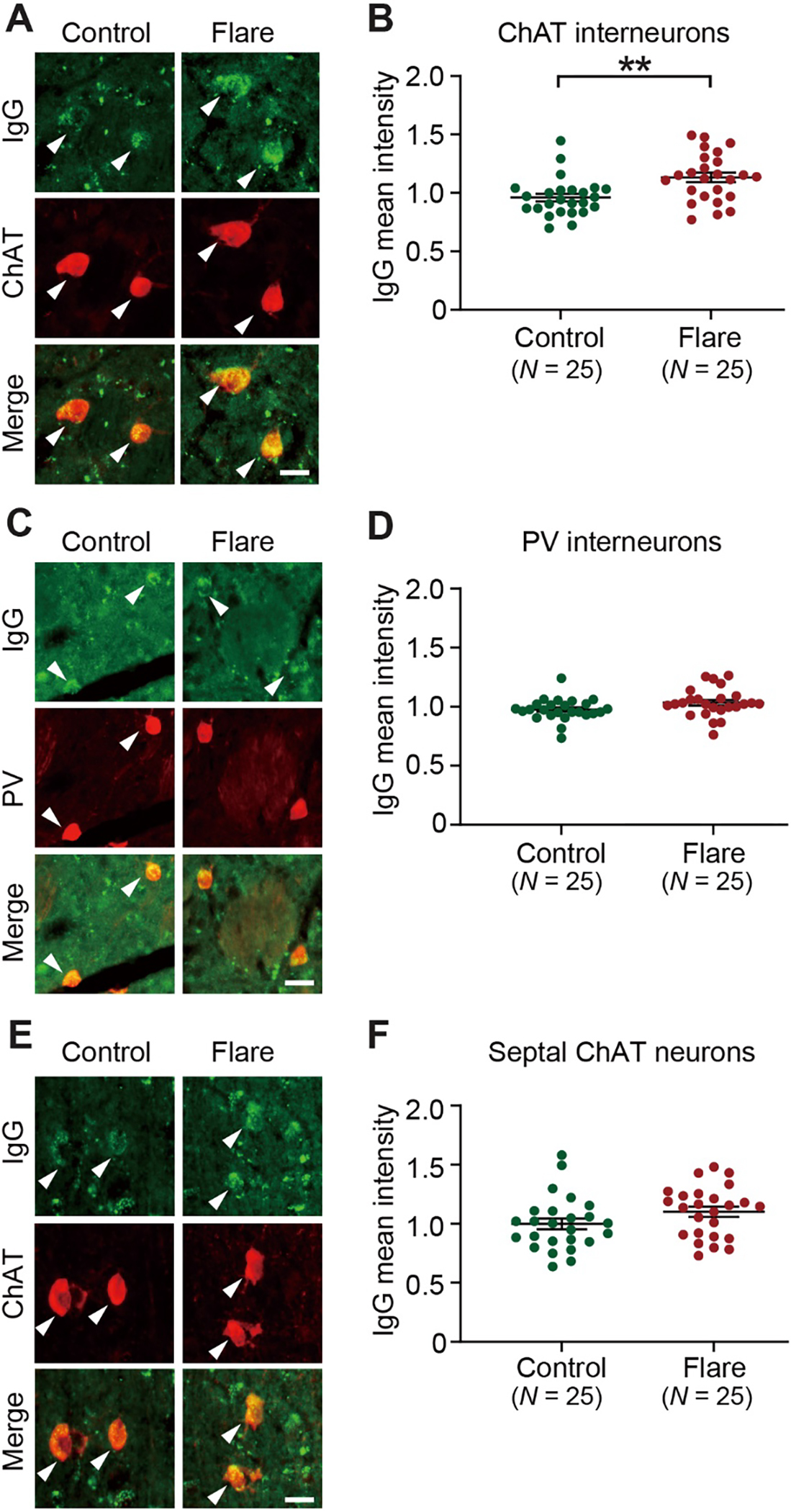
A. Representative images of immunohistochemical staining for human IgG (green) and choline acetyltransferase (ChAT, red) after incubation of mouse brain slices with diluted PANS flare and control plasmas. Arrowheads indicate human IgG binding to ChAT-positive interneurons in the mouse striatum. B. IgG from PANS “flare” plasmas showed elevated binding to ChAT interneurons relative to IgG from control plasmas (2-tailed independent sample t-test: t[48] = 3.263, p = 0.002; Cohen’s d = 0.922). C. Representative images of immunohistochemical staining of human IgG (green) and parvalbumin (PV, red). Arrowheads indicate human IgG binding to PV-positive interneurons. D. Plasma IgG binding to PV interneurons did not significantly differ between PANS “flare” and control groups (2-tailed independent sample t-test: t[48] = 1.915, p = 0.061). E. Representative images of immunohistochemical staining of human IgG (green) and choline acetyltransferase (ChAT, red) in the medial septal nuclei. Arrowheads indicate human IgG binding to ChAT-positive neurons. F. Plasma IgG binding to septal ChAT neurons did not significantly differ between PANS “flare” and controls (2-tailed independent sample t-test: t[48] = 1.627, p = 0.110). Data are shown as mean ± S.E.M. Each data point represents the mean value obtained from 6 mice treated with a single plasma. **p < 0.01; Numbers in parentheses indicate sample size.. Scale bars: 20 μm.
Fig. 3. PANS IgG shows elevated binding to cholinergic interneurons in human brain slices.
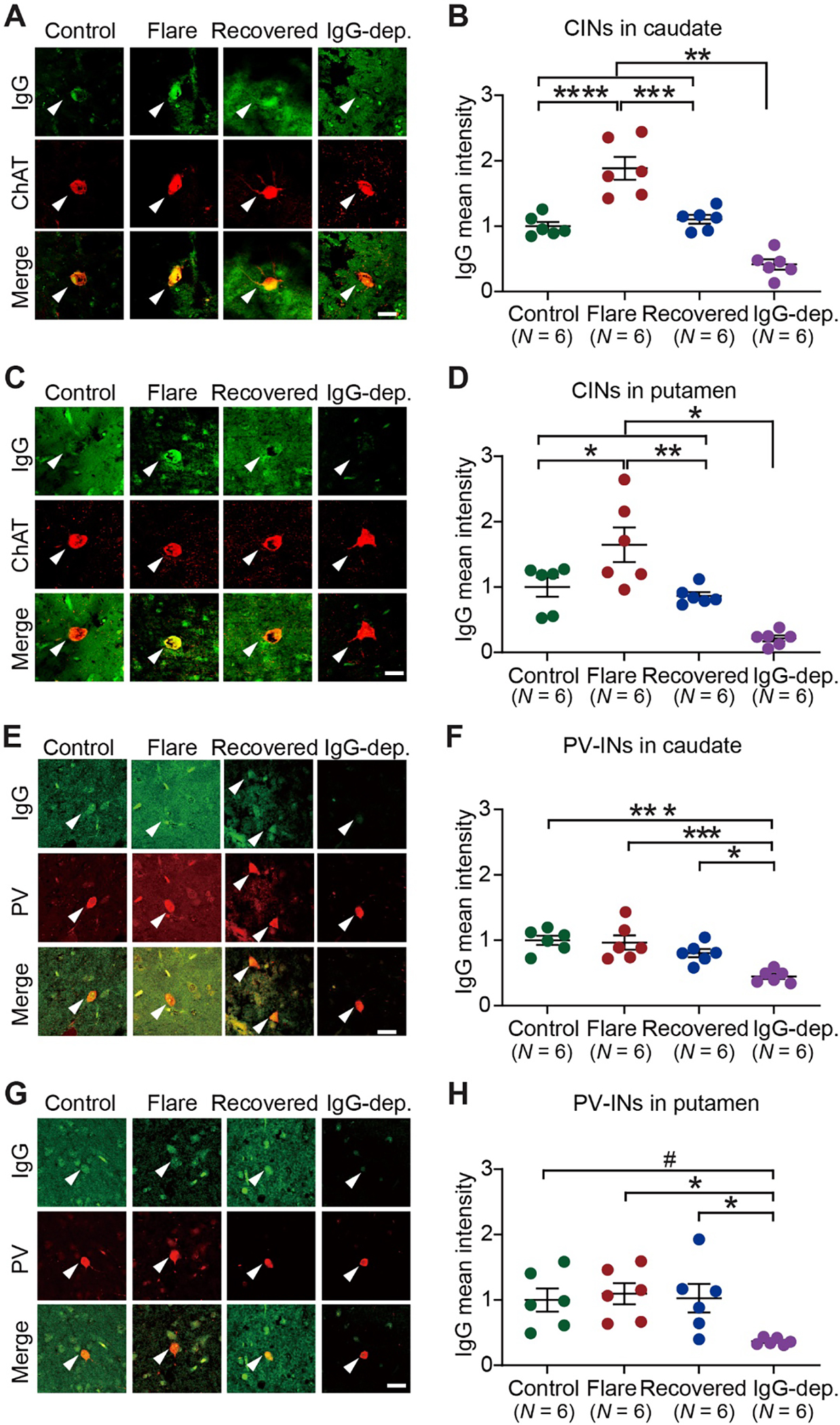
A, C. Representative images of immunohistochemical staining of human IgG (green) and choline acetyltransferase (ChAT, red) in human caudate (A) and putamen (C). Arrowheads indicate human IgG binding to CINs. See Figs S6 and S7 for additional images. B, D. PANS “flare” IgG showed elevated binding to CINs in human caudate and putamen. In caudate (B), a significant main effect was found (one-way ANOVA: F(3, 20) = 32.16, p < 0.0001, η2 = 0.828). Tukey’s post hoc tests revealed elevated IgG binding in the PANS “flare” group relative to the control group (Control vs Flare, p < 0.0001). This elevation was reversed in the “recovered” group (Flare vs Recovered, p = 0.0002). No difference was found between control and “recovered” samples (Control vs Recovered, p = 0.930). All three group showed higher IgG fluorescence intensity relative to PANS “flare” plasmas from which IgG was depleted (p < 0.01). In putamen (D), a significant main effect was found (one-way ANOVA: F(3, 20) = 14.26, p < 0.0001, η2 = 0.681). Tukey’s post hoc tests revealed elevated IgG binding in the PANS “flare” group relative to the control group (Control vs Flare, p = 0.037). This elevation was lost in the “recovered” group (Flare vs Recovered, p = 0.010). No difference was found between the control and “recovered” groups (Control vs Recovered, p = 0.930). All three group showed higher IgG fluorescence intensity relative to PANS “flare” plasmas from which IgG was depleted (p < 0.05). E, G. Representative confocal images of immunohistochemical staining of human IgG (green) and parvalbumin (PV, red) in human caudate (E) and putamen (G). Arrowheads indicate human IgG binding to PV-positive interneurons. F, H. PANS “flare” IgG did not show elevated binding to PV-positive interneurons. In caudate (F), a significant main effect was found (one-way ANOVA: F(3, 20) = 11.42, p = 0.0001, η2 = 0.631). IgG binding in the PANS “flare” group did not differ from that of the control group (Control vs Flare, p = 0.987) or the “recovered” group (Flare vs Recovered, p = 0.445). No difference was found between the control and “recovered” groups (Control vs Recovered, p = 0.278). All three group showed higher IgG fluorescence intensity relative to PANS “flare” plasmas from which IgG was depleted (Control vs IgG-dep, p = 0.0002; Flare vs IgG-dep, p = 0.0005; Recovered vs IgG-dep, p = 0.015). In putamen (H), a significant main effect was found (F(3, 20) = 4.440, p = 0.0151, η2 = 0.400). IgG binding in the PANS “flare” group did not differ from that of the control group (Control vs Flare, p = 0.975), or the “recovered” group (Flare vs Recovered, p = 0.990). No difference was found between the control and “recovered” groups (Control vs Recovered, p = 0.999). All three group showed higher IgG fluorescence intensity relative to PANS “flare” plasmas from which IgG was depleted (Control vs IgG-dep, p = 0.052; Flare vs IgG-dep, p = 0.022; Recovered vs IgG-dep, p = 0.041). Data are shown as mean ± S.E.M. Each data point represents mean value obtained from 2 post-mortem human brains for each plasma. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, #p = 0.052; Numbers in parentheses indicate sample size. Scale bars: 20 μm.
Fig. 4. PANS plasma reduces spontaneous activity in striatal cholinergic interneurons.

Acute mouse striatal slices were treated with plasma from control subjects (Con), PANS “flare” (Flare), PANS “recovered” (Recov.), and PANS flare plasmas from which IgG was depleted (IgG-dep.). A. Representative images of immunohistochemical staining for phospho-rpS6 (p-rpS6, green) and choline acetyltransferase (ChAT, red). Arrowheads indicate p-rpS6/ChAT co-labeled ChAT interneurons. B. A significant main effect was found (F[3, 20] = 5.673, p = 0.0056, η2 = 0.460). Control plasma did not significantly alter p-rpS6 relative to vehicle (one-sample t-test: t[5] = 1.879, p = 0.119). PANS “flare” plasma reduced phosphorylation levels of rpS6 in striatal CINs compared to control, at trend level (Con vs Flare, p = 0.079). This reduction was not seen in “recovered” plasma from the same subjects (Flare vs Recov, p = 0.046), or after IgG depletion in PANS “flare” plasma (Flare vs IgG-dep, p = 0.004). p-rpS6 levels did not differ between the control, “recovered”, or IgG-depleted groups (Con vs Recov, p = 0.993; Con vs IgG-dep, p = 0.500; Recov vs IgG-dep, p = 0.664). C. p-rpS6 levels correlated negatively with plasma IgG binding to striatal CINs (shown in Fig. 1A,B and Fig. 2A,B) after plasma incubation (R2 = 0.547, p = 0.004). D. p-rpS6 levels in CINs correlated negatively with CY-BOCS scores after plasma incubation (R2 = 0.355, p = 0.045). E. For each PANS “flare”-“recovered” pair, the difference in IgG binding to striatal CINs (Fig. 2A,B) correlated negatively with difference in p-rpS6 levels after plasma incubation (R2=0.767, p = 0.022). F. Representative images of immunohistochemical staining of phospho-rpS6 (p-rpS6, green) and parvalbumin (PV, red) in striatal slices after plasma treatment. Arrowheads indicate p-rpS6/PV co-staining. G. p-rpS6 levels in striatal PV interneurons remained unchanged after plasma incubation (one-way ANOVA: F(3, 20) = 0.306, p = 0.821). H. p-rpS6 levels did not correlate with plasma IgG binding to PV interneurons (shown in Fig. 1C, D and Fig. 2C, D) after plasma incubation (R2 = 0.029, p = 0.499). I. p-rpS6 levels in PV interneurons did not correlate with CY-BOCS scores after plasma incubation (R2 = 0.072, p = 0.393). J. For each PANS “flare”-“recovered” pair, the difference in IgG binding to striatal PV interneurons did not correlate with change in p-rpS6 levels after plasma incubation (R2 = 0.101, p = 0.314). Data are shown as mean ± S.E.M. Each data point represents mean value obtained from slices from 5 mice treated with a single plasma. Pairwise comparisons were performed using Tukey’s post hoc test. *p < 0.05, **p < 0.01, #p = 0.079; Numbers in parentheses indicate sample size. Correlation was determined by linear regression analysis using Pearson’s correlation (C, E, H and J) or Spearman’s correlation (D and I). Dotted lines in correlation analyses (C-E and H-J) indicate 95% confidence intervals. Scale bars: 20 μm.
2.4. Determination of IgG titers in plasma samples
Total IgG titers in plasma samples were determined using IgG (Total) Human ELISA Kit (Thermo Fisher Scientific, Rockland, IL) following manufacturer’s instructions and as described (Xu et al., 2021). More details are described in the Supplementary Information. Total IgG titers were higher in control samples than in PANS samples; there were no differences between genders or between PANS “flare” and “recovery” samples. All plasmas were diluted in 1×PBS + 0.1% BSA (bovine serum albumin) to 500 mg/dL IgG and randomized before testing. An equal amount of diluted plasma, and thus of IgG, was used in all assays.
2.5. Assessing phospho-rpS6 levels in acute mouse brain slices
Acute coronal mouse brain slices containing the striatum were prepared from male and female C57BL/6J mice as previously described (Ting et al., 2014; Xu et al., 2021). Briefly, mouse brains were quickly removed and placed in ice-cold oxygenated NMDG-aCSF (artificial cerebrospinal fluid, in mM: 92 NMDG, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 10 MgSO4, 0.5 CaCl2, 25 glucose, 2 thiourea, 3 sodium pyruvate and 5 sodium ascorbate, pH 7.35, saturated with 95% O2/5% CO2). Coronal slices (100 μm) through the striatum were cut using a Leica VT1000S vibratome (Leica Microsystems, Bannockburn, IL, USA) in the NMDG-aCSF solution. Slices were recovered in NMDG-aCSF for 10 min at 32°C before being transferred to regular aCSF (in mM: 119 NaCl, 2.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 2 CaCl2, 2 MgSO4 and 12.5 glucose) for 1 h at 30 °C under constant oxygenation with 95% O2/5% CO2. After recovery, slices were treated with plasma samples (6.25 mg/dL diluted in aCSF) or the aCSF control for 1 h at 30°C. After treatment, slices were fixed in cold 4% paraformaldehyde (PFA) in 1×PBS (pH 7.4) containing 5 mM NaF for 1 h at 4 °C, followed by immunohistochemistry.
2.6. Immunohistochemistry and image quantification
Paraformaldehyde-fixed mouse striatal slices (20 μm coronal sections) and formalin-fixed human brain slices (50 μm coronal sections of caudate and putamen) were used to examine plasma IgG deposition, as described (Xu et al., 2021). Brain sections were blocked in freshly prepared 0.1% Sudan Black B (in 70% ethanol) for 10 min at RT to reduce autofluorescence (Baschong et al., 2001). After washes, sections were incubated in blocking buffer (1×PBS + 0.3% Triton X-100 supplemented with 5% normal donkey serum) for 1h at RT, and then incubated with plasma (1.25 mg/dL each in blocking buffer) overnight at 4°C. The next day, sections were washed in blocking buffer and incubated with an anti-human IgG antibody and specific neuronal markers (ChAT and PV, Supplementary Table 3) overnight at 4 °C, followed by incubation with fluorophore-conjugated secondary antibodies (Supplementary Table 3) for 1 h at RT. After washes, sections were mounted in Vectashield HardSet Mounting Medium (Vector Laboratories), coverslipped, and stored at 4 °C.
For plasma IgG binding to mouse neurons (Figs. 1 and 2), each plasma was tested in brain sections from 6 mice (both male and female), with 4–6 images collected randomly from the dorsal striatum of each mouse, without overlap, using an Axio Scope A1 fluorescent microscope with a 10×/0.45 NA objective (Zeiss, Germany). For plasma IgG binding to human brain slices (Fig. 3), each plasma was tested in brain sections from 2 subjects, with 6 images collected, without overlap, in each section. Images were captured by sequential scanning of sections on an Olympus Fluoview FV-1000 confocal microscope with a 20×/0.85NA objective (Olympus, Japan).
Fig. 2. IgG binding to cholinergic interneurons in adult mouse brain is reduced in plasmas drawn from PANS subjects during symptom recovery.
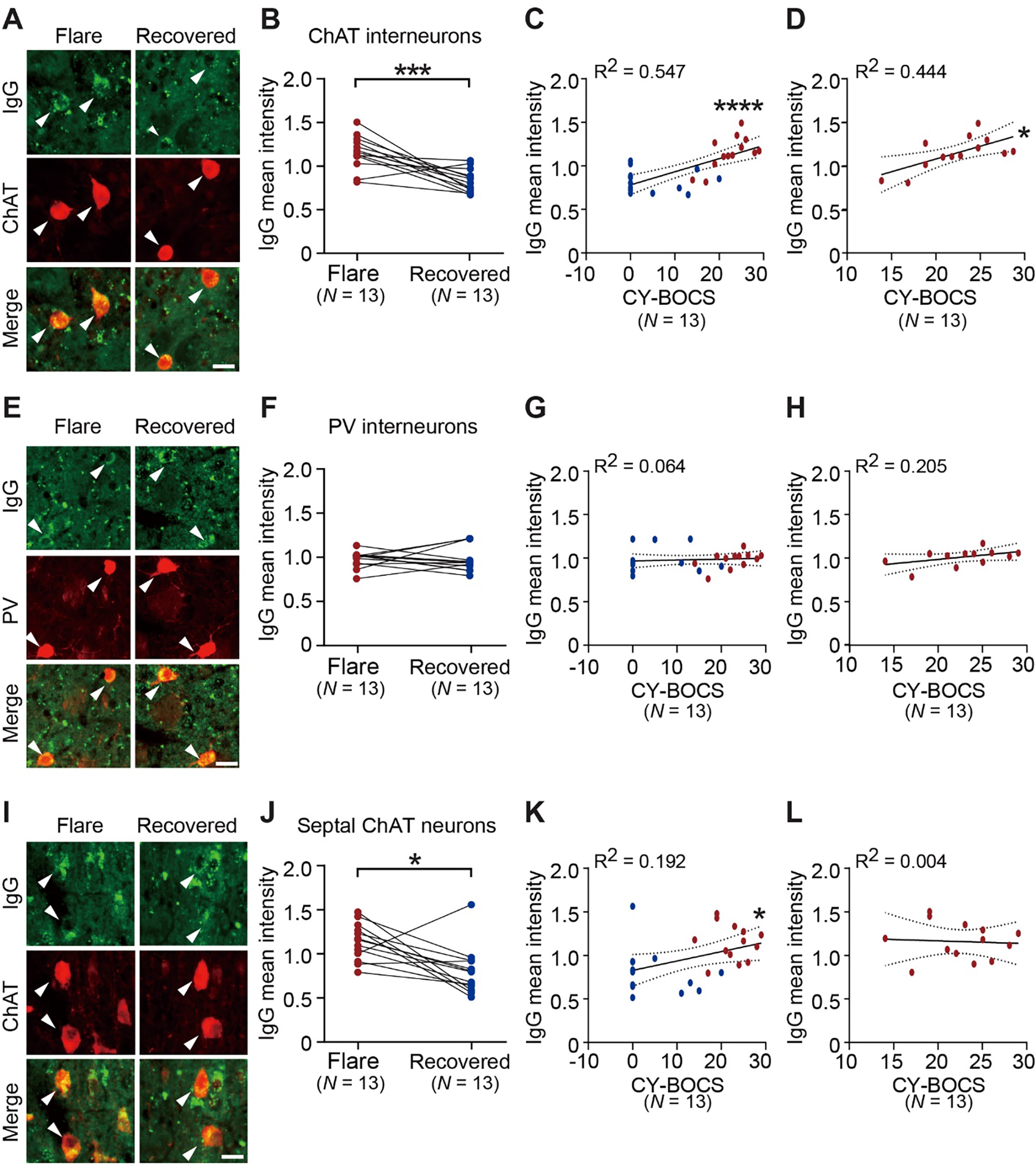
A. Representative images of immunohistochemical staining of human IgG (green) and choline acetyltransferase (ChAT, red) in the mouse striatum. Arrowheads indicate human IgG binding to ChAT-positive interneurons. B. IgG binding to ChAT interneurons was decreased during symptom recovery, relative to plasma collected during symptom flare (paired t-test: t[12] = 4.534, p = 0.0007; Cohen’s d = 1.258). C. IgG binding to striatal ChAT interneurons correlated with CY-BOCS scores (Spearman’s correlation: R2 = 0.547, p < 0.0001) in all the cases shown in B. D. PANS “flare” IgG binding to striatal ChAT interneurons correlated with CY-BOCS scores (Pearson’s correlation: R2 = 0.444, p = 0.013). E. Representative images of immunohistochemical staining of human IgG (green) and parvalbumin (PV, red). Arrowheads indicate human IgG binding to PV-positive interneurons. F. IgG binding to PV interneurons did not differ between PANS “flare” and “recovered” groups (Wilcoxon paired signed-rank test: W = −3, p = 0.946). G. IgG binding to striatal PV interneurons did not correlate with CY-BOCS scores (Spearman’s correlation: R2 = 0.064, p = 0.202). H. PANS “flare” IgG binding to striatal PV interneurons did not correlate with CY-BOCS scores (Pearson’s correlation: R2 = 0.205, p = 0.121). I. Representative images of immunohistochemical staining of human IgG (green) and choline acetyltransferase (ChAT, red). Arrowheads indicate human IgG binding to ChAT-positive neurons in the medial septum (septal ChAT neurons). J. IgG binding to septal ChAT neurons was decreased in the “recovered” samples relative to PANS “flare” (Wilcoxon paired signed-rank test: W = −69, p = 0.013; r(equivalent) = 0.473). K. Across all samples, IgG binding to septal ChAT neurons showed a slight but significant correlation with CY-BOCS scores (Spearman’s correlation: R2 = 0.192, p = 0.025). L. PANS “flare” IgG binding to septal ChAT neurons did not correlate with CY-BOCS scores (Pearson’s correlation: R2 = 0.004, p = 0.833). Data are shown as mean ± S.E.M. Each data point represents mean value obtained from 6 mice for each plasma. *p < 0.05, ***p < 0.001, ****p < 0.0001; Numbers in parentheses indicate sample size. Dotted lines in correlation analyses (C, D, G, H, K and L) indicate 95% confidence intervals. Scale bars: 20 μm.
To assay the effect of PANS plasma on CIN activity (Fig. 4), acute mouse striatal slices treated with plasma (as above) were immunostained with anti-phospho-rpS6 (see Supplementary Table 3), following the same procedure. Each plasma was tested in brain sections from 5 mice, with 6 images chosen randomly without overlap collected in each section using an Olympus Fluoview FV-1000 confocal microscope with a 20×/0.85NA objective. Acquisition settings were kept constant for all image acquisitions within each experiment.
Automated quantitation of mean fluorescence intensity within each cell was achieved using Fiji ImageJ from NIH (https://imagej.net/Fiji/Downloads) with batch processing as described (Xu et al., 2021). Briefly, images of neuronal marker immunostaining (ChAT or PV) were thresholded and used to generate regions of interest (ROIs) corresponding to cell bodies of the selected cell type. These ROIs were then overlayed on the corresponding images of human IgG immunostaining (Figs. 1–3) or phospho-rpS6 immunostaining (Fig. 4), and immunostaining within each cellular ROI was quantified.
2.7. Data analysis
Plasma samples were processed in multiple batches. All plasmas were diluted to 500 mg/dL and randomized before testing. To minimize batch effects, PANS “Flare” plasmas, age- and gender-matched controls, and PANS “Recovered” draws (when available) were balanced within each batch. Batch effects on raw values were small in most cases (Supplementary Fig. 1); nevertheless, to reduce variability, data were normalized to the mean value of the control samples processed in the same batch (Fig. 1–3) or the aCSF-treated control from the same batch (Fig. 4) for analysis. All data are aggregated by plasma sample (i.e. the n for each analysis is the number of plasma samples, not the number of images, slices, or mice) and expressed as mean ± S.E.M. All raw and normalized values are shown in Supplementary Table 4. Statistical analyses were performed using SPSS Statistics 28 (IBM, New York, NY) or Prism 9.4 (GraphPad Software, La Jolla, California). Significance (p < 0.05) was determined by two-tailed t-test (unpaired or paired), one-sample t-test, or one-way analysis of variance (ANOVA) with post-hoc Tukey’s test for normally-distributed data, or by Wilcoxon paired signed-rank test and Kruskal-Wallis H test for non-normally-distributed data (data normality was evaluated using the Shapiro-Wilk test in Prism 9.4). Pearson or Spearman correlation was used to examine relationships between measures. Test-retest repeatability was evaluated using intraclass correlation coefficients, as described (Koo and Li, 2016). Effect sizes were calculated where significant differences were found between groups. Specifically, Cohen’s d and eta squared (η2) were used for parametric data; whereas r(equivalent) was used for non-parametric data (analyzed by Wilcoxon paired signed-rank test) (Rosenthal, 1991; Rosenthal and Rubin, 2003). Chi-square was used to examine categorical (positive or negative) differences between groups, followed by evaluation of Cramer’s V (Cohen, 2013) and odds ratios (Szumilas, 2010) as measures for effect size. The specific test used in each analysis is indicated in the corresponding figure legend. The details of all statistical analyses are shown in Supplementary Table 5.
3. Results
3.1. Plasma antibodies from children with PANS.
Children with PANS had lower total IgG titers in plasma collected during a symptom flare than matched controls (t[48] = 2.228, p = 0.031). In the subset of children for whom paired blood draws during a flare and during a period of recovery were available, IgG titers did not differ between flare and recovery plasma samples (2-tailed paired t-test: t[12] = 0.450, p = 0.661). Titers of PANS flare (N = 25) or PANS flare-recovery pairs (N = 13 each group) correlate with symptom severity as measured by CY-BOCS scores (not shown).
3.2. Elevated binding of antibodies from PANS plasma to mouse striatal cholinergic interneurons
Plasma IgG from children with PANS, collected during symptom flare, showed elevated binding to adult mouse striatal CINs, relative to plasma from age- and gender-matched controls (Fig. 1A–B; Cohen’s d = 0.922). This effect size is large, but somewhat smaller than that seen in our previous study (Xu et al., 2021) of children with strictly defined PANDAS who responded to IVIG (d = 1.502). There were no effects of gender or age at sample draw (not shown); we therefore did not include these covariates in subsequent analyses, for simplicity. IgG titers did not correlate with values of IgG binding to mouse striatal CINs (not shown).
To categorize subjects as ‘positive’ or ‘negative’ for CIN binding, we initially used the 95th percentile of binding in the control group controls as a cutoff, as is commonly done in other contexts (Agmon-Levin et al., 2014). Chi-square analysis revealed a trend towards more positive cases in the PANS flare group than in the control group (Supplementary Fig. 2B; p = 0.123). Next, we plotted a receiving operating characteristic curve (ROC) to determine an optimal cutoff value (Supplementary Fig. 2C). The area under the curve (AUC = 0.749) indicated an overall acceptable accuracy of CIN binding as a test for PANS (Hosmer Jr et al., 2013) and identified the optimal cutoff value as the 88th percentile of the control group. Chi-square analysis using this cutoff value showed the PANS flare group had more positive cases relative to the control group (p = 0.0002; Supplementary Fig. 2D).
Next, we re-analyzed individual images, categorizig each individual cell as ‘positive’ or ‘negative’ for IgG binding based on signal-to-background ratio (≥ 1.5) and found the PANS flare group had higher percentage of IgG+-ChAT+ cells than controls (p = 0.007; Supplementary Fig. 2E), consistent with a previous study in which individual cells were manually categorized (Frick et al., 2018) and with the intensity-based analysis presented here (Fig. 1B). Using this measure, and setting a cutoff value at the 95th percentile of the control distribution (Supplementary Fig. 2F), chi-square analysis showed increased IgG-positive cells in the PANS flare group (p = 0.017). As before, we obtained the optimal cutoff value based on the ROC (Supplementary Fig. 2G). The AUC (0.757) indicated an overall acceptable accuracy of the test; the optimal cutoff value was at the 88th percentile of the control group. Chi-square analysis using this cutoff confirmed that the PANS flare group had significantly more positive cases (p = 0.001; Supplementary Fig. 2H).
No statistically significant group differences were found in IgG binding to parvalbumin (PV)-expressing striatal interneurons (Fig. 1C–D) or to cholinergic neurons in the medial septum (Fig. 1E–F), although trends were seen in both cases.
We selected 6 PANS “flare” cases with relatively high plasma antibody binding to mouse striatal CINs, available “recovered” draws, and corresponding age- and gender-matched controls (see Supplementary Table 1) for further analysis. As PANS is a pediatric condition, we repeated the IgG binding assay in this subset using sections from juvenile mouse brains. As in adult brains, PANS flare IgG showed elevated binding to CINs but not to PV interneurons or ChAT neurons in the medial septum (Supplementary Fig. 3). IgG binding to CINs in adult and juvenile mouse tissue was correlated.
The elevated binding of PANS flare IgG to CINs, but not to PV interneurons, was further confirmed in mouse striatal slice cultures (Supplementary Fig. 4A, B, E, F).
3.3. Decreased plasma IgG binding to CINs in “recovered” draws
For a subset of these relapsing and remitting PANS cases, plasma was available both during symptom flare and during a period of symptomatic recovery (see Methods for details). We examined the difference in plasma IgG binding to adult mouse CINs bewteen symptom flare and recovery samples in these subjects (N = 13). Antibody binding to CINs was reduced in recovery samples and paralleled symptom improvement (Fig. 2A–B; d = 1.258). IgG binding to CINs correlated with symptom severity, as measured using the Child Yale-Brown Obsessive-Compulsive Scale (CY-BOCS (Scahill et al., 1997); Fig. 2C–D). There was no similar change in plasma IgG binding to PV interneurons (Fig. 2E–F) or correlation between IgG binding to PV interneurons in adult mouse brains and CY-BOCS scores (Fig. 2G–H). Unexpectedly, there was a reduction in plasma IgG binding to adult septal cholinergic neurons in conjunction with symptom improvement (Fig. 2I–J). A weak but statistically significant correlation was found between IgG binding to septal cholinergic neurons and CY-BOCS scores (Fig. 2K–L). Total IgG titer did not correlate with IgG binding to CINs in the flare-recovery samples (not shown).
We repeated this flare-recovered comparison in juvenile mouse sections in the six selected samples (Supplementary Table 1). We found the same binding patterns in juvenile mouse brains, i.e. the “recovered” samples showed decreased binding to CINs and septal cholinergic neurons, but not to PV interneurons (Supplementary Fig. 3 IgG binding in adult and juvenile mouse brain sections were moderately correlated in CINs and PV interneurons, with a trend-level correlation (based on Cohen’s guidelines (Cohen, 2013)) in septal cholinergic neurons (Supplementary Fig. 3D, H, L). We evaluated repeatability using the intraclass correlation coefficient (ICC) and found moderate consistency between results from adult and juvenile mouse brain slices (Koo and Li, 2016).
Finally, we examined the flare-recovered comparison in slice cultures. CIN binding was again normalized in matched plasma samples at recovery draws (Supplementary Fig. 4A, C). Both Pearson’s correlation and ICC indicated moderate consistency between results from slice cultures and brain sections (Supplementary Fig. 4D). No significant difference was found in IgG binding to PV interneurons in slice cultures. The values obtained in the different assays showed a trend towards a positive correlation (Supplementary Fig. 4E, G, H).
3.4. PANS IgG shows elevated binding to CINs, but not to PV interneurons, in normal human brains
We replicated the IgG binding analysis in the selected 6 PANS “flare” cases, matched recovery samples, and healthy controls (Supplementary Table 1) on post mortem human brain tissue. We additionally tested PANS “flare” plasmas from which IgG was depleted using Protein A/G agarose beads; IgG depletion was verified using an anti-human IgG antibody on a dot blot (Supplementary Fig. 5).
In the caudate, one-way ANOVA revealed a significant main effect of group (η2 = 0.828), with significantly elevated IgG binding to CINs in the PANS “flare” group but not in “recovery” samples, relative to controls (Fig. 3A–B). All plasma-treated brain sections showed higher IgG immunostaining than the IgG-depleted group (Fig. 3A–B). Similar results were seen with IgG binding to CINs in the putamen (η2 = 0.681; Fig. 3C–D). In PV interneurons, in contrast, significant main effects of group were driven by the IgG-depleted group; there were no differences between control and PANS plasmas, during “flare” or “recovered”, in caudate (Fig. 3E–F) or putamen (Fig. 3G–H).
To assess the reproducibility and reliability of our assays, we examined the relationship of IgG binding to interneurons in the mouse (Fig. 1) and human brain (Fig. 3); these values correlated strongly (Supplementary Fig. 6). Good to excellent repeatibility was also revealed by ICCs. To assess the possibility that our results are confounded by autofluorescence, which can be a problem in immuofluorescent studies in human post-mortem tissue despite the use of autofluorescence quenching, we replicated binding to human caudate using an enyzyme-based colorimetric approach. This again showed significantly elevated IgG binding to CINs in the PANS “flare” group relative to controls, which was reversed in in “recovery” samples (Supplementary Fig. 7).
3.5. PANS plasma IgG reduces CIN activity
We next tested the effect of PANS IgG on interneuron function, using phosphorylation of ribosomal protein S6 (p-rpS6) as a marker of spontaneous neural activity (Bertran-Gonzalez et al., 2012; Knight et al., 2012; Pirbhoy et al., 2016; Xu et al., 2021). Acute mouse striatal slices were incubated with plasma from the 6 selected PANS subjects and age- and gender-matched controls described above. In CINs, there was a significant main effect of pretreatment (one-way ANOVA, p = 0.006, η2 = 0.460). Control plasma did not significantly alter p-rpS6 levels, relative to vehicle. PANS “flare” plasma treatment significantly lowered p-rpS6 levels relative to control; this was not seen in “recovered” plasma or after IgG depletion. Neither “recovered” plasma nor IgG-depleted PANS “flare” plasma reduced p-rpS6 in CINs relative to ACSF (Fig. 4A–B).
Across the control, PANS “flare”, and “recovered” groups, plasma IgG binding to CINs correlated negatively with p-rpS6 levels after plasma pretreatment (Fig. 4C). p-rpS6 levels also showed a weak correlation with CY-BOCS scores in PANS “recovered” samples (Fig. 4D). Importantly, the difference between PANS “flare” and “recovered” IgG binding to CINs in the same subjects correlated with the difference in p-rpS6 levels after plasma incubation (Fig. 4E). These results indicate that PANS “flare” IgG binding negatively regulates the activity of striatal CINs.
No group difference was found in the activity of PV-expressing interneurons by one-way ANOVA (Fig. 4F–G). No correlations were found between PV p-rpS6 levels and other measures (Fig. 4H–J).
We used whole-cell patch recording in acute mouse brain slices from ChATBAC-eGFP transgenic mice (Gamage et al., 2020) to confirm the effect of PANS plasma on CIN activity. Because this experiment uses a large amount of serum it was performed with a single selected PANS sample that showed the highest IgG binding to mouse striatal CINs and the matched control (Fig. 1 andSupplementary Table 1), as a confirmatory test. CINs were readily identified by their size and morphology and confirmed by their electrophysiological properties (Supplementary Fig. 8A–C). PANS and control plasmas did not significantly affect intrinsic membrane properties of CINs, although there was a trend towards hyperpolarized resting membrane protetial (RMP) after treatment with the PANS plasma (not shown).
CINs receive various synaptic inputs, including glutamate and dopamine (DA) (Lim et al., 2014), and we have previously shown PANDAS serum to alter response to these transmitters (Xu et al., 2021). We examined CIN response to bath application of agonists for these receptors. As predicted, the ionotropic glutamate agonist AMPA produced a robust increase in action potential frequency in control plasma-pretreated CINs; this was significantly attenuated after pretreatment with PANS plasma (Supplementary Fig. 8D–E). Similarly, bath application of DA increased action potential frequency in control plasma-pretreated, but not PANS plasma-pretreated, CINs (Supplementary Fig. 8F–G).
4. Discussion
PANS, like PANDAS, has been proposed to derive from the production of autoantibodies that cross-react with targets in the brain, including in the basal ganglia, following infection in a susceptible individual, leading to neuroinflammation and subsequent motor and behavioral abnormalities (Chan and Frankovich, 2019; Swedo and Williams, 2017; Williams and Swedo, 2015). In support of this post-infectious autoimmune hypothesis, high rates of autoimmune disorders and inflammatory conditions have been reported in children with PANS and PANDAS, and in their first-degree relatives (Calaprice et al., 2017; Frankovich et al., 2015a; Gromark et al., 2019; O’Dor et al., 2022). Meta-analysis documents an increase in proinflammatory cytokines (most clearly TNFα, but probably also IL-17 and IL-1β) in children with OCD and movement disorder symptoms (Fabricius et al., 2022), though negative findings have also been reported (Cosco et al., 2019). Serum/plasma immunoglobulin levels have been reported to be altered in PANS/PANDAS subjects, with lower levels of IgG, IgA, and IgM, and higher levels of IgE (Calaprice et al., 2017; Frankovich et al., 2015a; Gromark et al., 2019; Leonardi et al., 2023; Murphy et al., 2015). Neuroimaging suggests that patients with PANS and PANDAS display changes to the basal ganglia region consistent with neuroinflammation; one PET study suggested microglial activation in the basal ganglia during the acute phase of illness (Cabrera et al., 2019; Giedd et al., 2000; Giedd et al., 1996; Kumar et al., 2015; Zheng et al., 2020).
We have previously described binding of PANDAS antibodies to striatal CINs, using sera collected over several years at the National Institute of Mental Health (Frick et al., 2018; Xu et al., 2021). To test whether this binding pattern generalizes to PANS, a more heterogeneous diagnosis, and to samples collected at other clincial sites, we here performed similar analyses in plasma from children with relapsing and remitting PANS, assessed at the Immune Behavioral Health Clinic at Stanford University. We find elevated binding of antibodies from children with PANS with evidence of Streptococcal infection, collected during symptom flare, to striatal CINs but not to two other neuron types, in both adult mouse and human brain (Figs. 1 and 3; Supplementary Fig. 7) as well as in juvenile mouse brain sections and adult mouse brain slice cultures (Supplementary Figs. 2 and 3). These results replicate our previous findings in patients with strictly-defined, IVIG-responsive PANDAS (Frick et al., 2018; Williams et al., 2016; Xu et al., 2021). The consistency and specificity of elevated antibody binding to CINs, despite the differences in diagnosis, geography, and sample collection (Stanford PANS clinic vs NIMH; PANS plasma vs PANDAS serum), increases confidence in the finding. Unlike our previous work with PANDAS, we find a trend towards increased antibody binding to PV interneurons and cholinergic septal neurons, suggesting that antibody binding to these other cell types might be relevant in a subset of the more heterogeneous PANS population.
Elevated IgG binding is not seen in samples collected from a subset of these subjects during a period of symptom recovery; binding correlates with symptom severity (as measured by CY-BOCS scores; Fig. 2). This is similar to what we have reported previously after IVIG treatment in PANDAS (Frick et al., 2018; Xu et al., 2021); however, in the PANS cases analyzed here, patients were treated clinically using a variety of interventions, and the order of and time between “flare” and “recovery” blood draws varies. Of note, we also find a significant reduction in antibody binding to cholinergic neurons in the medial septum at recovery in these subjects (Fig. 2), which was not observed in our previous study of PANDAS (Xu et al., 2021); this may reflect differences in the patient populations or in the treatments administered.
The molecular targets of these IgG antibodies remain to be elucidated. A number of potential antibody targets (including D1R and D2R) have been described in Sydenham chorea (SC), Tourette syndrome, and PANDAS. Some autoantibody targets may be shared among these disorders; others are likely to be distinct. Antibodies binding to D1R and D2R have been more consistently reported in SC (Ben-Pazi et al., 2013; Cox et al., 2013; Kirvan et al., 2007; Kirvan et al., 2003; Kirvan et al., 2006b) than in PANDAS (Brilot et al., 2011; Dale et al., 2012; Morris et al., 2009; Singer et al., 2005). We previously examined binding of antibodies to D1- and D2-expressing medium spiny neurons in the striatum and found no difference between PANDAS and control sera (Xu et al., 2021). It remains possible that PANS and PANDAS-associated antibodies bind to D2 receptors on CINs; D2Rs are known to be expressed at the postsynaptic sites in the majority of CINs (Dawson et al., 1988; Yan et al., 1997).
Here, as in our previous work (Xu et al., 2021), we find that PANS antibody binding to CINs reduces their activity, indexed using an activity-dependent molecular marker, p-rpS6 (Fig. 4) and confirmed using single-cell patch clamp electrophysiology (Supplementary Fig. 8). This effect on CIN activity is not seen with plasma collected during recovery, or in flare plasma from which IgG has been depleted. This supports the conclusion that IgG binding to CINs, as opposed to other activities in the flare plasma, is responsible for CIN inhibition.
Precisely how PANS antibody binding affects CINs, and how binding leads to reduced activity, remains to be determined. Previous studies in other contexts have shown various PANDAS and SC antibodies to decrease cyclic adenosine monophosphate (cAMP) levels and to induce calcium calmodulin protein kinase II (CaMKII) activity and dopamine release (Cox et al., 2013; Cunningham and Cox, 2016; Kirvan et al., 2006b). The relationship of these findings to the alteration of CIN activity shown here merit further study.
We have used phosphorylation of rpS6 to index neuronal activity (Biever et al., 2015; Meyuhas, 2008, 2015), but rpS6 is an important regulator of a range of cellular processes and may directly participate in pathophysiologically relevant events. rpS6 is regulated by phosphorylation at 2 pairs of sites: Ser235/Ser236 and Ser240/Ser244 (Bertran-Gonzalez et al., 2012) and is regulated by a number of cell signaling pathways, including S6 kinases (S6Ks) protein phosphatase 1 (PP1); these molecules are in turn regulated by the Akt-mammalian target of rapamycin (mTOR)-S6K signaling cascade (Biever et al., 2015; Meyuhas, 2008, 2015) and by cAMP-PKA through dopamine- and cAMP-regulated phosphoprotein, 32 kDa (DARPP-32) (Greengard, 2001; Hemmings et al., 1984). This network of signaling pathways is regulated by dopamine as well as by neural activity. It will be important to further characterize the effects of PANS and PANDAS autoantibody binding in CINs on these and related cellular signaling mechanisms.
Although few in number (about 1% of the total striatal cell population), CINs are key modulators of striatal function, releasing acetylcholine (ACh) across broad striatal territories (Apicella, 2007; Burguiere et al., 2015; Lim et al., 2014; Poppi et al., 2021; Rapanelli et al., 2017a; Tepper and Bolam, 2004). Several ACh receptors (AChRs) are expressed in GABAergic SPNs and interneurons in the striatum (Goldberg et al., 2012; Lim et al., 2014). Interestingly, inhibitory M4 mAChRs are expressed at higher levels in SPNs of the direct pathway (dSPNs) than in Spns of the indirect pathway (Hersch et al., 1994; Kreitzer, 2009; Yan et al., 2001). Thus, inhibition of CINs may result in an imbalance between the direct and indirect pathways (Lanciego et al., 2012; Nelson and Kreitzer, 2014). CINs are known to co-release glutamate (Higley et al., 2011) and GABA (Granger et al., 2016; Nelson et al., 2014), and to regulate dopamine release (Threlfell et al., 2012; Zhou et al., 2001). They may also modulate neuroinflammation: activation of α7 nAChRs and M3 mAChRs decreases microglial activation and cytokine (TNFα and IL-6) secretion in animal models (Binning et al., 2020; Dash et al., 2016; Gamage et al., 2020; Pannell et al., 2016). Thus, CIN dysregulation may alter basal ganglia information processing by multiple mechanisms.
Previous work has highlighted the contribution of CINs to symptomatology in OCD and TS. CINs are reduced in number in post-mortem tissue from individuals with TS (Kataoka et al., 2010; Lennington et al., 2016), as well as in TS-derived basal ganglia organoids (Brady et al., 2022). In animal models, selective depletion of striatal CINs results in repetitive behavioral pathology (Rapanelli et al., 2017b; Xu et al., 2015) and increased functional connectivity between frontal cortical areas and the motor region of the striatum (Martos et al., 2017). Thus, reduced activity by PANS antibody binding to CINs may have a range of effects across relevant brain circuitries.
There are several limitations to this study. First, although we extend our previous findings (obtained in strictly-defined PANDAS cases) to a more heterogeneous population (relapsing and remitting PANS, from a different clinical setting), and confirmed the binding patterns in both adult and juvenile mouse brain sections and adult mouse brain slice cultures, these PANS cases were selected for a history of Streptococcal infections. Unselected PANS cases may be still more heterogeneous; this will be examined in future work. Importantly, an autoimmune pathophysiology may not be restricted to PANS and PANDAS, but may occur in a broader subset of OCD cases. Anti-basal ganglia antibodies have been reported in idiopathic OCD (Bhattacharyya et al., 2009; Cox et al., 2015; Dale et al., 2005; Pearlman et al., 2014). Further work is required to examine whether elevated antibody binding to CINs is also present in non-PANS/PANDAS OCD or in other diagnostic groups.
Second, our immunofluorescence-based ex vivo assay is semi-quantitative and has significant background. Background fluorescence may derive from autoantibodies in control plasma, from nonspecific interactions between IgG and the target tissue, or from IgG-independent sources of fluorescence. Of note, IgG-depleted plasmas retains 10–30% of the signal of the control groups in the binding to human brain (Fig. 3). Autofluorescence may be caused by components other than IgG species in human serum/plasma samples (Wolfbeis and Leiner, 1985). This issue was overcomed by using enzyme-based double staining (Supplementary Fig. 6). However, while enzymatic IHC (e.g. HRP-DAB reaction) is widely used in the literature, there are concerns about the usage of DAB staining for quantification in light microscopy. It has been shown that the brown product from HRP-DAB reaction is actually a scatterer rather than an absorber of light, and thus the optical density does not follow a linear relationship with its concentration (van der Loos, 2008). Our confirmation of elevated PANS IgG binding to CINs in both mouse and human brain tissue despite these limitations is encouraging.
Third, we have focused on three neuron types (CINs, PV interneurons, and septal cholinergic neurons). We cannot exclude the possibility that PANDAS/PANS IgG may bind to other cells and in other brain regions – indeed, we think this is likely to be the case.
Fourth, the samples sizes were small in some experiments due to limited supplies of clinical plasma samples, human brain tissue, and the time-consuming nature for some of the work. A subset of control, PANS flare, and recovery samples was tested in the IgG binding to juvenile mouse brain, adult brain slice cultures, human brain, and spontaneous neural activity using p-rpS6 as a molecular marker (Figs. 3 and 4; Supplementary Figs. 3, 4 and 7). Whole-cell recording of CINs was only conducted on a single control-PANS pair (Supplementary Fig. 8). Nevertheless, results in these experiments were in agreement with our hypothesis and supportive of findings in the full cohort.
The identity of antibody targets and key signaling pathways affected by PANDAS/PANS antibody binding to CINs remain to be elucidated. The recent development of high-throughput screening technology (Wang et al., 2022; Wang et al., 2021) may facilitate the discovery of molecular targets of these autoantibodies. Given the etiological and phenomenological heterogeneity of these conditions, we expect that distinct autoantibodies will be present in different PANDAS/PANS cases. The current work and our previous findings point to CINs as a plausible locus of pathology in PANDAS/PANS, providing a framework to test candidate pathogenic autoantibodies.
In conclusion, this work in PANS replicates the antibody binding pattern and functional consequences on cellular activity previously seen in PANDAS (Frick et al., 2018; Xu et al., 2021), with elevated IgG binding to striatal CINs in both mouse and human brain, and consequent decreased CIN activity in an ex vivo assay. Antibody binding to CINs (but not to PV interneurons) and reduction of CIN activity parallels symptom improvement during recovery. These finding are in agreement with the hypothesis that at least a subset of PANS cases have an immunological pathogenesis. Striatal CINs may be a locus of interest to investigate the pathophysiology in PANS.
Supplementary Material
Highlights.
PANS IgG drawn at flare shows elevated binding to CINs in both mouse and human brain.
PANS IgG binding to CINs reduces their activity using p-rpS6 as a marker.
Elevated IgG binding to CINs is resolved in the same subject during symptom recovery.
Reduction of PANS IgG binding to CINs parallels symptom improvement during recovery.
Striatal CINs may be a locus of interest to investigate the pathophysiology in PANS.
Acknowledgements
We thank Dr. Soraya Scuderi and Betsy D’Amico for technical support. We also thank laboratory members for helpful discussions and critical reading of the manuscript. We thank the Lucile Packard Foundation for Children’s Health and the Dollinger family for the Stanford Dollinger PANS Biomarker Discovery Core which supported the research infrastructure to collect and distribute PANS blood samples. We thank the Stanford IBH clinic staff, parents, and children (patients and controls) for their time and effort in supporting this research.
Funding
This work was funded by NIH grants R01NS101104 (CP), R21MH109700 (CP), R01MH118453 (FV), R00NS114166 (AC), R01NS133434(AC), by the Colton Center for Autoimmunity at Yale (CP), and by the Department of Mental Health and Addiction Services of the State of Connecticut through its support of the Ribicoff Research Facilities at the Connecticut Mental Health Center. The content reflects the views of the authors and does not represent the official views of the National Institutes of Health or the State of Connecticut.
Abbreviations
- ACh
acetylcholine
- AChRs
acetylcholine receptors
- aCSF
artificial cerebrospinal fluid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ANOVA
analysis of variance
- AP
alkaline phosphatase
- AUC
area under the curve
- cAMP
cyclic adenosine monophosphate
- CaMKII
calcium calmodulin protein kinase II
- ChAT
Choline Acetyltransferase
- CINs
cholinergic interneurons
- CY-BOCS
Child Yale-Brown Obsessive-Compulsive Scale
- DA
dopamine
- DAB
diaminobenzidine
- eGFP
enhanced green fluorescent protein
- ELISA
enzyme-linked immunosorbent assay
- HRP
horseradish peroxidase
- ICC
intraclass correlation coefficient
- IgG
Immunoglobulin G
- IL-6
interleukin 6
- IVIG
intravenous immunoglobulin
- mAChRs
muscarinic AChRs
- mTOR
mammalian target of rapamycin
- OCD
obsessive-compulsive disorder
- PANDAS
Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcus
- PANS
Pediatric Acute-onset Neuropsychiatric Syndrome
- PV
Parvalbumin
- ROC
receiver operating characteristic curve
- rpS6
ribosomal protein S6
- SC
Sydenham chorea
- S.E.M.
standard error of the mean
- TH
tyrosine hydroxylase
- TMB
3,3’,5,5’-tetramethylbenzidine
- TNFα
tumor necrosis factor alpha
- TS
Tourette syndrome
Footnotes
Declaration of Competing Interest
Dr. Pittenger has served as a consultant and received research funding in the past year for Biohaven Pharmaceuticals, Transcend Therapeutics, Ceruvia Lifesciecnes, Freedom Biosciences, and Nobilis Therapeutics, and royalties from Oxford University Press, all for work unrelated to the current results. He is an inventor on a patent applications related to the use of neurofeedback and of psychedelic drugs for the treatment of OCD, also unrelated to this work. Dr. Che has received research funding from Duraviva Pharma for work unrealted to the current results. All other authors report no competing interests. The authors have declared that no conflict of interest exists.
Appendix A. Supplementary data
Supplementary data to this article can be found online at the journal website (https://www.sciencedirect.com/journal/brain-behavior-and-immunity)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
Data will be made available on request.
References
- Agmon-Levin N, Damoiseaux J, Kallenberg C, Sack U, Witte T, Herold M, Bossuyt X, Musset L, Cervera R, Plaza-Lopez A, Dias C, Sousa MJ, Radice A, Eriksson C, Hultgren O, Viander M, Khamashta M, Regenass S, Andrade LE, Wiik A, Tincani A, Rönnelid J, Bloch DB, Fritzler MJ, Chan EK, Garcia-De La Torre I, Konstantinov KN, Lahita R, Wilson M, Vainio O, Fabien N, Sinico RA, Meroni P, Shoenfeld Y, 2014. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis 73, 17–23. doi: 10.1136/annrheumdis-2013-203863 [DOI] [PubMed] [Google Scholar]
- Allen AJ, Leonard HL, Swedo SE, 1995. Case study: a new infection-triggered, autoimmune subtype of pediatric OCD and Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 34, 307–311. doi: 10.1097/00004583-199503000-00015 [DOI] [PubMed] [Google Scholar]
- Apicella P, 2007. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci 30, 299–306. doi: 10.1016/j.tins.2007.03.011 [DOI] [PubMed] [Google Scholar]
- Baschong W, Suetterlin R, Laeng RH, 2001. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM). J Histochem Cytochem 49, 1565–1572. doi: 10.1177/002215540104901210 [DOI] [PubMed] [Google Scholar]
- Ben-Pazi H, Stoner JA, Cunningham MW, 2013. Dopamine receptor autoantibodies correlate with symptoms in Sydenham’s chorea. PLoS One 8, e73516. doi: 10.1371/journal.pone.0073516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Chieng BC, Laurent V, Valjent E, Balleine BW, 2012. Striatal cholinergic interneurons display activity-related phosphorylation of ribosomal protein S6. PLoS One 7, e53195. doi: 10.1371/journal.pone.0053195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Khanna S, Chakrabarty K, Mahadevan A, Christopher R, Shankar SK, 2009. Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive-compulsive disorder. Neuropsychopharmacology 34, 2489–2496. doi: 10.1038/npp.2009.77 [DOI] [PubMed] [Google Scholar]
- Biever A, Valjent E, Puighermanal E, 2015. Ribosomal Protein S6 Phosphorylation in the Nervous System: From Regulation to Function. Front Mol Neurosci 8, 75. doi: 10.3389/fnmol.2015.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binning W, Hogan-Cann AE, Yae Sakae D, Maksoud M, Ostapchenko V, Al-Onaizi M, Matovic S, Lu WY, Prado MAM, Inoue W, Prado VF, 2020. Chronic hM3Dq signaling in microglia ameliorates neuroinflammation in male mice. Brain Behav Immun 88, 791–801. doi: 10.1016/j.bbi.2020.05.041 [DOI] [PubMed] [Google Scholar]
- Brady MV, Mariani J, Koca Y, Szekely A, King RA, Bloch MH, Landeros-Weisenberger A, Leckman JF, Vaccarino FM, 2022. Mispatterning and interneuron deficit in Tourette Syndrome basal ganglia organoids. Mol Psychiatry 27, 5007–5019. doi: 10.1038/s41380-022-01880-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilot F, Merheb V, Ding A, Murphy T, Dale RC, 2011. Antibody binding to neuronal surface in Sydenham chorea, but not in PANDAS or Tourette syndrome. Neurology 76, 1508–1513. doi: 10.1212/WNL.0b013e3182181090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguiere E, Monteiro P, Mallet L, Feng G, Graybiel AM, 2015. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol 30, 59–65. doi: 10.1016/j.conb.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera B, Romero-Rebollar C, Jiménez-Ángeles L, Genis-Mendoza AD, Flores J, Lanzagorta N, Arroyo M, de la Fuente-Sandoval C, Santana D, Medina-Bañuelos V, Sacristán E, Nicolini H, 2019. Neuroanatomical features and its usefulness in classification of patients with PANDAS. CNS Spectr 24, 533–543. doi: 10.1017/s1092852918001268 [DOI] [PubMed] [Google Scholar]
- Calaprice D, Tona J, Parker-Athill EC, Murphy TK, 2017. A Survey of Pediatric Acute-Onset Neuropsychiatric Syndrome Characteristics and Course. J Child Adolesc Psychopharmacol 27, 607–618. doi: 10.1089/cap.2016.0105 [DOI] [PubMed] [Google Scholar]
- Chain JL, Alvarez K, Mascaro-Blanco A, Reim S, Bentley R, Hommer R, Grant P, Leckman JF, Kawikova I, Williams K, Stoner JA, Swedo SE, Cunningham MW, 2020. Autoantibody Biomarkers for Basal Ganglia Encephalitis in Sydenham Chorea and Pediatric Autoimmune Neuropsychiatric Disorder Associated With Streptococcal Infections. Front Psychiatry 11, 564. doi: 10.3389/fpsyt.2020.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Frankovich J, 2019. Infections, Antibiotics, and Mental Health Deteriorations. J Child Adolesc Psychopharmacol 29, 647–648. doi: 10.1089/cap.2019.0100 [DOI] [PubMed] [Google Scholar]
- Chang K, Frankovich J, Cooperstock M, Cunningham MW, Latimer ME, Murphy TK, Pasternack M, Thienemann M, Williams K, Walter J, Swedo SE, 2015. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol 25, 3–13. doi: 10.1089/cap.2014.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 2013. Statistical power analysis for the behavioral sciences. Academic press. [Google Scholar]
- Cosco TD, Pillinger T, Emam H, Solmi M, Budhdeo S, Matthew Prina A, Maes M, Stein DJ, Stubbs B, Carvalho AF, 2019. Immune Aberrations in Obsessive-Compulsive Disorder: a Systematic Review and Meta-analysis. Mol Neurobiol 56, 4751–4759. doi: 10.1007/s12035-018-1409-x [DOI] [PubMed] [Google Scholar]
- Cox CJ, Sharma M, Leckman JF, Zuccolo J, Zuccolo A, Kovoor A, Swedo SE, Cunningham MW, 2013. Brain human monoclonal autoantibody from sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: implications in human disease. J Immunol 191, 5524–5541. doi: 10.4049/jimmunol.1102592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CJ, Zuccolo AJ, Edwards EV, Mascaro-Blanco A, Alvarez K, Stoner J, Chang K, Cunningham MW, 2015. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 25, 76–85. doi: 10.1089/cap.2014.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW, Cox CJ, 2016. Autoimmunity against dopamine receptors in neuropsychiatric and movement disorders: a review of Sydenham chorea and beyond. Acta Physiol (Oxf) 216, 90–100. doi: 10.1111/apha.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RC, Heyman I, Giovannoni G, Church AW, 2005. Incidence of anti-brain antibodies in children with obsessive-compulsive disorder. Br J Psychiatry 187, 314–319. doi: 10.1192/bjp.187.4.314 [DOI] [PubMed] [Google Scholar]
- Dale RC, Merheb V, Pillai S, Wang D, Cantrill L, Murphy TK, Ben-Pazi H, Varadkar S, Aumann TD, Horne MK, Church AJ, Fath T, Brilot F, 2012. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain 135, 3453–3468. doi: 10.1093/brain/aws256 [DOI] [PubMed] [Google Scholar]
- Dash PK, Zhao J, Kobori N, Redell JB, Hylin MJ, Hood KN, Moore AN, 2016. Activation of Alpha 7 Cholinergic Nicotinic Receptors Reduce Blood-Brain Barrier Permeability following Experimental Traumatic Brain Injury. J Neurosci 36, 2809–2818. doi: 10.1523/jneurosci.3197-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, Filloux FM, Wamsley JK, 1988. Evidence for dopamine D-2 receptors on cholinergic interneurons in the rat caudate-putamen. Life Sci 42, 1933–1939. doi: 10.1016/0024-3205(88)90492-4 [DOI] [PubMed] [Google Scholar]
- Ercan TE, Ercan G, Severge B, Arpaozu M, Karasu G, 2008. Mycoplasma pneumoniae infection and obsessive-compulsive disease: a case report. J Child Neurol 23, 338–340. doi: 10.1177/0883073807308714 [DOI] [PubMed] [Google Scholar]
- Fabricius RA, Sørensen CB, Skov L, Debes NM, 2022. Cytokine profile of pediatric patients with obsessive-compulsive and/or movement disorder symptoms: A review. Front Pediatr 10, 893815. doi: 10.3389/fped.2022.893815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankovich J, Swedo S, Murphy T, Dale RC, Agalliu D, Williams K, Daines M, Hornig M, Chugani H, Sanger T, Muscal E, Pasternack M, Cooperstock M, Gans H, Zhang Y, Cunningham M, Bernstein G, Bromberg R, Willett T, Brown K, Farhadian B, Chang K, Geller D, Hernandez J, Sherr J, Shaw R, Latimer E, Leckman J, Thienemann M, 2017. Clinical Management of Pediatric Acute-Onset Neuropsychiatric Syndrome: Part II-Use of Immunomodulatory Therapies. J Child Adolesc Psychopharmacol 27, 574–593. doi: 10.1089/cap.2016.0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankovich J, Thienemann M, Pearlstein J, Crable A, Brown K, Chang K, 2015a. Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: presenting characteristics of the first 47 consecutive patients. J Child Adolesc Psychopharmacol 25, 38–47. doi: 10.1089/cap.2014.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankovich J, Thienemann M, Rana S, Chang K, 2015b. Five youth with pediatric acute-onset neuropsychiatric syndrome of differing etiologies. J Child Adolesc Psychopharmacol 25, 31–37. doi: 10.1089/cap.2014.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick LR, Rapanelli M, Jindachomthong K, Grant P, Leckman JF, Swedo S, Williams K, Pittenger C, 2018. Differential binding of antibodies in PANDAS patients to cholinergic interneurons in the striatum. Brain Behav Immun 69, 304–311. doi: 10.1016/j.bbi.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick LR, Williams K, Pittenger C, 2013. Microglial dysregulation in psychiatric disease. Clin Dev Immunol 2013, 608654. doi: 10.1155/2013/608654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage R, Wagnon I, Rossetti I, Childs R, Niedermayer G, Chesworth R, Gyengesi E, 2020. Cholinergic Modulation of Glial Function During Aging and Chronic Neuroinflammation. Front Cell Neurosci 14, 577912. doi: 10.3389/fncel.2020.577912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MA, Snider LA, Leitman SF, Werden R, Swedo SE, 2005. Treatment of Sydenham’s chorea with intravenous immunoglobulin, plasma exchange, or prednisone. J Child Neurol 20, 424–429. doi: 10.1177/08830738050200050601 [DOI] [PubMed] [Google Scholar]
- Gerentes M, Pelissolo A, Rajagopal K, Tamouza R, Hamdani N, 2019. Obsessive-Compulsive Disorder: Autoimmunity and Neuroinflammation. Curr Psychiatry Rep 21, 78. doi: 10.1007/s11920-019-1062-8 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE, 2000. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry 157, 281–283. doi: 10.1176/appi.ajp.157.2.281 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Leonard HL, Richter D, Swedo SE, 1996. Case study: acute basal ganglia enlargement and obsessive-compulsive symptoms in an adolescent boy. J Am Acad Child Adolesc Psychiatry 35, 913–915. doi: 10.1097/00004583-199607000-00017 [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Ding JB, Surmeier DJ, 2012. Muscarinic modulation of striatal function and circuitry. Handb Exp Pharmacol, 223–241. doi: 10.1007/978-3-642-23274-9_10 [DOI] [PubMed] [Google Scholar]
- Granger AJ, Mulder N, Saunders A, Sabatini BL, 2016. Cotransmission of acetylcholine and GABA. Neuropharmacology 100, 40–46. doi: 10.1016/j.neuropharm.2015.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, 2001. The neurobiology of dopamine signaling. Biosci Rep 21, 247–269. doi: 10.1023/a:1013205230142 [DOI] [PubMed] [Google Scholar]
- Gromark C, Harris RA, Wickström R, Horne A, Silverberg-Mörse M, Serlachius E, Mataix-Cols D, 2019. Establishing a Pediatric Acute-Onset Neuropsychiatric Syndrome Clinic: Baseline Clinical Features of the Pediatric Acute-Onset Neuropsychiatric Syndrome Cohort at Karolinska Institutet. J Child Adolesc Psychopharmacol 29, 625–633. doi: 10.1089/cap.2018.0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjari P, Oldmark MH, Fernell E, Jakobsson K, Vinsa I, Thorsson M, Monemi M, Stenlund L, Fasth A, Furuhjelm C, Johnels J, Gillberg C, Johnson M, 2022. Paediatric Acute-onset Neuropsychiatric Syndrome (PANS) and intravenous immunoglobulin (IVIG): comprehensive open-label trial in ten children. BMC Psychiatry 22, 535. doi: 10.1186/s12888-022-04181-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC Jr., Nairn AC, Aswad DW, Greengard P, 1984. DARPP-32, a dopamine- and adenosine 3’:5’-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. II. Purification and characterization of the phosphoprotein from bovine caudate nucleus. J Neurosci 4, 99–110. doi: 10.1523/jneurosci.04-01-00099.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI, 1994. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci 14, 3351–3363. doi: 10.1523/jneurosci.14-05-03351.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL, 2011. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One 6, e19155. doi: 10.1371/journal.pone.0019155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW Jr, Lemeshow S, Sturdivant RX, 2013. Applied logistic regression. John Wiley & Sons. [Google Scholar]
- Johnson M, Ehlers S, Fernell E, Hajjari P, Wartenberg C, Wallerstedt SM, 2021. Anti-inflammatory, antibacterial and immunomodulatory treatment in children with symptoms corresponding to the research condition PANS (Pediatric Acute-onset Neuropsychiatric Syndrome): A systematic review. PLoS One 16, e0253844. doi: 10.1371/journal.pone.0253844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM, 2010. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol 518, 277–291. doi: 10.1002/cne.22206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirvan CA, Cox CJ, Swedo SE, Cunningham MW, 2007. Tubulin is a neuronal target of autoantibodies in Sydenham’s chorea. J Immunol 178, 7412–7421. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Heuser JS, Cunningham MW, 2003. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med 9, 914–920. doi: 10.1038/nm892 [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Kurahara D, Cunningham MW, 2006a. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham’s chorea. Autoimmunity 39, 21–29. doi: 10.1080/08916930500484757 [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW, 2006b. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol 179, 173–179. doi: 10.1016/j.jneuroim.2006.06.017 [DOI] [PubMed] [Google Scholar]
- Knight ZA, Tan K, Birsoy K, Schmidt S, Garrison JL, Wysocki RW, Emiliano A, Ekstrand MI, Friedman JM, 2012. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell 151, 1126–1137. doi: 10.1016/j.cell.2012.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo TK, Li MY, 2016. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 15, 155–163. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, 2009. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci 32, 127–147. doi: 10.1146/annurev.neuro.051508.135422 [DOI] [PubMed] [Google Scholar]
- Kumar A, Williams MT, Chugani HT, 2015. Evaluation of basal ganglia and thalamic inflammation in children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection and tourette syndrome: a positron emission tomographic (PET) study using 11C-[R]-PK11195. J Child Neurol 30, 749–756. doi: 10.1177/0883073814543303 [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Luquin N, Obeso JA, 2012. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med 2, a009621. doi: 10.1101/cshperspect.a009621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, Huttner A, Pletikos M, Sestan N, Leckman JF, Vaccarino FM, 2016. Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biol Psychiatry 79, 372–382. doi: 10.1016/j.biopsych.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi L, Lorenzetti G, Carsetti R, Piano Mortari E, Guido CA, Zicari AM, Förster-Waldl E, Loffredo L, Duse M, Spalice A, 2023. Immunological characterization of an Italian PANDAS cohort. Front Pediatr 11, 1216282. doi: 10.3389/fped.2023.1216282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SA, Kang UJ, McGehee DS, 2014. Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci 6, 22. doi: 10.3389/fnsyn.2014.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martos YV, Braz BY, Beccaria JP, Murer MG, Belforte JE, 2017. Compulsive Social Behavior Emerges after Selective Ablation of Striatal Cholinergic Interneurons. J Neurosci 37, 2849–2858. doi: 10.1523/jneurosci.3460-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O, 2008. Physiological roles of ribosomal protein S6: one of its kind. Int Rev Cell Mol Biol 268, 1–37. doi: 10.1016/s1937-6448(08)00801-0 [DOI] [PubMed] [Google Scholar]
- Meyuhas O, 2015. Ribosomal Protein S6 Phosphorylation: Four Decades of Research. Int Rev Cell Mol Biol 320, 41–73. doi: 10.1016/bs.ircmb.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Morer A, Lázaro L, Sabater L, Massana J, Castro J, Graus F, 2008. Antineuronal antibodies in a group of children with obsessive-compulsive disorder and Tourette syndrome. J Psychiatr Res 42, 64–68. doi: 10.1016/j.jpsychires.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Morris CM, Pardo-Villamizar C, Gause CD, Singer HS, 2009. Serum autoantibodies measured by immunofluorescence confirm a failure to differentiate PANDAS and Tourette syndrome from controls. J Neurol Sci 276, 45–48. doi: 10.1016/j.jns.2008.08.032 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Patel PD, McGuire JF, Kennel A, Mutch PJ, Parker-Athill EC, Hanks CE, Lewin AB, Storch EA, Toufexis MD, Dadlani GH, Rodriguez CA, 2015. Characterization of the pediatric acute-onset neuropsychiatric syndrome phenotype. J Child Adolesc Psychopharmacol 25, 14–25. doi: 10.1089/cap.2014.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP, Kreitzer AC, 2014. Striatal cholinergic interneurons Drive GABA release from dopamine terminals. Neuron 82, 63–70. doi: 10.1016/j.neuron.2014.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Kreitzer AC, 2014. Reassessing models of basal ganglia function and dysfunction. Annu Rev Neurosci 37, 117–135. doi: 10.1146/annurev-neuro-071013-013916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dor SL, Homayoun S, Downer OM, Hamel MA, Zagaroli JS, Williams KA, 2022. A Survey of Demographics, Symptom Course, Family History, and Barriers to Treatment in Children with Pediatric Acute-Onset Neuropsychiatric Disorders and Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal Infections. J Child Adolesc Psychopharmacol 32, 476–487. doi: 10.1089/cap.2022.0063 [DOI] [PubMed] [Google Scholar]
- Orlovska S, Vestergaard CH, Bech BH, Nordentoft M, Vestergaard M, Benros ME, 2017. Association of Streptococcal Throat Infection With Mental Disorders: Testing Key Aspects of the PANDAS Hypothesis in a Nationwide Study. JAMA Psychiatry 74, 740–746. doi: 10.1001/jamapsychiatry.2017.0995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell M, Meier MA, Szulzewsky F, Matyash V, Endres M, Kronenberg G, Prinz V, Waiczies S, Wolf SA, Kettenmann H, 2016. The subpopulation of microglia expressing functional muscarinic acetylcholine receptors expands in stroke and Alzheimer’s disease. Brain Struct Funct 221, 1157–1172. doi: 10.1007/s00429-014-0962-y [DOI] [PubMed] [Google Scholar]
- Pavone P, Bianchini R, Parano E, Incorpora G, Rizzo R, Mazzone L, Trifiletti RR, 2004. Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Pediatr Neurol 30, 107–110. doi: 10.1016/s0887-8994(03)00413-2 [DOI] [PubMed] [Google Scholar]
- Pearlman DM, Vora HS, Marquis BG, Najjar S, Dudley LA, 2014. Anti-basal ganglia antibodies in primary obsessive-compulsive disorder: systematic review and meta-analysis. Br J Psychiatry 205, 8–16. doi: 10.1192/bjp.bp.113.137018 [DOI] [PubMed] [Google Scholar]
- Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, Swedo SE, 1999. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 354, 1153–1158. doi: 10.1016/s0140-6736(98)12297-3 [DOI] [PubMed] [Google Scholar]
- Pirbhoy PS, Farris S, Steward O, 2016. Synaptic activation of ribosomal protein S6 phosphorylation occurs locally in activated dendritic domains. Learn Mem 23, 255–269. doi: 10.1101/lm.041947.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppi LA, Ho-Nguyen KT, Shi A, Daut CT, Tischfield MA, 2021. Recurrent Implication of Striatal Cholinergic Interneurons in a Range of Neurodevelopmental, Neurodegenerative, and Neuropsychiatric Disorders. Cells 10. doi: 10.3390/cells10040907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Frick LR, Pittenger C, 2017a. The Role of Interneurons in Autism and Tourette Syndrome. Trends Neurosci 40, 397–407. doi: 10.1016/j.tins.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Frick LR, Xu M, Groman SM, Jindachomthong K, Tamamaki N, Tanahira C, Taylor JR, Pittenger C, 2017b. Targeted Interneuron Depletion in the Dorsal Striatum Produces Autism-like Behavioral Abnormalities in Male but Not Female Mice. Biol Psychiatry 82, 194–203. doi: 10.1016/j.biopsych.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H, Cameron DJ, 2012. Lyme disease and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): an overview. Int J Gen Med 5, 163–174. doi: 10.2147/ijgm.S24212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, 1991. Meta-analytic procedures for social research, Rev. ed. Sage Publications, Inc, Thousand Oaks, CA, US. [Google Scholar]
- Rosenthal R, Rubin DB, 2003. r equivalent: A simple effect size indicator. Psychol Methods 8, 492–496. doi: 10.1037/1082-989x.8.4.492 [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF, 1997. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 36, 844–852. doi: 10.1097/00004583-199706000-00023 [DOI] [PubMed] [Google Scholar]
- Sigra S, Hesselmark E, Bejerot S, 2018. Treatment of PANDAS and PANS: a systematic review. Neurosci Biobehav Rev 86, 51–65. doi: 10.1016/j.neubiorev.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Singer HS, Gilbert DL, Wolf DS, Mink JW, Kurlan R, 2012. Moving from PANDAS to CANS. J Pediatr 160, 725–731. doi: 10.1016/j.jpeds.2011.11.040 [DOI] [PubMed] [Google Scholar]
- Singer HS, Hong JJ, Yoon DY, Williams PN, 2005. Serum autoantibodies do not differentiate PANDAS and Tourette syndrome from controls. Neurology 65, 1701–1707. doi: 10.1212/01.wnl.0000183223.69946.f1 [DOI] [PubMed] [Google Scholar]
- Singer HS, Loiselle CR, Lee O, Minzer K, Swedo S, Grus FH, 2004. Anti-basal ganglia antibodies in PANDAS. Mov Disord 19, 406–415. doi: 10.1002/mds.20052 [DOI] [PubMed] [Google Scholar]
- Singer HS, Mascaro-Blanco A, Alvarez K, Morris-Berry C, Kawikova I, Ben-Pazi H, Thompson CB, Ali SF, Kaplan EL, Cunningham MW, 2015. Neuronal antibody biomarkers for Sydenham’s chorea identify a new group of children with chronic recurrent episodic acute exacerbations of tic and obsessive compulsive symptoms following a streptococcal infection. PLoS One 10, e0120499. doi: 10.1371/journal.pone.0120499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedo SE, 2002. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). Mol Psychiatry 7 Suppl 2, S24–25. doi: 10.1038/sj.mp.4001170 [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leckman JF, Rose NR, 2012. From Research Subgroup to Clinical Syndrome: Modifying the PANDAS Criteria to Describe PANS (Pediatric Acute-onset Neuropsychiatric Syndrome). Pediatr Therapeut 2, 113. doi: 10.4172/2161-0665.1000113 [DOI] [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK, 1998. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 155, 264–271. doi: 10.1176/ajp.155.2.264 [DOI] [PubMed] [Google Scholar]
- Swedo SE, Williams KA, 2017. PANDAS as a Poststreptococcal Autoimmune Neuropsychiatric Form of OCD. In: Pittenger C, Pittenger C (Eds.), Obsessive-compulsive Disorder: Phenomenology, Pathophysiology, and Treatment. Oxford University Press, pp. 311–320. [Google Scholar]
- Szumilas M, 2010. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry 19, 227–229. [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP, 2004. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14, 685–692. doi: 10.1016/j.conb.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ, 2012. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64. doi: 10.1016/j.neuron.2012.04.038 [DOI] [PubMed] [Google Scholar]
- Ting JT, Daigle TL, Chen Q, Feng G, 2014. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol 1183, 221–242. doi: 10.1007/978-1-4939-1096-0_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis MD, Hommer R, Gerardi DM, Grant P, Rothschild L, D’Souza P, Williams K, Leckman J, Swedo SE, Murphy TK, 2015. Disordered eating and food restrictions in children with PANDAS/PANS. J Child Adolesc Psychopharmacol 25, 48–56. doi: 10.1089/cap.2014.0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loos CM, 2008. Multiple immunoenzyme staining: methods and visualizations for the observation with spectral imaging. J Histochem Cytochem 56, 313–328. doi: 10.1369/jhc.2007.950170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EY, Dai Y, Rosen CE, Schmitt MM, Dong MX, Ferré EMN, Liu F, Yang Y, González-Hernández JA, Meffre E, Hinchcliff M, Koumpouras F, Lionakis MS, Ring AM, 2022. High-throughput identification of autoantibodies that target the human exoproteome. Cell Rep Methods 2. doi: 10.1016/j.crmeth.2022.100172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EY, Mao T, Klein J, Dai Y, Huck JD, Jaycox JR, Liu F, Zhou T, Israelow B, Wong P, Coppi A, Lucas C, Silva J, Oh JE, Song E, Perotti ES, Zheng NS, Fischer S, Campbell M, Fournier JB, Wyllie AL, Vogels CBF, Ott IM, Kalinich CC, Petrone ME, Watkins AE, Dela Cruz C, Farhadian SF, Schulz WL, Ma S, Grubaugh ND, Ko AI, Iwasaki A, Ring AM, 2021. Diverse functional autoantibodies in patients with COVID-19. Nature 595, 283–288. doi: 10.1038/s41586-021-03631-y [DOI] [PubMed] [Google Scholar]
- Williams KA, Swedo SE, 2015. Post-infectious autoimmune disorders: Sydenham’s chorea, PANDAS and beyond. Brain Res 1617, 144–154. doi: 10.1016/j.brainres.2014.09.071 [DOI] [PubMed] [Google Scholar]
- Williams KA, Swedo SE, Farmer CA, Grantz H, Grant PJ, D’Souza P, Hommer R, Katsovich L, King RA, Leckman JF, 2016. Randomized, Controlled Trial of Intravenous Immunoglobulin for Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections. J Am Acad Child Adolesc Psychiatry 55, 860–867. doi: 10.1016/j.jaac.2016.06.017 [DOI] [PubMed] [Google Scholar]
- Wolfbeis OS, Leiner M, 1985. Mapping of the total fluorescence of human blood serum as a new method for its characterization. Analytica Chimica Acta 167, 203–215. doi: 10.1016/S0003-2670(00)84422-0 [DOI] [Google Scholar]
- Xu J, Liu RJ, Fahey S, Frick L, Leckman J, Vaccarino F, Duman RS, Williams K, Swedo S, Pittenger C, 2021. Antibodies From Children With PANDAS Bind Specifically to Striatal Cholinergic Interneurons and Alter Their Activity. Am J Psychiatry 178, 48–64. doi: 10.1176/appi.ajp.2020.19070698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kobets A, Du JC, Lennington J, Li L, Banasr M, Duman RS, Vaccarino FM, DiLeone RJ, Pittenger C, 2015. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci U S A 112, 893–898. doi: 10.1073/pnas.1419533112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Flores-Hernandez J, Surmeier DJ, 2001. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience 103, 1017–1024. doi: 10.1016/s0306-4522(01)00039-2 [DOI] [PubMed] [Google Scholar]
- Yan Z, Song WJ, Surmeier J, 1997. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol 77, 1003–1015. doi: 10.1152/jn.1997.77.2.1003 [DOI] [PubMed] [Google Scholar]
- Zheng J, Frankovich J, McKenna ES, Rowe NC, MacEachern SJ, Ng NN, Tam LT, Moon PK, Gao J, Thienemann M, Forkert ND, Yeom KW, 2020. Association of Pediatric Acute-Onset Neuropsychiatric Syndrome With Microstructural Differences in Brain Regions Detected via Diffusion-Weighted Magnetic Resonance Imaging. JAMA Netw Open 3, e204063. doi: 10.1001/jamanetworkopen.2020.4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA, 2001. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci 4, 1224–1229. doi: 10.1038/nn769 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


