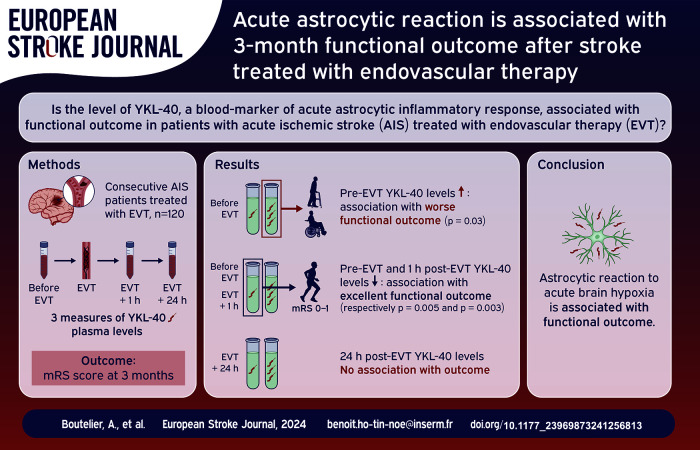
Abstract
Introduction:
More than 50% of large vessel occlusion (LVO) acute ischemic stroke (AIS) patients treated with endovascular therapy (EVT) remain severely disabled at 3 months. We hypothesized that acute astrocytic inflammatory response may play a pivotal role in post-AIS brain changes associated with poor functional outcome. We proposed to evaluate the level of YKL-40, a glycoprotein mainly released by reactive astrocytes.
Patients and methods:
A monocentric prospective cohort study was conducted on consecutive LVO AIS patients treated with EVT. Three blood samples (before, within 1 and 24-hour post-EVT) were collected to measure plasma YKL-40 concentrations. Functional outcome was assessed according to the modified Rankin Scale (mRS) score at 3 months.
Results:
Between 2016 and 2020, 120 patients were included. The plasma concentration of YKL-40 before EVT was statistically and independently associated with 3-month worse functional outcome (adjusted cOR, 1.59; 95% CI [1.05–2.44], p = 0.027) but not the two following samples 1-hour and 24-hour post-EVT. Accordingly, we found that excellent functional outcome was associated with a lower level of YKL-40 before and within 1 h after EVT (p = 0.005 and p = 0.003, respectively) but not when measured 24 h after EVT (p = 0.2).
Discussion and conclusion:
This study suggests that the astrocytic reaction to acute brain hypoxia, especially before recanalization, is associated with worse functional outcome. Such early biomarker of the astrocytic response in AIS may optimize individualized care in the future.
Clinical Trial Registration-URL:
http://www.clinicaltrials.gov. Unique identifier: NCT02900833.
Keywords: Astrocytic reaction, inflammation, ischemic stroke, endovascular therapy
Graphical abstract.
Introduction
Endovascular therapy (EVT) has revolutionized the treatment of acute ischemic stroke (AIS) due to large vessel occlusion (LVO) with better functional outcomes (FO), yet despite high rate of successful recanalization, more than 50% of EVT-treated AIS patients remain severely disabled at 3 months. 1 One explanation for this discrepancy may be the occurrence of ischemia-induced brain response (often referred to as “neuroinflammation”), including astrocytic and microglial reaction triggering the production of several proinflammatory signal molecules, which participate in the immune system activation leading to brain damage. 2 The glycoprotein YKL-40, a 40 kDa plasma protein with three N-terminal amino acids being Y (tyrosine), K (lysine), and L (leucine), is predominantly secreted, in the brain, by reactive astrocytes 3 and was previously found to be elevated in AIS patients. 4 Whether dynamic changes in the astrocytic reaction occur in EVT-treated AIS patients remains unknown, and so does the potential relationship of this phenomenon to functional outcome in these patients. In the present study, using YKL-40 plasma levels, we investigated the longitudinal evolution of the astrocytic inflammatory response in EVT-treated patients for AIS and its relationship with functional outcome.
Patients and methods
Patient information was collected prospectively using a standardized questionnaire (Endovascular Treatment in Ischemic Stroke registry). Patients or their legal surrogate provided informed consent for study participation. The local ethics committee approved this research protocol (CPP Ile de France VII, ID-RCB number: 2015-A01856-43). Clinical Trial Registration-URL: http://www.clinicaltrials.gov. Unique identifier: NCT02900833.
Patient data collection
All consecutive patients with AIS due to LVO treated by EVT at the Rothschild foundation hospital between April 2016 and April 2020, and for whom all three blood samples were available were included in this study (Figure 1). Patients’ clinical, radiological, biological and treatment characteristics were collected prospectively. Functional outcome was assessed by neurologists with the modified Rankin Scale (mRS) at 3 months, during face-to-face interviews or via telephone conversations with the patients or their relatives.
Figure 1.
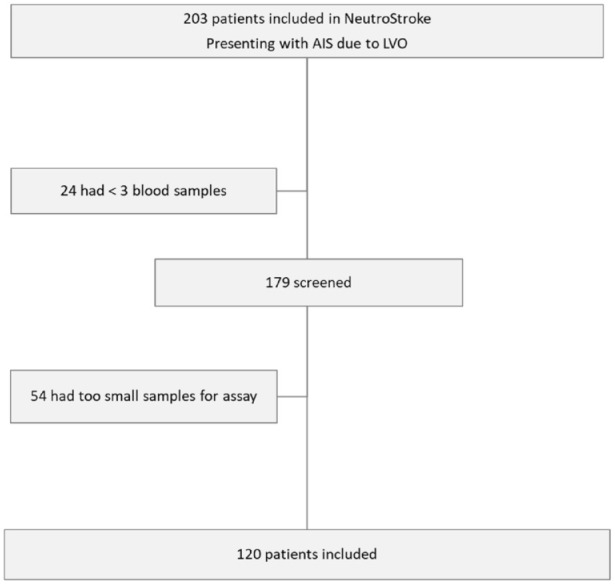
Flow-chart of patients included in the study.
Plasma collection and YKL-40 quantification
Blood was collected in EDTA (BD Vacutainer 4 ml, K3E 7.2 mg) from the femoral artery for the first two samplings (at the beginning of EVT and within 1 h after EVT), and from a venous brachial puncture at 24-hour post-EVT. Plasma was collected after two centrifugations (10 min ×2000 g at 20°C and 15 min ×2500 g at 20°C) within 15 min after blood withdrawal and immediately frozen at −80°C for further analysis. YKL-40 was quantified using a commercial ELISA kit (R&D systems, DY2599).
Clinical outcome definitions
Excellent outcome and favorable outcomes were defined as a mRS score between 0 and 1 and between 0 and 2, respectively, or equal to pre-stroke mRS. Unfavorable outcome was defined as a mRS score > 1 and different to pre-stroke mRS when compared to excellent, or a mRS score > 2 and different to pre-stroke mRS when compared to favorable outcome.
Statistical analysis
Categorical variables were expressed as frequencies and percentages. Quantitative variables were expressed as mean (standard deviation, SD), or median (interquartile range, IQR) for non-normal distribution. Normality of distributions was assessed graphically and by using the Shapiro-Wilk test.
Comparison of YKL-40 at three times (before EVT, within 1 h after EVT and 24 h after EVT) was performed using a linear mixed regression model with subject as random effect to take into account the repeated measure by patient.
Associations of YKL-40 assessed at three times with functional outcomes were investigated by using ordinal logistic regression models for shift 3-month mRS score after combined 5 and 6 together and by using logistic regression models for binary outcomes (favorable and excellent FO) after examined the log-linearity assumption. We assessed the shape of associations (log-linearity assumption) by using restricted cubic spline functions and the proportional odds assumptions by using score Chi-Square test and we found no deviation. Logistic regression models were further adjusted on pre-specified covariates (age, admission NIHSS, and intravenous thrombolysis (IVT)). Finally, we assessed the heterogeneity in associations of YKL-40 with favorable and excellent outcomes, according to IVT status by including the corresponding interaction term into the multivariable logistic or ordinal logistic regression models (adjusted on age and admission NIHSS score). Using an increase of 1 SD in YKL-40 (calculated among overall study population), we derived from logistic regression models, odds ratio (ORs) or common ORs (cORs) for 1-point mRS worsening as effect size measures, with their 95% confidence intervals (CIs).
Statistical testing was done at the two-tailed α level of 0.05. Data were analyzed using the SAS software package, release 9.4 (SAS Institute, Cary, NC).
Results
Between April 2016 and April 2020, 120 patients had the YKL-40 plasma level available at the 3 time-points and were finally included in the present study (Figure 1).
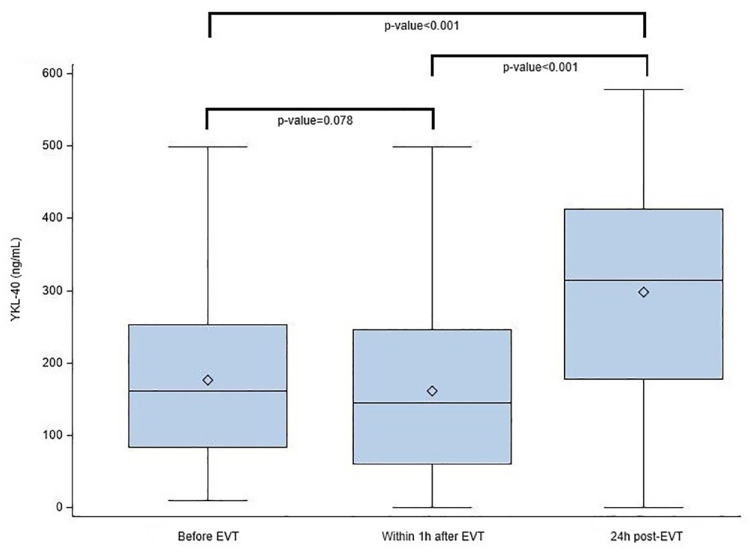
Main patient’s characteristics, treatment and outcomes are reported in Table 1. The YKL-40 plasma concentration at 24 h was significantly higher compared to measures before and within 1 h after EVT (p < 0.001; Figure 2).
Table 1.
Patient characteristics (n = 120).
| Characteristics | |
|---|---|
| Age, year mean (SD) | 67.4 ± 15.9 |
| Men, n (%) | 66 (55.0) |
| Medical history | |
| Hypertension, n (%) | 72/120 (60.0) |
| Diabetes, n (%) | 23/120 (19.2) |
| Dyslipidemia, n (%) | 30/120 (25.0) |
| Current smoking, n (%) | 32/120 (26.7) |
| Previous stroke or TIA, n (%) | 23/120 (19.2) |
| Pre-stroke mRS > 1 | 39/120 (32.5) |
| Antithrombotic treatment | 61/119 (51.3) |
| Current AIS event | |
| Baseline NIHSS score, median (IQR) 1 | 17 (9–20) |
| Baseline ASPECTS, median (IQR) 2 | 7 (5–9) |
| IVT | 58/120 (48.3) |
| First-line EVT strategy | |
| Stent retriever alone | 8/118 (6.8) |
| Contact aspiration alone | 57/118 (48.3) |
| Stent retriever and contact aspiration | 53/118 (44.9) |
| YKL-40 plasma levels | |
| Before EVT, mean (SD), ng/ml 3 | 177 (111) |
| Within 1 h after EVT, mean (SD), ng/ml 3 | 161 (112) |
| 24 h after EVT, mean (SD) ng/ml 3 | 298 (142) |
| Functional outcomes at 3 months | |
| Favorable outcome, n (%) | 51/110 (46.4) |
| Excellent outcome, n (%) | 30/110 (27.3) |
Values expressed as no/total no. (%) unless otherwise indicated. ASPECTS: Alberta stroke program early computed tomography score; TIA: transient ischemic attack; IQR: interquartile range; IVT: intravenous thrombolysis; NIHSS: national institutes of health stroke scale; SD: standard deviation.
2 missing data.
9 missing data.
1 missing data.
Figure 2.
Plasma YKL-40 levels according to time of sampling (expressed in ng/ml). YKL-40: 40 kDa plasma protein with 3 N-terminal amino acids being Y (tyrosine), K (lysine); EVT: endovascular therapy.
Early plasma YKL-40 level is associated with 3-month functional outcome
In univariate ordinal shift analysis, higher values of YKL-40 at each time point of assessment were associated with worse functional outcome (unadjusted cORs for mRS increase [95% CI] associated with one SD increase in YKL-40 of 2.17 [1.43–2.94] before EVT, 2.05 [1.43–3.03] within 1 h after EVT and 1.67 [1.19–2.38] at 24 h after EVT. After adjustment on age, admission NIHSS and IVT status, only association between YKL-40 before EVT and 3-month mRS remained significant (adjusted cOR, 1.61; 95% CI [ 1.05–2.44], p = 0.026 while adjusted cOR for within 1 h after EVT and 24 h after EVT were 1.47, 95% CI [0.97–2.22] and 1.32, 95% CI [0.88–1.92] respectively).
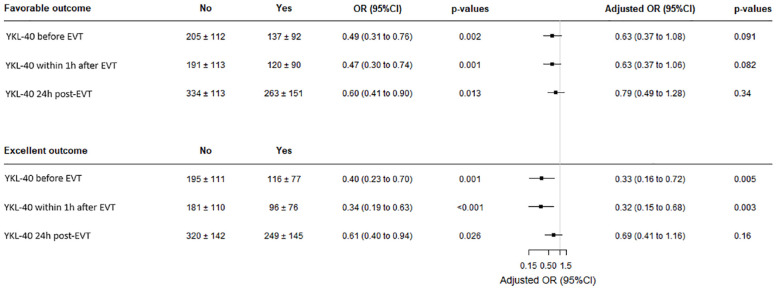
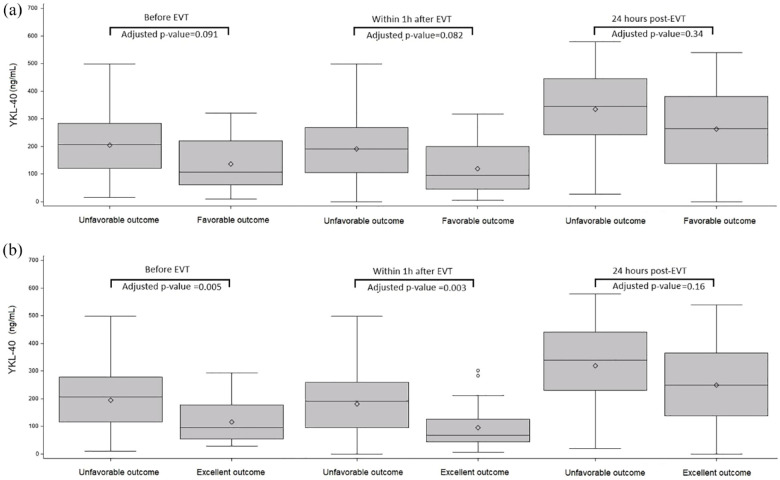
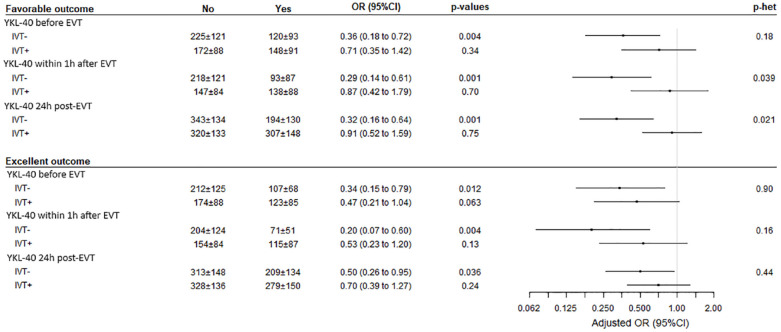
On the other hand, when binary outcomes were analyzed, favorable outcome was associated with a lower level of YKL-40 at each time point (Figure 4), with an OR [95% CI] per one SD increase in YKL-40 of 0.49 [0.31–0.76] (p = 0.002) before EVT, 0.47 [0.30–0.74] (p = 0.001), within 1 h after EVT; and 0.61 [0.41–0.90] (p = 0.013) at 24 h after EVT. After adjustment for age, admission NIHSS and IVT, associations were slightly attenuated and did not reach the significance level (Figures 3(a) and 4). Lower values of YKL-40 at each time point were also associated with excellent outcome in univariate analysis (Figure 4). After adjustment for age, admission NIHSS and IVT, the association of lower value of YKL-40 before EVT and within 1 h after EVT with excellent outcome remained significant with an adjusted OR (aOR) per one SD increase in YKL-40 of 0.33 (95% CI [0.16–0.72], p = 0.005) before EVT; and 0.32 (95% CI [0.15–0.68], p = 0.003) within 1 h after EVT. The association of lower value of YKL-40 24 h after EVT with excellent outcomes was not significant (aOR = 0.69; 95% CI [0.41–1.16], p = 0.16) (Figures 3(b) and 4).
Figure 4.
Association of YKL-40 at each time-point with favorable and excellent outcomes. YKL-40 levels are expressed in ng/ml. YKL-40: 40 kDa plasma protein with 3 N-terminal amino acids being Y (tyrosine), K (lysine); EVT: endovascular therapy; OR: odds ratio.
OR and p-values obtained using logistic regression model before and after adjustment on age, admission NIHSS, and IVT.
Figure 3.
Distribution of YKL-40 at each time-point of assessment according to favorable outcome (a) and excellent outcome (b). YKL-40: 40 kDa plasma protein with 3 N-terminal amino acids being Y (tyrosine), K (lysine); EVT: endovascular therapy; favorable outcome: modified Rankin Scale score 0–2 at 3 months; excellent outcome: modified Rankin Scale score 0–1 at 3 months.
p-values for logistic regression model after adjustment on age, admission NIHSS and IVT.
Early plasma YKL-40 level and 3-month functional outcomes according to IVT
As shown in Figure 5, a significant heterogeneity in the association of YKL-40 values within 1 h after EVT and 24 h after EVT with favorable outcome was observed according to IVT status. Lower values of YKL-40 were associated with favorable outcome in patients without IVT (OR per one SD increase in YKL-40, 0.37 [95% CI, 0.17–0.81] within 1 h after EVT and 0.37 [ 95% CI, 0.17–0.78 for post-EVT]). In patients with IVT, favorable outcome occurred irrespective of YKL-40 levels. Regarding the excellent outcome, the differences in effect sizes (OR) according to IVT were attenuated and did not reach the significance levels.
Figure 5.
Association of YKL-40 at each time-point with favorable and excellent outcomes according to IVT prior to EVT. YKL-40 levels are expressed in ng/ml. YKL-40: 40 kDa plasma protein with 3 N-terminal amino acids being Y (tyrosine), K (lysine); EVT: endovascular therapy; cOR: cumulative odds ratio, OR: odds ratio; p-het: p-value of heterogeneity. OR, cOR and p-values obtained using logistic regression model for binary outcomes 3-month mRS score after combined 5 and 6 together, after adjustment on age and admission NIHSS.
Discussion
In this prospective homogenous cohort of 120 AIS patients treated with EVT, we observed that acute YKL-40 plasma levels before EVT were independently associated with functional outcome at 3 months.
A previous study already observed, in a cohort of 105 non-LVO AIS patients, an increased YKL-40 level within 12 h and at 24 h of admission and a correlation with 3 months functional outcome. 5 We show here that this association is observed before EVT but not anymore at 24 h. In accordance with this last study, 5 we observed a 24 h increase in YKL-40 plasma level, but, to our knowledge, our study is the first one acknowledging acute plasma temporal dynamic of YKL-40 at 3 time-points in the first 24 h, allowing us to show that critical release of YKL-40 begins at the hyper-acute phase, even before EVT.
As reviewed recently, 2 several studies have demonstrated that focal ischemia and neuronal death could lead to a global immune brain response and that acute non-specific innate immune systemic activation biomarkers were correlated with functional outcome.6–8 In our study, the association between functional outcome and YKL-40 plasma level is independent of baseline clinical AIS severity when assessed before EVT, suggesting that the intensity of the hyper-acute astrocytic inflammatory response could reflect the hypoxia-induced brain damage. Interestingly, it seems that IVT may mitigate the deleterious pathophysiological processes associated with YKL-40 plasma release since the association between YKL-40 levels and 3-month functional outcomes are significantly reduced in the IVT-treated patient population. These last results are in accordance with our previous study where we found that IVT reduced the neutrophil activation in post-EVT AIS patients. 8
YKL-40 is a non-specific molecule and known to be expressed by many cell types including macrophages. 9 It is believed to be involved in T2-helper cytotoxicity and tissue remodeling, especially in cancer and systemic inflammatory diseases. 9 In brain pathologies, YKL-40 is mainly expressed by astrocytes10,11 and associated with an astrocytic inflammatory response to ongoing acute or chronic brain injuries such as neurodegenerative diseases, acute stroke and neuroinfectious diseases. 3 This nonspecific expression of YKL-40 may, at least in part, explain the lack of association with the 3-month neurological outcomes when assessed 24-hour post-EVT. In fact, at 24 h this biomarker may not only reflect the ongoing astrocytic inflammatory response but also the post-AIS systemic inflammation.
It has been shown in vitro that YKL-40 is involved in astrocyte differentiation and astrogliogenesis. 12 However, whether YKL-40 is a surrogate biomarker of reactive astrocytes or an effector that could become a therapeutic target remains to be established. 3
Several limitations must be acknowledged. Despite its prospective design, the present study is observational, potentially leading to confounding biases in the analysis, and pertains only to a small population of EVT-treated AIS patients. Moreover, the puncture sites are different between the two peri-procedure time-points (before and 1-hour post-EVT), withdrawn from the femoral artery and the third one, 24-hour post EVT, withdrawn on a brachial vein which may induce a slight difference in the plasma content. However, our pilot study based on a high-quality and homogeneous cohort of patients highlights for the first time that the critical astrocytic inflammatory response may start at the hyper-acute phase of AIS, even before recanalization, suggesting that future neuroprotective strategies should be administered concomitantly or even before recanalization therapies to hope a maximal efficacy. Finally, such early biomarker may help in selecting patients for an individualized neuroprotective-based care in the future.
Conclusion
This study suggests that the astrocytic reaction to acute brain hypoxia, especially before recanalization, is associated with worse functional outcome. These results reinforce the need to invest future research in neuroprotective strategies associated with recanalization therapies to reduce the dire consequences of acute ischemic stroke to brain survival and functions.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Agence Nationale de la Recherche, Grant/Award Number: ANR-16-RHUS-0004 (RHU TRT_cSVD) ANR-18-RHUS-0001 (RHU Booster) and ANR-22-CE17-0032 (INFLAME).
Ethical approval: The ethics committee of CPP Ile de France VII approved this study (REC number:2015-A01856-43).
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Guarantor: Dr Benoit Ho-Tin-Noe, INSERM U1144, Optimisation Thérapeutique en Neuropsychopharmacologie, Paris, France; benoit.ho-tin-noe@inserm.fr
Contributorship: BHTN, JPD, AB, MM researched literature and conceived the study. JPD was involved in protocol development, gaining ethical approval, patient recruitment and data analysis. AB wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs: Ada Boutelier  https://orcid.org/0009-0001-4998-3904
https://orcid.org/0009-0001-4998-3904
Francois Delvoye  https://orcid.org/0000-0002-0697-2156
https://orcid.org/0000-0002-0697-2156
References
- 1. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 2. Shi K, Tian DC, Li ZG, et al. Global brain inflammation in stroke. Lancet Neurol 2019; 18: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 3. Li F, Liu A, Zhao M, et al. Astrocytic chitinase-3-like protein 1 in neurological diseases: potential roles and future perspectives. J Neurochem 2023; 165: 772–790. [DOI] [PubMed] [Google Scholar]
- 4. Xu X, Ma H, Xu J, et al. Elevation in circulating YKL-40 concentration in patients with cerebrovascular disease. Bosn J Basic Med Sci 2014; 14: 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park HY, Jun CD, Jeon SJ, et al. Serum YKL-40 levels correlate with infarct volume, stroke severity, and functional outcome in acute ischemic stroke patients. PLoS One 2012; 7: e51722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boisseau W, Desilles JP, Fahed R, et al. Neutrophil count predicts poor outcome despite recanalization after endovascular therapy. Neurology 2019; 93: e467–e475. [DOI] [PubMed] [Google Scholar]
- 7. Whiteley W, Wardlaw J, Dennis M, et al. The use of blood biomarkers to predict poor outcome after acute transient ischemic attack or ischemic stroke. Stroke 2012; 43: 86–91. [DOI] [PubMed] [Google Scholar]
- 8. Maïer B, Di Meglio L, Desilles JP, et al. Neutrophil activation in patients treated with endovascular therapy is associated with unfavorable outcomes and mitigated by intravenous thrombolysis. J Neurointerv Surg 2024; 16: 131–137. [DOI] [PubMed] [Google Scholar]
- 9. Yeo IJ, Lee CK, Han SB, et al. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol Ther 2019; 203: 107394. [DOI] [PubMed] [Google Scholar]
- 10. Bonneh-Barkay D, Wang G, Starkey A, et al. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J Neuroinflammation 2010; 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Querol-Vilaseca M, Colom-Cadena M, Pegueroles J, et al. YKL-40 (Chitinase 3-like I) is expressed in a subset of astrocytes in Alzheimer’s disease and other tauopathies. J Neuroinflammation 2017; 14: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh SK, Bhardwaj R, Wilczynska KM, et al. A complex of nuclear factor I-X3 and STAT3 regulates astrocyte and glioma migration through the secreted glycoprotein YKL-40. J Biol Chem 2011; 286: 39893–39903. [DOI] [PMC free article] [PubMed] [Google Scholar]