Abstract
Introduction:
Current guidelines indicate prolonged cardiac rhythm monitoring for atrial fibrillation screening in patients with cryptogenic ischemic stroke (IS) or transient ischemic attack (TIA). This study aimed to assess the incidence of cryptogenic IS/TIA eligible for such investigation, and to estimate the number of patients potentially concerned in whole France annually.
Methods:
All cryptogenic acute IS/TIA cases ⩾35 years old were retrieved from the population-based Dijon Stroke Registry, France (2013–2020). Patients eligible for prolonged cardiac rhythm monitoring were defined after excluding those who died in-hospital or within the first 30 days, or with preexisting major impairment. Annual incidence rates of eligible cryptogenic IS/TIA were calculated by age groups and sex. The total number of eligible patients in France was estimated by standardization to age- and sex-specific incidence.
Results:
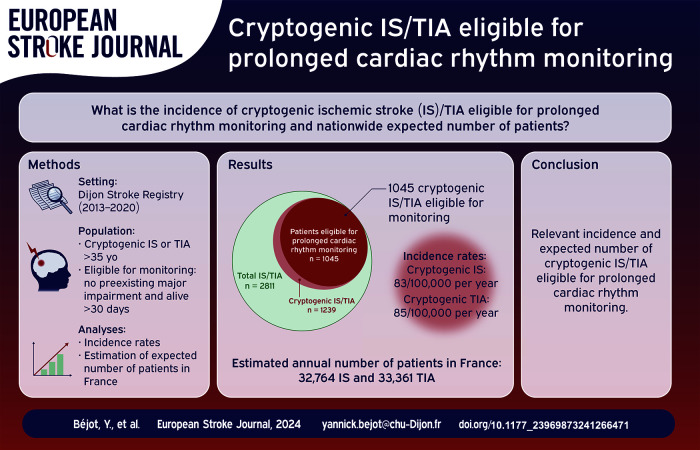
Among 2811 IS/TIA patients recorded in the Dijon Stroke Registry, 1239 had cryptogenic IS/TIA of whom 1045 were eligible for prolonged cardiac rhythm monitoring (517 IS and 528 TIA, mean age 73.6 ± 14.6 years old, 55.4% women). Crude incidence rates of eligible cryptogenic IS/TIA were 169/100,000 per year (95% CI: 159–179) in overall sexes, 83/100,000 per year (95% CI: 76–91) for IS, and 85/100,000 per year (95% CI: 78–93) for TIA. The total number of patients with cryptogenic IS/TIA eligible for prolonged cardiac rhythm monitoring in France was estimated to be 66,125 (95% CI: 65,622–66,630) for the calendar year 2022, including 32,764 (95% CI: 32,410–33,120) with IS and 33,361 (95% CI: 33,004–33,721) with TIA.
Conclusions:
This study demonstrated a high incidence of cryptogenic IS/TIA eligible for prolonged cardiac rhythm monitoring. Estimates at a national level pointed out the large number of patients who may require access to such atrial fibrillation screening, with attention to be paid on regarding organization of care networks and related costs.
Keywords: Ischemic stroke, transient ischemic attack, cryptogenic stroke, atrial fibrillation, population-based study, prolonged cardiac rhythm monitoring, incidence
Graphical abstract.
Introduction
Despite improvements in etiological phenotyping, a high proportion of ischemic stroke (IS) and transient ischemic attack (TIA) remains of undetermined origin after the initial diagnostic work-up. According to population-based studies, the prevalence of cryptogenic IS ranges from 22% to 52%. 1 This proportion could be even greater in TIA patients, although less information is available in the literature. One major underlying cause of cryptogenic IS/TIA is covert atrial fibrillation (AF), the diagnosis of which has a major impact in terms of secondary prevention, as prescription of oral anticoagulants is indicated to reduce the risk of recurrent events. 2 Diagnosis of covert AF remains challenging, although the development of new technologies including external or implantable (ICM) long-lasting cardiac rhythm monitoring have been shown to dramatically increase its detection in patients with cryptogenic IS/TIA, with detection rates ranging from 15% to 30% according to the duration of recording.3–7 Therefore, recent evidence-based recommendations from the European Stroke Organization indicated the use of ICM in adult patients with IS or TIA of undetermined origin to identify subclinical AF. 8 Such a recommendation implies setting up dedicated patient care pathways, with major impact on resource requirements and costs to the healthcare system.
To help guiding the current and future needs, this population-based study aimed to determine age and sex specific incidence of cryptogenic IS/TIA eligible for prolonged cardiac rhythm monitoring, and to illustrate the burden of this issue by estimating the number of patients potentially concerned in whole France annually.
Methods
Study population and case-collection
This study was based on data obtained from the Dijon Stroke Registry, the methodology of which has been described elsewhere. 9 Briefly, this population-based registry complies with the defined criteria for conducting high-quality incidence stroke and TIA studies, 10 and the guidelines for the reporting of incidence and prevalence studies in neuroepidemiology according to Standards of Reporting of Neurological Disorders (STROND). 11 All cases of acute symptomatic stroke and TIA among residents of the city of Dijon (Burgundy, France, currently 162,000 inhabitants) were prospectively collected thanks to overlapping sources of information to ensure the exhaustiveness of identification of hospitalized and non-hospitalized cases of symptomatic cerebrovascular events. Cases were registered based on a systematic review of medical records from following sources: (1) Hospital admission and emergency department registers, including the Dijon University Hospital where the only stroke unit is located, and private hospitals of the city; (2) Computerized hospital discharge diagnostic codes using the international Classification of Diseases, tenth revision (ICD-10) (I61, I62, I63, I64, G45, G46, and G81); (3) Medical records of patients identified from a computer-generated list of all requests for brain and cerebral vascular imaging in public and private centers; (4) Direct transmission from general practitioners and private neurologists of the city to identify stroke patients from home or nursing homes; (5) death certificates to identify deaths out of hospital.
The final adjudication of cases was made by senior neurologists trained in stroke assessment according to the World Health Organization (WHO) diagnostic criteria, 12 based on all available information. The timing of data extraction relative to the event for IS/TIA classification was 3 months. For this study, we restricted analysis to patients with IS and TIA, after excluding patients with intracerebral or subarachnoid hemorrhage. TIA was defined according to the WHO classical epidemiological definition, that is, sudden development of acute loss of focal cerebral or ocular function with symptoms lasting <24 h, presumed to be due to embolic or thrombotic vascular disease, 12 as previously described, 13 and as recommended by criteria for population-based stroke and TIA incidence studies. 10 Additionally, to comply with the criteria for advanced studies, 10 we identified TIA without evidence of brain infarct based on available imaging. Hence, these patients are classified as TIA patients according to the 2002 TIA Working Group definition, whereas those with transient symptoms but ischemic lesion on imaging are considered as IS. 14
The cause of IS and TIA was determined by the TOAST (trial of ORG 10,172 in acute stroke treatment) classification. 15 According to it, cryptogenic stroke/TIA was defined as an ischemic cerebrovascular event not attributable to a definite cardioembolic source, large artery atherosclerosis, small artery disease, or other identified causes, after the initial diagnostic work-up of the cerebrovascular event. This definition included cases with either negative or incomplete evaluation. Adjudication was made by a senior investigator of the Dijon Stroke Registry. In our Registry, patients <60 years with a patent foramen ovale (PFO) with an associated atrial septal aneurysm (ASA) or a large interatrial shunt, or those with PFO and documented paradoxical embolism were classified as cardioembolic IS or IS from other cause. In addition, to take into account potential sources of IS/TIA (although considered as cryptogenic in TOAST classification), we collected following conditions: atheromatous stenosis of artery supplying the territory involved by IS or TIA, but not qualifying for large artery atheroma according to TOAT classification (i.e., stenosis less than 50%); PFO/ASA not having criteria to be considered as cardioembolic/other cause as described above; active cancer since it can be associated with increased risk of hypercoagulability. We also considered patients with an indication for long-term anticoagulation. Although in these patients searching for AF may be relevant for etiological phenotyping, in routine practice clinicians are reluctant to perform long-term cardiac rhythm monitoring in them in the absence of consequence in terms of therapeutic strategies.
For the present analysis, we considered the time period from January 1, 2013 to December 31, 2020. We included patients aged ⩾35 years old with either first-ever (i.e., first episode occurring in a patient’s life) or recurrent IS or TIA. We chose using this age cut-off to consider both the criteria of the CRYSTAL-AF study that included cryptogenic IS/TIA patients aged 40 and over, 4 and the updated criteria for conducting high-quality incidence stroke and TIA studies that strongly recommend standard methods for the presentation of incidence rates according to mid-decades age bands. 10
Data collected
Functional status of patients before IS/TIA was assessed by the modified Rankin Scale score (mRS). Preexisting dementia defined as a cognitive decline sufficient to interfere with independence in activities of daily living was evaluated as previously described based on interviews with patients, their relatives or their general practitioner, as well as the review of medical files.16,17 For this analysis we defined patients with major impairment as those with either premorbid IS/TIA mRS score of 5, or with preexisting dementia and living in an institution. In-hospital and 30-day case-fatality were retrieved from a systematic review of death certificates obtained from the French national database. Information was available for all patients. Patients in whom prolonged cardiac rhythm monitoring for at least 24 h (either in-hospital continuous electrocardiographic monitoring or Holter monitoring) was not performed during diagnostic work-up, or information was missing, were identified for sensitivity analyses. Patients were considered not to have benefited from this exam if it had not been performed within 3 months after IS/TIA, which corresponded to the date of final adjudication of cases.
Statistical analysis
Annual incidence rates of cryptogenic IS and TIA eligible for prolonged cardiac rhythm monitoring were calculated according to age groups, and sex, and were expressed per 100,000 people with 95% confidence intervals (CIs) assuming a Poisson distribution. Denominators concerning the population of Dijon aged ⩾35 years old were based on yearly census data provided by the French National Institute of Statistics. The estimation of the number of patients with cryptogenic IS and TIA in France was obtained by standardization to age- and sex-specific incidence. The 2022 French population distribution was obtained from the French National Institute of Statistics. In sensitivity analyses, we considered the proportion of patients who did not had prolonged cardiac rhythm monitoring for each sex and age groups. We assumed that 4% of patients would have been diagnosed with AF if this exam had been performed, according to the literature.3–6,18 We then subtracted these patients with a potential diagnosis of AF from our nationwide estimates. Statistical analysis was performed with STATA@13 software (StataCorp LP, College Station, Texas, USA).
Ethics
The Dijon Stroke Registry was approved by following national ethics boards: the Comité d’Evaluation des Registres (French National Committee of Registers), Santé Publique France (French Institute for Public Health Surveillance), and the Commission Nationale Informatique et Liberté (French data protection authority). In accordance with the French legislation, boards waived the need for written patient consent.
Results
Between January 2013 and December 2020, 2811 acute ischemic cerebrovascular events in adults ⩾35 years old were recorded in the Dijon Stroke Registry, including 1731 IS and 1080 TIA cases. Among them, 1239 were cryptogenic IS/TIA (Figure 1). After exclusion of 81 cases corresponding to recurrences occurring less than 3 years after the index recorded IS/TIA event, we considered 1158 patients (610 IS and 548 TIA). Among these patients, we successively excluded those who died during hospitalization or during the first 30 days following IS/TIA, and those with preexisting major impairment. Final analysis was performed on the 1045 remaining patients (517 IS and 528 TIA, mean age 73.6 ± 14.6 years old, 55.4% women) who were considered as cryptogenic IS/TIA eligible for additional prolonged cardiac rhythm monitoring (Figure 1). Among TIA patients, 32 (6.1%) had an ischemic lesion visible on brain imaging.
Figure 1.
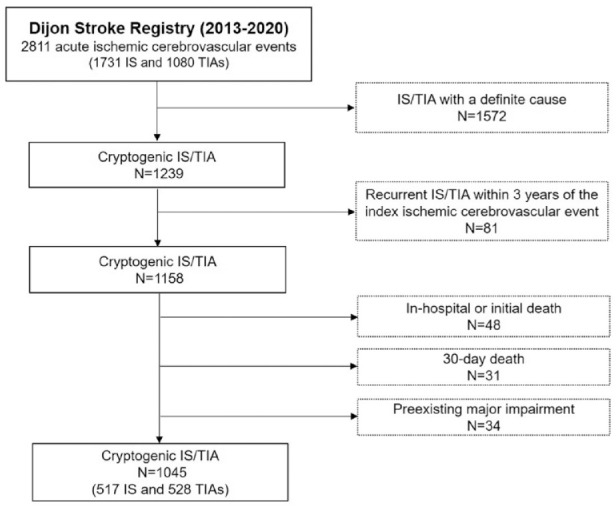
Study flow chart.
Distribution of patients according to age groups and sex is shown in Table 1. Crude incidence rates of eligible cryptogenic IS/TIA in adults ⩾35 years old were 169/100,000 per year (95% CI: 159–179) in overall sexes, 168/100,000 per year in men (95% CI: 153–184) and 169/100,000 per year in women (95% CI: 155–183). Corresponding rates were 83 (76–91) in overall sexes, 87 (77–99) in men, and 80 (71–90) in women for IS, and were 85 (78–93) in overall sexes, 81 (71–92) in men, and 89 (79–99) in women for TIA.
Table 1.
Annual incidence rates of cryptogenic IS/TIA eligible for prolonged cardiac rhythm monitoring in adults ⩾35 years old in Dijon, France, from 2013 to 2020 (expressed as n/100,000/year).
| Sex and age groups | N at risk | Cryptogenic IS/TIA eligible for prolonged cardiac rhythm monitoring |
Cryptogenic IS eligible for prolonged cardiac rhythm monitoring |
Cryptogenic TIA eligible for prolonged cardiac rhythm monitoring |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Rates | 95% CI | N | Rates | 95% CI | N | Rates | 95% CI | ||
| Men | ||||||||||
| 35–40 | 35,189 | 8 | 22.7 | 9.8–44.8 | 3 | 8.5 | 1.8–24.9 | 5 | 14.2 | 4.6–33.2 |
| 40–45 | 34,502 | 18 | 52.2 | 30.9–82.5 | 3 | 8.7 | 1.8–25.4 | 15 | 43.5 | 24.3–71.7 |
| 45–50 | 33,052 | 22 | 66.6 | 41.7–100.8 | 13 | 39.3 | 20.9–67.3 | 9 | 27.2 | 12.5–51.7 |
| 50–55 | 31,090 | 32 | 102.9 | 70.4–145.3 | 19 | 61.1 | 36.8–95.4 | 13 | 41.8 | 22.3–71.5 |
| 55–60 | 30,397 | 40 | 131.6 | 94.0–179.2 | 19 | 62.5 | 37.6–97.6 | 21 | 69.1 | 42.8–105.6 |
| 60–65 | 28,322 | 53 | 187.1 | 140.2–244.8 | 34 | 120.0 | 83.1–167.8 | 19 | 67.1 | 40.4–104.8 |
| 65–70 | 26,094 | 58 | 222.3 | 168.8–287.3 | 32 | 122.6 | 83.9–173.1 | 26 | 99.6 | 65.1–146.0 |
| 70–75 | 18,778 | 50 | 266.3 | 197.6–351.0 | 23 | 122.5 | 77.6–183.8 | 27 | 143.8 | 94.8–209.2 |
| 75–80 | 15,181 | 38 | 250.3 | 177.1–343.6 | 17 | 112.0 | 65.2–179.3 | 21 | 138.3 | 85.6–211.5 |
| 80–85 | 12,384 | 64 | 516.8 | 398.0–659.9 | 37 | 298.8 | 210.4–411.8 | 27 | 218.0 | 143.7–317.2 |
| ⩾85 | 12,035 | 83 | 689.7 | 549.3–854.9 | 42 | 349.0 | 251.5–471.7 | 41 | 340.7 | 244.5–462.2 |
| Total | 277,024 | 466 | 168.2 | 153.3–184.2 | 242 | 87.4 | 76.7–99.1 | 224 | 80.9 | 70.6–92.2 |
| Women | ||||||||||
| 35–40 | 35,520 | 9 | 25.3 | 11.6–48.1 | 4 | 11.3 | 3.1–28.8 | 5 | 14.1 | 4.6–32.9 |
| 40–45 | 35,030 | 9 | 25.7 | 11.7–48.8 | 6 | 17.1 | 6.3–37.3 | 3 | 8.6 | 1.8–25.0 |
| 45–50 | 35,064 | 16 | 45.6 | 26.1–74.1 | 7 | 20.0 | 8.0–41.1 | 9 | 25.7 | 11.7–48.7 |
| 50–55 | 34,822 | 26 | 74.7 | 48.8–109.4 | 12 | 34.5 | 17.8–60.2 | 14 | 40.2 | 22.0–67.5 |
| 55–60 | 35,824 | 28 | 78.2 | 51.9–113.0 | 9 | 25.1 | 11.5–47.7 | 19 | 53.0 | 31.9–82.8 |
| 60–65 | 34,871 | 27 | 77.4 | 51.0–112.7 | 15 | 43.0 | 24.1–70.9 | 12 | 34.4 | 17.8–60.1 |
| 65–70 | 33,743 | 46 | 136.3 | 99.8–181.8 | 21 | 62.2 | 38.5–95.1 | 25 | 74.1 | 47.9–109.4 |
| 70–75 | 25,347 | 54 | 213.0 | 160.0–278.0 | 27 | 106.5 | 70.2–155.0 | 27 | 106.5 | 70.2–155.0 |
| 75–80 | 20,987 | 77 | 366.9 | 289.5–458.6 | 34 | 162.0 | 112.2–226.4 | 43 | 204.9 | 148.3–276.0 |
| 80–85 | 21,026 | 95 | 451.8 | 365.6–552.3 | 52 | 247.3 | 184.7–324.3 | 43 | 204.5 | 148.0–275.5 |
| ⩾85 | 30,588 | 192 | 627.7 | 542.0–723.0 | 88 | 287.7 | 230.7–354.4 | 104 | 340.0 | 277.8–412.0 |
| Total | 342,822 | 579 | 168.9 | 155.4–183.2 | 275 | 80.2 | 71.0–90.3 | 304 | 88.7 | 79.0–99.2 |
| Men and women | ||||||||||
| 35–40 | 70,709 | 17 | 24.0 | 14.0–38.5 | 7 | 9.9 | 4.0–20.4 | 10 | 14.1 | 6.8–26.0 |
| 40–45 | 69,532 | 27 | 38.8 | 25.6–56.5 | 9 | 12.9 | 5.9–24.6 | 18 | 25.9 | 15.3–40.9 |
| 45–50 | 68,116 | 38 | 55.8 | 39.5–76.6 | 20 | 29.4 | 17.9–45.3 | 18 | 26.4 | 15.7–41.8 |
| 50–55 | 65,912 | 58 | 88.0 | 66.8–113.8 | 31 | 47.0 | 32.0–66.8 | 27 | 41.0 | 27.0–59.6 |
| 55–60 | 66,221 | 68 | 102.7 | 79.7–130.2 | 28 | 42.3 | 28.1–61.1 | 40 | 60.4 | 43.2–82.3 |
| 60–65 | 63,193 | 80 | 126.6 | 100.4–157.6 | 49 | 77.5 | 57.4–102.5 | 31 | 49.1 | 33.3–69.6 |
| 65–70 | 59,837 | 104 | 173.8 | 142.0–210.6 | 53 | 88.6 | 66.3–115.9 | 51 | 85.2 | 63.5–112.1 |
| 70–75 | 44,125 | 104 | 235.7 | 192.6–285.6 | 50 | 113.3 | 84.1–149.4 | 54 | 122.4 | 91.9–159.7 |
| 75–80 | 36,168 | 115 | 318.0 | 262.5–381.7 | 51 | 141.0 | 105.0–185.4 | 64 | 177.0 | 136.3–226.0 |
| 80–85 | 33,410 | 159 | 475.9 | 404.8–555.9 | 89 | 266.4 | 213.9–327.8 | 70 | 209.5 | 163.3–264.7 |
| ⩾85 | 42,623 | 275 | 645.2 | 571.2–726.1 | 130 | 305.0 | 254.8–362.2 | 145 | 340.2 | 287.1–400.3 |
| Total | 619,846 | 1045 | 168.6 | 158.5–179.1 | 517 | 83.4 | 76.4–90.9 | 528 | 85.2 | 78.1–92.8 |
After age and sex standardization to the French population, the total number of patients with cryptogenic IS/TIA eligible for prolonged cardiac rhythm monitoring was estimated to be 66,124 (95% CI: 65,622–66,630) for the calendar year 2022, including 32,182 men (95% CI: 31,830–32,535) and 33,943 women (95% CI: 33,583–34,306) (Table 2). In details, the number of cryptogenic IS was 32,764 (95% CI: 32,410–33,120), and that of cryptogenic TIA was 33,361 (95% CI: 33,004–33,721) (Supplemental Tables 1 and 2).
Table 2.
Estimated number of patients with cryptogenic IS/TIA eligible for prolonged cardiac rhythm monitoring in France for calendar year 2022.
| Age groups | Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|
| 2022 French population | Incidence rates (Dijon Stroke Registry) | Estimated number of patients in France | 95% CI | 2022 French population | Incidence rates (Dijon Stroke Registry) | Estimated number of patients in France | 95% CI | |
| 35–40 | 2,038,490 | 22.7 | 463 | 422–507 | 2,161,802 | 25.3 | 548 | 503–596 |
| 40–45 | 2,074,941 | 52.2 | 1083 | 1019–1149 | 2,164,498 | 25.7 | 556 | 511–604 |
| 45–50 | 2,134,631 | 66.6 | 1421 | 1348–1497 | 2,186,078 | 45.6 | 998 | 937–1062 |
| 50–55 | 2,192,825 | 102.9 | 2257 | 2165–2352 | 2,261,574 | 74.7 | 1689 | 1609–1772 |
| 55–60 | 2,158,015 | 131.6 | 2840 | 2737–2946 | 2,279,864 | 78.2 | 1782 | 1700–1867 |
| 60–65 | 1,998,819 | 187.1 | 3740 | 3621–3862 | 2,177,958 | 77.4 | 1686 | 1606–1768 |
| 65–70 | 1,826,825 | 222.3 | 4061 | 3937–4188 | 2,077,890 | 136.3 | 2833 | 2730–2939 |
| 70–75 | 1,709,892 | 266.3 | 4553 | 4422–4687 | 1,997,938 | 213.0 | 4256 | 4129–4386 |
| 75–80 | 1,125,175 | 250.3 | 2816 | 2713–2922 | 1,398,856 | 366.9 | 5132 | 4993–5274 |
| 80–85 | 747,549 | 516.8 | 3863 | 3742–3987 | 1,054,963 | 451.8 | 4767 | 4633–4904 |
| ⩾85 | 737,195 | 689.7 | 5084 | 4945–5226 | 1,544,879 | 627.7 | 9697 | 9505–9892 |
| Total | 18,744,357 | 32,182 | 31,830–32,535 | 21,306,300 | 33,943 | 33,583–34,306 | ||
Cardiac rhythm monitoring for at least 24 h was not performed during the initial diagnostic work-up, or information was missing, in 397 (38.0%) patients (24.6% in IS, and 51.1% in TIA patients). In sensitivity analysis, according to the assumption of a 4% rate of potential AF diagnosis in these patients, the number of remaining cryptogenic IS/TIA patients eligible for prolonged cardiac rhythm monitoring was estimated at 65,138 (95% CI: 64,639–65,640), including 32,451 (95% CI: 32,099–32,806) IS and 32,687 (95% CI: 32,334–33,043) (Supplemental Tables 3 and 4).
Finally, among the 1045 patients with cryptogenic IS/TIA according to TOAST classification, eligible for additional prolonged cardiac rhythm monitoring, we identified 140 (13.4%) with at least one condition that might be regarded as a potential cause, or who required long-term anticoagulation for another indication than IS/TIA (Supplemental Figure 1).
Discussion
This study demonstrated a high incidence of cryptogenic IS/TIA in adults >35 years old, eligible for prolonged cardiac rhythm monitoring. Estimates at a national level pointed out the large number of patients who may require access to such AF screening, with attention to be paid on regarding organization of care networks and related costs.
Our study relied on a population-based registry that allowed for a comprehensive collection of cases of IS and TIA, regardless of their management, thanks to multiple sources of information. Unlike administrative hospital-based databases, our analysis also considered non-hospitalized patients with ischemic cerebrovascular events, who are nevertheless managed in outpatient medical pathways and may require prolonged cardiac rhythm monitoring as part of the etiological assessment. Of note, these patients accounted for 8.2% of IS, and 27.3% of TIA (data not shown), and they would not have been identified in hospital administrative databases, thus highlighting the significant underestimation of the incidence (and number) of IS, and even more so of TIA, from these sources. 19 Moreover, in our registry, all suspected cases of IS/ TIA were confirmed after an epidemiological investigation and validation by a senior stroke-trained neurologist, thus overcoming the imperfect sensitivity and specificity of administrative databases for identifying stroke cases.20,21
This study offered the opportunity to provide data about age- and sex- specific incidence rates of cryptogenic IS/TIA, that could be helpful for clinicians and policy-makers to estimate the current and anticipate the future needs for prolonged cardiac rhythm monitoring in a context of increasing number of stroke as a consequence of aging population and demographic transition.22,23 Of note, although we used a cut-off age of 35 years for reasons described in the methods, the prevalence of AF in young (<60 years old) patients with cryptogenic IS/TIA is lower than in older. ESO guidelines recommends screening for all patients, regardless of age, but identifying factors for better selecting patients for access to long-lasting cardiac rhythm monitoring would make sense in terms of relevance and care prioritization. Several such factors and scores have been proposed to select patients at higher risk of AF on prolonged cardiac rhythm monitoring, most of them including age as one of the main determinants of AF risk. However, in SAFAS study we previously conducted, we suggested that a combination of biomarkers such as galectin-3, NT-proBNP, and osteoprotegerin with left atrial indexed volume resulted in a good predictive model for AF detected after stroke, whereas age did not appear as an independent predictor of AF. 24 Moreover, few scores have been developed and validated specifically for ICM patients, and we demonstrated in a cohort of 384 cryptogenic stroke patients with ICM 25 that both the previously described HAVOC and Brown ESUS-AF (combining age and left atrial dilatation) scores, although recommended in an ESC Working Group paper to select patients, 26 lack sensitivity. This is probably due to the fact that they are based mainly on cohorts in which AF was diagnosed by ECG or external Holter monitoring (i.e., patients with a high AF burden). Therefore we still lack specific scores that may help to safely exclude low-risk patients from prolonged cardiac rhythm monitoring.
To refine our estimates, in addition to excluding patients who died soon after their cerebrovascular event, we considered patients with severe disability prior to the event, defined as those with premorbid IS/TIA mRS score of 5, or with preexisting dementia and living in an institution. Although ESO guidelines recommend prolonged cardiac rhythm monitoring with ICM in patients with cryptogenic IS/TIA without mentioning distinction with regard to prior condition, 8 patients with already severely impaired autonomy are usually excluded from invasive diagnostic workup in routine clinical practice. Additionally, the inclusion of patients with premorbid mRS score of 4 can be discussed. Indeed, in a context of limited resources, the search for AF using prolonged cardiac rhythm monitoring, especially ICM, could be considered excessive in these patients who are already severely handicapped. On the other hand, AF diagnosis has important therapeutic implications, aimed at reducing the risk of stroke recurrence, which in these patients could worsen their condition or lead to death. In our study, only a low proportion of patients (6.2%) had a premorbid mRS score of 4, thus limiting the impact on our results.
We also chose to exclude cases corresponding to recurrences occurring less than 3 years after the index recorded cryptogenic IS/TIA event. Hence, the maximum lifespan of current ICM is around 3 years, and we considered that if a patient had a recurrent IS/TIA during this period, he would not have benefited from the implantation of a second device over this period.
Finally, although commonly used, the TOAST classification is often considered too restrictive, resulting in a high proportion of IS/TIA being classified as cryptogenic. To attempt to take into account this issue, we identified patients with least one condition that might be regarded as a potential cause, despite being classified as cryptogenic cerebrovascular events. We also identified patients who required long-term anticoagulation for another indication than IS/TIA. Taken together, these patients account for only a limited proportion of cryptogenic IS/TIA.
Several limitations must be acknowledged. Because of the population-based setting, prolonged cardiac rhythm monitoring for at least 24 h was not performed or information was missing in some patients. To account for it, we made the assumption that performing such examination in these patients would have resulted in a diagnosis of AF in 4% of cases, consistently with literature data on rate of AF detection based on 24 h-holter or telemetry.3–6,18 We applied this hypothesis in sensitivity analyses to avoid an overestimation effect. As a result, estimates were only slightly altered. Of note, as the timing of data extraction relative to the event for stroke classification was 3 months, we cannot exclude that some patients did indeed undergo this exam later. Furthermore, we chose not excluding patients with severe disability immediately after the stroke. Hence, although 3.3% of patients included in our analysis had a mRS score of 5 at discharge, and 10.4% had a mRS score of 4 (data not presented), it was not possible to rule out a delayed functional recovery in the absence of longer term follow-up information. Thus, if we had excluded them, we would have underestimated the number of patients eligible for prolonged cardiac rhythm monitoring. Of note, given the small percentage of patients concerned, we can reasonably assume that this limitation had little impact on the overall results. Additionally, there are certainly other reasons why a practitioner might choose not to opt for implementing prolonged cardiac rhythm monitoring in a patient, such as a severe associated illness threatening short-term prognosis or a patient's refusal, we were not able to take into account. Finally, despite rigorous age and sex standardization, caution must be exercised regarding our nationwide estimates of the number of cryptogenic IS/TIA eligible for prolonged cardiac rhythm monitoring, as differences in the health status of Dijon residents compared with the rest of France cannot be totally ruled out.
To conclude, this study pointed out the high number of patients with cryptogenic IS/TIA eligible for prolonged cardiac rhythm monitoring according to current guidelines. Our findings highlighted the need to consequently adapt healthcare systems to meet current and future needs regarding etiological diagnostic workup of IS/TIA patients.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873241266471 for Incidence and nationwide estimates of cryptogenic ischemic stroke or TIA eligible for prolonged cardiac rhythm monitoring by Yannick Béjot, Gauthier Duloquin and Charles Guenancia in European Stroke Journal
Acknowledgments
None.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Charles Guenancia reports personal fees from MicroPort CRM, Medtronic, Astra-Zeneca, BMS, Pfizer, Abbott, outside the submitted work. Yannick Béjot reports personal fees from BMS, Pfizer, Medtronic, Amgen, Servier, NovoNordisk, Novartis, outside the submitted work. Other authors: none.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Dijon Stroke Registry is supported by Santé Publique France (French Institute for Public Health Surveillance), Institut national de la santé et de la recherche médicale (INSERM), and University Hospital of Dijon. The Dijon Stroke Registry received a grant from Medtronic for the present analysis. Sponsors had no roles in the preparation of data or the manuscript.
Ethical approval: The Dijon Stroke Registry was approved by following national ethics boards: the Comité d’Evaluation des Registres (French National Committee of Registers), Santé Publique France (French Institute for Public Health Surveillance), and the Commission Nationale Informatique et Liberté (French data protection authority).
Informed consent: In accordance with the French legislation, boards waived the need for written patient consent.
Guarantor: YB.
Contributorship: Concept and design of the study: YB; Data acquisition: GD, YB; Data analysis: YB; Interpretation of data: YB, CG; Manuscript draft: YB; Manuscript revision: YB, CG; Manuscript approval: YB, GD, CG.
ORCID iDs: Yannick Béjot  https://orcid.org/0000-0001-7848-7072
https://orcid.org/0000-0001-7848-7072
Charles Guenancia  https://orcid.org/0000-0002-3554-7714
https://orcid.org/0000-0002-3554-7714
Data availability statement: The authors declare that all supporting data are available within the article.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Béjot Y, Daubail B, Giroud M. Epidemiology of stroke and transient ischemic attacks: current knowledge and perspectives. Rev Neurol (Paris) 2016; 172: 59–68. [DOI] [PubMed] [Google Scholar]
- 2. Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: a European Stroke Organisation guideline. Eur Stroke J 2019; 4: 198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370: 2467–2477. [DOI] [PubMed] [Google Scholar]
- 4. Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014; 370: 2478–2486. [DOI] [PubMed] [Google Scholar]
- 5. Sposato LA, Field TS, Schnabel RB, et al. Towards a new classification of atrial fibrillation detected after a stroke or a transient ischaemic attack. Lancet Neurol 2024; 23: 110–122. [DOI] [PubMed] [Google Scholar]
- 6. Buck BH, Hill MD, Quinn FR, et al. Effect of implantable vs prolonged external electrocardiographic monitoring on atrial fibrillation detection in patients with ischemic stroke: the PER DIEM randomized clinical trial. JAMA 2021; 325: 2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brachmann J, Morillo CA, Sanna T, et al. Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: three-year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol 2016; 9: e003333. [DOI] [PubMed] [Google Scholar]
- 8. Rubiera M, Aires A, Antonenko K, et al. European Stroke Organisation (ESO) guideline on screening for subclinical atrial fibrillation after stroke or transient ischaemic attack of undetermined origin. Eur Stroke J 2022; 7: VI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinguet V, Duloquin G, Thibault T, et al. Pre-existing brain damage and association between severity and prior cognitive impairment in ischemic stroke patients. J Neuroradiol 2023; 50: 16–21. [DOI] [PubMed] [Google Scholar]
- 10. Feigin V, Norrving B, Sudlow CLM, et al. Updated criteria for population-based stroke and transient ischemic attack incidence studies for the 21st century. Stroke 2018; 49: 2248–2255. [DOI] [PubMed] [Google Scholar]
- 11. Bennett DA, Brayne C, Feigin VL, et al. Development of the Standards of Reporting of Neurological Disorders (STROND) checklist: a guideline for the reporting of incidence and prevalence studies in neuroepidemiology. Neurology 2015; 85: 821–828. [DOI] [PubMed] [Google Scholar]
- 12. WHO MONICA Project Principal Investigators. The World Health Organization Monica Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol 1988; 41: 105–114. [DOI] [PubMed] [Google Scholar]
- 13. Béjot Y, Brenière C, Graber M, et al. Contemporary epidemiology of transient ischemic attack in Dijon, France (2013–2015). Neuroepidemiology 2017; 49: 135–141. [DOI] [PubMed] [Google Scholar]
- 14. Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack—proposal for a new definition. N Engl J Med 2002; 347: 1713–1716. [DOI] [PubMed] [Google Scholar]
- 15. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 16. Béjot Y, Duloquin G, Crespy V, et al. Influence of preexisting cognitive impairment on clinical severity of ischemic stroke: the Dijon stroke registry. Stroke 2020; 51: 1667–1673. [DOI] [PubMed] [Google Scholar]
- 17. Graber M, Garnier L, Mohr S, et al. Influence of pre-existing mild cognitive impairment and dementia on post-stroke mortality. The Dijon stroke registry. Neuroepidemiology 2020; 54: 490–497. [DOI] [PubMed] [Google Scholar]
- 18. Bernstein RA, Kamel H, Granger CB, et al. Effect of long-term continuous cardiac monitoring vs usual care on detection of atrial fibrillation in patients with stroke attributed to large- or small-vessel disease: the STROKE-AF randomized clinical trial. JAMA 2021; 325: 2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lecoffre C, de Peretti C, Gabet A, et al. National trends in patients hospitalized for stroke and stroke mortality in France, 2008 to 2014. Stroke 2017; 48: 2939–2945. [DOI] [PubMed] [Google Scholar]
- 20. Aboa-Eboulé C, Mengue D, Benzenine E, et al. How accurate is the reporting of stroke in hospital discharge data? A pilot validation study using a population-based stroke registry as control. J Neurol 2013; 260: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giroud M, Hommel M, Benzenine E, et al. Positive predictive value of French hospitalization discharge codes for stroke and transient ischemic attack. Eur Neurol 2015; 74: 92–99. [DOI] [PubMed] [Google Scholar]
- 22. Béjot Y, Bailly H, Graber M, et al. Impact of the ageing population on the burden of stroke: the Dijon stroke registry. Neuroepidemiology 2019; 52: 78–85. [DOI] [PubMed] [Google Scholar]
- 23. Brainin M, Feigin VL, Norrving B, et al. Global prevention of stroke and dementia: the WSO declaration. Lancet Neurol 2020; 19: 487–488. [DOI] [PubMed] [Google Scholar]
- 24. Didier R, Garnier L, Duloquin G, et al. Distribution of atrial cardiomyopathy markers and association with atrial fibrillation detected after ischaemic stroke in the SAFAS study. Stroke Vasc Neurol 2024; 9: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grygorowicz C, Benali K, Serzian G, et al. Value of HAVOC and Brown ESUS-AF scores for atrial fibrillation on implantable cardiac monitors after embolic stroke of undetermined source. J Stroke Cerebrovasc Dis 2024; 33: 107451. [DOI] [PubMed] [Google Scholar]
- 26. Dilaveris PE, Antoniou CK, Caiani EG, et al. ESC Working Group on e-Cardiology Position Paper: accuracy and reliability of electrocardiogram monitoring in the detection of atrial fibrillation in cryptogenic stroke patients: in collaboration with the Council on Stroke, the European Heart Rhythm Association, and the Digital Health Committee. Eur Heart J Digit Health 2022; 3: 341–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873241266471 for Incidence and nationwide estimates of cryptogenic ischemic stroke or TIA eligible for prolonged cardiac rhythm monitoring by Yannick Béjot, Gauthier Duloquin and Charles Guenancia in European Stroke Journal