Abstract
Introduction:
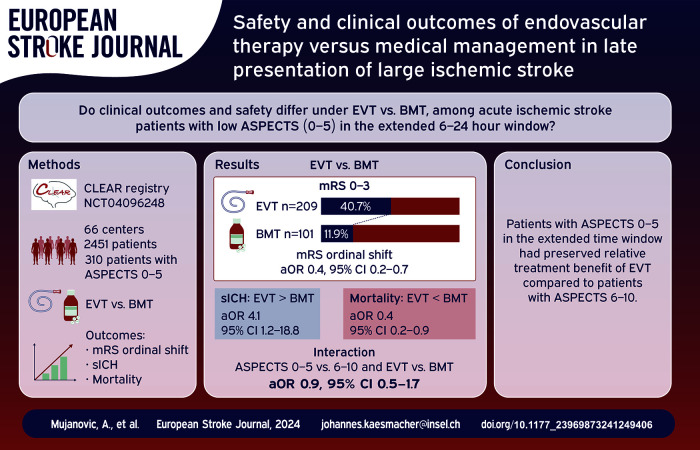
The benefit of endovascular therapy (EVT) among stroke patients with large ischemic core (ASPECTS 0–5) in the extended time window outside of trial settings remains unclear. We analyzed the effect of EVT among these stroke patients in real-world settings.
Patients and methods:
The CT for Late Endovascular Reperfusion (CLEAR) study recruited patients from 66 centers in 10 countries between 01/2014 and 05/2022. The extended time-window was defined as 6–24 h from last-seen-well to treatment. The primary outcome was shift of the 3-month modified Rankin scale (mRS) score. Safety outcomes included symptomatic intracranial hemorrhage (sICH) and mortality. Outcomes were analyzed with ordinal and logistic regressions.
Results:
Among 5098 screened patients, 2451 were included in the analysis (median age 73, 55% women). Of patients with ASPECTS 0–5 (n = 310), receiving EVT (n = 209/310) was associated with lower 3-month mRS when compared to medical management (median 4 IQR 3–6 vs 6 IQR 4–6; aOR 0.4, 95% CI 0.2–0.7). Patients undergoing EVT had higher sICH (11.2% vs 4.0%; aOR 4.1, 95% CI 1.2–18.8) and lower mortality (31.6% vs 58.4%, aOR 0.4; 95% CI 0.2–0.9) compared to medically managed patients. The relative benefit of EVT was comparable between patients with ASPECTS 0 and 5 and 6–10 in the extended time window (interaction aOR 0.9; 95% CI 0.5–1.7).
Conclusion:
In the extended time window, patients with ASPECTS 0–5 may have preserved relative treatment benefit of EVT compared to patients with ASPECTS 6–10. These findings are in line with recent trials showing benefit of EVT among real-world patients with large ischemic core in the extended time window.
Trial registration number:
clinicaltrials.gov; Unique identifier: NCT04096248
Keywords: Endovascular therapy, mechanical thrombectomy, best medical treatment, large ischemic core, extended time-window
Graphical abstract.
Introduction
Present stroke treatment guidelines recommend endovascular therapy (EVT) as the standard of care for patients with acute ischemic stroke due to large vessel occlusion (LVO).1–3 Patients presenting with small ischemic core on admission, defined as Alberta Stroke Program Early CT Score (ASPECTS) 6–10, are routinely treated with EVT; however, the ASPECTS threshold below which there is no clinical benefit from EVT is not established as most pivotal EVT trials excluded patients presenting with large ischemic core (ASPECTS 0–5). 4
Recent randomized controlled trials (RCT) enrolled patients with large ischemic core to receive either best medical treatment (BMT) or EVT. Five of these trials (Recovery by Endovascular Salvage for Cerebral Ultra-Acute Embolism–Japan Large Ischemic Core Trial [RESCUE-Japan LIMIT], Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients with a Large Infarct Core [ANGEL-ASPECT], A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke [SELECT2], Efficacy, Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window [TENSION] and LArge Stroke Therapy Evaluation [LASTE]) reported benefit of EVT over BMT in patients with low ASPECTS, while one RCT (Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischemic Stroke [TESLA]) did not show superiority of EVT over BMT in this subpopulation.5–10 However, the majority of patients included in these RCTs presented ⩽6 h of symptom onset and with ASPECTS 3–5. Moreover, patients included into RCTs usually present a highly selected subpopulation and may not be representative of real-world settings. 11 Some observational studies using multi-center data have also shown benefit of EVT among patients with ASPECTS 3–5 presenting >6 h.12,13 However, other observational studies in the extended time window showed disparate results with limited sample size.14–17 Therefore, it still remains unclear if patients with ASPECTS 0–5 who present in the extended time-window (6–24 h after symptom onset) might benefit from EVT in a real-world setting. This multicenter study aimed to evaluate the clinical outcomes and safety of EVT among acute ischemic stroke patients presenting with low ASPECTS in the extended time window.
Methods
CT for Late Endovascular Reperfusion (CLEAR) was a multicenter study of consecutive acute ischemic stroke patients undergoing EVT or BMT for LVO in the extended time window (clinicaltrials.gov, NCT04096248). 18 CLEAR recruited patients from 15 centers in 5 countries that were treated from January 2014 until December 2020. After the initial paper, there was an expansion to a total of 66 sites, of whom 21 sites contributed consecutive data on patients with large ischemic core up to May 2022. 18 The extended time window was defined as 6–24 h from last-seen-well (TLSW) to treatment. Rating of ASPECTS was based on either the last non-contrast CT or diffusion-weighted MRI before intervention. The study period was during a time before and after the DAWN and DEFUSE-3 trial results when EVT was not routinely offered at centers in Europe in the extended window. Selection criteria and neuroimaging protocols were site-adjudicated. 18 Institutional Research Board or ethics committees’ approval were obtained from all enrolling sites. This study was conducted according to the Declaration of Helsinki and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Anonymized study data are available from the corresponding author upon reasonable request and presented research plan.
Population and outcomes
Inclusion criteria for the present study were: (1) prestroke modified Rankin Scale (pre-mRS) score 0–3; (2) baseline National Institutes of Health Stroke Scale (NIHSS) score ⩾ 6; (3) TLSW-to-Treatment time within 6–24 h; (4) LVO of the internal carotid artery or middle cerebral artery; (5) available data on the 3-month modified Rankin Scale (mRS) score. For the primary analysis, we included only patients presenting with ASPECTS 0–5, and for the secondary analysis we included patients across all ASPECTS (0–10).
The primary outcome of interest was the ordinal shift of the mRS score at 3 months. Secondary outcomes were independent ambulation, defined as mRS score 0–3 at the 3 month follow-up. Safety outcomes included rates of symptomatic intracranial hemorrhage (sICH), defined as intracranial hemorrhage associated with deterioration in NIHSS ⩾ 4, and mortality at 3 months.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics, with continuous data reported as median (interquartile range) and categorical data reported as counts (%). The Mann-Whitney U test was used for continuous and Fisher exact test for categorical variables. Mixed effects regression models were used to account for the heterogeneity between study sites by using random-intercept adjusted for clustering at the site level. Independent ambulation at 3 months was estimated by both ordinal and logistic regressions. Regression analyses were adjusted for baseline and potential pathophysiological covariates which could influence patient outcome: age, sex, NIHSS on admission, pre-stroke mRS, occlusion site, hypertension, atrial fibrillation, diabetes mellitus, TLSW-to-treatment time, intravenous thrombolysis and treatment modality. For the secondary analysis, which included patients with ASPECTS 0–10, we added a multiplicative interaction term between the ASPECTS (dichotomized as 0–5 vs 6–10) and treatment (EVT vs BMT). The odds ratios of the interaction term describe the change of the association between EVT versus BMT and outcome if ASPECTS 0–5. In the logistic regression analysis, an odds ratio < 1 for the interaction term indicates the association is less strong than expected when considering only the main effects, while odds ratio > 1 indicates the association is stronger than expected. Only patients with all available data were included in the analysis (complete case analysis). For sensitivity purposes, we used multiple imputations (Multivariate Imputation by Chained Equations, MICE method) to account for missing data, after which the whole dataset was included in the regression analysis. Results are reported as adjusted odds ratios (aOR) with corresponding 95% confidence intervals (CI). All statistical analyses were conducted in R (v4.0.0 R Foundation for Statistical Computing, Vienna, Austria).
Results
Among 5098 patients assessed for eligibility, we included 2451 patients in our analysis (Figure S1). For the primary analysis only patients with ASPECTS 0–5 were considered (n = 310). The median age of this cohort was 72 years (IQR 59–82), 53.5% were female and 67% underwent EVT. When stratified across treatment arms, those who underwent EVT were younger (68 years vs 80 years, p < 0.001), less likely to have hypertension (61.2% vs 79.2%, p = 0.002) or atrial fibrillation (33.1% vs 47.5%; p = 0.03), have lower pre-mRS (0, IQR 0–1 vs 1, IQR 0–3; p < 0.001), more likely to be transfer patients (57.7% vs 15.8%, p < 0.001), have an ICA occlusion (41.6% vs 21.8%, p = 0.003), shorter TLSW-to-Treatment time (11 h 30 min vs 14 h 5 min, p = 0.001) and were more likely to have received IVT (19.6% vs 4.0%, p < 0.001) as shown in Table 1. Patients in the BMT arm were more likely to undergo CT on admission, while EVT-treated patients more often had MRI on admission (CT vs MRI rates per treatment arms: 97% vs 3%; 61% vs 39%; respectively). The distribution of ASPECTS across treatment arms is shown in Table S1.
Table 1.
Baseline characteristics of patients with low ASPECTS.
| Variable | Overall | BMT | EVT | p | Missing (%) | |
|---|---|---|---|---|---|---|
| N (%) | 310 | 101 | 209 | |||
| BASELINE | ||||||
| Age (median [IQR]) | 72 [59, 82] | 80 [68, 85] | 68 [55, 79] | <0.001 | 0 | |
| Female Sex (%) | 166 (53.5) | 60 (59.4) | 106 (50.7) | 0.188 | 0 | |
| Hypertension (%) | 208 (67.1) | 80 (79.2) | 128 (61.2) | 0.002 | 0 | |
| Atrial fibrillation (%) | 99 (38.8) | 48 (47.5) | 51 (33.1) | 0.029 | 17.7 | |
| Diabetes mellitus (%) | 58 (18.7) | 23 (22.8) | 35 (16.7) | 0.263 | 0 | |
| NIHSS at admission (median [IQR]) | 19 [15, 22] | 19 [16, 24] | 18 [15, 22] | 0.158 | 0 | |
| pre-mRS (median [IQR]) | 0 [0, 1] | 1 [0, 3] | 0 [0, 1] | <0.001 | 0 | |
| Patient arrival (%) | Direct admission | 170 (56.3) | 85 (84.2) | 85 (42.3) | <0.001 | 2.6 |
| Transfer | 132 (43.7) | 16 (15.8) | 116 (57.7) | |||
| Occlusion site (%) | ICA | 109 (35.2) | 22 (21.8) | 87 (41.6) | 0.003 | 0 |
| M1 MCA | 178 (57.4) | 67 (66.3) | 111 (53.1) | |||
| M2 MCA | 21 (6.8) | 11 (10.9) | 10 (4.8) | |||
| M3 MCA | 2 (0.6) | 1 (1.0) | 1 (0.5) | |||
| Imaging on admission (%) | CT | 126 (40.9) | 73 (73.7) | 53 (25.4) | <0.001 | 0.6 |
| CTP | 97 (31.5) | 23 (23.2) | 74 (35.4) | |||
| MRI | 85 (27.6) | 3 (3.0) | 82 (39.2) | |||
| ASPECTS (median [IQR]) | 5 [3, 5] | 4 [2, 5] | 5 [4, 5] | <0.001 | 0 | |
| Last known well to CT time (h) (median [IQR]) | 11.35 [8.63, 14.80] | 13.95 [9.63, 16.63] | 10.60 [8.05, 13.70] | <0.001 | 0 | |
| Last known well to treatment time (h) (median [IQR]) | 11.87 [9.08, 15.56] | 14.08 [8.86, 17.15] | 11.50 [8.85, 14.18] | 0.001 | 0 | |
| TREATMENT | ||||||
| Intravenous thrombolysis (%) | 45 (14.5) | 4 (4.0) | 41 (19.6) | <0.001 | 0.0 | |
| OUTCOME | ||||||
| NIHSS at discharge (median [IQR]) | 14 [8, 21] | 15 [10, 24] | 14 [8, 21] | 0.308 | 31.6 | |
| sICH (%) | 27 (8.9) | 4 (4.0) | 23 (11.2) | 0.066 | 1.6 | |
| mRS, ordinal at 3 months (median [IQR]) | 4 [3, 6] | 6 [4, 6] | 4 [3, 6] | <0.001 | 0 | |
| mRS 0–3 at 3 months (%) | 97 (31.3) | 12 (11.9) | 85 (40.7) | <0.001 | 0 | |
| mRS 0–2 at 3 months (%) | 52 (16.8) | 7 (6.9) | 42 (21.5) | 0.002 | ||
| mRS 0–1 at 3 months (%) | 18 (5.8) | 2 (2.0) | 16 (7.7) | 0.081 | ||
| Mortality (%) | 125 (40.3) | 59 (58.4) | 66 (31.6) | <0.001 | 0 | |
BMT: best medical therapy; EVT: endovascular therapy; NIHSS: National Institutes of Health Stroke Scale; pre-mRS: pre-stroke modified Rankin Scale score; ICA: internal carotid artery; MCA: middle cerebral artery; ASPECTS: Alberta Stroke Program Early Computed Tomography Score; sICH: symptomatic intracranial hemorrhage.
Main outcomes
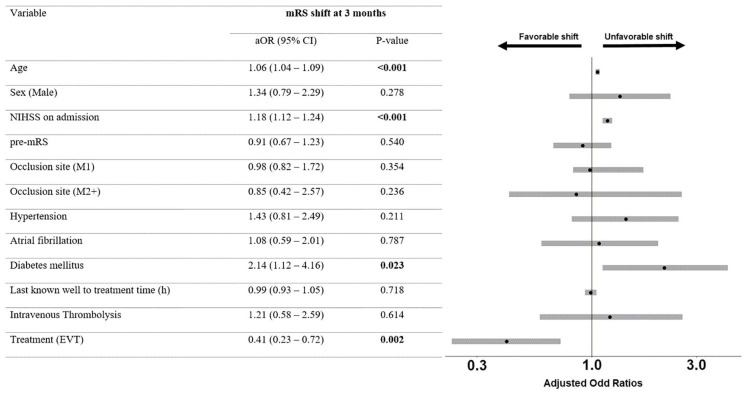
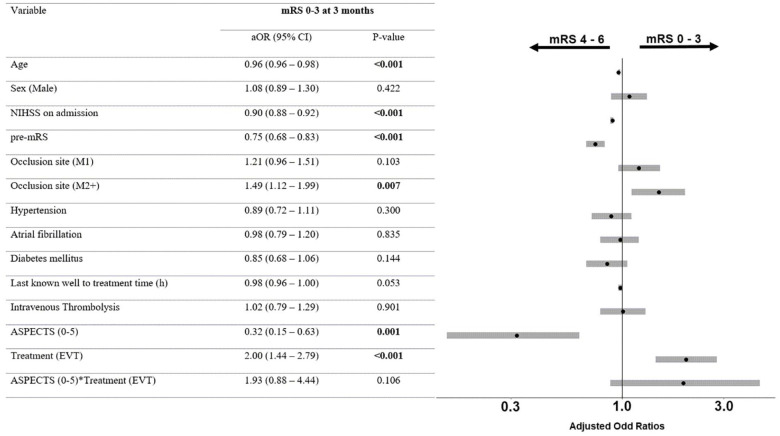
Patients with low ASPECTS who underwent EVT were more likely to achieve independent ambulation at 3 months compared to patients undergoing BMT (mRS 0–3: 40.7% vs 11.9%, p < 0.001). After adjustment for the prespecified confounders, there was a significant association between mRS ordinal shift and EVT (aOR 0.4, 95% CI 0.2–0.7; Figures 1 and 2). The aOR for independent ambulation at 3 months was 5.0, 95% CI 2.2–12.6. Sensitivity analysis with imputed data showed comparable results (Table S2). Patients with low ASPECTS undergoing EVT had higher likelihood for sICH (aOR 4.1, 95% CI 1.2–18.8) and lower likelihood for mortality at 3 months (aOR 0.4, 95% CI 0.2–0.9). Comparable results were obtained with imputed data (Table S3).
Figure 1.
Adjusted ordinal regression among patients with low ASPECTS.
mRS: modified Rankin Scale; aOR: adjusted odds ratios; CI: confidence interval; NIHSS: National Institutes of Health Stroke Scale; pre-mRS: pre-stroke modified Rankin Scale score; EVT: endovascular therapy. Odds ratios in ordinal regression > 1 indicate an unfavorable shift on the mRS, while odds ratios < 1 indicate a favorable shift on the mRS.
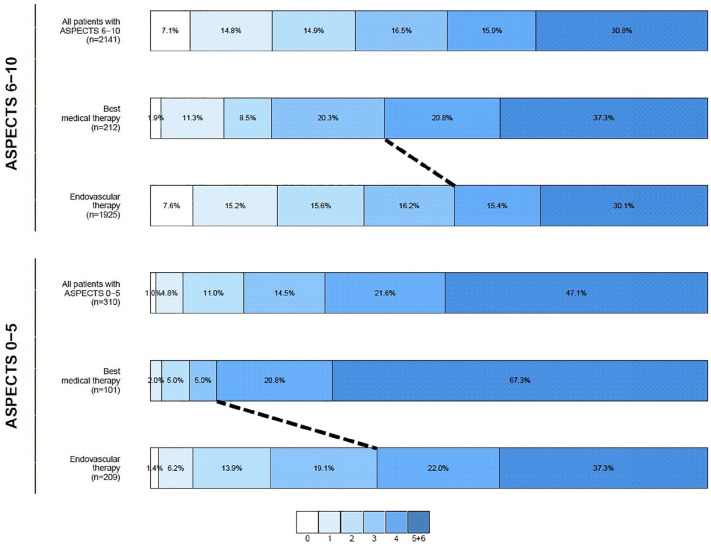
Figure 2.
Modified Rankin Scale score shift at 3-month follow-up.
ASPECTS: Alberta Stroke Program Early Computed Tomography Score. Ordinal shift on the mRS (dashed black line for independent ambulation) was observed among patients with both high and low ASPECTS. After adjustment, the association between endovascular therapy and independent ambulation remained (ordinal regression aOR 0.4, 95% CI 0.2–0.7).
Interaction effect
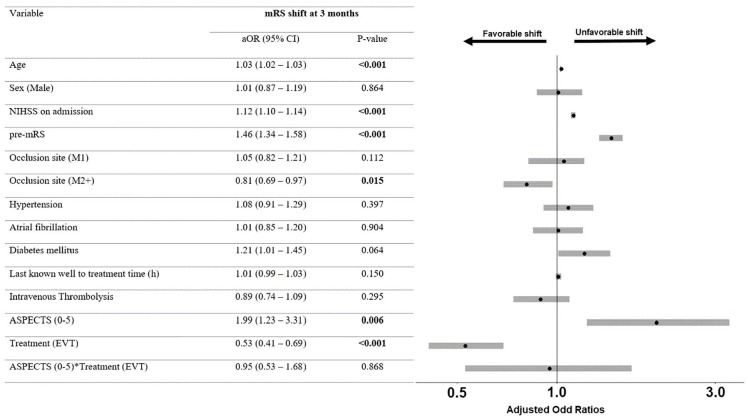
Patients with both low and high ASPECTS (n = 2451) were included in the secondary analysis. When comparing patients with low versus high ASPECTS, patients with lower ASPECTS were younger (72 years vs 73 years, p = 0.04), had higher NIHSS score at baseline (19, IQR 15–22 vs 16, IQR 11–20; p < 0.001), were directly admitted to the treating center (56.3% vs 45.1%; p < 0.001), had an ICA occlusion (35.2% vs 24.6%; p < 0.001) and received BMT (32.6% vs 9.9%; p < 0.001, Table S4). In the adjusted analysis, the interaction term ASPECTS × Treatment was neither associated with mRS shift at 3 months (aOR 0.9, 95% CI 0.5–1.7, Figure 3), nor with independent ambulation at 3 months (aOR 1.9, 95% CI 0.9–4.4, Figure 4). Analysis with multiple imputations for missing data showed comparable results (Table S5). The interaction term remained non-significant for both safety outcomes (aOR 0.6, 95% CI 0.1–3.2 and 0.7, 95% CI 0.4–1.5 for sICH and mortality, respectively; Table S6).
Figure 3.
Adjusted ordinal regression among all patients.
mRS: modified Rankin Scale; aOR: adjusted odds ratios; CI: confidence interval; NIHSS: National Institutes of Health Stroke Scale; pre-mRS: pre-stroke modified Rankin Scale score; ASPECTS: Alberta Stroke Program Early Computed Tomography Score; EVT: endovascular therapy. Odds ratios in ordinal regression > 1 indicate an unfavorable shift on the mRS, while odds ratios < 1 indicate a favorable shift on the mRS.
Figure 4.
Adjusted logistic regression among all patients.
mRS: modified Rankin Scale; aOR: adjusted odds ratios; CI: confidence interval; NIHSS: National Institutes of Health Stroke Scale; pre-mRS: pre-stroke modified Rankin Scale score; EVT: endovascular therapy. After adjustment for the prespecified confounders, the interaction term ASPECTS × Treatment was not associated with independent ambulation at 3 months (aOR 1.9, 95% CI 0.9–4.4).
Discussion
The main findings of this study are: (1) In routine clinical practice, EVT is associated with higher rates of independent ambulation among acute ischemic stroke patients presenting with anterior large vessel occlusion and low ASPECTS. (2) In comparison to patients with low ASPECTS receiving BMT, patients undergoing EVT had lower mortality rates but increased rates of symptomatic intracranial hemorrhage. (3) Although the overall outcome of patients with low ASPECTS is poor, the relative benefit of endovascular therapy may be preserved among patients with low ASPECTS.
Treatment benefit of EVT
The RESCUE Japan Limit trial showed higher likelihood of achieving independent ambulation in the EVT versus BMT group (RR 2.4, 95% CI 1.3–4.4). 5 The same results were shown by the ANGEL ASPECT and SELECT2 trials (RR 1.5, 95% CI 1.2–1.9 and RR 2.1, 95% CI 1.4–2.9, respectively).6,7 The TENSION trial was stopped prematurely for efficacy and reported comparable results for mRS 0–3 (aOR 2.8, 95% CI 1.5–5.5). 9 Meta-analyses showed that EVT was significantly associated with reduced disability (OR 1.7, 95% CI 1.4–2.1) and higher rates of independent ambulation (RR 1.8, 95% CI 1.3–2.5). 17 Preliminary results from the TESLA trial did not show superiority of EVT over BMT, 8 whereas the LASTE trial demonstrated superiority of EVT over BMT for patients with ASPECTS 0–5 presenting in the 7-h window, most of whom were selected by MRI. 10
However, the number of patients presenting in the extended time window across these trials varies. In RESCUE Japan Limit, only 30% of patients were admitted within 6–24 h after TLSW. 5 In ANGEL ASPECT and SELECT2, these numbers were higher (60%–70%); however, these two trials enrolled patients with ASPECTS 3–5, and ANGEL ASPECT enrolled ASPECTS 0–2 with 70–100 ml core volume.5–7 On the other hand, the median onset-to-groin-puncture time in TENSION was 4 h 12 min and the median onset-to-randomization time was 2 h. Similarly, the LASTE trial only enrolled patients in the early window (onset-to-last-known-well < 7 h) with median onset-to-imaging time of 2 h 50 min.9,10 Moreover, there was heterogeneity across the design of these trials. 19 Selection criteria differed according to the imaging modality; some trials used automated volumetric methods, two trials enrolled patients mainly by assessing MRI mismatch, only three trials had international recruitment, mortality in the control groups differed and deviations from the intended intervention were common.19,20 Additionally, patients with large ischemic core are thought to be fast progressors, and the potential benefit of EVT among patients who present in an extended window with potentially more established infarct has not been fully explored in real-world setting yet.
In the present analysis, we observed preserved beneficial association of EVT with better outcomes among patients with low and high ASPECTS in the extended time window. Our data suggest a potentially beneficial effect which was reported in RCTs that spanned a heterogeneous population with variable definitions of “large core” in various time windows. This finding is consistent with the increasing evidence that support EVT treatment in different ischemic stroke patient subpopulations. Concurrently, rates of independent ambulation seemed lower in large ischemic core patients who received BMT only.
Ischemic penumbra could also be a potentially relevant factor when deciding to pursue EVT among this patient subpopulation. The SELECT2 trial showed no heterogeneity of EVT treatment effect among patient with and without penumbra, across different mismatch profiles (p > 0.5 for all thresholds). 21 The beneficial effect of EVT was preserved even among late-presenting patients with no or minimal penumbra. While the presence of penumbra increases the probability of independent ambulation, it should not be the determining factor for pursuing EVT. Ischemic core volume, on the other hand, seems to be an important indicator for treatment outcome, especially among patients with volume > 150 ml. 21 Results from the upcoming pooled analysis of individual patient-level data from large core trials (MAGNA collaboration) will likely provide more data on these associations. 22
Observational data
An analysis of the German Stroke Registry showed that patients with low ASPECTS who achieve successful reperfusion might benefit from EVT even when receiving it up to 17 h from symptom onset. 12 Subanalysis of the STAR registry reported that both low ASPECTS and extended window were independently associated with a lower odds of achieving good outcome. 13 In the present analysis, we observed that patients with low ASPECTS who received EVT had higher likelihood of achieving independent ambulation when compared to BMT. We also noticed a tendency by the treating team to choose younger patients with less comorbidities to undergo EVT. The percentage of patients with ASPECTS 4–5 were 93% and 55% in the EVT and BMT group, respectively. These differences between patients undergoing EVT and BMT are expected in the real-world setting and underline true differences in management and patient selection across centers before RCT data became available. To mitigate this selection bias, we adjusted our analysis for differences in baseline status. Even after adjustment for these differences, an association between EVT and favorable mRS shift was preserved showing potential efficacy of EVT in this subgroup of real-world stroke patients. Association between older age, preexisting comorbidities and poor outcome could be linked with an already compromised state of general health and overall decreased functional reserve in older age.15,16
EVT also seems to be cost-effective for patients with large ischemic core.23,24 Data from several European countries, United States and China have shown that incremental cost-effectiveness can range up to US$11,000 per quality-adjusted life-years gained.23,24 This association on cost-effectiveness was preserved even at different thresholds of willingness to pay, providing additional evidence of beneficial EVT effect across different healthcare systems.
Safety concerns
In four large-core RCTs (RESCUE Japan Limit, ANGEL ASPECT, SELECT2 and TENSION), rates of sICH at 24–48 h and all-cause mortality at 90 days were comparable between the two treatment arms. In the meta-analysis of the first three trials, there was a higher risk of sICH in the EVT arm (RR 1.9, 95% CI 1.1–3.7) and no difference in 3-month mortality (RR 0.9, 95% CI 0.8–1.1). 17 According to another study-level meta-analysis, sICH risk was higher in the EVT- compared to the BMT-arm (RR 1.8, 95% CI 0.9–3.5, p = 0.07). 17 In the sub-analysis of observational studies only, patients undergoing EVT had lower 3-month mortality risk (RR 0.6, 95% CI 0.5–0.7; p < 0.001).
We also observed a higher risk for sICH among patients with low ASPECTS undergoing EVT. This could be due to reperfusion injury with the sudden restoration of blood flow in a large infarct area, which subsequently leads to blood extravasation around the ischemic tissue. It could also be due to other individual factors that can mediate higher sICH risk (e.g. poor status at admission, older age, presence of edema, worse collateral status).25,26 Despite the beneficial treatment effect of EVT, the prognosis of patients with low ASPECTS remains overall poor. Two thirds of patients with low ASPECTS were treated with EVT; however, only one-third achieved independent ambulation. This implies that most patients will remain disabled despite receiving treatment. Therefore, selection criteria for EVT in this subgroup of stroke patients should not be based purely on ASPECTS. ASPECTS are based on anatomical structures and this results in unequal coverage of brain tissue by individual ASPECT regions. 27 Further, this leads to disparity in weighing different brain regions without consideration on their eloquence. 27 ASPECTS should be considered only as a part of a broader diagnostic approach alongside other imaging and clinical characteristics that are used for EVT selection. This could maximize benefits over general treatment-related risks.
Limitations
The retrospective study design limits the generalization of our results. Patients undergoing EVT were in general younger, had better pre-stroke independence, fewer comorbidities and were more likely to receive intravenous thrombolysis. However, this selection bias is reflective of real-world management of large-core patients before RCT data were available. Even after adjustment for these confounders, the relative treatment benefit of EVT was still preserved. We cannot exclude that our analyses were adjusted for all relevant confounders and it remains unclear if these adjustments appropriately mitigated the present selection bias. Multiple centers included in this study used different imaging tools, scanners and protocols which likely contributed to selection bias. This heterogeneity is reflective of real-world practice and differences between the centers were adjusted for in the analysis. The number of patients with ASPECTS 0–2 in our analyses was small; therefore we advise caution when extrapolating our results to these subgroups. 28 As our study was conducted prior to the results of multiple large core trials, changes in selection treatment paradigms may incur differing results as patients with larger ischemic core are considered for EVT.
Conclusion
In this multi-center study of real-world patients with large ischemic score who underwent endovascular therapy in the extended time window, there was a preserved relative treatment benefit of endovascular therapy comparable to patients with small ischemic core. Patients with large ischemic core in the extended time window might be a subpopulation of ischemic stroke patients who would benefit from endovascular therapy.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873241249406 for Safety and clinical outcomes of endovascular therapy versus medical management in late presentation of large ischemic stroke by Adnan Mujanovic, Daniel Strbian, Jelle Demeestere, João Pedro Marto, Volker Puetz, Raul G Nogueira, Mohamad Abdalkader, Simon Nagel, Jean Raymond, Marc Ribo, Patrik Michel, Shinichi Yoshimura, Osama O Zaidat, Simon Winzer, Santiago Ortega-Gutierrez, Sunil A Sheth, James E Siegler, Anne Dusart, Diogo C Haussen, Hilde Henon, Bettina L Serrallach, Mahmoud H Mohammaden, Markus A Möhlenbruch, Marta Olive-Gadea, Ajit S Puri, Nobuyuki Sakai, Piers Klein, Liisa Tomppo, Francois Caparros, João Nuno Ramos, Mouhammad Jumaa, Syed Zaidi, Tomas Dobrocky, Nicolas Martinez-Majander, Stefania Nannoni, Flavio Bellante, Aaron Rodriguez-Calienes, Sergio Salazar-Marioni, Pekka Virtanen, Daniel PO Kaiser, Rita Ventura, Jessica Jesser, Alicia C Castonguay, Muhammad M Qureshi, Hesham E Masoud, Milagros Galecio-Castillo, Manuel Requena, Riikka Lauha, Wei Hu, Eugene Lin, Zhongrong Miao, Daniel Roy, Hiroshi Yamagami, David J Seiffge, Davide Strambo, Peter A Ringleb, Robin Lemmens, Urs Fischer, Thanh N Nguyen and Johannes Kaesmacher in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873241249406 for Safety and clinical outcomes of endovascular therapy versus medical management in late presentation of large ischemic stroke by Adnan Mujanovic, Daniel Strbian, Jelle Demeestere, João Pedro Marto, Volker Puetz, Raul G Nogueira, Mohamad Abdalkader, Simon Nagel, Jean Raymond, Marc Ribo, Patrik Michel, Shinichi Yoshimura, Osama O Zaidat, Simon Winzer, Santiago Ortega-Gutierrez, Sunil A Sheth, James E Siegler, Anne Dusart, Diogo C Haussen, Hilde Henon, Bettina L Serrallach, Mahmoud H Mohammaden, Markus A Möhlenbruch, Marta Olive-Gadea, Ajit S Puri, Nobuyuki Sakai, Piers Klein, Liisa Tomppo, Francois Caparros, João Nuno Ramos, Mouhammad Jumaa, Syed Zaidi, Tomas Dobrocky, Nicolas Martinez-Majander, Stefania Nannoni, Flavio Bellante, Aaron Rodriguez-Calienes, Sergio Salazar-Marioni, Pekka Virtanen, Daniel PO Kaiser, Rita Ventura, Jessica Jesser, Alicia C Castonguay, Muhammad M Qureshi, Hesham E Masoud, Milagros Galecio-Castillo, Manuel Requena, Riikka Lauha, Wei Hu, Eugene Lin, Zhongrong Miao, Daniel Roy, Hiroshi Yamagami, David J Seiffge, Davide Strambo, Peter A Ringleb, Robin Lemmens, Urs Fischer, Thanh N Nguyen and Johannes Kaesmacher in European Stroke Journal
Acknowledgments
We would like to thank Oscar Bolanos of Medtronic.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: U.Fischer reported research support of the Swiss National Science Foundation and the Swiss Heart Foundation; research grants from Medtronic (BEYOND SWIFT, SWIFT DIRECT) and from Stryker, Rapid medical, Penumbra and Phenox (DISTAL); consultancies for Medtronic, Stryker, and CSL Behring; participation in an advisory board for Alexion/Portola, Boehringer Ingelheim, Biogen and Acthera; member of a clinical event committee (CEC) of the COATING study (Phenox) and member of the data and safety monitoring committee (DSMB) of the TITAN, LATE_MT and IN EXTREMIS trials; presidency of the Swiss Neurological Society. All fees are paid to institutions. T.Dobrocky reported MicroVention consultancy. H.Henon reported grants from SANOFI-AVENTIS U.S. LLC. D.Haussen reported consultancy for Vesalio, Cerenovus, Stryker, Brainomix, Poseydon Medical, and Chiesi USA; DSMB from Jacobs Institute; stock options in Viz AI. J.Kaesmacher reported grants from Swiss Academy of Medical Sciences/Bangerter Foundation, Swiss Stroke Society, Clinical Trials Unit Bern. J. Siegler reported grants from Medtronic and Philips. D.Kaiser reported grants from Joachim Herz Foundation. N.Martinez-Majander reported grants from Finnish Medical Foundation. J.P.Marto reported consulting and speaker fees from Amicus Therapeutics and Boehringer Ingelheim. P.Michel reported grants from University of Lausanne, Swiss National Science Foundation. M.Möhlenbruch reported grants from Medtronic, Stryker, MicroVention. S.Nagel reported consultancy for Brainomix; speaker Boehringer Ingelheim, Pfizer. T.Nguyen discloses research support from Medtronic to her institution; advisory board Idorsia, Brainomix, ArunaBio; Associate Editor of Stroke. R.Nogueira reported consultancy for Biogen, Brainomix, Corindus, Cerenovus, Stryker, Medtronic, Ceretrieve, Anaconda, Biomed, Vesalio, Imperative Care, NeuroVasc Technologies, Viz AI, Genentech, Prolong Pharmaceuticals, Perfuze, Phenox, RapidPulse; stock options Viz AI, Vesalio, Perfuze, Corindus, Brainomix, Ceretrieve; grants from Cerenovus, Stryker. V.Puetz reported lecturer fee for Daiichi Sankyo. M.Ribo reported consultancy for Medtronic, MiniMed, Cerenovus, AptaTargets, Stryker, Philips; stock holdings in Methinks, Nora, Anaconda Biomed. P.Ringleb reported travel support from Bayer, Bristol Myers Squibb; consultancy for Daiichi Sankyo Company, Boehringer Ingelheim. S.Sheth reported consultancy for Imperative Care, Viz AI, Penumbra, Motif Neurosciences; NIH grant. S. Yoshimura reported research grants from Medico’s Hirata, Medtronic, and Terumo; and lecturer fees from Medtronic, Kaneka, Stryker, Daiichi Sankyo, Bristol-Meyers Squibb, and Johnson & Johnson. Sakai reported a research grant from Japan Lifeline, Kaneka, Medtronic, Terumo and TG Medical; lecturer’s fees from Asahi-Intec, Kaneka, Medtronic, Stryker and Terumo; advisory boards for Johnson&Johnson, Medtronic and Terumo. H.Yamagami reported research grants from Bristol-Myers Squibb; lecturer’s fees from Stryker, Medtronic, J&J, Bayer, Daiichi Sankyo, Bristol-Myers Squibb, Otuska Pharmaceutical; advisory board for Daiichi Sankyo. D. Strbian reported research grant from the governmental educational funding (Finland), hospital research funds (HUS), unrestricted educational grant (Boehringer Ingelheim), whereas all funds were handled by hospital-based institution; partner of the consortium funding for the PROOF trial (EU), LVO check (EU), and electric impedance tomography project (Jane ja Aatos Erkon Säätiö); consultancies and advisory scientific board for Orion, Herantis Pharma, Boehringer Ingelheim, Portola, Alexion, AstraZeneca, Bristol-Meyers-Squibb, Janssen; member of the DSMB on the ENDOLOW trial. All other authors declare no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Society of Vascular and Interventional Neurology and Medtronic.
Ethical approval: This study received ethics committee approval from all enrolling sites.
Informed consent: Written informed consent was waived because of the retrospective nature of this study and because the research was considered no more than minimal risk.
Guarantor: T.N. Nguyen and J. Kaesmacher.
Contributorship: A. Mujanovic contributed to conception and design, analysis and interpretation of data, and writing of the original draft. D. Strbian contributed to conception and design, analysis and interpretation of data. J. Demeestere contributed to conception and design, analysis of data, and writing of the original draft. J.P. Marto contributed to design and critical revision of the manuscript for important intellectual content. U. Fischer contributed to conception and design, critical revision of the publication for important intellectual content. T.N. Nguyen contributed to conception and design, critical revision of the publication for important intellectual content, and supervision. J. Kaesmacher contributed to conception and design, critical revision of the publication for important intellectual content, and supervision. All other authors contributed substantially to data acquisition, interpretation, and critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript.
ORCID iD: Adnan Mujanovic  https://orcid.org/0000-0002-6839-7134
https://orcid.org/0000-0002-6839-7134
Daniel Strbian  https://orcid.org/0000-0001-9095-2344
https://orcid.org/0000-0001-9095-2344
Jelle Demeestere  https://orcid.org/0000-0001-8186-0237
https://orcid.org/0000-0001-8186-0237
João Pedro Marto  https://orcid.org/0000-0003-2277-5950
https://orcid.org/0000-0003-2277-5950
Markus A Möhlenbruch  https://orcid.org/0000-0002-5075-704X
https://orcid.org/0000-0002-5075-704X
Nobuyuki Sakai  https://orcid.org/0000-0002-3289-1210
https://orcid.org/0000-0002-3289-1210
Piers Klein  https://orcid.org/0000-0001-7468-137X
https://orcid.org/0000-0001-7468-137X
Liisa Tomppo  https://orcid.org/0000-0002-9369-5846
https://orcid.org/0000-0002-9369-5846
João Nuno Ramos  https://orcid.org/0000-0001-9678-3422
https://orcid.org/0000-0001-9678-3422
Nicolas Martinez-Majander  https://orcid.org/0000-0001-8489-7051
https://orcid.org/0000-0001-8489-7051
Flavio Bellante  https://orcid.org/0000-0002-8718-9250
https://orcid.org/0000-0002-8718-9250
Sergio Salazar-Marioni  https://orcid.org/0000-0002-2722-7542
https://orcid.org/0000-0002-2722-7542
Pekka Virtanen  https://orcid.org/0000-0002-9547-4940
https://orcid.org/0000-0002-9547-4940
Jessica Jesser  https://orcid.org/0000-0002-1236-8828
https://orcid.org/0000-0002-1236-8828
Manuel Requena  https://orcid.org/0000-0002-5671-6484
https://orcid.org/0000-0002-5671-6484
David J Seiffge  https://orcid.org/0000-0003-3890-3849
https://orcid.org/0000-0003-3890-3849
Thanh N Nguyen  https://orcid.org/0000-0002-2810-1685
https://orcid.org/0000-0002-2810-1685
Supplemental material: Supplemental material for this article is available online.
References
- 1. Powers W, Rabinstein A, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke Association. 2019; 50: e344–e418. DOI: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 2. Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) - European Society for minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J 2019; 4: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nguyen TN, Castonguay AC, Siegler JE, et al. Mechanical thrombectomy in the late presentation of anterior circulation large vessel occlusion stroke: a guideline from the Society of Vascular and Interventional Neurology guidelines and practice standards committee. Stroke Vasc Interv Neurol 2023; 3: e000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 5. Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. New Engl J Med 2022; 386: 1303–1313. [DOI] [PubMed] [Google Scholar]
- 6. Huo X, Ma G, Tong X, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. New Engl J Med 2023; 388: 1272–1283. [DOI] [PubMed] [Google Scholar]
- 7. Sarraj A, Hassan AE, Abraham MG. Trial of endovascular thrombectomy for large ischemic strokes. New Engl J Med 2024; 390: 388–1271. [DOI] [PubMed] [Google Scholar]
- 8. Zaidat OO, Kasab SA, Sheth S, et al. TESLA trial: rationale, protocol, and design. Stroke Vasc Interv Neurol 2023; 3: 1–9. [Google Scholar]
- 9. Bendszus M, Fiehler J, Subtil F, et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: multicentre, open-label, randomised trial. Lancet 2023; 6736: 1–11. [DOI] [PubMed] [Google Scholar]
- 10. Costalat V, Lapergue B, Albucher J, et al. Evaluation of acute mechanical revascularization in large stroke (ASPECTS ⩽5) and large vessel occlusion within 7 h of last-seen-well: the LASTE multicenter, randomized, clinical trial protocol. Int J Stroke 2024; 19: 114–119. [DOI] [PubMed] [Google Scholar]
- 11. Yaghi S, Siegler JE, Nguyen TN. Pitfalls of randomized controlled trials in stroke: how can we do better? Stroke Vasc Interv Neurol 2023; 3: 1–5. [Google Scholar]
- 12. Broocks G, Hanning U, Bechstein M, et al. Association of thrombectomy with functional outcome for patients with ischemic stroke who presented in the extended time window with extensive signs of infarction. JAMA Netw Open 2022; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Almallouhi E, Al Kasab S, Hubbard Z, et al. Outcomes of mechanical thrombectomy for patients with stroke presenting with low Alberta Stroke Program Early Computed Tomography Score in the early and extended window. JAMA Netw Open 2021; 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zaidat OO, Liebeskind DS, Jadhav AP, et al. Impact of age and Alberta stroke program early computed tomography score 0 to 5 on mechanical thrombectomy outcomes: analysis from the STRATIS registry. Stroke 2021; 52: 2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaesmacher J, Chaloulos-Iakovidis P, Panos L, et al. Mechanical thrombectomy in ischemic stroke patients with Alberta Stroke Program Early Computed Tomography Score 0-5. Stroke 2019; 50: 880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Virtanen P, Tomppo L, Martinez-Majander N, et al. Thrombectomy in acute ischemic stroke in the extended time window: real-life experience in a high-volume center. J Stroke Cerebrovasc Dis 2022; 31: 1–7. [DOI] [PubMed] [Google Scholar]
- 17. Li Q, Abdalkader M, Siegler JE, et al. Mechanical thrombectomy for large ischemic stroke: a systematic review and meta-analysis. Neurology 2023; 101: E922–E932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen TN, Abdalkader M, Nagel S, et al. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol 2022; 79: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Starikova N, Räty S, Strbian D, et al. Endovascular thrombectomy for anterior circulation large vessel occlusion stroke: an evolution of trials. Semin Neurol 2023; 43: 397–407. [DOI] [PubMed] [Google Scholar]
- 20. Thomalla G. One treatment to heal them all: thrombectomy also benefits stroke with large ischemic core. Clin Neuroradiol 2023; 33: 267–269. [DOI] [PubMed] [Google Scholar]
- 21. Sarraj A, Hassan A, Abraham M, et al. Endovascular thrombectomy for large ischemic stroke across ischemic injury and penumbra profiles. JAMA 2024; 331: 750–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarraj A. Mechanical thrombectomy for large brain infarction (MAGNA collaboration). In: European stroke organisation conference (ESOC), München, Germany. [Google Scholar]
- 23. Pan Y, Huo X, Jin A, et al. Cost-effectiveness of endovascular therapy for acute ischemic stroke with large infarct in China. J Neurointerv Surg 2024; 16: 453–458. [DOI] [PubMed] [Google Scholar]
- 24. Moreu M, Scarica R, Pérez-García C, et al. Mechanical thrombectomy is cost-effective versus medical management alone around Europe in patients with low ASPECTS. J Neurointerv Surg 2023; 15: 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Broocks G, Hanning U, Flottmann F, et al. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain 2019; 142: 1399–1407. [DOI] [PubMed] [Google Scholar]
- 26. Broocks G, Kniep H, Schramm P, et al. Patients with low Alberta stroke program early CT score (ASPECTS) but good collaterals benefit from endovascular recanalization. J Neurointerv Surg 2020; 12: 747–752. [DOI] [PubMed] [Google Scholar]
- 27. Schröder J, Thomalla G. A critical review of Alberta stroke program early CT score for evaluation of acute stroke imaging. Front Neurol 2016; 7: 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun D, Guo X, Nguyen T, et al. Alberta stroke program early computed tomography score, infarct core volume, and endovascular therapy outcomes in patients with large infarct: a secondary analysis of the ANGEL-ASPECT Trial. JAMA Neurol 2024; 81: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873241249406 for Safety and clinical outcomes of endovascular therapy versus medical management in late presentation of large ischemic stroke by Adnan Mujanovic, Daniel Strbian, Jelle Demeestere, João Pedro Marto, Volker Puetz, Raul G Nogueira, Mohamad Abdalkader, Simon Nagel, Jean Raymond, Marc Ribo, Patrik Michel, Shinichi Yoshimura, Osama O Zaidat, Simon Winzer, Santiago Ortega-Gutierrez, Sunil A Sheth, James E Siegler, Anne Dusart, Diogo C Haussen, Hilde Henon, Bettina L Serrallach, Mahmoud H Mohammaden, Markus A Möhlenbruch, Marta Olive-Gadea, Ajit S Puri, Nobuyuki Sakai, Piers Klein, Liisa Tomppo, Francois Caparros, João Nuno Ramos, Mouhammad Jumaa, Syed Zaidi, Tomas Dobrocky, Nicolas Martinez-Majander, Stefania Nannoni, Flavio Bellante, Aaron Rodriguez-Calienes, Sergio Salazar-Marioni, Pekka Virtanen, Daniel PO Kaiser, Rita Ventura, Jessica Jesser, Alicia C Castonguay, Muhammad M Qureshi, Hesham E Masoud, Milagros Galecio-Castillo, Manuel Requena, Riikka Lauha, Wei Hu, Eugene Lin, Zhongrong Miao, Daniel Roy, Hiroshi Yamagami, David J Seiffge, Davide Strambo, Peter A Ringleb, Robin Lemmens, Urs Fischer, Thanh N Nguyen and Johannes Kaesmacher in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873241249406 for Safety and clinical outcomes of endovascular therapy versus medical management in late presentation of large ischemic stroke by Adnan Mujanovic, Daniel Strbian, Jelle Demeestere, João Pedro Marto, Volker Puetz, Raul G Nogueira, Mohamad Abdalkader, Simon Nagel, Jean Raymond, Marc Ribo, Patrik Michel, Shinichi Yoshimura, Osama O Zaidat, Simon Winzer, Santiago Ortega-Gutierrez, Sunil A Sheth, James E Siegler, Anne Dusart, Diogo C Haussen, Hilde Henon, Bettina L Serrallach, Mahmoud H Mohammaden, Markus A Möhlenbruch, Marta Olive-Gadea, Ajit S Puri, Nobuyuki Sakai, Piers Klein, Liisa Tomppo, Francois Caparros, João Nuno Ramos, Mouhammad Jumaa, Syed Zaidi, Tomas Dobrocky, Nicolas Martinez-Majander, Stefania Nannoni, Flavio Bellante, Aaron Rodriguez-Calienes, Sergio Salazar-Marioni, Pekka Virtanen, Daniel PO Kaiser, Rita Ventura, Jessica Jesser, Alicia C Castonguay, Muhammad M Qureshi, Hesham E Masoud, Milagros Galecio-Castillo, Manuel Requena, Riikka Lauha, Wei Hu, Eugene Lin, Zhongrong Miao, Daniel Roy, Hiroshi Yamagami, David J Seiffge, Davide Strambo, Peter A Ringleb, Robin Lemmens, Urs Fischer, Thanh N Nguyen and Johannes Kaesmacher in European Stroke Journal